Introduction
Concurrent chemoradiotherapy (CCRT) is the main
treatment for locally advanced cervical cancer (1–3).
Neoadjuvant chemotherapy (NAC) was widely employed until CCRT
became standard, and favorable results have been reported (4–6).
However, the efficacy of NAC has not been confirmed by some
researchers (7–11), therefore its value remains unclear.
Neoadjuvant intraarterial chemotherapy (IA-NAC) is another method
of delivering NAC as an alternative to systemic chemotherapy.
IA-NAC has been reported to achieve beneficial results that cannot
be obtained by systemic chemotherapy or CCRT (12–15),
but conclusive evidence is limited, and a standard IA-NAC regimen
has not been established. We have employed IA-NAC to treat advanced
cervical cancer since 1991 and have used three regimens over that
period. The results obtained with each of these regimens were
examined in the present study.
Materials and methods
Patients
Between January 1991 and April 2006, 55 patients
with stage IIB–IIIB primary cervical cancer were enrolled in the
study. All patients gave their written informed consent to
treatment. Eligibility criteria were as follows: age <75 years,
no distant metastasis, WHO performance status of 0–2, no previous
treatment and adequate renal, pulmonary, hepatic, bone marrow and
cardiac function. The mean patient age was 53.5 years (range
28–74). Table I summarizes the
FIGO clinical stage and tumor histology. There were 32 patients in
FIGO stage IIB, 1 patient in stage IIIA and 22 patients in stage
IIIB. There were 41 squamous cell carcinomas, 5 adenosquamous
carcinomas, 7 adenocarcinomas and 2 other tumors.
 | Table I.FIGO stage and tumor histology of the
eligible patients. |
Table I.
FIGO stage and tumor histology of the
eligible patients.
| Stage | IIB | IIIA | IIIB | Total |
|---|
| Histology | | | | |
| Squamous | 23 | 1 | 17 | 41 |
| Adenosquamous | 4 | 0 | 1 | 5 |
| Adeno | 5 | 0 | 2 | 7 |
| Others | 0 | 0 | 2 | 2 |
| Total | 32 | 1 | 22 | 55 |
Neoadjuvant intraarterial chemotherapy
(IA-NAC)
Angiography was performed to detect the tumor
feeding vessels before IA-NAC. Catheters were inserted by the
Seldinger's technique and were advanced into each of the uterine
arteries superselectively, when possible. In the event difficulty
was encountered in catheterizing either of the uterine arteries,
the catheter tip was placed in the internal iliac artery instead.
Anticancer agents were injected over 20–30 min. After treatment,
the catheters were removed, and sandbags were placed to apply firm
pressure to the groin for 6 h. Hydration with normal saline and 5%
dextrose was started from 3 h before IA-NAC and was continued to
maintain a urine output >100 ml/h for 24 h.
The following three different regimens were employed
for IA-NAC in chronological order. i) Between January 1991 and
December 1996, BMP therapy [bleomycin (20 mg), mitomycin-C (10 mg)
and cisplatin (80 mg/m2)] was administered to 12
patients. They included 9 patients with squamous cell carcinoma, 2
patients with adenosquamous carcinoma and 1 patient with
adenocarcinoma. ii) Between January 1997 and March 2000, either of
the following two regimens was administered depending on the tumor
histology. PAMF therapy [cisplatin (80 mg/m2),
epirubicin hydrochloride (60 mg/m2), mitomycin-C (20 mg)
and 5-fluorouracil (500 mg)] was administered to 20 patients with
squamous cell carcinoma and 2 patients with other histological
types. PACF therapy [cisplatin (80 mg/m2), epirubicin
hydrochloride (60 mg/m2), cyclophosph-amide (500 mg) and
5-fluorouracil (500 mg)] was administered to 2 patients with
adenosquamous carcinoma and 2 patients with adenocarcinoma. iii)
Between April 2000 and April 2006, CDDP + THP therapy [cisplatin
(100 mg/m2) and pirarubicin hydrochloride (40 mg)] was
administered to 12 patients with squamous cell carcinoma, 1 patient
with adenosquamous carcinoma and 4 patients with
adenocarcinoma.
IA-NAC was administered for 1–3 courses every 3
weeks. The number of courses given depended on the tumor response
and operability determined by MRI and pelvic examination.
Assessment of response
The complete blood count and renal and liver
function tests were performed before each course of IA-NAC. Tumor
dimensions were measured by MRI before and after therapy. Patients
were evaluated for tumor response according to the Response
Evaluation Criteria in Solid Tumors (RECIST).
Additional therapy
Type III radical hysterectomy (16) with pelvic lymphadenectomy was
performed in patients who responded to IA-NAC, when possible.
Patients who did not respond to IA-NAC and could not tolerate or
refused surgery received pelvic radiotherapy, including external
irradiation (Linac 50.4 Gy) and brachytherapy delivered by a remote
control afterloading system using 60Co (RALS 20–40 Gy). After
radical hysterectomy, patients who had poor prognostic factors,
including a positive resection margin, lymph node metastases,
vessel invasion, parametrial infiltration and vaginal invasion,
also received postoperative adjuvant radiation therapy (Linac
45–50.4 Gy).
Follow-up
After treatment, patients were reviewed every 3
months for 3 years, then every 6 months for the next 2 years and
annually thereafter. At each follow-up visit, general and
gynecologic examinations and cervical cytology were performed.
Computed tomography and MRI were conducted once or twice a year.
The median follow-up period for all the patients was 63.2 months
(range 5–182).
Statistical analysis
Overall survival was defined as the time from the
start of the first course of IA-NAC to death from any cause or to
the date of last contact. The Kaplan-Meier method was used to
describe overall survival, and the log-rank test was used to
examine differences in survival between the different regimens. We
initially examined the difference in survival between the squamous
cell carcinoma group and the ‘adenosquamous carcinoma +
adenocarcinoma’ group, after which survival with each anticancer
regimen was subjected to analysis.
The Chi-square test was used for the comparison of
the response rate and the hematological toxicity of each anticancer
regimen. All statistical tests were performed two-sided, and P=0.05
was accepted as statistically significant.
Results
Number of courses
Eight of the 55 patients (14.5%) received 1 course
of IA-NAC, 36 patients (65.5%) received 2 courses and 11 patients
(20.0%) received 3 courses (mean 2.1).
Clinical response
The response rate achieved with IA-NAC was 90.2% for
squamous cell carcinoma, 60% for adenosquamous carcinoma and 42.9%
for adenocarcinoma, and a significant difference was observed
between squamous cell carcinoma and the other histological types
(P<0.01). Pathological CR (complete disappearance of the tumor
confirmed by postoperative histological examination) was only
achieved in 3 patients with squamous cell carcinoma, i.e., in 5.5%
(3/55) of the entire study population.
Response rates were also compared between the
different IA-NAC regimens. After excluding 2 patients with special
tumors, the subjects were classified into 41 patients with squamous
cell carcinoma and 12 patients with adenosquamous carcinoma or
adenocarcinoma (Table II). In
patients with squamous cell carcinoma, the response rate achieved
with BMP, PAMF and CDDP + THP therapy was 77.8, 95 and 91.7%,
respectively. The response rate was lower with BMP than with the
other two regimens, but a significant difference was not observed
(P=0.345). In patients with adenosquamous carcinoma or
adenocarcinoma, the response rate achieved with BMP, PACF and CDDP
+ THP was 33.3, 100 and 25%, respectively. The response to PACF was
significantly higher compared to the other two regimens (P=0.047),
although the number of patients treated was small.
 | Table II.Response rate of squamous cell
carcinoma or adenosquamous carcinoma + adenocarcinoma. |
Table II.
Response rate of squamous cell
carcinoma or adenosquamous carcinoma + adenocarcinoma.
| Histological
type | Regimen | Total | CR | PR | SD | PD | Response rate
(%) |
|---|
| Squamous (n=41) | | | | | | | |
| BMP | 9 | 2 | 5 | 2 | 0 | 77.8 |
| PAMF | 20 | 6 | 13 | 1 | 0 | 95.0 |
| CDDP + THP | 12 | 6 | 5 | 1 | 0 | 91.7 |
| Ad. Sq + Ad
(n=12) | | | | | | | |
| BMP | 3 | 0 | 1 | 2 | 0 | 33.3 |
| PACF | 4 | 0 | 4 | 0 | 0 | 100.0 |
| CDDP + THP | 5 | 0 | 1 | 4 | 0 | 25.0 |
Other therapy
After IA-NAC, type III radical hysterectomy was
performed in 81.3% (26/32) and 43.5% (10/22) of the patients with
stage IIB and IIIB disease, respectively, and radical hysterectomy
was performed in 65.5% (36/55) of the patients. Among the 36
patients who underwent surgery, radiotherapy was performed as
postoperative adjuvant therapy in 28 patients. Patients who did not
respond to IA-NAC or who refused surgery after IA-NAC and only
received radiotherapy accounted for 34.5% (19/55) of the
subjects.
Adverse events due to IA-NAC
Table III shows the
hematological toxicity of each regimen. Adverse effects were
evaluated using the National Cancer Institute – Common Toxicity
Criteria (NCI-CTC) version 3.0. The data were collected
retrospectively from the patient files.
 | Table III.Hematological toxicity of each
regimen. |
Table III.
Hematological toxicity of each
regimen.
| Regimen | Parameter | Grade
|
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| BMP (n=12) | | | | | | |
| WBC | 1 | 3 | 7 | 1 | 0 |
| Platelets | 6 | 3 | 3 | 0 | 0 |
| Hemoglobin | 0 | 1 | 10 | 1 | 0 |
| PAMF (n=22) | | | | | | |
| WBC | 0 | 0 | 3 | 13 | 6 |
| Platelets | 4 | 3 | 5 | 9 | 1 |
| Hemoglobin | 0 | 1 | 13 | 7 | 1 |
| PACF (n=4) | | | | | | |
| WBC | 0 | 0 | 2 | 2 | 0 |
| Platelets | 4 | 0 | 0 | 0 | 0 |
| Hemoglobin | 0 | 1 | 3 | 0 | 0 |
| CDDP + THP
(n=17) | | | | | | |
| WBC | 2 | 2 | 8 | 5 | 0 |
| Platelets | 15 | 2 | 0 | 0 | 0 |
| Hemoglobin | 0 | 2 | 14 | 1 | 0 |
Grade 3 and 4 toxicity was significantly more common
with PAMF than with the other regimens for the WBC count, platelet
count and hemoglobin (P<0.01, P<0.01 and P<0.01,
respectively). With regard to nonhematological toxicities, renal
and hepatic dysfunction caused by IA-NAC was never worse than grade
1.
Specific complications of IA-NAC included gluteal
pain in 5 patients (9.1%), gluteal skin pigmentation in 2 patients
(3.6%) and vesical necrosis in 2 patients (3.6%). Vesical necrosis
occurred in 1 patient each after treatment with PAMF and CDDP +
THP. The uterine artery was employed for superselective infusion in
both patients, but a feeding vessel to the bladder could not be
completely excluded by angiography at the time of treatment.
Survival
The survival rate was examined separately for
patients with squamous cell carcinoma and for those with
adenosquamous carcinoma or adenocarcinoma. The 5-year survival rate
was 72.9% for patients with squamous cell carcinoma and 50% for
patients with adenosquamous carcinoma or adenocarcinoma. Although
survival was more favorable in the squamous cell carcinoma group,
the difference was not significant (P=0.056) (Fig. 1).
When the survival achieved with each anticancer
regimen was examined, the 5-year survival rate of patients with
squamous cell carcinoma was 66.7% with BMP, 69.3% with PAMF and
83.3% with CDDP + THP therapy. Thus, CDDP + THP therapy was the
most effective, although the difference was not significant
compared to the other two regimens (P=0.337) (Fig. 2). In patients with adenosquamous
carcinoma or adenocarcinoma, the 5-year survival rate was 33.3%
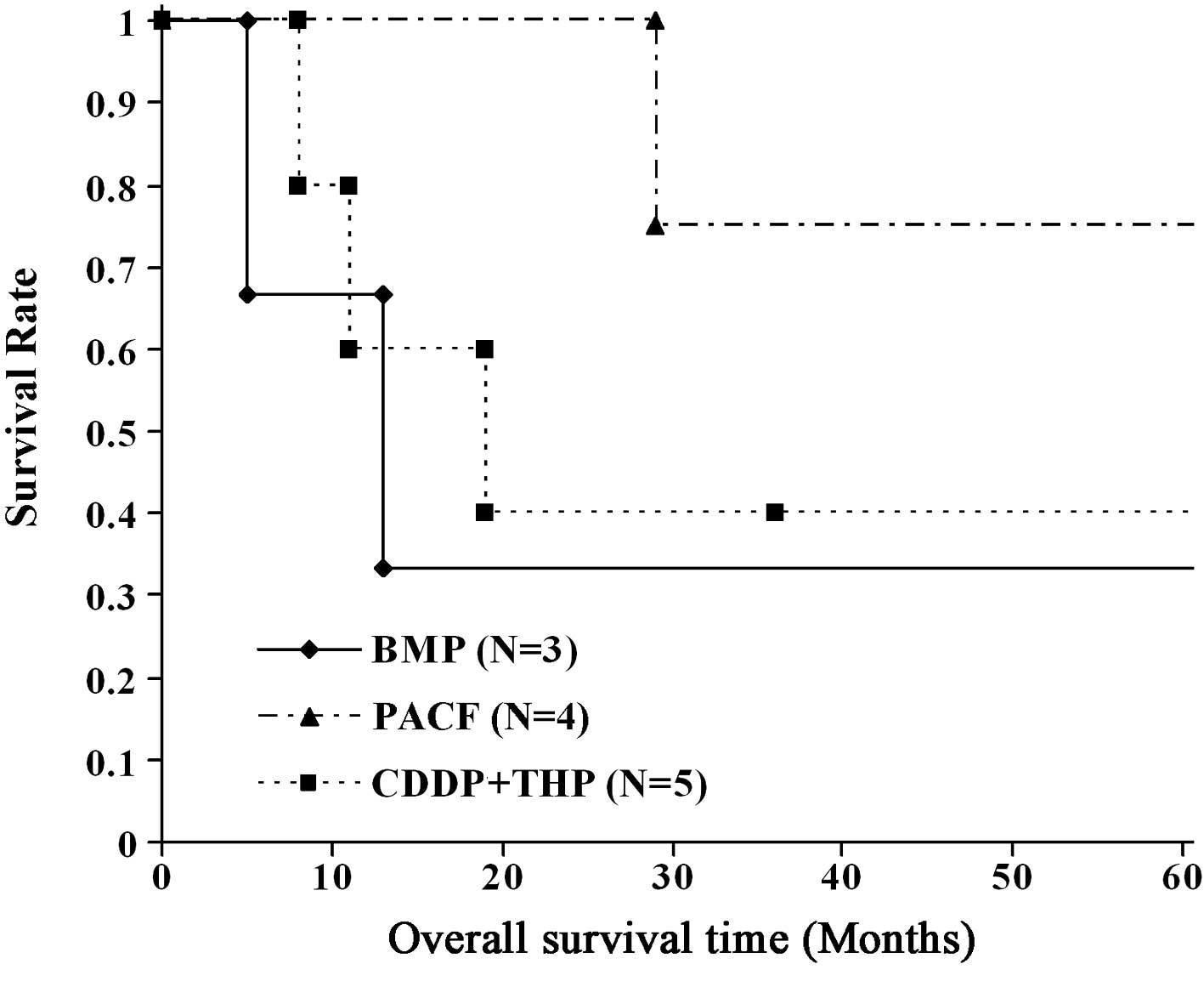
with BMP, 75% with PACF and 20% with CDDP + THP therapy. PACF
therapy was the most effective, although the difference in outcome
compared with the other two regimens was not significant (P=0.184)
(Fig. 3).
Discussion
NAC was commonly employed for cervical cancer until
CCRT became a standard treatment, and favorable results have been
obtained (4–6). However, the efficacy of NAC has not
been confirmed (7). There have
also been negative reports concerning the efficacy of NAC for
advanced cervical cancer (stage III or IV) (8–11),
therefore its value remains controversial.
IA-NAC is employed to obtain higher tissue drug
concentrations by direct infusion of anticancer agents into the
feeding artery of a tumor to maximize the anticancer activity and
minimize adverse effects related to drugs entering the systemic
circulation. The first report on IA-NAC was published in 1950 by
Bierman et al (17), and
its use in the gynecological field was reported by Cromer et
al in 1952 (18). One of the
reasons for the lack of popularity of IA-NAC is presumed to be the
need for special procedures, such as arterial catheterization. The
fact that there are no prospective studies providing conclusive
evidence may be another reason, but favorable results that are
unlikely to be achieved with systemic chemotherapy or CCRT have
been reported (12–15).
The present study revealed that the response rate of
squamous cell carcinoma to IA-NAC was very high at 90.2%.
Investigation of survival also showed a high 5-year survival rate
of 72.9% in patients with stage IIB–IIIB squamous cell carcinoma,
which was improved compared to the previously reported results for
chemoradiation (19). A standard
IA-NAC regimen has not been established and the use of various
anticancer agents has been reported (12–15).
We compared three IA-NAC regimens that were employed in
chronological order. In the patients with squamous cell carcinoma,
PAMF and CDDP + THP therapy achieved a response rate of ≥90%. The
5-year survival rate achieved by CDDP + THP therapy was higher than
those for BMP or PAMF therapy, although significant differences
were not observed (P=0.337) (Fig.
2), and the hematological toxicity of CDDP + THP therapy was
significantly less severe than that of PAMF therapy (Table III). This suggests that it would be
reasonable to use CDDP + THP therapy. On the other hand, studies of
systemic chemotherapy for adenocarcinoma of the cervix have shown
that the response rate of advanced or recurrent cancer to NAC
ranges between 20 and 50% (20–22),
and is relatively low (50–80%) even for early stage cancer
(23–25). In our study of IA-NAC, the response
rate of adenosquamous carcinoma and adenocarcinoma was 60 and
42.9%, respectively, and these rates were significantly lower than
that for squamous cell carcinoma. All 4 patients that were
administered PACF therapy achieved a partial response, and 3 of
them achieved 5-year survival. However, the number of patients
treated was small, therefore the efficacy of PACF therapy should be
further investigated.
Vesical necrosis occurred in 2 patients as a
specific adverse effect of intraarterial therapy and it was
considered to be attributable to high concentrations of anticancer
agents reaching the bladder after superselective infusion into the
uterine artery. When the presence of a feeding vessel for the
bladder cannot be excluded by angiography before IA-NAC, the
internal iliac artery should be selected instead for intraarterial
infusion.
In conclusion, our study showed a high response rate
and a high 5-year survival rate in patients with stage IIB–IIIB
squamous cell carcinoma. Therefore, IA-NAC seems to be a reasonable
treatment option for such tumors, and CDDP + THP therapy seems to
be the most effective of the regimens we tested. Even in patients
with stage IIB–IIIB adenosquamous carcinoma or adenocarcinoma, a
5-year survival rate of 50% was obtained. However, the number of
patients treated was small, therefore the efficacy of PACF therapy
should be further investigated.
References
|
1.
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and paraaortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Whitney CW, Sause W, Bundy BN, Malfetano
JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL and Liao SY:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.PubMed/NCBI
|
|
4.
|
Sardi JE, Giaroli A, Sananes C, Ferreira
M, Soderini A, Bermudez A, Snaidas L, Vighi S, Gomez Rueda N and di
Paola G: Long-term follow-up of the first randomized trial using
neoadjuvant chemotherapy in stage Ib squamous carcinoma of the
cervix: the final results. Gynecol Oncol. 67:61–69. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Benedetti-Panici P, Greggi S, Colombo A,
Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F,
Zola P, Mangioni C and Landoni F: Neoadjuvant chemotherapy and
radical surgery versus exclusive radiotherapy in locally advanced
squamous cell cervical cancer: results from the Italian
multi-center randomized study. J Clin Oncol. 20:179–188. 2002.
View Article : Google Scholar
|
|
6.
|
Neoadjuvant Chemotherapy for Locally
Advanced Cervical Cancer Meta-analysis Collaboration: Neoadjuvant
chemotherapy for locally advanced cervical cancer: a systematic
review and meta-analysis of individual patient data from 21
randomised trials. Eur J Cancer. 39:2470–2486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chang TC, Lai CH, Hong JH, Hsueh S, Huang
KG, Chou HH, Tseng CJ, Tsai CS, Chang JT, Lin CT, Chang HH, Chao
PJ, Ng KK, Tang SG and Soong YK: Randomized trial of neoadjuvant
cisplatin, vincristine, bleomycin, and radical hysterectomy versus
radiation therapy for bulky stage IB and IIA cervical cancer. J
Clin Oncol. 18:1740–1747. 2000.
|
|
8.
|
Kumar L, Kaushal R, Nandy M, Biswal BM,
Kumar S, Kriplani A, Singh R, Rath GK and Kochupillai V:
Chemotherapy followed by radiotherapy versus radiotherapy alone in
locally advanced cervical cancer: a randomized study. Gynecol
Oncol. 54:307–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sundfor K, Trope CG, Hogberg T, Onsrud M,
Koern J, Simonsen E, Bertelsen K and Westberg R: Radiotherapy and
neoadjuvant chemotherapy for cervical carcinoma. A randomized
multicenter study of sequential cisplatin and 5-fluorouracil and
radiotherapy in advanced cervical carcinoma stage 3B and 4A.
Cancer. 77:2371–2378. 1996. View Article : Google Scholar
|
|
10.
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araujo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991.PubMed/NCBI
|
|
11.
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology
Association. J Clin Oncol. 13:444–451. 1995.
|
|
12.
|
Park SY, Kim BG, Kim JH, Lee JH, Lee ED,
Lee KH, Park KB, Lee BH and Kim KH: Phase I/II study of neoadjuvant
intraarterial chemotherapy with mitomycin-C, vincristine, and
cisplatin in patients with stage IIb bulky cervical carcinoma.
Cancer. 76:814–823. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Scarabelli C, Zarrelli A, Gallo A and
Visentin MC: Multimodal treatment with neoadjuvant intraarterial
chemotherapy and radical surgery in patients with stage IIIB–IVA
cervical cancer. A preliminary study. Cancer. 76:1019–1026.
1995.
|
|
14.
|
Sugiyama T, Nishida T, Hasuo Y, Fujiyoshi
K and Yakushiji M: Neoadjuvant intraarterial chemotherapy followed
by radical hysterectomy and/or radiotherapy for locally advanced
cervical cancer. Gynecol Oncol. 69:130–136. 1998. View Article : Google Scholar
|
|
15.
|
Yamakawa Y, Fujimura M, Hidaka T, Hori S
and Saito S: Neoadjuvant intraarterial infusion chemotherapy in
patients with stage IB2–IIIB cervical cancer. Gynecol Oncol.
77:264–270. 2000.
|
|
16.
|
Piver MS, Rutledge F and Smith JP: Five
classes of extended hysterectomy for women with cervical cancer.
Obstet Gynecol. 44:265–272. 1974.PubMed/NCBI
|
|
17.
|
Bierman HR, Byron RL Jr, Miller ER and
Shimkin MB: Effects of intra-arterial administration of nitrogen
mustard. Am J Med. 8:5351950. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cromer JK, Bateman JC, Berry GN, Kennelly
JM, Klopp CT and Platt LI: Use of intra-arterial nitrogen mustard
therapy in the treatment of cervical and vaginal cancer. Am J
Obstet Gynecol. 63:538–548. 1952.PubMed/NCBI
|
|
19.
|
Toita T, Moromizato H, Ogawa K, Kakinohana
Y, Maehama T, Kanazawa K and Murayama S: Concurrent
chemoradiotherapy using high-dose-rate intracavitary brachytherapy
for uterine cervical cancer. Gynecol Oncol. 96:665–670. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Umesaki N, Fujii T, Nishimura R, Tanaka T,
Nishida M, Fushiki H, Takizawa K, Yamamoto K, Hasegawa K and Izumi
R: Phase II study of irinotecan combined with mitomycin-C for
advanced or recurrent squamous cell carcinoma of the uterine
cervix: the JGOG study. Gynecol Oncol. 95:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dimopoulos MA, Papadimitriou CA, Sarris K,
Aravantinos G, Kalofonos C, Gika D, Gourgoulis GM, Efstathiou E,
Skarlos D and Bafaloukos D: Combination of ifosfamide, paclitaxel,
and cisplatin for the treatment of metastatic and recurrent
carcinoma of the uterine cervix: a phase II study of the Hellenic
Cooperative Oncology Group. Gynecol Oncol. 85:476–482. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Papadimitriou CA, Sarris K, Moulopoulos
LA, Fountzilas G, Anagnostopoulos A, Voulgaris Z, Gika D,
Giannakoulis N, Diakomanolis E and Dimopoulos MA: Phase II trial of
paclitaxel and cisplatin in metastatic and recurrent carcinoma of
the uterine cervix. J Clin Oncol. 17:761–766. 1999.PubMed/NCBI
|
|
23.
|
Iwasaka T, Fukuda K, Hara K, Yokoyama M,
Nakao Y, Uchiyama M and Sugimori H: Neoadjuvant chemotherapy with
mitomycin C, etoposide, and cisplatin for adenocarcinoma of the
cervix. Gynecol Oncol. 70:236–240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lissoni A, Gabriele A, Gorga G, Tumolo S,
Landoni F, Mangioni C and Sessa C: Cisplatin-, epirubicin- and
paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann
Oncol. 8:969–972. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Benedetti-Panici P, Greggi S, Scambia G,
Salerno MG, Amoroso M, Maneschi F, Cutillo G, Caruso A, Capelli A
and Mancuso S: Locally advanced cervical adenocarcinoma: is there a
place for chemo-surgical treatment? Gynecol Oncol. 61:44–49. 1996.
View Article : Google Scholar : PubMed/NCBI
|