Introduction
Mechanical ventilators are indispensable for
patients with many types of respiratory failure so that vital
organs can be adequately oxygenated. However, excessive mechanical
ventilation may cause serious complications, one of which is
ventilator-induced lung injury (VILI) (1–3).
VILI leads to an increase of cytokine and/or chemokine production
and alveolar-capillary permeability, promoting protein-rich edema
formation, thus impairing gas exchange (4–6).
In addition, it is generally agreed that VILI induces multiple
organ failure (MOF), which can ultimately prove fatal. It has been
suggested that VILI may trigger release of inflammatory mediators
into the circulation, thereby exacerbating a pro-inflammatory
systemic environment and eventually leading to detrimental effects
in distal organs (7–9).
Pulmonary cells are overstretched when volumes in
some areas in the lung are increased by mechanical ventilation
(10,11). The effects of cyclic stretching
have been studied in various cell systems (12–18). Studies in vivo and in
vitro have suggested that cell stretching induces gene
expression and protein production of various inflammatory
mediators, such as tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-6, and IL-8 (19,20). Recently, it was shown that the
expressions of early response genes [early growth response gene
(EGR)1, heat shock protein (HSP)70, IL-1β, IL-6, and macrophage
inflammatory protein (MIP)-2] were changed by over-distension of
the lungs in adult and newborn rats, before the appearance of overt
lung injury (21). In those
studies, however, the cells were stretched for only a few specific
time periods, and the expressions of the genes/proteins of
inflammatory mediators were often assessed only after stretching
had been terminated or significant lung injury had occurred. None
of the studies investigated the time courses of these changes in
expression. Although it is now well known that specific mediators
are involved in the pathogenesis of stretch-induced lung injury,
only fragmentary evidence of their role in vivo and in
vitro has been obtained (20–23). In other words, although the steady
state of molecular systems causing VILI has been clarified to some
extent, further understanding of the dynamics of these systems is
still required.
The aim of this study was to examine time-courses of
the gene expression and protein production of IL-6 in human
pulmonary artery endothelial cells (HPAECs) when subjected to
cyclic stretching. HPAECs were chosen for four reasons: i) To our
knowledge, production of IL-6 protein by HPAECs subjected to cyclic
stretching has not been investigated in detail in vitro. ii)
The inflammatory mediators produced by HPAECs located on the
surface of the pulmonary artery would easily pass into circulating
blood. iii) Our previous study showed that the strain on HPAECs was
nearly equal to that on a flexible silicoelastic membrane on which
HPAECs were cultured (24). iv)
Among cell lines derived from normal tissues in the lung, only
HPAECs were available. IL-6 was chosen because our preliminary
experiments had shown that, among various inflammatory mediators,
IL-6 protein was that mainly produced by HPAECs subjected to cyclic
stretching (25).
Materials and methods
Cell culture
HPAECs (CC-2530; Lonza) were cultured in a growth
medium, EGM-2 Bullet kit™, consisting of EBM-2 and SingleQuots™
(human epidermal growth factor, heparin, hydrocortisone, FBS,
ascorbic acid, vascular endothelial growth factor (VEGF),
insulin-like growth factor (R3-IGF)-1 and GA-1000 (50 mg/ml
gentamicin, 50 μg/ml amphotericin B; CC-3162; Lonza). The
concentrations of the added factors in SingleQuots are
unpublished.
HPAECs passaged 7 times were seeded on 6-well
flexible silicoelastic membrane culture plates (BioFlex®
Culture Plates; Flexcell International) at a density of
2×104 cells/cm2. The seeded area of the
culture plate was where uniform strain was imposed on the cells
(Fig. 1). Pre-culture was
performed for 48 h (21% O2, 5% CO2, 37°C),
after which the cells adhered tightly to the membrane.
Cyclic stretching
A flexcell strain unit (Flexercell® FX-4000T™
Tension System and BioFlex® Loading Stations™; Flexcell
International) provided uniform strain on a membrane surface by
application of vacuum pressure to a silicoelastic membrane
(26). Deformation of the
membrane led to the elongation of the cells adhering to its surface
(Fig. 2).
Under our experimental conditions, the cyclic
stretching comprised 20% elongations with a square waveform of 15
cycles/min, and the ratio of stretching to relaxation was 1:2. The
20% elongation is the maximum achievable with the FX-4000T™ using a
circular loading post. Cyclic stretching that exceeds 15%
elongation has been reported to be pathological (27–29). The waveform, cycle and ratio
employed are equivalent to the clinical standards of mechanical
ventilation, and thus mimicked excessive mechanical
ventilation.
The longest stretching duration of 48 h was employed
for two reasons. One is that the compliance of the membrane
remained constant until stretching for 72 h (29), and the other is that the provider
of the medium (Lonza) recommends replacement of the medium every 48
h.
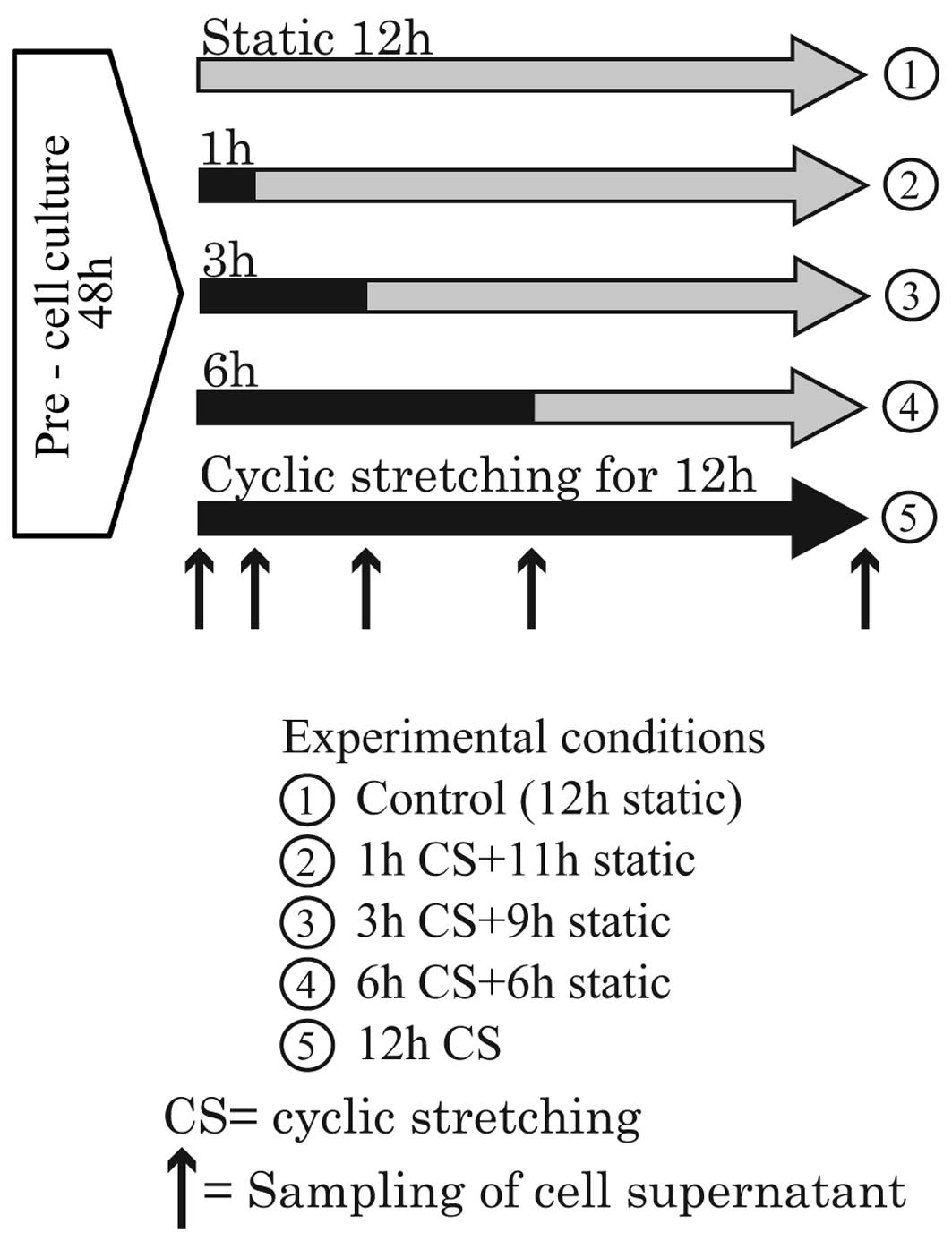
Production of cell cytokines
After pre-culture, the cells were stretched for
various durations. The experiments were broadly divided into two
groups because only four flexible bottom culture plates can be set
simultaneously in the Loading Stations™. Thus, four different
durations of stretching are possible in a single experiment. In the
first group, the durations were 1, 3, 6 and 12 h. During the
experiments, the cell supernatant was sampled at the time points
indicated by the up-pointing arrows in Fig. 3. In the second group, the
durations were 12, 24, 36 and 48 h. During the experiments, the
cell supernatant was sampled at the time points indicated by the
up-pointing arrows in Fig. 4.
The supernatants of the cells that had been
stretched in the two groups of experiments were assayed for the
protein concentrations of IL-1β, IL-6 and IL-8 by ELISA (KHC0011,
KHC0061, KHC0081; Invitrogen) to clarify whether they had been
increased by cyclic stretching.
Real-time RT-PCR
After pre-culture, the cells were stretched for 5,
10 and 30 min and 1 and 3 h. cDNA was synthesized from total-RNA,
extracted from the cells before and after stretching (Fig. 5), using an iScript™ cDNA Synthesis
kit (Bio-Rad Laboratories, Inc.). The real-time RT-PCR reaction was
performed using a DNA Engine Opticon® system (Bio-Rad
Laboratories, Inc.) with primers for IL-6. Reaction data were
normalized relative to the expression of GAPDH.
Statistical analysis
All measured experimental data are presented as
fold-change (means ± SD) relative to control samples. The
Kruskal-Wallis and Scheffe tests were used for statistical
comparisons of the expression levels of both the IL-6 gene and
protein. Significance was accepted at P<0.05. All experiments
were repeated 5 times independently (n=5).
Results
Stretching duration and IL-6 protein
production
The fold changes shown in Fig. 6 were obtained by dividing the
measured IL-6 protein levels by the IL-6 protein level before the
start of stretching (0 h). The fold change in unstretched (control)
cells appeared to be increased at 3, 6 and 12 h after the start of
stretching, but the increase was not statistically significant.
When the cells were stretched, many significant differences were
found in the IL-6 protein levels at 3, 6 and 12 h relative to those
in unstretched cells at the same time points. To show these
increases more clearly, other fold changes were calculated by
dividing the IL-6 protein levels in the stretched cells by those in
the unstretched cells at the same time points (Fig. 7). When the cells were stretched
for 1 h and subsequently unstretched, the degree of increase in
protein expression remained unchanged from 0 to 12 h. When the
cells were stretched for 3 h and subsequently unstretched, the fold
change at 3 h was significantly higher than at any other time
point. When the cells were stretched for 6 h and subsequently
unstretched, the fold changes at 3 and 6 h were significantly
higher than that at 0 h, and the fold changes at 3 and 12 h were
significantly higher than that at 1 h. When the cells were
stretched for 12 h, the fold changes at 3, 6 and 12 h were
significantly higher than those at 0 and 1 h.
These results showed that at least 3 h of stretching
was necessary to increase the expression of IL-6 protein, and that
when stretching was discontinued after 3 h of stretching the
protein expression did not increase further.
Expression of IL-1β protein was not detected at any
time point, and the level of IL-8 protein showed no significant
changes during cyclic stretching (data not shown).
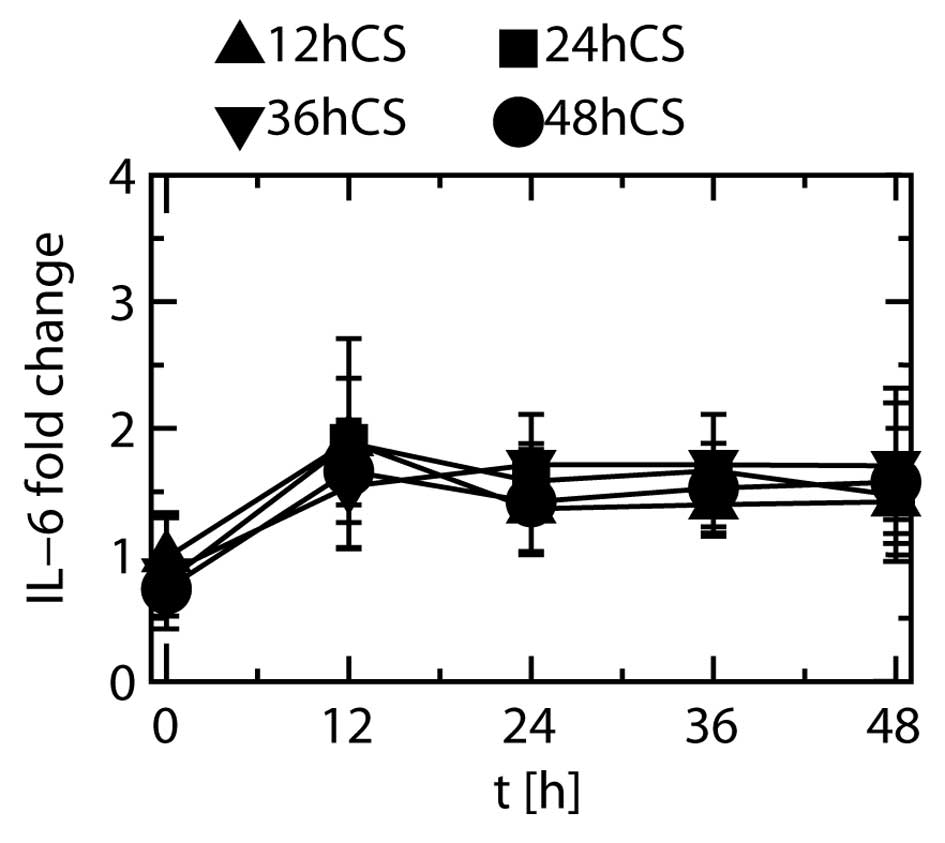
Stretching duration and IL-6 protein
production
Fold changes in the expression of IL-6 when the
cells were stretched for 12, 24, 36 and 48 h are depicted in
Fig. 8. Most of the fold changes
observed in the stretched cells showed no significant differences
relative to that at 0 h, irrespective of stretching duration.
IL-1β protein was not detected at any time point,
and the level of IL-8 protein did not show any significant changes
during cyclic stretching (data not shown).
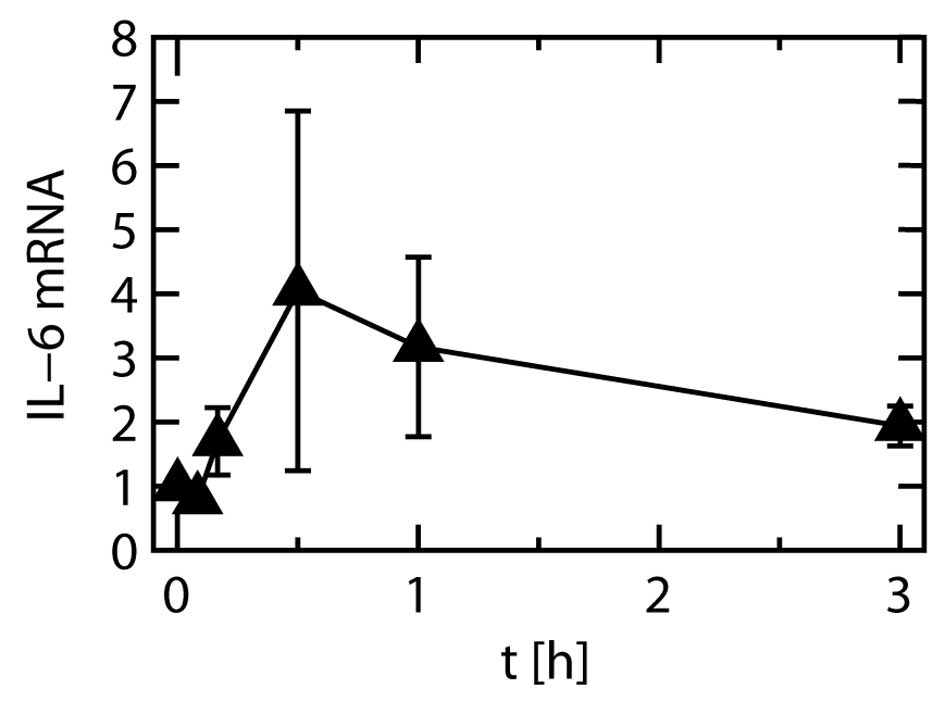
Gene expression
The expression level of the IL-6 gene were
significantly increased by stretching at all time points other than
1 min, relative to the levels before the start of stretching. The
level peaked at 30 min after the start of stretching (Fig. 9).
Discussion
The levels of IL-6 mRNA and protein peaked at 30 min
and at 3 h of stretching, respectively. The difference in the peak
times probably reflects the process of IL-6 protein synthesis after
the appearance of IL-6 mRNA.
IL-6 protein usually decays in serum in vivo
(30,31), but under our in vitro
experimental conditions it did not do so, even without stretching.
One possible explanation for this finding is that in vivo
the IL-6 protein is eliminated by certain enzymes, which are
lacking under in vitro conditions. Furthermore, the IL-6
protein is probably released from HPAECs without any stimulation
when they are cultured in medium, as has been reported for the
culture of human AG01522 fibroblasts (32).
Our finding that even when stretching was continued
for 12 h, there was no further increase in the IL-6 protein level
compared to 3 h of stretching, was in accordance to the finding
that IL-6 mRNA expression was considerably reduced after 3 h of
stretching in comparison to its peak (Fig. 9). As IL-6 protein expression was
maximal after a specific duration (3 h) of cyclic stretching, it
appears that timing is important when investigating interactions
among inflammatory mediators and/or their precursors.
Acknowledgements
This study was supported in part by a
grant from Kitasato University School of Allied Health Sciences
nos. 2010-1049 and 2011-1050 to K.K., no. 2011-1033 to M.N. and a
scientific research grant from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (no. 23792084) to K.K.
References
|
1.
|
JC PerkerLA HernandezKJ PeevyMechanisms of
ventilator-induced lung injuryCrit Care
Med21131143199310.1097/00003246-199301000-000248420720
|
|
2.
|
L PinhuT WhiteheadT EvansM
GriffithsVentilator-associated lung
injuryLancet361332340200310.1016/S0140-6736(03)12329-X12559881
|
|
3.
|
V LionettiFA RecchiaVM RanieriOverview of
ventilator-induced lung injury mechanismsCurr Opin Crit
Care118286200510.1097/00075198-200502000-0001315659950
|
|
4.
|
LN TremblayAS SlutskyVentilator-induced
injury: from barotraumas to biotraumaProc Assoc Am
Physicians11048248819989824530
|
|
5.
|
JD RicardD DreyfussG
SaumonVentilator-induced lung injuryEur Respir J
Suppl422S9S200310.1183/09031936.03.0042010312945994
|
|
6.
|
LN TremblayAS SlutskyVentilator-induced
injury: from the bench to the bedsideIntensive Care
Med322433200610.1007/s00134-005-2817-816231069
|
|
7.
|
AS SlutskyLN TremblayMultiple system organ
failure. Is mechanical ventilation a contributing factor?Am J
Respir Crit Care
Med15717211725199810.1164/ajrccm.157.6.97090929620897
|
|
8.
|
VM RanieriPM SuterC TortorellaEffect of
mechanical ventilation on inflammatory mediators in patients with
acute respiratory distress syndrome: a randomized controlled
trialJAMA2825461199910.1001/jama.282.1.54
|
|
9.
|
FB PlotzAS SlutskyAJ van VughtCJ
HeijnenVentilator-induced lung injury and multiple system organ
failure: a critical review of facts and hypothesesIntensive Care
Med3018651872200410.1007/s00134-004-2363-915221129
|
|
10.
|
J MeadT TakishimaD LeithStress
distribution in lungs: a model of pulmonary elasticityJ Appl
Physiol2859660819705442255
|
|
11.
|
JJ MariniNew opinions for the ventilator
management of acute lung injuryNew Horiz148950319938087570
|
|
12.
|
I KomuroT KaidaY ShibazakiM KurabayashiY
KatohE HohF TakakuY YazakiStretching cardiac myocytes stimulates
protooncogene expressionJ Biol Chem2653595359819902105950
|
|
13.
|
T IbaS MaitzT FurbertO RosalesMD WidmannB
SpillaneT ShinT SonodaBE SumpioEffect of cyclic stretch on
endothelial cells from different vascular bedCirc
Shock3519319819911777956
|
|
14.
|
JE ScottSY YangE StanikJE
AndersonInfluence of strain on [3H]thymidine incorporation,
surfactant-related phospholipid synthesis, and cAMP levels in fetal
type II alveolar cellsAm J Respir Cell Mol Biol82582651993
|
|
15.
|
F LyallMR DeehanIA GreerF BoswellWC
BrownGT McInnesMechanical stretch increases protooncogene
expression and phosphoinositide turnover in vascular smooth muscle
cellsJ Hypertens1211391145199410.1097/00004872-199410000-00003
|
|
16.
|
DL WangBS WungYJ ShyyCF LinYJ ChaoS UsamiS
ChienMechanical strain induces monocyte chemotactic protein-1 gene
expression in endothelial cell. Effects of mechanical strain on
monocyte adhesion to endothelial cellsCirc
Res77294302199510.1161/01.RES.77.2.2947614716
|
|
17.
|
J PuginI DunnP JollietD TassauxJL
MagnenatLP NicodJC ChevroletActivation of human macrophages by
mechanical ventilation in vitroAm J
Physiol275L1040L105019989843840
|
|
18.
|
NE VlahakisMA SchroederAH LimperRD
HubmayrStretch induces cytokine release by alveolar epithelial
cells in vitroAm J Physiol277L167L173199910409244
|
|
19.
|
A von BethmannF BraschKM MullerS
UhligBarotrauma-induced cytokine and eicosanoid release from the
isolated perfused and ventilated mouse lungAm J Respir Crit Care
Med153A5301996
|
|
20.
|
L TremblayF ValenzaSP RibeiroJ LiAS
SlutskyInjurious ventilatory strategies increase cytokines and
c-fos m-RNA expression in an isolated rat lung modelJ Clin
Invest99944952199710.1172/JCI1192599062352
|
|
21.
|
IB CoplandBP KavanaghD EngelbertsC
McKerlieJ BelikM PostEarly changes in lung gene expression due to
high tidal volumeAm J Respir Crit Care
Med16810511059200312816737
|
|
22.
|
IB CoplandM PostStretch-activated
signaling pathways responsible for early response gene expression
in fetal lung epithelial cellsJ Cell
Physiol210133143200710.1002/jcp.2084016998809
|
|
23.
|
JD RicardD DreyfussG SaumonProduction of
inflammatory cytokines in ventilator-induced lung injury: a
reappraisalAm J Respir Crit Care
Med16311761180200110.1164/ajrccm.163.5.200605311316656
|
|
24.
|
K KobayashiA KishiM TanakaH InaokaS
NebuyaY FukuokaH KobayashiM NoshiroComparison of strains on a
silicoelastic membrane and on cells adhering to the membraneTrans
Jpn Soc Med Biol Eng474644692009(In Japanese)
|
|
25.
|
K KobayashiM TanakaH InaokaS NebuyaY
FukuokaK KokuboH KobayashiM NoshiroTemporal changes in gene
expressions and cytokine productions caused by stretching of normal
human pulmonary artery endothelial cellsEur Respir J36Suppl
54432s433s2010
|
|
26.
|
JP Vande GeestES Di MartinoDA VorpAn
analysis of the complete strain field within Flexercell membranesJ
Biomech3719231928200415519600
|
|
27.
|
KG BirukovJR JacobsonAA FloresSQ YeAA
BirukovaAD VerinJG GarciaMagnitude-dependent regulation of
pulmonary endothelial cell barrier function by cyclic stretchAm J
Physiol Lung Cell Mol
Physiol285L785L797200310.1152/ajplung.00336.200212639843
|
|
28.
|
JE WoodellM LaBergeEM Langan IIIRH
HildermanIn vitro strain-induced endothelial cell dysfunction
determined by DNA synthesisProc Inst Mech Eng
H2171320200310.1243/09544110376259769212578215
|
|
29.
|
DJ TschumperlinJ OswariAS
MarguliesDeformation-induced injury of alveolar epithelial cells.
Effect of frequency, duration, and amplitudeAm J Respir Crit Care
Med162357362200410.1164/ajrccm.162.2.980700310934053
|
|
30.
|
T SaitohE KokueM ShimodaThe impact of
acute phase response on the plasma clearance of antipyrine,
theophylline, phenytoin and nifedipine in rabbitJ Vet Pharmacol
Ther23153158200010.1046/j.1365-2885.2000.00266.x11110102
|
|
31.
|
BP RamakersM de GoeijJG van der HoevenWH
PetersP PickkersInflammation-induced hepatotoxicity in
humansShock31151156200910.1097/SHK.0b013e31818335ff18636040
|
|
32.
|
L MariottiA FacoettiD AlloniA BertolottiE
RanzaA OttolenghiEffects of ionizing radiation on cell-to-cell
communicationRadiat Res174280289201010.1667/RR1889.120726722
|