Introduction
Breast cancer is the most frequent cancer in women
(1). Evidence suggests that
polyunsaturated fatty acids (PUFAs), including arachidonic acid
(AA) and linolenic acid (LA) but also eicosapentaenoic acid (EPA)
and docosahexaenoic acid (DHA), influence breast cancer
proliferation (2–4), differentiation (3), and prognosis (5). Clinical and research data in the
past 20 years reveal that cyclooxygenase (COX)2 is overexpressed in
a variety of malignant tumors (6–8)
and are linked to apoptosis resistance (9), invasive tumor behavior (10) and the poor prognosis of breast
cancer (8,11,12). However, the mechanism of
unsaturated fatty acids upon breast cancer still remains
unclear.
Ca2+ is one of most important signal
transduction elements in cells ranging from bacteria to neurons.
The molecular identity of the membrane protein that serves to
enable capacitative Ca2+ entry (CCE) following the
functional depletion of intracellular Ca2+ store or
activation of G-protein (13) has
yet to be determined with any certainty, but the canonical,
vertebrate TRPs (TRPC) are widely believed to fulfil this vital
role in many cell types (14,15). Cell-specific, differential
expression of TRPC has been described in many excitable and
non-excitable cell types, including prostate cancer cells (16) and breast cancer cells (17,18) where they contribute and mediate
the Ca2+ entry in response to multiple physico-chemical
stimuli (19,20).
The PUFA, AA has been proposed to activate TRPC, in
many mammalian cell types, including endothelial (21,22), epithelial and smooth muscle
(23) cells, but the direct
evidence for this in these cells is lacking and the proposals are
based primarily upon findings in Drosophila TRP and TRP-like
(TRPL) (24) and mammalian TRPV
channels (25) where AA and LA
induce activation, leading to Ca2+ entry.
COX acts to degrade AA. In addition, high cellular
levels of COX are commonly used as a marker for malignant breast
cancer (6,10,12). This suggests that AA and/or its
degenerate products may play a role in this pathological
process.
In this study, we found the functional expression of
TRPC3 in human MCF-7 breast cancer cell-mediated Ca2+
entry. Native TRPC channels in MCF-7 cells were inhibited by PUFA.
Ca2+ entry via activated TRPC was enhanced when PUFA
were absent, suggesting a double-gating mechanism for TRPC that may
be involved in MCF breast cancer cell proliferation and
invasion.
Materials and methods
Cell culture
MCF-7 cells were grown in DMEM medium containing 10%
fetal calf serum and 1% penicillin/streptomycin serum as described
(9). Cells were plated onto
ø13-mm coverslips and used when 60–70% confluent.
Calcium imaging
The growth medium was removed and cells were rinsed
once in Earle’s balanced salts solution (EBSS; Invitrogen).
Calcium-green of 50 μg AM (C3012; Invitrogen) or Fura-2 AM (F1221;
Invitrogen) were dissolved in 20 μl 20% pluronic acid in DMSO (0.01
g in 50 μl DMSO stock). Before the experiment, mixtures of 1 μl dye
preparation in 200 μl EBSS was applied and cells were incubated for
60 min. Prior to placing the coverslip into the recording chamber,
coverslips were rinsed in EBSS to remove residual dye. Data
acquisition and analysis were performed via OpenLab v.3.1.7
(Improvision Ltd., Coventry, UK). A CCD camera (ORCA-AG; Hamamatsu
Ltd., Japan) was used to capture the fluorescent image by using
Fura-2-AM and calcium green. In the experiments performed using
Fura-2, fluorescent intensities were measured with
dual-sequential-wavelength excitation at 340 and 380 nm, and
emission at 510 nm. Changes in Ca2+ concentration were
expressed as ratios of 340/380. Fluorescent intensity of calcium
green-1 was measured with a single wavelength excitation at 488 nm
and emission at 528 nm. Changes in the Ca2+
concentration were expressed as ΔF/F, where F was the fluorescence
intensity when cells were at rest, and ΔF was the change in
fluorescence during stimulation.
iRNA and plasmid of hCOX2
Stealth siRNA (Invitrogen) was obtained from
Invitrogen. MCF-7 cells were passaged onto coverslips in 500 μl
Opti-MEM (Invitrogen) one day before transfection and reached about
40–50% confluence at the time of transfection. siRNA of 20 pmol
(against TRPC3) or the siRNA negative control complex, with a 1:125
final dilution of Lipofectamine 2000 (Invitrogen) was used
according to the manufacturer’s instructions. The knockdown effects
were examined at 48 h and the results were compared with control
and control without knockdown. Results were collected from 3
different batches of MCF-7 cells. Human hCOX2 plasmids were
obtained from Professor R. Kulmacz (University of Texas Health
Science Center at Houston). Cells were transfected with hCOX2 by
Lipofectamine 2000. The effects of transfection were examined by
western blot analysis at 24 and 48 h.
RT-PCR and immunostaining
RT-PCR experiments followed standard protocols.
Primers were designed with primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi)
for TRPC1 (NM_003304/92 bp), TRPC3 (NM_003305/157 bp), TRPC4
(NM_016179/191 bp), TRPC5 (NM_012471/108 bp), TRPC7 (NM_020389/135
bp) and the α1C subunit (NM_000719/194 bp), α1G subunit
(AH_007322/135 bp) and α1H subunit of VGCCs (NM_021098/123 bp).
Antibodies against TRPC1, 3, 4 and 5 were the kind gift from
Professor W.P. Schilling (Case Western University, Cleveland, OH,
USA). The peptide sequence (26)
used to generate the antibody against TRPC3 was
RRRRLQKDIEMGMGN.
Cell cycle analysis
After removal of methanol, cells were treated with a
Coulter DNA-Prep reagent kit (Beckman-Coulter, France). Cells were
resuspended in 40 μl of a lysing and permeabilizing reagent and 400
μl of a propidium iodide solution containing RNAse. Flow cytometry
analyses were performed using a Coulter Epics Elite ESP flow
cytometer (Beckman-Coulter) equipped with a 488 nm argon laser
running at 15 mW. The red DNA fluorescence signal was analyzed as
total (area) vs. peak signal, in order to eliminate doublets and
aggregates. Data were recorded for at least 10,000 events. Cell
cycle distribution was analyzed with the Multicycle-AV software
(Phoenix Flow Systems, San Diego, CA, USA).
Cell survival and proliferation
Cell proliferation was determined using the
tetrazolium salt reduction method (MTT). Cells were seeded at
4×104 cells/well on a 24-well plate (for a given
condition on three separate experiments) and allowed to start
growth for 48 h. Drugs and AA were added for 24 and 48 h at the
concentrations indicated in the figure legends. Cells were
incubated at 37°C with the tetrazolium salt
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] and
metabolically active cells reduced the dye to purple formazan.
After 45 min of incubation at 37°C, the medium was discarded and
MTT formazan crystals were solubilized with DMSO. Absorbance was
measured in a multiwell plate spectrophotometer at 570 nm
(Molecular Devices model Thermomax microplate reader, Les Ulis,
France). For easier comparison between conditions, results obtained
for proliferation were normalized. The means were then calculated
on the daily calculated ratios. The mean of each triplicate was
used to create a data point for comparing cell growth in different
conditions.
Cell migration/invasion in vitro
Invasion and migration were analyzed in BD Falcon
24-well plates with 8-μm pore size polyethylene terephtalate
membrane BD BioCoat cell culture inserts. For the invasion assay,
the membrane was covered with a film of Matrigel
(Becton-Dickinson). The upper compartment was seeded with
4×104 viable cells in DMEM with 5% FBS ± drugs/AA and
the lower compartment was filled with DMEM supplemented with 10%
FBS as a chemo-attractant ± drugs/AA. After 48 h at 37°C, cells
remaining on the upper side on the membrane were removed with a
cotton swab and the cells that had migrated and were attached to
the lower side were stained with hematoxylin for 2 min and counted
on the total surface of the insert using light microscopy at x200
magnification.
Solution and chemicals
EBSS for calcium imaging recording contained: NaCl
116.3 mM, NaH2PO4 1 mM, KCl 5.3 mM,
MgCl2 1 mM, CaCl2 1.8 mM, NaHCO3
26 mM, D-glucose 5.5 mM, HEPES 10 mM. The EGTA solution for calcium
imaging recording contained: NaCl 116.3 mM,
NaH2PO4 1 mM, KCl 5.3 and 1.8 mM,
NaHCO3 26 mM, D-glucose 5.5 mM, HEPES 10 mM, EGTA 0.2
mM. All solutions were prepared fresh on the day of
experimentation.
Chemicals dissolved in ethanol or DMSO were made up
as 1,000 times stock solutions. All 1X chemical solutions were made
with an appropriate bath solution on the day of experimentation.
Solutions such as TG, AA, ETYA, were kept in the dark and on ice
and added into a syringe which led to the recording chamber when
necessary. The solvent, ethanol at the same dilution, was tested
alone in controls and had no effects on calcium entry evoked either
by store depletion or OAG stimulation.
Results
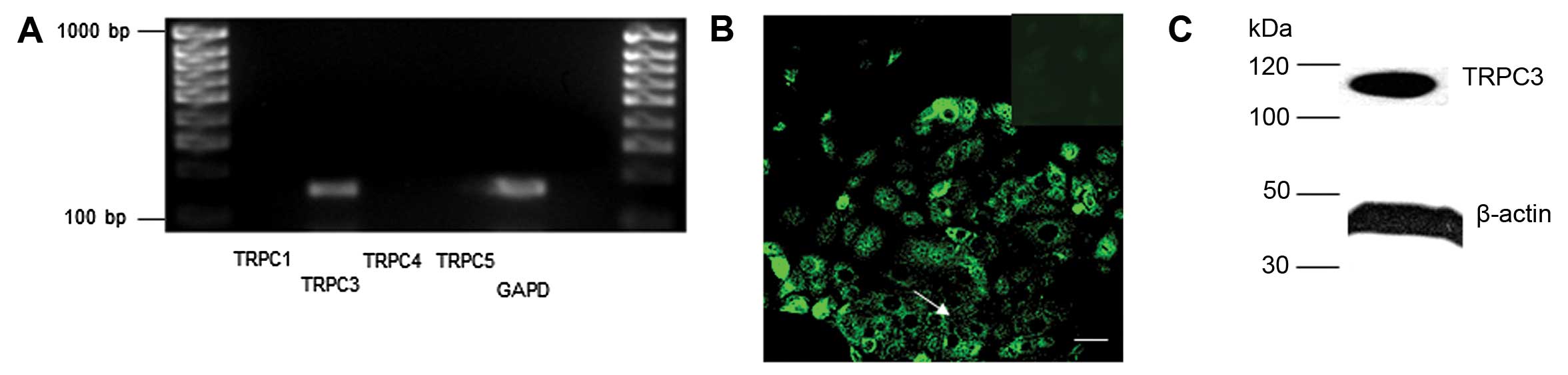
TRPC3 and the α1H subunit of the voltage
gated calcium channel (VGCC) expressed in MCF-7 breast cancer
cells
Expression of the TRPC3, α1H (T type) but not the
α1C or α1G subunits of the voltage-gated calcium channel (VGCC) was
observed in MCF-7 cells (Fig.
1).
The spatial distribution of TRPC3 protein in MCF-7
cells was determined by immunohistochemistry, as previously
reported in other cell types (27). Abundant, non-discrete TRPC3
expression throughout the cell cytoplasm and at the cell surface
was observed (Fig. 1B). Western
blot analysis consistently demonstrated abundant expression of
TRPC3 in MCF-7 cells (Fig.
1C).
Native TRPC3 in MCF-7 breast cancer cells
activated by DAG and store depletion
Recombinant human TRPC3 can be directly activated by
diacylglycerol (DAG) (28,29).
In MCF-7 cells, OAG (100 μM), a synthetic DAG, induced a
significant intracellular Ca2+ elevation in an
extracellular calcium-dependent manner (Fig. 2). Recent reports have confirmed
that TRPC3 could also mediate store operated calcium entry (SOCs)
(30). Activation of native TRPC3
via store depletion by SERCA pump inhibitors, such as TG, has been
reported in HSG (31) and
epithelial cells (32). In MCF-7
cells, store depletion by TG (1 μM) and EGTA bath led to
significant Ca2+ elevations (Fig. 2A).
 | Figure 2.Polyunsaturated fatty acids inhibited
Ca2+ entry through TRPC3 channels. (A) Ca2+
entry was evoked by store depletion with thapsigargin (TG, 1 μM)
and EGTA (blue line). 2-APB (pink line, 100 μM) induced a transient
Ca2+ elevation following a rapid decrease.
[Ca2+]i elevation was inhibited by
arachidonic acid (AA, yellow line, 10 μM), linonic acid (LA, 10 μM)
and ETYA (brown line, 20 μM). (B) The dashed horizontal line
indicates the basal Ca2+ level. TG (1 μM) elevated
cellular Ca2+ levels that were rapidly reduced to below
basal levels by wash out with EGTA (200 μM). Application of
arachidonic acid (AA, 10 μM) prevented the overshooting response
seen in (A) and only returned Ca2+ to the basal level,
indicating a block of the TRPC-mediated Ca2+ entry
pathway by AA. Wash off of AA with EBSS caused an overshooting
response to occur that could be blocked, as previously, by
application of AA (10 μM). (C) Log dose response curve of varying
concentrations of AA upon the OAG-induced Ca2+
elevation. Responses were quantified as the area under the
Ca2+ response curve and normalized by determining the
area in the presence of AA, expressed as a fraction of the control
response. Curve fit was with a single sigmoidal function. The inlet
shows an example of different concentration-dependent effects of AA
upon Ca2+ entry elicited by OAG (50 μM). (D) Western
blot analysis demonstrates that protein levels of TRPC3 channels
gradually diminished after 48 h. (E) Ca2+ entry evoked
by store depletion was significantly reduced 48 h after incubation
of MCF-7 cells with RNAi against TRPC3, . Results were averaged
from 3 coverslips except where indicated. |
AA and LA directly inhibited calcium
entry via native TRPC3
The Ca2+ elevation induced by store
depletion was almost quenched by bath application of 10 μM AA
(Fig. 2A, yellow line) or 10 μM
LA (Fig. 2A, blue line). 2APB, as
a common antagonist of TRPC3, at the concentration of 100 μM,
caused a transient calcium peak followed by decay (Fig. 2A, pink line) to a level similar to
that in the presence of AA or LA. In addition, the CCE following
Ca2+ replacement after EGTA, could be prevented by 10 μM
AA and CCE was observed after removal, by washout, of the AA
inhibition (Fig. 2B).
Effect of PUFA on TRPC3 not via the
degenerated products of PUFA
Exogenous AA can freely penetrate membranes, but
will be challenged and degraded by endogenous intracellular
cyclooxygenases (COX) or lipooxygenases (LOX). To exclude the
possibility that the inhibition effect is due to degenerated
metabolites of AA rather than AA itself, 14-eicosatetraynoic acid
(ETYA, 20 μM), a competitive analogue of AA resistant to LOX and
other degradative enzymes, was used to mimic the effect of AA.
ETYA, like AA, inhibited CCE induced by store depletion (Fig. 2A, brown line), suggesting that
PUFA interacts directly with TRPC channels.
The effect of fatty acids on ion channels is
sometimes attributed to non-specific influences upon cell membranes
(33,34). Polyunsaturated fatty acids may,
for example, increase membrane fluidity. AA at a concentration
between 25–100 μM directly affected channel proteins via a change
of the lipid environment (34).
We observed that application of 500 μM AA induced a massive,
irreversible Ca2+ elevation, presumably following the
collapse of cell integrity. We therefore maintained PUFA
concentrations below the threshold (15 μM) for such effects.
Additionally, CCE was also inhibited by the double-bonds
unsaturated fatty acid, e.g. LA (5 and 10 μM) and the mono-bond
unsaturated fatty acid, oleic acid (10 μM). However, the saturated
fatty acid, stearic acid (20 μM), had no effect upon induced
CCE.
To confirm that CCE in MCF-7 cells was mediated via
TRPC3 channels, we examined Ca2+ entry in MCF-7 cells by
knocking down TRPC3 (shealth RNAi; Invitrogen) (Fig. 2D and E). A negative control iRNA
was utilized as a control to discount the non-specific effect of
TRPC3 iRNA. Transfected cells gradually lost the TRPC3 channels and
after 48 h, detectable TRPC3 protein was almost eliminated
(Fig. 2D). In parallel to the
decrease of TRPC3 protein, calcium elevation evoked by store
depletion was also significantly reduced; suggesting that calcium
elevation induced by store depletion was mediated via the TRPC3
channels.
Dose response of AA inhibition upon
calcium entry via TRPC3
Ca2+ entry in MCF-7 cells could be evoked
by application of OAG (Fig. 2C).
AA in a range from 0.1 to 20 μM was employed to study the
inhibitory effect of AA on OAG induced Ca2+ elevation. A
single, sigmoidal dose-response curve was fitted according to data
(Fig. 2C) and gave an
IC50 of AA upon OAG induced Ca2+ elevation of
1.78±0.17 μM.
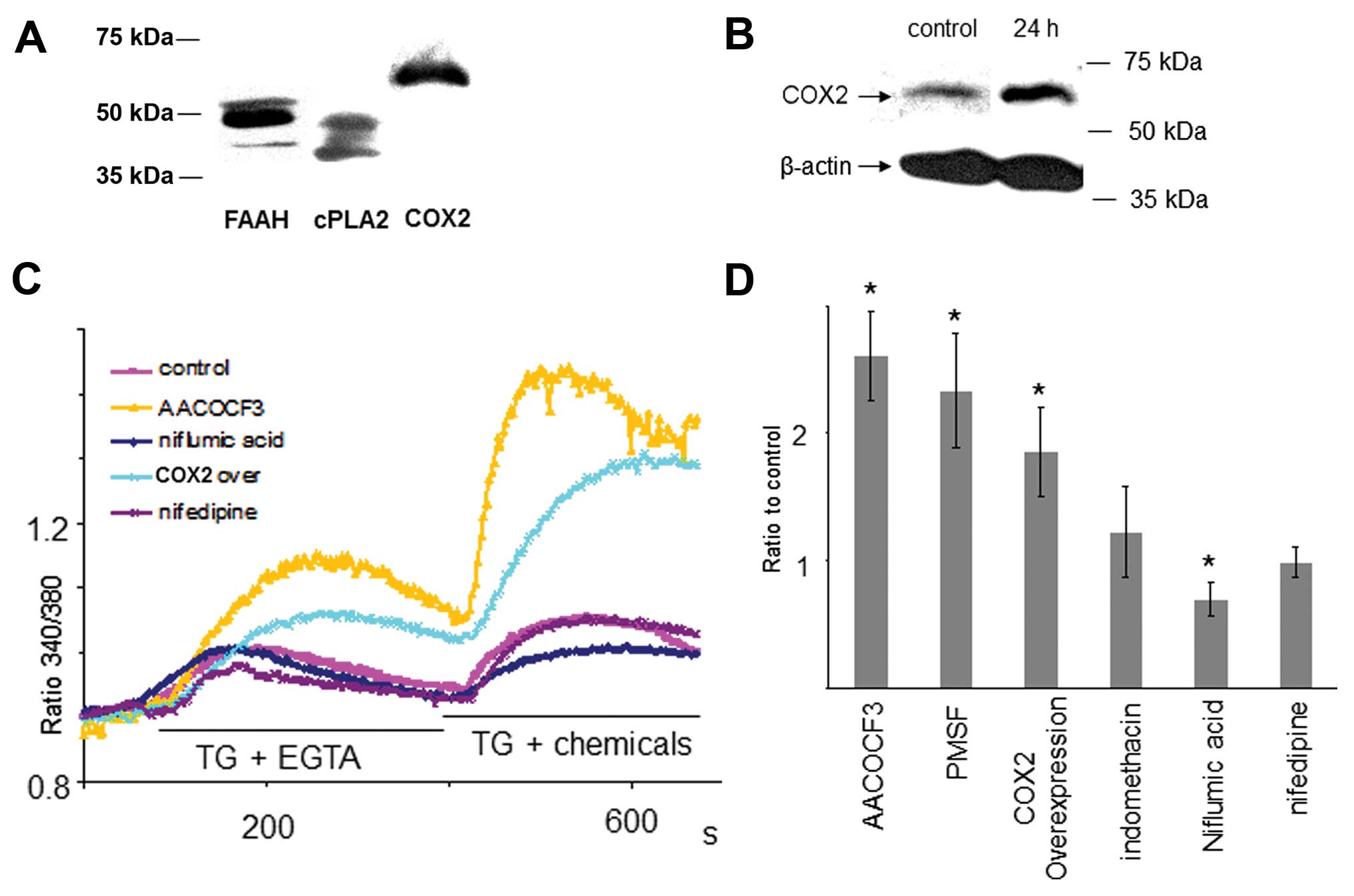
Regulation of endogenous AA in MCF-7 and
its effect on Ca2+ entry via TRPC3
There are generally two principal sources in AA
generation, i) cytosolic phospholipase A2 (PLA2)
releasing AA from appropriate phospholipids (35–37) and ii) fatty acid amide hydrolase
(FAAH) degenerating endogenous anandamide and relatives into AA.
The AA metabolism diverges down two main pathways, the COX and LOX
pathways (38).
cPLA2, FAAH and COX2 were demonstrated to be present
in MCF-7 breast cancer cells by western blot analyses (Fig. 3A). Accordingly, reduction of
endogenous PUFA could be achieved by either inhibition of cPLA2,
FAAH or overexpression of COX2. Inhibition COX2 or LOX could induce
the elevation of endogenous PUFA. Consistent with our results
above, reduction of endogenous PUFA by the cPLA2 antagonist
(AACOCF3), FAAH antagonist (PMSF), and overexpression of COX2
significantly enhanced Ca2+ entry via TRPC3. Niflumic
acid, the antagonist of COX2, reduces degeneration of PUFA,
resulting in elevation of endogenous PUFA. Ca2+ entry
via TRPC3 was reduced by pre-incubation with niflumic acid
(Fig. 3D).
Effect of TRPC antagonism on MCF-7 cell
proliferation and invasion
The common antagonist of TRP channels, 2-APB, was
used to investigate the effects of these channels on the
proliferation of MCF-7 breast cancer cells. 2-APB (50 μM)
significantly reduced the S phase of the cell cycle (Fig. 4A-i), and cell proliferation (MTT
test; 100 μM), whilst D609, an inhibitor of phospholipase C, had no
effect (Fig. 4A-ii). 2-APB also
reduced MCF-7 cell migration and invasion in a dose-dependent
manner (Fig. 4A-iii and iv). The
effects of increasing concentration of AA on cell migration are
shown in Fig. 4B. Taken together,
these results suggest that cellular AA inhibits Ca2+
entry via TRPC3, thus interfering with the Ca2+
requirement for cell proliferation and invasion.
Discussion
In this study, we found that TRPC3 channels were
highly expressed in MCF-7 breast cancer cells. Not only DAG but
also store depletion activated TRPC3 to mediate Ca2+
entry. [Ca2+]i was thereafter crucial to
determine breast cancer cell proliferation and migration. PUFAs,
such as AA and LA, but not saturated fatty acids, inhibited
Ca2+ entry mediated by TRPC3. Channel activation and
removal of PUFAs were required to allow Ca2+ entry via
TRPC. Endogenous local PUFAs regulated by cellular generation and
degeneration pathways therefore played an important role in
mediating [Ca2+]i via TRPC. PUFAs as well as
the TRPC3 antagonist attenuated breast cancer cell proliferation
and migration, suggesting a mechanism in which PUFAs restrain
breast cancer via inhibition of TRPC channels.
TRP channels play a key role in the regulation of
intracellular Ca2+ in multiple cell types e.g. sperm
(39), smooth muscle (40) and prostate cancer cells (41) in response to multiple stimuli. At
least four of the TRPC family members (TRPC1, 2, 4 and 5) are known
to be activated by store depletion, while TRPC3 and 6 are generally
regarded to be activated by DAG (28,29). However, native TRPC3 in different
cells e.g. avian pre-B cells (42), ROS 17/2.8 (43) and PS1 prostate cancer cells
(44) are also reported to be
activated by store depletion. The proportion of activation in these
cells, induced by DAG or by store depletion, may vary with
different levels of TRPC3 expression. It has been reported that
expression levels of the TRPC3 channel might determine the actual
activation mechanism (45).
Inconsistent to previous studies, we found that TRPC3 in MCF-7
breast cancer cells are activated by DAG as well as by store
depletion. The expression of TRP in MCF-7 cells may vary (18) due to the culture methods. In hBDC,
TRPC1, TRPC6, TRPM7, TRPM8 and TRPV6 channels were overexpressed in
hBDA compared to the adjacent non-tumoral tissue. TRPC1, TRPM7 and
TRPM8 expression strongly correlated with proliferative parameters,
and TRPV6 was mainly overexpressed in invasive breast cancer cells
(18). However, in the MCF-7 cell
line in our laboratory, TRPC1 was not expressed.
PUFAs have been found to directly activate
Drosophila TRP and TRPL (24) as well as TRPV in vascular
endothelial cells (25). However,
there is little evidence to show that PUFA directly activates
native TRPC in mammalian tissues rather than in expression systems.
Interestingly, Ca2+ entry mediated by AA has been
reported (46–48), but it is ascribed to a novel
receptor-activated calcium entry pathway, ARC (49,50), as there appeared to be mutual
antagonism of CCE and ARC (51–53). In our experiment, the application
of PUFA alone did not cause calcium elevation, and selective TRPC3
iRNA inhibited the calcium elevation in response to store
depletion. We therefore ruled out the possibility of calcium entry
via ARC. Other studies on the effect of AA on voltage-gated Kv
channels suggest that AA closed Kv channels by introducing
conformational alternations in selective filter regions of the
channel (54). How PUFAs interact
with the TRPC3 protein within internal membranes, at present, is
still unclear.
The lack of specific antagonists for TRP channels
creates difficulties in determining the molecular basis of TRP
channels. 2-APB has a broad effect on TRPC, IP3 and TRPV. The
silent response of MCF-7 cells to CAP, the most potent TRPV
agonist, ruled out the possible involvement of TRPV in the
mediation of calcium entry in MCF-7 cells. Consistently, western
blot analyses against TRPV1 in MCF-7 cells failed to detect the
expected protein, compared to positive controls from rat brain. In
addition, the potent inhibition of RNAi against TRPC3 on the
Ca2+ entry induced by store depletion suggests that the
inhibition of AA on Ca2+ entry occurred via its effect
on TRPC3.
The concentration of free AA in resting cells is
universally described as low but varies from cell to cell, AA
concentrations in the resting leukocyte have been measures at 0.5–1
μM as opposed to at 15 μM in the islets of Langerhans (55). This resting concentration can be
varied dynamically by either increases, for example via activation
of G-protein coupled receptors and phospholipase A2 (56), or decreases via AA degenerative
pathways mediated principally by COX and LOX. As there are multiple
buffer systems for AA, the free intracellular AA concentrations
correlating with the bath AA concentration are not easily
determined.
As expected, reduction of endogenous AA by
inhibition of cPLA2, FAAH and overexpression of COX2 increase
Ca2+ entry evoked by store depletion. However, the
effect of increasing endogenous AA by inhibition of COX2 and/or LOX
was not of significance, suggesting some unknown mechanism. In
addition, inhibition of FAAH was complicated, because AEA alone
causes calcium elevations (data not shown).
In addition, COX2 has been shown to be overexpressed
in a variety of malignant tumors (6,7)
and linked to apoptosis resistance (9), invasive tumor behavior (10) and the poor prognosis of breast
cancer (12). Our findings
suggest a mechanism whereby AA, through direct inhibition of
Ca2+ entry via TRPC3 channels may play a role in cancer
cell proliferation and invasion.
In conclusion, we have demonstrated that
polyunsaturated fatty acids directly inhibit TRPC3 in MCF-7 cells
and is a potent mechanism for regulating Ca2+ entry and
[Ca2+]i. Our data suggests a role for such
regulation in breast cancer metastasis. Thus, TRPC3 appears as a
new mediator of breast cancer cell migration/invasion and
represents a potential target for a new class of anticancer
agents.
Abbreviations:
|
TRP
|
transient receptor potential;
|
|
PUFA
|
polyunsaturated fatty acids;
|
|
CCE
|
capacitative Ca2+
entry;
|
|
VGCC
|
voltage-gated calcium channel;
|
|
SER
|
smooth endoplasmic reticulum;
|
|
TG
|
tharpsigargin;
|
|
AA
|
arachidonic acid;
|
|
LA
|
linoleic acid;
|
|
COX
|
cyclooxygenases;
|
|
LOX
|
lipooxygenases;
|
|
ETYA
|
14-eicosatetraynoic acid;
|
|
EPA
|
eicosapentaenoic acid;
|
|
DHA
|
docosahexaenoic acid.
|
Acknowledgements
This study was supported by grants
from the Scientific Research Projects of Scientific and Technology
Bureau of Shanghai (09411952800) to H.Z. This study was partly
supported in part by the Dean’s Initiative Fund, University of
Birmingham and BBSRC to Y.G.
References
|
1.
|
B WeigeltLJ van’t VeerHard-wired genotype
in metastatic breast cancerCell
Cycle3756757200410.4161/cc.3.6.92315153810
|
|
2.
|
M NoguchiDP RoseM EarashiI MiyazakiThe
role of fatty acids and eicosanoid synthesis inhibitors in breast
carcinomaOncology52265271199510.1159/0002274717777237
|
|
3.
|
H ChamrasA ArdashianD HeberJA GlaspyFatty
acid modulation of MCF-7 human breast cancer cell proliferation,
apoptosis and differentiationJ Nutr
Biochem13711716200210.1016/S0955-2863(02)00230-912550055
|
|
4.
|
L De PetrocellisD MelckA PalmisanoThe
endogenous cannabinoid anandamide inhibits human breast cancer cell
proliferationProc Natl Acad Sci USA958375838019989653194
|
|
5.
|
LD YeeDC YoungTJ RosolAM VanbuskirkSK
ClintonDietary (n-3) polyunsaturated fatty acids inhibit
HER-2/neu-induced breast cancer in mice independently of the PPARγ
ligand rosiglitazoneJ Nutr135983988200515867269
|
|
6.
|
B SinghA LucciRole of cyclooxygenase-2 in
breast cancerJ Surg
Res108173179200210.1006/jsre.2002.653212472107
|
|
7.
|
S ZhaWR GageJ SauvageotCyclooxygenase-2 is
up-regulated in proliferative inflammatory atrophy of the prostate,
but not in prostate carcinomaCancer Res6186178623200111751373
|
|
8.
|
E HoriaBA WatkinsComplementary actions of
docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness
in MDA-MB-231 breast cancer
cellsCarcinogenesis28809815200710.1093/carcin/bgl18317052999
|
|
9.
|
JY LiouN AleksicSF ChenTJ HanSK ShyueKK
WuMitochondrial localization of cyclooxygenase-2 and
calcium-independent phospholipase A(2) in human cancer cells:
Implication in apoptosis resistanceExp Cell
Res3067584200510.1016/j.yexcr.2005.01.01115878334
|
|
10.
|
B ArunP GossThe role of COX-2 inhibition
in breast cancer treatment and preventionSemin
Oncol312229200410.1053/j.seminoncol.2004.03.04215179621
|
|
11.
|
HJ MurffXO ShuH LiDietary polyunsaturated
fatty acids and breast cancer risk in Chinese women: a prospective
cohort studyInt J
Cancer12814341441201110.1002/ijc.2570320878979
|
|
12.
|
C DenkertKJ WinzerS HauptmannPrognostic
impact of cyclooxygenase-2 in breast cancerClin Breast
Cancer4428433200410.3816/CBC.2004.n.00615023244
|
|
13.
|
DJ BeechK MurakiR FlemmingNon-selective
cationic channels of smooth muscle and the mammalian homologues of
Drosophila TRPJ
Physiol559685706200410.1113/jphysiol.2004.06873415272031
|
|
14.
|
B NiliusStore-operated Ca2+
entry channels: still elusive!Sci STKE 2004e362004
|
|
15.
|
C MontellPhysiology, phylogeny, and
functions of the TRP superfamily of cation channelsSci STKE 2001:
re1200110.1126/stke.2001.90.re111752662
|
|
16.
|
V SydorenkoY ShubaS
ThebaultReceptor-coupled, DAG-gated Ca2+-permeable
cationic channels in LNCaP human prostate cancer epithelial cellsJ
Physiol548823836200312724346
|
|
17.
|
Y El HianiA AhidouchM RoudbarakiS GueninG
BruleH Ouadid-AhidouchCalcium-sensing receptor stimulation induces
nonselective cation channel activation in breast cancer cellsJ
Membr Biol211127137200617041782
|
|
18.
|
I Dhennin-DuthilleM GautierM FaouziHigh
expression of transient receptor potential channels in human breast
cancer epithelial cells and tissues: correlation with pathological
parametersCell Physiol Biochem28813822201110.1159/000335795
|
|
19.
|
B MinkeB CookTRP channel proteins and
signal transductionPhysiol Rev82429472200211917094
|
|
20.
|
DE ClaphamTRP channels as cellular
sensorsNature426517524200310.1038/nature0219614654832
|
|
21.
|
A MottolaS AntoniottiD LovisoloL
MunaronRegulation of noncapacitative calcium entry by arachidonic
acid and nitric oxide in endothelial cellsFASEB
J1920752077200516204355
|
|
22.
|
B NiliusG DroogmansIon channels and their
functional role in vascular endotheliumPhysiol
Rev8114151459200111581493
|
|
23.
|
LC NuttleAL LigonKR FarrellRL
HesterInhibition of phospholipase A2 attenuates functional
hyperemia in the hamster cremaster muscleAm J
Physiol276H1289H1294199910199854
|
|
24.
|
S ChybP RaghuRC HardiePolyunsaturated
fatty acids activate the Drosophila light-sensitive channels
TRP and TRPLNature397255259199910.1038/16703
|
|
25.
|
H WatanabeJ VriensJ PrenenG DroogmansT
VoetsB NiliusAnandamide and arachidonic acid use
epoxyeicosatrienoic acids to activate TRPV4
channelsNature424434438200310.1038/nature0180712879072
|
|
26.
|
M GoelWG SinkinsWP SchillingSelective
association of TRPC channel subunits in rat brain synaptosomesJ
Biol Chem2774830348310200210.1074/jbc.M20788220012377790
|
|
27.
|
MC BunielWP SchillingDL KunzeDistribution
of transient receptor potential channels in the rat carotid
chemosensory pathwayJ Comp
Neurol464404413200310.1002/cne.1079812900933
|
|
28.
|
C ZittAG ObukhovC StrubingExpression of
TRPC3 in Chinese hamster ovary cells results in calcium-activated
cation currents not related to store depletionJ Cell
Biol13813331341199710.1083/jcb.138.6.1333
|
|
29.
|
T HofmannAG ObukhovM SchaeferC HarteneckT
GudermannG SchultzDirect activation of human TRPC6 and TRPC3
channels by
diacylglycerolNature397259263199910.1038/167119930701
|
|
30.
|
E KaznacheyevaL GlushankovaV
BugajSuppression of TRPC3 leads to disappearance of store-operated
channels and formation of a new type of store-independent channels
in A431 cellsJ Biol Chem2822365523662200710.1074/jbc.M608378200
|
|
31.
|
X LiuBC BandyopadhyayBB SinghK GroschnerIS
AmbudkarMolecular analysis of a store-operated and
2-acetyl-sn-glycerol-sensitive non-selective cation channel.
Heteromeric assembly of TRPC1-TRPC3J Biol
Chem2802160021606200510.1074/jbc.C40049220015834157
|
|
32.
|
BC BandyopadhyayWD SwaimX LiuRS RedmanRL
PattersonIS AmbudkarApical localization of a functional
TRPC3/TRPC6-Ca2+-signaling complex in polarized
epithelial cells. Role in apical Ca2+ influxJ Biol
Chem2801290812916200515623527
|
|
33.
|
H MevesModulation of ion channels by
arachidonic acidProg
Neurobiol43175186199410.1016/0301-0082(94)90012-47526418
|
|
34.
|
H SchmittH MevesModulation of neuronal
calcium channels by arachidonic acid and related substancesJ Membr
Biol145233244199510.1007/BF002327157563024
|
|
35.
|
EA DennisDiversity of group types,
regulation, and function of phospholipase A2J Biol
Chem269130571306019948175726
|
|
36.
|
EJ AckermannES KempnerEA
DennisCa(2+)-independent cytosolic phospholipase A2 from
macrophage-like P388D1 cells. Isolation and characterizationJ Biol
Chem269922792331994
|
|
37.
|
EA DennisThe growing phospholipase A2
superfamily of signal transduction enzymesTrends Biochem
Sci2212199710.1016/S0968-0004(96)20031-39020581
|
|
38.
|
VE SteeleET HawkJL VinerRA LubetMechanisms
and applications of non-steroidal anti-inflammatory drugs in the
chemoprevention of cancerMutat Res523–524137144200312628511
|
|
39.
|
MK JungnickelH MarreroL BirnbaumerJR
LemosHM FlormanTrp2 regulates entry of Ca2+ into mouse
sperm triggered by egg ZP3Nat Cell
Biol3499502200110.1038/3507457011331878
|
|
40.
|
SZ XuDJ BeechTrpC1 is a membrane-spanning
subunit of store-operated Ca(2+) channels in native vascular smooth
muscle cellsCirc Res888487200111139478
|
|
41.
|
F Vanden AbeeleL LemonnierS ThebaultTwo
types of store-operated Ca2+ channels with different
activation modes and molecular origin in LNCaP human prostate
cancer epithelial cellsJ Biol Chem2793032630337200415138280
|
|
42.
|
JW Putney JrM TrebakG VazquezB WedelGS
BirdSignalling mechanisms for TRPC3 channelsNovartis Found
Symp258123133discussion 133–139, 155–159, 263–266, 2004
|
|
43.
|
C BaldiG VazquezJC CalvoR BolandTRPC3-like
protein is involved in the capacitative cation entry induced by
1alpha,25-dihydroxy-vitamin D3 in ROS 17/2.8 osteoblastic cellsJ
Cell Biochem90197205200310.1002/jcb.1061212938168
|
|
44.
|
S ThebaultA ZholosA
EnfissiReceptor-operated Ca2+ entry mediated by
TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell lineJ
Cell Physiol204320328200515672411
|
|
45.
|
G VazquezBJ WedelM TrebakG St John BirdJW
Putney JrExpression level of the canonical transient receptor
potential 3 (TRPC3) channel determines its mechanism of activationJ
Biol Chem2782164921654200310.1074/jbc.M30216220012686562
|
|
46.
|
TJ ShuttleworthArachidonic acid activates
the noncapacitative entry of Ca2+ during
[Ca2+]i oscillationsJ Biol
Chem271217202172519968702966
|
|
47.
|
TJ ShuttleworthJL ThompsonMuscarinic
receptor activation of arachidonate-mediated Ca2+ entry
in HEK293 cells is independent of phospholipase CJ Biol
Chem2733263632643199810.1074/jbc.273.49.326369830003
|
|
48.
|
O MignenTJ ShuttleworthI(ARC), a novel
arachidonate-regulated, noncapacitative Ca(2+) entry channelJ Biol
Chem275911491192000
|
|
49.
|
O MignenJL ThompsonTJ
ShuttleworthCa2+ selectivity and fatty acid specificity
of the noncapacitative, arachidonate-regulated Ca2+
(ARC) channelsJ Biol Chem27810174101812003
|
|
50.
|
TJ ShuttleworthJL ThompsonO MignenARC
channels: a novel pathway for receptor-activated calcium entry
(Review)Physiology
(Bethesda)19355361200410.1152/physiol.00018.200415546853
|
|
51.
|
D LuoLM BroadGS BirdJW Putney JrMutual
antagonism of calcium entry by capacitative and arachidonic
acid-mediated calcium entry pathwaysJ Biol
Chem2762018620189200110.1074/jbc.M10032720011274150
|
|
52.
|
O MignenJL ThompsonTJ
ShuttleworthReciprocal regulation of capacitative and
arachidonate-regulated noncapacitative Ca2+ entry
pathwaysJ Biol
Chem2763567635683200110.1074/jbc.M10562620011470795
|
|
53.
|
GN HuangW ZengJY KimSTIM1
carboxyl-terminus activates native SOC, I(crac) and TRPC1
channelsNat Cell Biol810031010200610.1038/ncb145416906149
|
|
54.
|
D OliverCC LienM SoomT BaukrowitzP JonasB
FaklerFunctional conversion between A-type and delayed rectifier
K+ channels by membrane
lipidsScience304265270200410.1126/science.109411315031437
|
|
55.
|
AR BrashArachidonic acid as a bioactive
moleculeJ Clin Invest10713391345200110.1172/JCI1321011390413
|
|
56.
|
GY SunJ XuMD JensenPhospholipase A2 in
astrocytes: responses to oxidative stress, inflammation, and G
protein-coupled receptor agonistsMol
Neurobiol312742200510.1385/MN:31:1-3:02715953810
|