Introduction
Asthma is a group of complex diseases associated
with allergic airway inflammation, airway hyperresponsiveness (AHR)
to a variety of specific and nonspecific stimuli, chronic pulmonary
eosinophil infiltration, increased serum immunoglobulin E (IgE),
excessive mucus secretion and other features. At present, there is
no cure for asthma. Moreover, the frequency and severity of asthma
have increased steadily in developed nations (1). The pathophysiology of asthma is
thought to be mediated by activated CD4+ T lymphocytes,
which produce type 2 cytokines interleukin (IL)-4, IL-5, IL-9 and
IL-13 (2,3). Studies have revealed that IL-13 is
the most important mediator of asthma. Daily administration of
recombinant IL-13 (rIL-13) to the airways of unimmunized mice could
induce AHR, whereas blockade of IL-13 by a soluble IL-13α2-IgGFc
fusion protein (sIL-13α2-Fc) could result in a complete reversal of
such allergen-induced AHR (1,4).
In addition, therapeutic dosing with an anti-IL-13 monoclonal
antibody (mAb) could effectively reverse established AHR, and limit
asthmatic responses in a chronic mouse model of persistent asthma
(5). Moreover, neutralization of
IL-13 could inhibit the production of multiple cytokines,
chemokines, and matrix metalloproteinase-9 (MMP-9) (5). It has become increasingly clear that
IL-13 is a novel therapeutic target for the treatment of
asthma.
A variety of monoclonal antibodies and soluble
receptors blocking IL-13 have been developed for the treatment of
asthma, and some of them are currently being evaluated in clinical
development (6). These approaches
are mainly based on the development and administration of
functional recombinant protein antagonists that neutralize the
extracellular IL-13 function or block IL-13 signaling in target
cells. Although successful, these strategies also possess many
disadvantages, including difficulties in manufacturing active
recombinant protein, high-dose requirements, high costs for
manufactures and consumers, and the probable need for lifetime
treatment of the patient. Due to their relatively short half-lives,
recombinant proteins must be administered repeatedly by injection
once to several times a day (7).
Moreover, these approaches may have the risk of potential side
effects, such as infusion reactions and the production of
antibodies to the therapeutic agent (8). A peptide of IL-13 conjugated to
truncated hepatitis B core antigen has been developed and utilized
in mouse asthma models (9). The
mice immunized with IL-13 peptide-based vaccine displayed
suppression of IgE titers, airway inflammation, mucus production,
and AHR to ovalbumin (OVA) immunization and challenge. In the
present study, we developed a new mIL-13 derivative vaccine that
contained a keyhole limpet hemocyanin (KLH)-mIL-13 heterocomplex
(hereafter named ‘KLH-P’) without biological activity.
Asthma etiopathogenesis involves genetic
susceptibility, environmental factors, and clinical manifestations
that are closely associated with the presence of activated Th2
cytokine (IL-4, IL-5 and IL-13) (10). Numerous studies have provided
compelling evidence that IL-13 was necessary and sufficient to
induce all features of allergic asthma (11), and a therapeutic target for IL-13
is effective. KLH is a xenoantigen largely used in vivo as a
vaccine component with optimal carrier qualities, especially for
cancer vaccines (12). With the
use of KLH, a potent carrier, haptens and peptides would further
augment the immunogenicity in conjugate vaccines. This suggests
that the critical role of these conjugate vaccines may be the
induction of higher levels of T-cell immunity against KLH, which
leads to higher levels of Ab against the conjugated antigens. Based
on these findings, we constructed KLH-P by coupling mIL-13 peptide
to KLH by aldehyde treatment. In the heterocomplex, each KLH
carrier molecule is linked to a large number of mIL-13 peptides by
covalent and noncovalent bonds (13), which is devoid of the biological
activity of native IL-13 and is still immunogenic in mice.
The novel vaccine could trigger a B cell response
and generate Ab to mIL-13. To our knowledge, we have demonstrated
for the first time that KLH-P is able to induce high-titer IL-13
specific IgG with aluminum hydroxide as an adjuvant and prevent
mice from airway inflammation, epithelial cell proliferation with
goblet cell hyperplasia as well as other features of allergic
disease in a mouse model of acute asthma. Most importantly, this
study also showed that KLH-P could be a promising vaccine against
asthma.
Materials and methods
Reagents
A mouse IL-13 peptide was selected to generate a new
vaccine as previously reported (9). KLH and mIL-13 peptide were purchased
from China Peptides Co., Ltd.; mouse IL-13 protein from Invitrogen
Life Technologies (Carlsbad, CA, USA); goat anti-mouse IgG and
rabbit anti-mouse IgE were from Sigma-Aldrich (Schnelldorf,
Germany); the mIL-13 enzyme-linked immunosorbent assay (ELISA) kit,
the mIL-4 ELISA kit and the mIFN-γ ELISA kit were from R&D
Systems (Minneapolis, MN, USA); the total IgE ELISA kit from MD
Biosciences, Inc. (St. Paul, MN, USA); complete and incomplete
Freund’s adjuvant were from Sigma-Aldrich; OVA was from
Sigma-Aldrich; and aluminum hydroxide was from Pierce Chemical Co.
(Rockford, IL, USA).
Immunogenicity
The artificial antigen of IL-13 was prepared by
complexing mIL-13 with KLH by aldehyde treatment, as previously
reported (14). A control was
prepared by native KLH sample treated similarly and referenced in
the text as KLH. Three New Zealand white rabbits were
subcutaneously immunized with conjugates (KLH-P) and developed
satisfactory Ab titers, with the highest titer being 105
(data not shown).
Mice
Specific pathogen-free female BALB/c mice
(7–8-week-old) were purchased from Beijing Hua Fu Kang Biological
Technology Co., Ltd. (Beijing, China). All protocols used were
approved by the Institutional Animal Care and Use Committee of
Sichuan University (Chengdu, China).
Protocol of immunization, sensitization,
and challenge
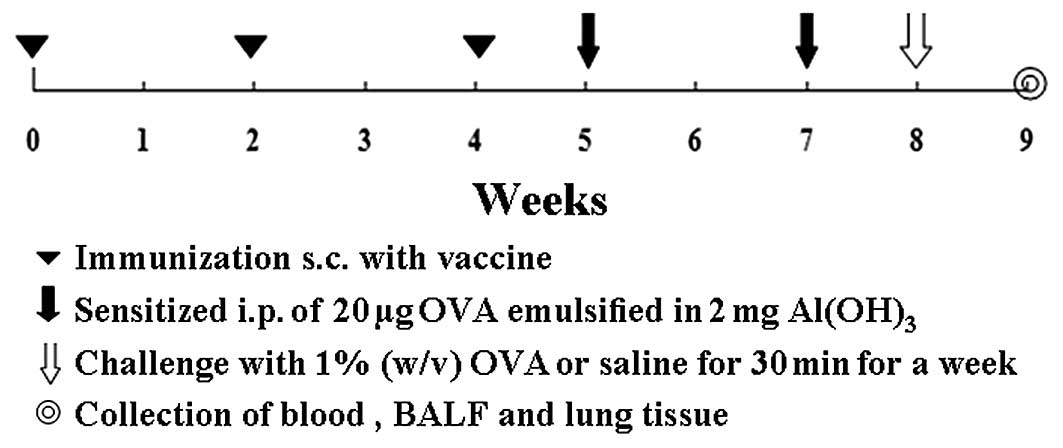
Mice were subsequently divided into four groups
(Fig. 1) including: i) KLH-P 10
μg (n=15), immunized subcutaneously with 10 μg vaccine, then
subjected to intraperitoneal sensitization and intranasal challenge
with OVA; ii) KLH-P 50 μg (n=15), immunized with 50 μg vaccine and
sensitized/challenged with OVA; iii) KLH (n=15), injected with KLH
and sensitized/challenged with OVA; iv) saline (n=15), injected
with saline and no exposure to OVA. In this protocol, mice were
immunized subcutaneously with vaccine on Weeks 0, 2 and 4 using
aluminum hydroxide as an adjuvant, and sensitized on Weeks 5 and 7,
by intraperitoneal injection of 20 μg OVA emulsified in 2 mg of
aluminum hydroxide in a total volume of 200 μl. After the initial
sensitization, the saline group of mice was only exposed to the
inhalation of a saline aerosol and three other groups were exposed
to an aerosol of 1% (w/v) OVA in saline using an ultrasonic
nebulizer (Pari-Boy; Pari-Werke, Starnberg, Germany) for 30 min for
Week 8.
Collection of blood and bronchoalveolar
lavage fluid (BALF) samples
Blood samples were collected on Weeks 1, 3, 5 and 7
before the succeeding OVA administration, and at the endpoint of
the experiment. Seventy-two hours after the last challenge, all
mice were sacrificed and their blood samples were collected. The
samples were centrifuged at 4°C for 10 min (1,200 rpm). Serum was
frozen and stored at −80°C for the measurement of cytokines and
antibodies by ELISA. BALF was collected as described previously
(15). Briefly, after the mice
were sacrificed, the chest cavity was exposed, the trachea was
carefully intubated with a cannula which was secured with
ligatures, 1 ml of cold normal saline was slowly infused into the
lungs and withdrawn. The BALF was centrifuged at 4°C for 10 min
(1,200 rpm). Supernatants of BALF were collected and stored at
−80°C until cytokine and leukotriene measurements were obtained.
Serum IL-13-specific IgG, total IgE, OVA-specific IgE, and cytokine
levels in BALF supernatants were assayed by an ELISA assay
following the manufacturer’s instructions.
Eosinophil cell counts in BALF
An eosinophil cell count in BALF was performed
automatically at the Chengdu GLP Center using an ADVIA2120
automated hematology analyzer (Siemens Healthcare Diagnostics,
Inc., Tarrytown, NY, USA).
ELISA
IL-13-specific IgG and OVA-specific IgE levels were
measured as described before (16). The levels of IL-13-specific IgG
and OVA-specific IgE levels were measured by coating microplates
with either mouse IL-13 (1 μg/ml) or OVA (2 μg/ml) at 4°C
overnight, incubating 2-fold serially diluted serum samples, flowed
by incubation with alkaline phosphatase-conjugated goat anti-mouse
IgG for IL-13-specific IgG assay or alkaline phosphatase-conjugated
rabbit anti-mouse IgE for OVA-specific IgE assay, and then adding
the substrate to develop the reaction. The concentration of IL-13,
IL-4 and IFN-γ in BALF supernatant were determined using commercial
ELISA kits according to the manufacturer’s specifications. The
concentration of total IgE in the serum was determined using
commercial ELISA kits according to the supplier’s instructions.
Pulmonary histology
Seventy-two hours after the last challenge, mice
were sacrificed and the lungs and trachea were filled with a
fixative (0.8% formalin, 4% acetic acid) intratracheally. Lungs
were removed and fixed with 10% (v/v) neutral-buffered formalin,
then dehydrated and embedded in paraffin. For the histological
examination, 5-μm sections of fixed embedded tissues were cut and
placed on glass slides, deparaffinized and stained with hematoxylin
and eosin (H&E) or alcian blue-periodic acid schiff (PAS) and
examined using an Olympus IX51 light microscope equipped with a CCD
camera. As previously described (15), the degree of peribronchiolar and
perivascular inflammation in H&E stained slides was evaluated
on a subjective indexed scale of 0 to 3: a value of 0 was adjudged
when no inflammation was detectable, a value of 1 for occasional
perivascular cuffing, a value of 2 for most bronchi or when vessels
were surrounded by a thin layer (1 to 5 cells) of inflammatory
cells, and a value of 3 when most bronchi or vessels were
surrounded by a thick layer (>5 cells) of inflammatory cells.
Scores were evaluated by three different observers blinded to the
experimental treatment. Glandular hyperplasia was observed using
the alcian blue-PAS combined staining in randomly selected bronchi.
Alcian blue-PAS stains mucin-secreted cells (goblet cells) and the
results were measured as a percentage of alcian blue-PAS positive
cells in the total airway epithelia of medium-sized airways.
Scoring was performed at a magnification of x200 by examining at
least 40 consecutive fields by one observer blinded to experimental
data.
Statistical analysis
Each experiment was performed three times with
similar results. All images acquired were characteristic of the
majority of analyzed tissues. Values were expressed as means ±
standard error of the mean (SEM). The significance of differences
between experimental groups was analyzed using one-way ANOVA
analysis of variance followed by Newman-Keuls multiple comparison
test. Statistical significance was set at P<0.05.
Results
KLH-P vaccination induction of
IL-13-specific IgG
First, we used three New Zealand white rabbits to
test the ability of the vaccine (KLH-P) to induce an IL-13-specific
IgG response after immunization with the vaccine in the presence of
adjuvant (alum or complete/incomplete Freund’s adjuvant, CFA/IFA).
All three rabbits immunized with the vaccine developed strong and
long-lasting IL-13-specific Ab, which reached high levels (titer up
to 105, data not shown) and sustained for 1 month before
any detectable decrease was measured. Mice were immunized with the
vaccine subcutaneously in the presence of an adjuvant (alum) and
generated satisfactory Ab titers. We used two distinct doses to
immunize mice to further our knowledge of the immunogenicity of
this vaccine. IL-13-specific IgG in serum response was displayed in
a dose-dependent manner within the dose range used. The result
indicated that 10 μg of vaccine given subcutaneously (s.c.) was
able to efficiently induce IgG responses (Fig. 2). During the period of the
experiment, 2 weeks after sensitization with OVA (Week 7),
IL-13-specific IgG titers slightly decreased when compared to those
at Week 5 immediately after the second immunization with the
vaccine, but remained high until the end of the study. The data
demonstrate that the KLH-P vaccine is immunogenic and displays
dose-dependent effects using aluminum hydroxide as adjuvant.
Total and OVA-specific IgE are reduced by
KLH-P vaccination
To determine whether KLH-P vaccine could modify an
OVA-specific T-helper 2 cell (Th2) response in vivo by
analyzing circulating IgE Ab levels 72 h after OVA challenge, sera
were collected on Weeks 0, 1, 3, 5, 7 and again at the endpoint of
the experiment (Week 9). Both the mean levels of serum total and
OVA-specific IgE antibodies in the vaccine-treated groups were
markedly reduced compared to those seen in the KLH groups (Fig. 3A and B). Total and OVA-specific
IgE levels were greatly increased after intraperitoneal
sensitization and intranasal challenge with OVA two times. However,
vaccination with KLH-P significantly suppressed total IgE (Week 7:
P<0.01, P<0.001 for KLH-P 10 and 50 μg, respectively; Week 9:
P<0.05 for KLH-P 50 μg) (Fig.
3A). In agreement with the inhibitory effect on total IgE,
immunization of KLH-P at 50 μg visibly reduced OVA-specific IgE
levels compared to the KLH group. After 2 times of sensitization
with OVA, even though IL-13-specific IgG levels slightly decreased
to some degree compared with those at Week 5, it still remained
103 at the endpoint of the study. In this experiment,
sera gathered at the endpoint with 5 single mice in each group were
analyzed to measure OVA-specific IgE (Week 9) (Fig. 3B). It appeared that the KLH-P
vaccine displayed a visible effect in inhibition of OVA-specific
IgE which was dose-dependent, although there was no statistical
significance observed.
KLH-P vaccine reduces eosinophil
percentage in BALF
To determine the contribution of this vaccine to
eosinophil recruitment after allergen challenge, BALF was collected
72 h after the last OVA aerosol challenge, and differential cell
counts were detected with an ADVIA2120 automated hematology
analyzer. OVA inhalation markedly (P<0.001) increased the
percentages of eosinophils compared with saline control. In
contrast, immunization with KLH-P vaccine in either dose
significantly (P<0.001) inhibited eosinophil recruitment to BALF
compared to the KLH group (Fig.
3C). These results suggest that KLH-P vaccination suppressed
the accumulation of eosinophils in BALF.
IL-13, IL-4 and IFN-γ levels in BALF
To assess whether IL-13, IL-4 and IFN-γ levels were
downregulated by this vaccine, BALF was collected and analyzed with
ELISA assays. IL-13 was increased in the BALF from mice exposed to
OVA. Most importantly, such an increase was inhibited by a previous
vaccination with KLH-P in either dose (P<0.001) (Fig. 4A). It’s worth noting that a
different vaccine dose did not significantly affect the mean levels
of IL-13 in BALF. The IL-4 (Fig.
4B) precludes any statistical significance despite the
appearance of a large decrease. When compared to normal mice
exposed only to saline, IFN-γ levels were apparently (P<0.05)
(Fig. 4C) increased in
OVA-sensitized and challenged mice. However, the mean level of
IFN-γ was not significantly modified in the group treated by
vaccine. Taking these results together, except for IL-13, the
current vaccine immunization did not produce significant effects on
cytokine levels compared with the KLH group.
KLH-P vaccine suppresses OVA-induced
tissue leukocyte influx and airway inflammation
To evaluate allergic airways inflammation, lung
tissues were collected 72 h after the last OVA exposure and
histological analyses were performed. Consistent with previous BALF
cytology, H&E staining of lung sections confirmed that the
accumulation of inflammatory cells in the airways was also
suppressed in mice that received KLH-P vaccine (Fig. 5A). Semi-quantitative analyses of
tissue inflammation were performed in each group, and a
peribronchial inflammation score was evaluated for 5–7 bronchi per
mouse (Fig. 5B). There was
evidence of significant peribronchial accumulation of inflammatory
cells in mice exposed to OVA, as representative examples are
presented in Fig. 5A. In
contrast, KLH-P vaccinated mice showed substantial attenuation in
the eosinophil rich leukocyte infiltration in the peribronchiolar
regions. Seventy-two hours after the last OVA challenge, the
inflammation scores of peribronchial as well as total lung were
increased significantly (P<0.001) in the KLH group compared with
scores in either dose of the KLH-P vaccine-treated group.
Additionally, the lung inflammation score observed after
immunization with the KLH-P vaccine in 10 μg dose is significantly
(P<0.01) lower than that of a group with a 50 μg dose. Taken
together, these results further confirmed that the KLH-P vaccine
significantly suppressed allergen-induced leukocyte influx and
airway inflammation.
The KLH-P vaccine suppresses airway
goblet cell metaplasia and mucus hypersecretion
Airway epithelial cell hyperplasia and mucus
secretion were assessed by an alcian blue-PAS combined staining. In
normal mice, goblet cells comprise less than 20% of cells in the
bronchial epithelium, and the number of goblet cells was greatly
decreased in the segmental bronchus (17). However, we observed an increased
number of goblet cells in the segmental bronchus, and prominent
mucus production by the airway epithelium in the KLH group compared
to the KLH-P vaccine-treated groups (Fig. 6A). We performed semi-quantitative
analysis of goblet cells in each group (Fig. 6B). Goblet cell metaplasia and
mucin hypersecretion were dramatically (P<0.001) inhibited in
the KLH-P vaccine treated groups. There was no significant
difference between the high-dose and low-dose groups (P>0.05).
PAS shows the red-purple cytoplasmic inclusion (neutral mucus) and
alcian blue shows the blue cytoplasmic inclusion (acid mucus).
Naturally, the same group samples or a single slice showed
different intensity staining neutral mucopolysaccharide (NMPS) and
acid mucopolysaccharide (AMPS). There is almost no neutral or acid
mucus production in the epithelial cell in saline control and
vaccinated mice compared a hypersecretion of acid mucus from goblet
cells in the KLH group. As a result, our findings have shown that
the KLH-P vaccine effectively suppressed allergen-induced airway
goblet cell metaplasia and mucus hypersecretion.
Discussion
Asthma is a lifelong disease that causes wheezing,
breathlessness, chest tightness, and coughing. It can limit a
person’s quality of life. The control of asthma symptomatology
requires a complex treatment plan that changes according to
presenting symptoms. Although most people with asthma may control
their symptoms and prevent asthma attacks by using medication, such
as inhaled corticosteroids. Recent studies have demonstrated that
adherence rates for asthmatic patients are particularly
problematic. Barriers to treatment include prolonged and complex
regimens, adverse effects, cost, and delayed onset of action. Less
than half of the patients could adhere to their prescribed regimens
(18,19), contributing to the poor clinical
outcomes in the treatment of asthma. Taking into account the
barriers of treatment-related therapy, a novel effective treatment
of asthma is strongly needed. In the present study, we aimed to
develop a novel vaccine to improve the efficacy and safety of
therapeutic strategies. The KLH-P vaccine has been constructed and
applied to asthmatic BALB/c mice sensitized and challenged with
OVA. We reported here that immunization of mice with this murine
KLH-P-induced polyclonal autoantibody neutralized excessive mIL-13
bioactivity and further inhibited the symptoms of experimental
model of murine asthma.
Immunization with our mIL-13 peptide kinoid induces
neutralizing auto-antibodies to mIL-13. We used two distinct doses
to further our knowledge of the immunogenicity of this vaccine. The
results indicate that just 10 μg of vaccine given s.c., rather than
100 μg given previously, was able to efficiently induce IgG
responses (Fig. 2). Although
there was a slight decrease of IL-13-specific IgG titers at Week 7
compared with those at Week 5, features of airway allergic
inflammatory response were prevented as long as the transient serum
remained above an effective threshold. The rapid decline of
neutralizing Abs after kinoid immunization (Fig. 2) may be due to the short life of B
cell memory in the absence of specific T cell help (20). Using the murine model of allergic
asthma and based on a preventative design, we further assessed the
efficacy of our IL-13 peptides kinoid vaccine in preventing total
and OVA-specific IgE amounts in serum, pulmonary eosinophilia,
allergic airway inflammation, and airway goblet cell hyperplasia
and mucus secretion.
IgE plays an important role in triggering the
allergic cascade, and anti-IgE therapy has confirmed its central
role in the inflammatory process of allergic asthma (21). Studies have demonstrated that
IL-13 contributes to IgE synthesis (22). Our results demonstrated that
vaccination with IL-13 peptides kiniod vaccine suppressed
allergen-induced total and OVA-specific IgE in a dose-dependent
manner. This is significant in order to prevent allergic symptoms.
Reduction in IgE levels leads to reduction in both basophil and
mast cell mediator response to allergen, and causes a decrease in
allergic inflammation by reducing the high-affinity IgE receptor on
mast cells and dendritic cells (23,24). Therefore, the suppression of Th2
responses IgE levels and eventually asthmatic symptoms may be
achieved through downregulation of IL-13. Collectively, the
effective suppression of total and OVA-specific IgE suggested our
IL-13 peptides kiniod vaccine has a strong potential in this
regard.
Airway eosinophilia is considered a cardinal feature
of allergic asthma (25). It is
thought that eosinophils contribute substantially not only to
airway remodeling (26), but also
to acute responses, such as goblet cell hyperplasia (27). As eosinophilia in the airway is an
important indicator of asthma, we performed eosinophil cell counts
in BALF and histopathological examination to assess the efficacy of
our IL-13 peptides kinoid vaccine on airway inflammation and goblet
cell hyperplasia. The results showed that immunization with two
different doses (10 or 50 μg) significantly suppressed the
accumulation of eosinophils in BALF (P<0.001 for both).
Furthermore, after immunization with the KLH-P
vaccine, we observed a dramatic suppression of allergen-induced
leukocyte influx, airway inflammation, and mucus production. Mucus
plugging has been recognized as a major factor contributing to
airway obstruction and mortality associated with acute severe
asthma (28–30). The continuity of surface goblet
cell granules and intraluminal mucus are an important source of the
secretions and contribute to the formation of mucus plugging in
asthma (30,31). It appears that mucus
hypersecretion is related to the pathogenesis of acute asthma
(30), and abnormal proliferation
of goblet cells is necessary for hypersecretion (31,32). The fact that acute airway
administration of IL-13 (32,33) or overproduction of IL13 in the
lung (34) could rapidly induce
mucus cell hyperplasia in nonimmunized mice and the formation of
Charcot-Leyden crystals (35)
indicates that IL-13 may play a role in promoting goblet cell
hyperplasia and mucus production. Further studies demonstrated that
IL-13 gene knockout (36) or
blockade of IL-13 by either administration of the soluble
IL-13α2-Ig (1,4) or through IL-13 gene targeting in
mice (36) prevents mucus
hypersecretion, and can reverse established goblet cell metaplasia.
Consistent with these observations, after immunization with the
KLH-P vaccine, we observed a dramatic suppression of
allergen-induced leukocyte influx, airway inflammation, and mucus
production, which is indicated by mucin-secreted goblet cells after
allergen challenge.
The mIL-13 kinoid immunization is safe. There were
no adverse reactions to the kinoid preparation. The lack of
autoimmune pathology due to the absence of T cell response after
other anti-cytokine immunizations was demonstrated in
kinoid-immunized mice, including TNF (37), VEGF (14), IFN-α (38), or other kinoids, minimizing the
risk of iatrogenic complications after kinoids immunization. Normal
development, a normal growth curve, and a lack of adverse clinical
features in kinoid-immunized mice compared to controls suggest that
this may represent an alternative strategy to passive anti-IL-13
mAb therapy or drugs that block IL-13 activity currently used in
patients.
Indeed, in our study anti-mIL-13 Abs did not
substitute for, but acted rather as an adjunct treatment in
addition to conventional treatments, as recommended in clinical
protocols for allergic asthma treatment. Particularly, after its
validation in humans, the vaccine could represent an alternative
prevention for patients with seasonal asthma. Furthermore, to
maintain an effective Ab threshold, the frequency of kinoid
boosters should be optimally determined by monitoring levels of
circulating Abs, maintaining the logic of metronomic scheduled
treatments (39).
Compared with passive immunization therapy, active
immunization with the kinoid vaccine described here would have the
following advantages. First, the generation of polyclonal
antibodies by active immunization excludes the formation of
anti-idiotypic antibodies, limiting the rate of therapeutic
failure. Second, the low frequency of boost immunization would
foster patient compliance. Finally, the proposed therapeutic
approach would represent a low cost.
In conclusion, our findings demonstrate that the
administration of IL-13 peptide kinoid vaccines triggers a strong
and transient Ab response to IL-13, which prevents the pathogenic
effects of this cytokine in an experimental murine model of acute
asthma induced by OVA. This may become an effective therapeutic
approach to allergic asthma treatment and may provide a new
therapeutic strategy for treating or vaccinating against other
diseases where overproduction of cytokines play a critical role in
pathogenesis.
Abbreviations:
|
IL-13
|
interleukin-13;
|
|
BALF
|
bronchoalveolar lavage fluids;
|
|
OVA
|
ovalbumin;
|
|
AHR
|
airway hyperresponsiveness;
|
|
IgE
|
immunoglobulin E;
|
|
IgG
|
immunoglobulin G;
|
|
mAb
|
monoclonal antibody;
|
|
MMP-9
|
matrix metalloproteinase-9;
|
|
KLH
|
keyhole limpet hemocyanin;
|
|
ELISA
|
enzyme-linked immunosorbent assay;
|
|
VEGF
|
vascular endothelial growth
factor;
|
|
TNF
|
tumor necrosis factor;
|
|
IL-4
|
interleukin-4;
|
|
IFN-γ
|
immunoreactive fibronectin-γ;
|
|
IFN-α
|
immunoreactive fibronectin-α;
|
|
H&E
|
hematoxylin and eosin;
|
|
PAS
|
periodic acid shiff;
|
|
CFA/IFA
|
complete/incomplete Freund’s
adjuvant;
|
|
NMPS
|
neutral mucopolysaccharide;
|
|
AMPS
|
acid mucopolysaccharide
|
Acknowledgements
This study was supported by the
Program for New Century Excellent Talents in University (NCET)
(NCET-09-0575) and the National Natural Science Foundation of China
(Grant no. 30973453).
References
|
1.
|
M Wills-KarpJ LuyimbaziX XuB
SchofieldInterleukin-13: central mediator of allergic
asthmaScience28222582261199810.1126/science.282.5397.22589856949
|
|
2.
|
FD FinkelmanSP HoganGK HersheyME
RothenbergM Wills-KarpImportance of cytokines in murine allergic
airway disease and human asthmaJ
Immunol18416631674201010.4049/jimmunol.090218520130218
|
|
3.
|
Q HamidM TulicImmunobiology of asthmaAnnu
Rev Physiol71489507200910.1146/annurev.physiol.010908.163200
|
|
4.
|
G GrünigM WarnockAE WakilRequirement for
IL-13 independently of IL-4 in experimental
asthmaScience2822261226319989856950
|
|
5.
|
G YangL LiA VolkTherapeutic dosing with
anti-interleukin-13 monoclonal antibody inhibits asthma progression
in miceJ Pharmacol Exp
Ther313815200510.1124/jpet.104.07613315644434
|
|
6.
|
MT KasaianDK MillerIL-13 as a therapeutic
target for respiratory diseaseBiochem
Pharmacol76147155200810.1016/j.bcp.2008.04.00218502398
|
|
7.
|
S HolgateA ChuchalinJ HebertEfficacy and
safety of a recombinant anti-immunoglobulin E antibody (omalizumab)
in severe allergic asthmaClin Exp
Allergy34632638200410.1111/j.1365-2222.2004.1916.x15080818
|
|
8.
|
WJ SandbornNew concepts in anti-tumor
necrosis factor therapy for inflammatory bowel diseaseRev
Gastroenterol Disord51018200515741928
|
|
9.
|
Y MaKT HayGlassAB BeckerNovel recombinant
interleukin-13 peptide-based vaccine reduces airway allergic
inflammatory responses in miceAm J Respir Crit Care
Med176439445200710.1164/rccm.200610-1405OC17556715
|
|
10.
|
M Wills-KarpImmunologic basis of
antigen-induced airway hyperresponsivenessAnnu Rev
Immunol17255281199910.1146/annurev.immunol.17.1.25510358759
|
|
11.
|
M Wills-KarpInterleukin-13 in asthma
pathogenesisImmunol
Rev202175190200410.1111/j.0105-2896.2004.00215.x
|
|
12.
|
PJ SabbatiniG RagupathiC HoodPilot study
of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus
QS21 in patients with epithelial ovarian, fallopian tube, or
peritoneal cancerClin Cancer
Res1341704177200710.1158/1078-0432.CCR-06-2949
|
|
13.
|
H Le BuanecD ZaguryStable immunogenic
product comprising antigenic heterocomplexesUS Patent 7972603.
Filed Septemper 16, 2003; issued July 5, 2011
|
|
14.
|
FH RadH Le BuanecS PaturanceVEGF kinoid
vaccine, a therapeutic approach against tumor angiogenesis and
metastasesProc Natl Acad Sci
USA10428372842200710.1073/pnas.061102210417301234
|
|
15.
|
YG KwakCH SongHK YiInvolvement of PTEN in
airway hyperresponsiveness and inflammation in bronchial asthmaJ
Clin Investig11110831092200310.1172/JCI1644012671058
|
|
16.
|
Y MaAG MaZ PengA potential immunotherapy
approach: mucosal immunization with an IL-13 peptide-based
virus-like particle vaccine in a mouse asthma
modelVaccine2580918099200710.1016/j.vaccine.2007.09.00917935839
|
|
17.
|
JY MaS MedicherlaI KerrR MangaduAA
ProtterLS HigginsSelective p38-α mitogen-activated protein kinase
inhibitor attenuates lung inflammation and fibrosis in IL-13
transgenic mouse model of asthmaJ Asthma Allergy131442008
|
|
18.
|
H MilgromB BenderL AckersonP BowryB SmithC
RandNoncompliance and treatment failure in children with asthmaJ
Allergy Clin
Immunol9810511057199610.1016/S0091-6749(96)80190-48977504
|
|
19.
|
C RandR WiseM NidesMetered-dose inhaler
adherence in a clinical trialAm Rev Respir
Dis14615591564199210.1164/ajrccm/146.6.15591456575
|
|
20.
|
D GrayH SkarvallB-cell memory is
short-lived in the absence of
antigenNature3367073199810.1038/336070a03263573
|
|
21.
|
S HolgateT CasaleS WenzelJ BousquetY
DenizC ReisnerThe anti-inflammatory effects of omalizumab confirm
the central role of IgE in allergic inflammationJ Allergy Clin
Immunol115459465200510.1016/j.jaci.2004.11.05315753888
|
|
22.
|
CL EmsonSE BellA JonesW WisdenAN
McKenzieInterleukin (IL)-4-independent induction of immunoglobulin
(Ig) E, and perturbation of T cell development in transgenic mice
expressing IL-13J Exp
Med188399404199810.1084/jem.188.2.3999670052
|
|
23.
|
LA BeckGV MarcotteD MacGlashanA TogiasS
SainiOmalizumab-induced reductions in mast cell FcεRI expression
and functionJ Allergy Clin Immunol114527530200415356552
|
|
24.
|
C PrussinDT GriffithKM BoeselH LinB
FosterTB CasaleOmalizumab treatment downregulates dendritic cell
FcεRI expressionJ Allergy Clin Immunol11211471154200315753888
|
|
25.
|
MM EpsteinDo mouse models of allergic
asthma mimic clinical disease?Int Arch Allergy
Immunol13384100200010.1159/00007613114726635
|
|
26.
|
JJ LeeD DiminaMP MaciasDefining a link
with asthma in mice congenitally deficient in
eosinophilsScience30517731776200410.1126/science.109947215375267
|
|
27.
|
K DabbaghK TakeyamaHM LeeIF UekiJA
LausierJA NadelIL-4 induces mucin gene expression and goblet cell
metaplasia in vitro and in vivoJ Immunol16262336237199910229869
|
|
28.
|
C AgustiK TakeyamaLO CardellGoblet cell
degranulation after antigen challenge in sensitized guinea pigs.
Role of neutrophilsAm J Respir Crit Care
Med15812531258199810.1164/ajrccm.158.4.98010419769289
|
|
29.
|
M DunnillThe pathology of asthma, with
special reference to changes in the bronchial mucosaJ Clin
Pathol132733196010.1136/jcp.13.1.2713818688
|
|
30.
|
S ShimuraY AndohM HaraguchiK
ShiratoContinuity of airway goblet cells and intraluminal mucus in
the airways of patients with bronchial asthmaEur Respir
J913951401199610.1183/09031936.96.090713958836649
|
|
31.
|
T AikawaS ShimuraH SasakiM EbinaT
TakishimaMarked goblet cell hyperplasia with mucus accumulation in
the airways of patients who died of severe acute asthma
attackChest101916921199210.1378/chest.101.4.9161555462
|
|
32.
|
T NakanoH InoueS FukuyamaNiflumic acid
suppresses interleukin-13-induced asthma phenotypesAm J Respir Crit
Care Med17312161221200610.1164/rccm.200410-1420OC16528019
|
|
33.
|
A KibeH InoueS FukuyamaDifferential
regulation by glucocorticoid of interleukin-13-induced
eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in
mouse airwaysAm J Respir Crit Care
Med1675056200310.1164/rccm.211008412502476
|
|
34.
|
PC FulkersonCA FischettiLM HassmanNM
NikolaidisME RothenbergPersistent effects induced by IL-13 in the
lungAm J Respir Cell Mol
Biol35337346200610.1165/rcmb.2005-0474OC16645178
|
|
35.
|
Z ZhuRJ HomerZ WangPulmonary expression of
interleukin-13 causes inflammation, mucus hypersecretion,
subepithelial fibrosis, physiologic abnormalities, and eotaxin
productionJ Clin Investig103779788199910.1172/JCI5909
|
|
36.
|
DM WalterJJ McIntireG BerryCritical role
for IL-13 in the development of allergen-induced airway
hyperreactivityJ
Immunol16746684675200110.4049/jimmunol.167.8.466811591797
|
|
37.
|
H Le BuanecL DelavalléeN BessisTNFα kinoid
vaccination-induced neutralizing antibodies to TNFα protect mice
from autologous TNFα-driven chronic and acute inflammationProc Natl
Acad Sci USA10319442194472006
|
|
38.
|
D ZaguryH Le BuanecA MathianIFNα kinoid
vaccine-induced neutralizing antibodies prevent clinical
manifestations in a lupus flare murine modelProc Natl Acad Sci
USA106529452992009
|
|
39.
|
RS KerbelBA KamenThe anti-angiogenic basis
of metronomic chemotherapyNat Rev
Cancer4423436200410.1038/nrc136915170445
|