Introduction
Oral squamous-cell carcinoma (OSCC) is a major cause
of morbidity and mortality worldwide, accounting for 275,000 new
cases and more than 120,000 deaths annually (1). Despite therapeutic and diagnostic
advances, patients are often diagnosed at advanced stages, and
mortality rates are still increasing (2). This highlights the need for continued
efforts to discover suitable biomarkers for early disease diagnosis
and for the improved understanding of disease pathogenesis as a
first step towards improving treatment. Considering these issues,
it is imperative to study OSCC at the genetic level and to
characterize the genetic changes responsible for carcinogenesis and
tumour behavior.
Since cancer has the specific potential of rapid and
unlimited growth, oxidative stress, which is characterized by an
imbalance between the presence of relatively high levels of toxic
reactive species principally consisting of reactive oxygen species
(ROS) and antioxidative defense mechanisms, is a common feature in
a wide range of solid tumors, including OSCC (3,4).
Some studies have found that cells undergoing neoplastic
transformation show marked changes in metabolism, resulting in an
increase in oxidative stress in cancerous cells (5,6).
Enhanced oxidizing status in transforming cells is thought to
induce DNA damage, leading to genetic lesions that initiate
tumorigenicity and facilitate immortalization, enhance cell
proliferation and sustain subsequent local and systemic tumor
progression (7–9). Additionally, recent studies revealed
that oxidative stress is not merely toxic due to ROS production by
metabolism, but also serves an important regulatory role in
numerous oncogenic signaling pathways in cancerous cells (10,11).
Superoxide dismutase (SOD) is generally regarded as
one of the first lines of antioxidant defense in aerobic cells
(12). This enzyme is highly
efficient in protecting cells and tissues against oxidative stress
based on the potency of the cellular defense mechanism against ROS,
including true free radicals, superoxide anion
(O2−) and hydroxyl radical (OH−),
as well as non-radical compounds, such as hydrogen peroxide
(H2O2) (13). Substantial studies have reported
that the effects of SOD in human malignancies are associated with
tumor growth and drug resistance in vitro and in vivo
(14,15). To date, three distinct isoforms of
SOD and their distribution have been characterized in mammals
(16). Of these, extracellular
superoxide dismutase (EC-SOD) is the only isoform that is mainly
expressed in the extracellular space via binding with heparin
sulfate proteoglycans (17). Since
the extracellular space is known to have many potential sources of
ROS and to be a relatively more oxidized state than the interior of
cells, dysregulation of extracellular oxidant-moderating proteins,
including EC-SOD, is considered more important in cancer (18–20).
However, in contrast to the intracellular SODs, little is known
regarding EC-SOD in human tumors, including OSCC. The purpose of
the present study was therefore to determine EC-SOD protein
expression in a series of human primary OSCCs and oral premalignant
lesions (OPLs), and to correlate the expression with its clinical
relevance in patients with OSCC.
Materials and methods
Tissue specimens
Fifty-eight pairs of primary OSCC samples and
corresponding normal oral epithelium tissues were obtained at the
time of surgery, performed at Chiba University Hospital between
1998 and 2007. All patients provided their informed consent
according to the study protocol, which was reviewed and approved by
the institutional review board of Chiba University before any
procedures were performed. In addition, 20 samples from cases of
advanced OPLs pathologically diagnosed as leukoplakia with
epithelial dysplasia, i.e., mild (n=2), moderate (n=11) and severe
(n=7), in a high-risk oral site, such as the ventral-lateral tongue
or gingiva, were obtained as described above.
Histopathological diagnosis of each tumor specimen
was performed according to the International Histological
Classification of Tumors by the Department of Pathology, Chiba
University Hospital. Clinicopathological staging was determined by
the TNM classification of the International Union against
Cancer.
Immunohistochemistry
Immunohistochemical (IHC) staining was carried out
on 4-μm sections of paraffin-embedded specimens. The
clinicopathological characteristics of the OSCCs in this series are
summarized in Table I. Briefly,
after deparaffinization and hydration, the slides were pre-treated
in 10 mM sodium citrate buffer (pH 6.0) in a microwave oven for 5
min at 95°C. Endogenous peroxidase activity was quenched by a
30-min incubation in a mixture of 0.3% hydrogen peroxide solution
in 100% methanol. After being washed with PBS buffer, the sections
were incubated with primary antibody affinity-purified goat
antihuman EC-SOD polyclonal antibody (1:100 dilution; Santa Cruz
Biotechnology) at room temperature in a moist chamber for 2 h.
After being washed with PBS buffer, the slides were treated with
peroxidase-labeled secondary antibody for 1 h followed by color
development in 3,3′-diaminobenzidine tetrahydrochloride (Dako Japan
Inc.). Finally, the slides were lightly counterstained with
hematoxylin. As negative controls, the slides were incubated with
PBS instead of primary antibodies. To quantitate the state of
EC-SOD protein expression in these of components, we used
previously described Histo (H)-score systems (21,22).
In brief, the mean percentage of the epithelial cells that showed a
persistent EC-SOD signal was respectively determined in at least
five distinct fields in each section at a magnification of x400.
The intensity of the immunoreaction was scored as follows:
1+, weak; 2+, moderate; 3+,
intense. Three target cell types, normal, pre-malignant and
malignant epithelial cells, were identified for scoring. The
percentage of EC-SOD-positive cells and the staining intensity were
then multiplied to produce an EC-SOD H-score. Cases with a
cytoplasmic EC-SOD H-score exceeding 50.15 (maximum score of normal
tissues) were considered positive. Specimens were evaluated by two
independent pathologists, neither of whom had knowledge of patient
clinical status.
 | Table I.Correlation between cytoplasmic EC-SOD
protein expression and clinical calssification in OSCCs (n=58) and
OPLs (n=20). |
Table I.
Correlation between cytoplasmic EC-SOD
protein expression and clinical calssification in OSCCs (n=58) and
OPLs (n=20).
| Clinical
classification | Total | Result of
immunostaining: no. of patients (%)
| p-valuea |
|---|
| | Cytoplasmic EC-SOD
(−) | Cytoplasmic EC-SOD
(+) | |
|---|
| Age at surgery
(years) | | | | |
| <60 | 16 | 8 (50) | 8 (50) | |
| 60–69 | 20 | 9 (45) | 11 (55) | 0.9471 |
| ≥70 | 22 | 11 (50) | 11 (50) | |
| Gender | | | | |
| Male | 44 | 19 (43) | 25 (57) | 0.2244 |
| Female | 14 | 9 (64) | 5 (36) | |
| T-primary tumor | | | | |
| T1 | 5 | 4 (80) | 1 (20) | |
| T2 | 22 | 10 (45) | 12 (55) | 0.5393 |
| T3 | 14 | 7 (50) | 7 (50) | |
| T4 | 17 | 7 (41) | 10 (59) | |
| N-regional lymph
node | | | | |
| N (−) | 31 | 19 (61) | 12 (39) | 0.0397a |
| N (+) | 27 | 9 (33) | 18 (67) | |
| Stage | | | | |
| I | 5 | 4 (80) | 1 (20) | |
| II | 10 | 4 (40) | 6 (60) | 0.1896 |
| III | 12 | 8 (67) | 4 (33) | |
| IV | 31 | 12 (39) | 19 (61) | |
| Histopathological
type | | | | |
| Well
differentiated | 38 | 19 (50) | 19 (50) | 0.7866 |
| Moderately/poorly
differentiated | 20 | 9 (45) | 11 (55) | |
| Tumor site | | | | |
| Tongue | 32 | 16 (50) | 16 (50) | |
| Gingiva | 16 | 7 (44) | 9 (56) | 0.4375 |
| Oral floor | 3 | 2 (67) | 1 (33) | |
| Buccal
mucosa | 6 | 2 (33) | 4 (67) | |
| Lip | 1 | 1 (100) | 0 (0) | |
| Leukoplakia | | | | |
| Mild
dysplasia | 2 | 1 (50) | 1 (50) | |
| Moderate
dysplasia | 11 | 5 (45) | 6 (55) | 0.3981 |
| Severe
dysplasia | 7 | 1 (14) | 6 (86) | |
Statistical analysis
Statistical analysis of the OSCC tissue and
corresponding normal tissue was performed using Wilcoxon’s signed
rank sum test. The comparison OPLs and other tissue including
normal samples and OSCCs or the Mn-SOD-positive and Mn-SOD-negative
cases of OSCC were analyzed using the Mann-Whitney U-test.
Correlations between Mn-SOD H-score and clinicopathological
features were evaluated by Fisher’s exact test. Data are expressed
as the median values. p<0.05 was considered statistically
significant.
Results
EC-SOD expression in OSCCs and OPLs
IHC staining was performed using a series of OSCC
specimens, including 58 OSCCs with corresponding normal tissues and
20 OPLs that were histopathologically diagnosed as leukoplakia with
epithelial dysplasia. Considering that the evidence indicated that
the malignant transformation rate of oral leukoplakia with
dysplasia was apparently higher than that of oral leukoplakia
without dysplasia (23), patients
with advanced OPLs, defined as leukoplakia exhibiting epithelial
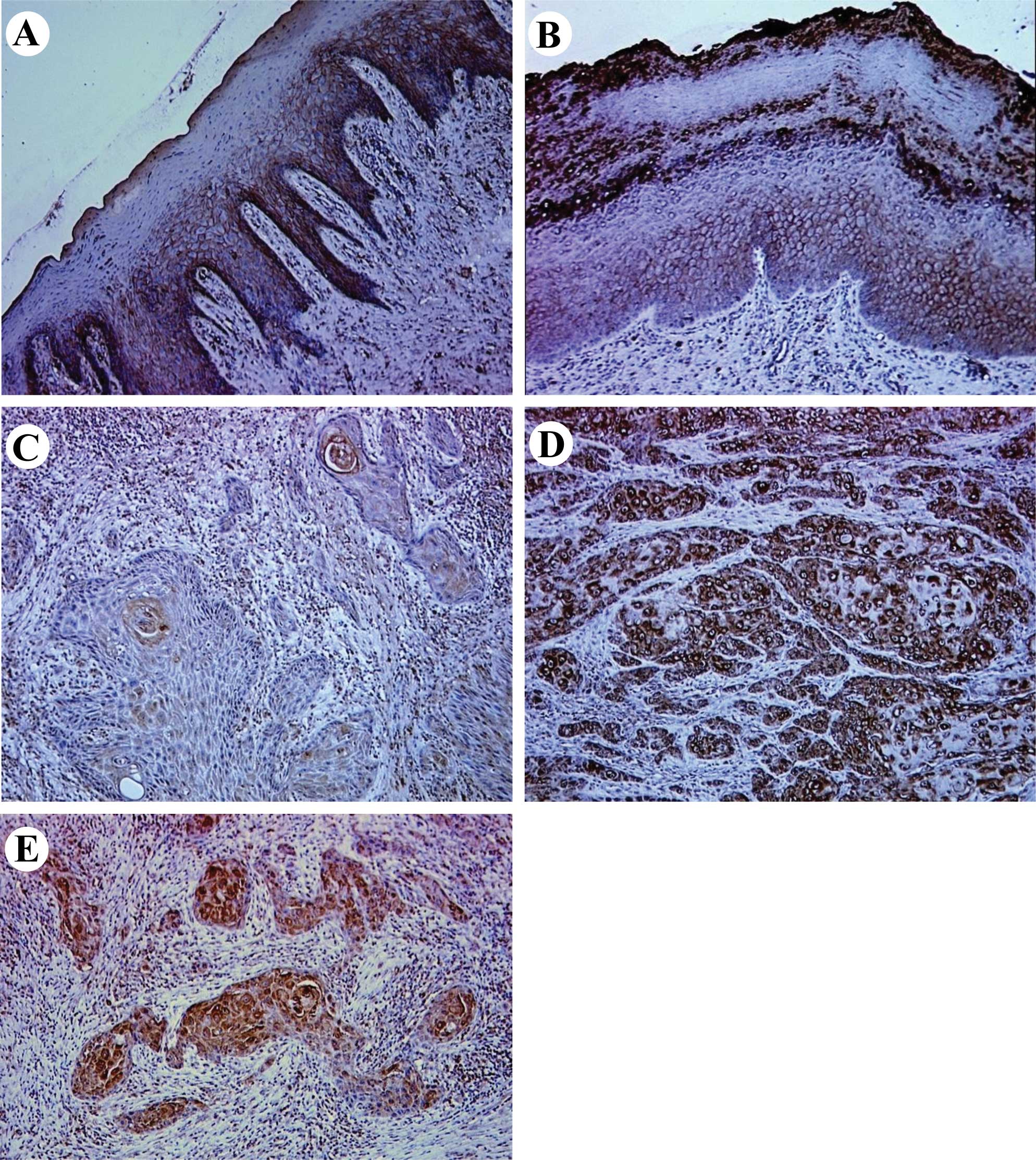
dysplasia, were eligible for the trial. Representative results for
EC-SOD protein expression in normal oral tissues, OPLs and primary
OSCCs are shown in Fig. 1.
Normal oral mucosal specimens exhibited consistently
strong EC-SOD immunoreaction on the plasma membrane of cells. In
OPLs, a loss of plasma membranous EC-SOD immunostaining was
observed in some cases, but many of the specimens revealed positive
immunoreactivity for EC-SOD on the plasma membrane of cells.
Notably, positive staining of the protein was also detected on the
cytoplasm of OPL cells in 65% of the specimens examined. Regarding
OSCCs, plasma membranous EC-SOD immunoreaction was largely lost in
the specimens examined (98%), whereas strong immunoreactivity of
the protein was observed on the cytoplasm of cancerous cells in 52%
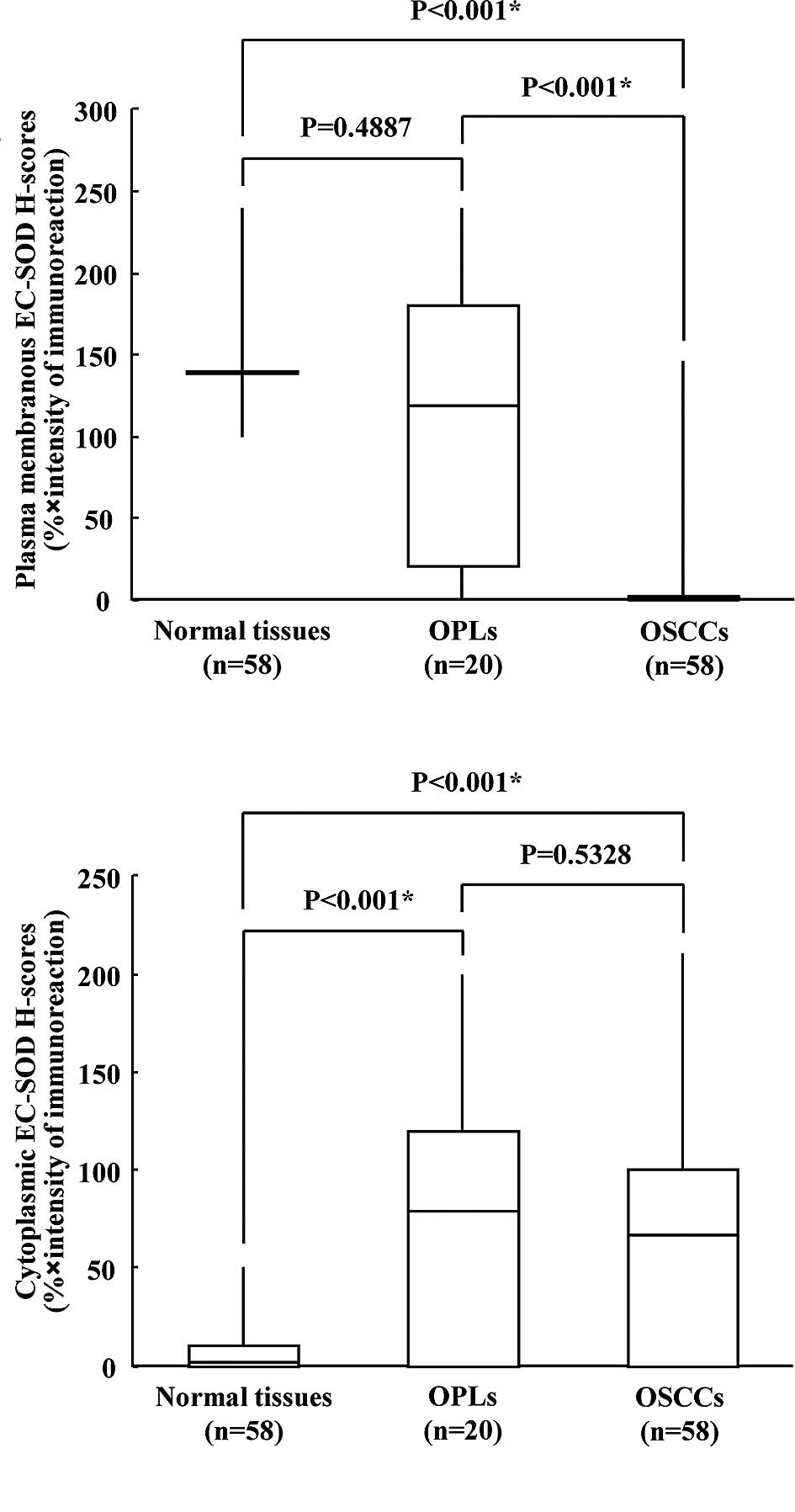
of the specimens (cytoplasmic EC-SOD-positive). According to the
H-scores, the expression levels of plasma membrane EC-SOD were
significantly reduced in OSCCs compared to their normal
counterparts (Wilcoxon’s signed rank sum test, p<0.001; Fig. 2A), whereas cytoplasmic EC-SOD
expression levels were considerably higher not only in OSCCs
(Wilcoxon’s signed rank sum test, p<0.001; Fig. 2B), but also in OPLs (Mann-Whitney
U-test, p<0.001) compared to normal tissues.
Correlation of EC-SOD expression with
clinicopathological parameters
In the present study, 52% of the OSCC patients were
characterized as EC-SOD-positive in the cytoplasmic compartments of
cancerous cells. The correlation between the clinicopathological
characteristics of the patients with OSCC and the status of
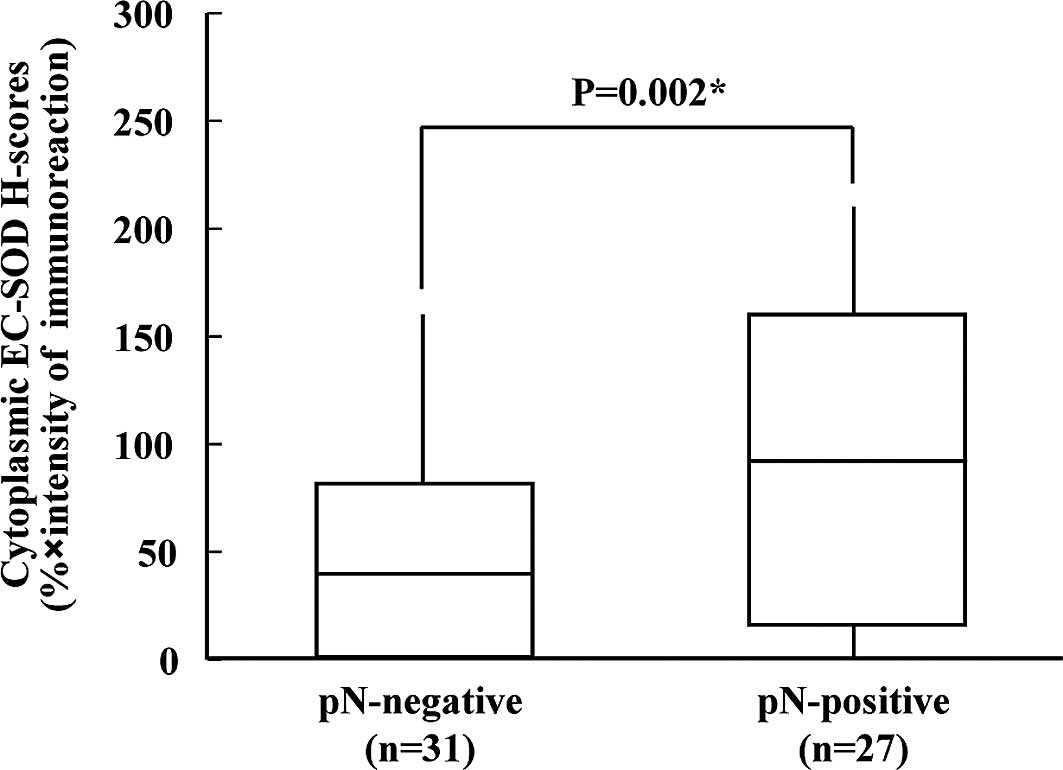
cytoplasmic EC-SOD expression is summarized in Table I. Positive EC-SOD expression of
cytoplasm was significantly associated with regional lymph node
metastasis (Fisher’s exact test, p=0.0397). The cytoplasmic EC-SOD
H-scores for tumors with lymph node metastasis and for those
without ranged from 0 to 210.55 (median 100.03) and from 0 to
160.02 (median 30.47), respectively. Cytoplasmic EC-SOD expression
levels were significantly higher in patients with OSCC who were
pN-positive, compared to those who were pN-negative (Mann-Whitney
U-test, p=0.002; Fig. 3).
Disscussion
In the present study, we initially characterized
EC-SOD protein expression in cancerous and pre-cancerous lesions of
the oral cavity using IHC analysis. Of particular interest is that,
although normal oral epithelium tissues consistently exhibited
positive expression of EC-SOD on the plasma membrane of cells in
all specimens, plasma membranous EC-SOD expression was
significantly down-regulated in almost all the OSCC specimens
examined (98%), indicating that EC-SOD may be critical for normal
functioning, and that the loss of the protein resultsan increased
risk of cancer development in cells. This notion is supported by a
number of previous studies in vitro and in vivo. Kim
et al demonstrated that EC-SOD transgenic mice showed
reduced incidence of tumors induced by
dimethylbenzanthracene/12-O-tetradecanoylphorbol-13-acetate
compared to controls (24). EC-SOD
overexpression significantly inhibited the growth of B16 melanomas
with high growth potential and decreased the metastatic behavior of
lung cancer cells in mice (25).
Moreover, a recent study revealed that EC-SOD inhibited the
invasive capacity of human prostate cancer cells. This was
correlated with reduced metalloproteinase activities (26), suggesting that EC-SOD may have the
potential to suppress aggressive tumor behavior.
Conversely, our study also identified altered
localization of EC-SOD protein at a high level of expression on the
cytoplasm of cancerous cells in 52% of the OSCC specimens examined
(p<0.001). Additionally, cytoplasmic EC-SOD overexpression was
associated with an aggressive phenotype of OSCC, including lymph
node metastasis (p=0.0397). The exact cause of the heterogeneous
distribution of EC-SOD in cancerous cells is not as yet clear;
however, the most likely cause is the polymorphic variant of the
EC-SOD gene, which involves a single nucleotide substitution (G to
C) and results in an amino acid change from arginine (Arg) to
glycine (Gly) at 213. This amino acid substitution decreases the
anchoring of EC-SOD to negatively charged polysaccharides,
including heparin (27).
Considering that EC-SOD localizes in extracellular spaces via
binding with heparin sulfate proteoglycans, this could be linked to
the EC-SOD heterogeneous localization in OSCC cells.
Subcellular localization of a protein often provides
important clues to its function (28), and the transition from the benign
state to the fully malignant one may involve a change in
subcellular presence. Some insight into the cytoplasmic mechanism
of EC-SOD associated with tumor aggressiveness can be gained from
the recent findings that manganese SOD, an intracellular SOD
isoform, enhances the invasive and migratory activity of tumor
cells though H2O2 production (29), and that overexpression of the
protein has been identified in various cancers together with an
association with poor prognosis (30,31).
Recently, we also found that manganese SOD up-regulation was
correlated with a high incidence of lymph node metastasis in OSCC
patients (32). Together, it is
possible that EC-SOD may function independently in cellular
components in cancer cells, acting as a tumor-suppressor on the
plasma membrane, but as a tumor-activator in the cytoplasm. Changes
in cytoplasmic EC-SOD protein expression were even detected in the
OPLs examined (65%), suggesting that the dysregulation of the
protein expression is a frequent and early event during oral
carcinogenesis.
In conclusion, our study provides the first
documentation that EC-SOD protein expression and subcellular
distribution is altered in both OPLs and OSCCs, and that positive
cytoplasmic EC-SOD expression is associated with an aggressive
tumor phenotype in OSCCs. The molecular mechanisms linked to the
aberrant expression of EC-SOD in OSCC cells remain unclear, and
further studies are warranted to elucidate the molecular
alterations involved in EC-SOD dysregulation in oral
carcinogenesis.
Acknowledgements
This study was partly supported by a
Grant-in-Aid for Scientific Research (No. 20791492) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan, and the Global COE Program (Global Center for Education and
Research in Immune System Regulation and Treatment), MEXT,
Japan.
References
|
1.
|
Ferlay J, Bray P, Pisani P and Parkin DM:
GLOBOCAN 2002: Cancer Incidence, Mortalityand Prevalence Worldwide.
IARC Cancer Base No. 5, version 2.0. IARC Press; Lyon: 2004
|
|
2.
|
La Vecchia C, Lucchini F, Negri E and Levi
F: Trends in oral cancer mortality in Europe. Oral Oncol.
40:433–439. 2004.
|
|
3.
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Klaunig JE and Kamendulis LM: The role of
oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol.
44:239–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Oberley LW, Oberley TD and Buettner GR:
Cell division in normal and transformed cells: the possible role of
superoxide and hydrogen peroxide. Med Hypoth. 7:21–42. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Spitz DS, Sim JE, Ridnour LA, Galoforo SS
and Lee YJ: Glucose deprivation-induced oxidative stress in human
tumor cells: a fundamental defect in metabolism? Ann NY Acad Sci.
899:349–362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Valko M, Izakovic M, Mazur M, Rhodes CJ
and Telser J: Role of oxygen radicals in DNA damage and cancer
incidence. Mol Cell Biochem. 266:37–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Franco R, Schoneveld O, Georgakilas AG and
Panayiotidis MI: Oxidative stress, DNA methylation and
carcinogenesis. Cancer Lett. 266:6–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gromadzińska J and Wasowicz W: The role of
reactive oxygen species in the development of malignancies. Int J
Occup Med Environ Health. 13:233–245. 2000.
|
|
10.
|
Kuznetsov AV, Smigelskaite J, Doblander C,
et al: Survival signaling by C-RAF: mitochondrial reactive oxygen
species and Ca2+ are critical targets. Mol Cell Biol. 28:2304–2313.
2008.PubMed/NCBI
|
|
11.
|
Cho HJ, Jeong HG, Lee JS, et al: Oncogenic
H-Ras enhances DNA repair through the Ras/phosphatidylinositol
3-kinase/Rac1 pathway in NIH3T3 cells. Evidence for association
with reactive oxygen species. J Biol Chem. 277:19358–19366. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Blokhina O, Virolainen E and Fagerstedt
KV: Antioxidants, oxidative damage and oxygen deprivation stress: a
review. Ann Bot. 91:179–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Liochev SI and Fridovich I: The effects of
superoxide dismutase on H2O2 formation. Free Radic Biol Med.
42:1465–1469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hileman EA, Achanta G and Huang P:
Superoxide dismutase: an emerging target for cancer therapeutics.
Expert Opin Ther Targets. 5:697–710. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kinnula VL and Crapo JD: Superoxide
dismutases in malignant cells and human tumors. Free Radic Biol
Med. 36:718–744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: a comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution
and expression. Free Radic Biol Med. 33:337–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sandström J, Karlsson K, Edlund T and
Marklund SL: Heparin-affinity patterns and composition of
extracellular superoxide dismutase in human plasma and tissues.
Biochem J. 294:853–857. 1993.PubMed/NCBI
|
|
18.
|
Moriarty-Craige SE and Jones DP:
Extracellular thiols and thiol/disulfide redox in metabolism. Annu
Rev Nutr. 24:481–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nakamura H, Masutani H and Yodoi J:
Extracellular thioredoxin and thioredoxin-binding protein 2 in
control of cancer. Semin Cancer Biol. 16:444–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rees MD, Kennett EC, Whitelock JM and
Davies MJ: Oxidative damage to extracellular matrix and its role in
human pathologies. Free Radic Biol Med. 44:1973–2001. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bilalovic N, Sandstad B, Golouh R, et al:
CD10 protein expression in tumor and stromal cells of malignant
melanoma is associated with tumor progression. Mod Pathol.
17:1251–1258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
McCarty KS Jr, Szabo E, Flowers JL, et al:
Use of monoclonal anti-estrogen receptor antibody in the
immunohistochemical evaluation of human tumors. Cancer Res.
46:4244–4248. 1986.PubMed/NCBI
|
|
23.
|
Amagasa T, Yamashiro M and Ishikawa H:
Oral leukoplakia related to malignant transformation. Oral Sci Int.
3:45–55. 2006. View Article : Google Scholar
|
|
24.
|
Kim SH, Kim MO, Gao P, et al:
Overexpression of extracellular superoxide dismutase (EC-SOD) in
mouse skin plays a protective role in DMBA/TPA-induced tumor
formation. Oncol Res. 15:333–341. 2005.PubMed/NCBI
|
|
25.
|
Wheeler MD, Smutney OM and Samulski RJ:
Secretion of extracellular superoxide dismutase from muscle
transduced with recombinant adenovirus inhibits the growth of B16
melanomas in mice. Mol Cancer Res. 1:871–881. 2003.PubMed/NCBI
|
|
26.
|
Chaiswing L, Zhong W, Cullen JJ, Oberley
LW and Oberley TD: Extracellular redox state regulates features
associated with prostate cancer cell invasion. Cancer Res.
68:5820–5826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sandström J, Nilsson P, Karlsson K and
Marklund SL: 10-fold increase in human plasma extracellular
superoxide dismutase content caused by a mutation in
heparin-binding domain. J Biol Chem. 269:19163–19166.
1994.PubMed/NCBI
|
|
28.
|
Horton P, Park KJ, Obayashi T, et al: WoLF
PSORT: protein localization predictor. Nucleic Acids Res.
35:585–587. 2007. View Article : Google Scholar
|
|
29.
|
Connor KM, Hempel N, Nelson KK, et al:
Manganese super-oxide dismutase enhances the invasive and migratory
activity of tumor cells. Cancer Res. 67:10260–10267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kim JJ, Chae SW, Hur GC, et al: Manganese
superoxide dismutase expression correlates with a poor prognosis in
gastric cancer. Pathobiology. 70:353–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Janssen AM, Bosman CB, Sier CF, et al:
Superoxide dismutases in relation to the overall survival of
colorectal cancer patients. Br J Cancer. 78:1051–1057. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yokoe H, Nomura H, Yamano Y, et al:
Characterization of intra-cellular superoxide dismutase alterations
in premalignant and malignant lesions of the oral cavity:
correlation with lymph node metastasis. J Cancer Res Clin Oncol.
135:1625–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|