Introduction
Patients with pancreatic cancer have a poor
prognosis with an overall 5-year survival rate of less than 5%
(1). More than 80% of patients
are diagnosed with pancreatic cancer at a locally advanced or
metastatic stage (2).
Understanding the molecular biology of pancreatic cancer, which can
involve migration and invasion may provide insights into the
development of novel tumor markers or new therapeutic
strategies.
Transforming growth factor-β (TGF-β) inhibits cell
proliferation and promotes tumor cell motility and invasion
(3,4). In comparison with non-tumor
pancreatic tissues, pancreatic tumors display increased TGF-β
expression (5,6). In addition, TGF-β treatment has been
demonstrated to induce epithelial-mesenchymal transition (EMT),
which is a key step in the progression of various cancers. During
EMT, cells undergo a developmental switch from a polarized,
epithelial phenotype to a mesenchymal phenotype (7).
Transcription factors snail and slug are key factors
for EMT. They downregulate the expression of various epithelial
factors, including E-cadherin, claudins and β-catenin, and
upregulate the expression of mesenchymal markers, including
N-cadherin, vimentin and fibronectin (8,9).
Smads also play important roles in the progression
of pancreatic cancer and Smad4 mutations are known to be present in
more than 50% of pancreatic cancer cases (10,11). Smad2/3 are considered to be key
regulators of pancreatic cancer. TGF-β activates the
phosphorylation of Smad2 and Smad3 and the activated Smad complex
binds to target gene promoters and regulates the transcriptional
responses to TGF-β in the nucleus (12–14).
We have previously shown that tumor necrosis
factor-α (TNF-α), hypoxia, paclitaxel, cisplatin,
CLOCK/brain-muscle-arnt-like-protein (BMAL) 1/2 and
polyinosinic-polycytidylic acid are upstream factors of the
differentiated embryo chondrocyte 2 (DEC2) (BHLHE41/Sharp1),
whereas its downstream factors include p53, Bax, Bim, vascular
endothelial growth factor (VEGF) and interferon β (IFN-β). We have
also demonstrated that DEC2 is involved in the regulation of
apoptosis, the response to hypoxia and the cell cycle in breast and
oral cancer and sarcoma cells (15–21). However, the roles of DEC2 in
pancreatic cancer remain unknown. In this study, we focused on the
role of DEC2 in pancreatic cancer BxPC-3 cells subjected to TGF-β
treatment and demonstrated that DEC2 has inhibitory effects on the
tumor progression of BxPC-3 cells.
Materials and methods
Cell culture and treatment
Human pancreatic cancer BxPC-3 cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were cultured in RPMI-1640 medium (Gibco-BRL,
Breda, The Netherlands) supplemented with 10% fetal bovine serum at
37°C in a humidified atmosphere of 95% air and 5% CO2.
For the experiments, the cells were incubated with or without
various concentrations of recombinant human TGF-β1 (R&D
Systems, Minneapolis, MN, USA) for 24 h.
Knockdown of DEC2 by interference
RNA
Short interference RNA (siRNA) against DEC2 was
synthesized by Qiagen (Hilden, Germany). The sequences of the sense
and anti-sense DEC2 siRNA and the negative control (scrambled)
siRNA were described previously (17). For the siRNA transfection
experiments, BxPC-3 cells were seeded at 5×104 cells per
35-mm well. After 24 h, the cells were transfected with the siRNA
using the Lipofectamine RNA iMAX reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). After being transfected, the
cells were incubated for 24 h and subjected to various
analyses.
DEC overexpression
DEC1 or DEC2 overexpression was induced using the
pcDNA vector as previously described (20). After being transfected, the cells
were incubated for 24 h and subjected to the migration or invasion
assay.
Western blotting
The cells were lysed using M-PER lysis buffer
(Thermo Scientific, Rockford, IL, USA) and their protein
concentrations (10 μg) were determined using the bicinchoninic acid
(BCA) assay. Their lysates were subjected to SDS-PAGE and the
proteins within them were transferred to PVDF membranes (Immobilion
P, Millipore, Tokyo, Japan), which were then incubated with
antibodies. The ECL, ECL-Plus or ECL-Advance western blotting
detection systems (Amersham Pharmacia Biotech, Uppsala, Sweden)
were used for detection.
Antibodies
During the western blotting, the membranes were
incubated with antibodies specific to DEC2 (1:40,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA, H-72), DEC1 (1:10,000;
Novus Biologicals Inc., Littleton, CO, USA), Smad3 (1:1,000;
Epitomics, Inc., Burlingame, CA, USA), pSmad3 (1:6,000; Epitomics,
Inc.), slug (1:3,000; Cell Signaling Technology, Inc., Beverley,
MA, USA), snail (1:3,000; Cell Signaling Technology, Inc.),
vimentin (1:10,000; Epitomics, Inc.), N-cadherin (1:10,000; ECM
Biosciences, Versailles, KY, USA), E-cadherin (1:1000; Takara Bio,
Inc.), claudin-1 (1:10,000; Invitrogen Life Technologies),
claudin-4 (1:20,000; Invitrogen Life Technologies) and actin
(1:30,000; Sigma), followed by horseradish peroxidase-conjugated
secondary antibody (Immuno Biological Laboratories Co., Ltd.,
Fujioka, Gunma, Japan). The Can Get Signal immunoreaction enhancer
solution (Toyobo Co., Ltd., Osaka, Japan) or the Immunoshot
immunoreaction enhancer solution (Cosmobio Co., Ltd., Tokyo, Japan)
was used to dilute the primary antibody.
Real-time polymerase chain reaction (PCR)
and reverse transcription-PCR
We prepared three independent RNA samples (n=3) for
real-time PCR from the BxPC-3 cells. Total RNA was isolated and
first-strand cDNA was synthesized as previously described (20). Real-time PCR was performed using
SYBR-Green Master Mix (Invitrogen Life Technologies). The sequences
of the primers for DEC1, DEC2 and 18S rRNA used in the real-time
PCR and the sizes of their products were previously described
(22). The sequences of the
primers for slug used for the real-time PCR were as follows:
slug-F, 5′-CCATTCCACGCCCAGCTA-3′ and R, 5′-TCACTCGCCC
CAAAGATGAG-3′. The amplified products of slug were 69 bp. The
sequences of the primers for DEC1, DEC2 and slug used for the
RT-PCR were as follows: DEC1-F, 5′-GTCTGTG AGTCACTCTTCAG-3′ and R,
5′-GAGTCTAGTTCTGTTTG AAGG-3′; DEC2-F, 5′-CACCTTTGACGTCTTTGGAG-3′
and R, 5′-GAGAGTGGGAATAGATGCAC-3′; slug-F, 5′-GAGCA
TTTGCAGACAGGTCA-3′ and R, 5′-TGAATTCCATGCTC TTGCAG-3′. The
amplified products of DEC1, DEC2 and slug were 534, 502 and 330 bp
in length, respectively. The cDNA for DEC1, DEC2 and slug were
amplified for up to 28 cycles. The PCR products were separated on
1.5% (w/v) agarose gels.
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay was performed using a kit from
Millipore, as described previously (16). Primers were designed to amplify a
Smad binding element containing a DNA fragment from the DEC2
promoter and their sequences were as follows: human DEC2-F,
5′-GAGGAAGTCGAGAGACCTTAA-3′ and R, 5′-CGCCAAAGGTACATGCACCA-3′.
Primers were also designed to amplify a DNA fragment containing the
E-box in the slug promoter and their sequences were as follows:
human slug-F, 5′-AGAGCAGAGCTTGTGCCTTC-3′ and R,
5′-GTGGGTTTGCTAATCCAAGG-3′.
Immunofluorescent staining
The cells were seeded in a 4-chamber slide glass and
incubated overnight. Then, they were washed with phosphate-buffered
saline (PBS) and fixed with 4% paraformaldehyde for 30 min, before
being permeabilized with 0.2% Triton X-100 in PBS for 10 min. The
permeabilized cells were then washed in PBS twice and treated with
5% normal horse serum in PBS for 30 min (to minimize the
non-specific adsorption of antibodies), before being incubated with
anti-slug (1:300) antibody at 4°C overnight. Next, the cells were
incubated for 1 h with goat anti-rabbit IgG antibody conjugated to
Alexa 488 dye (Molecular Probes Inc., Tokyo, Japan), while nuclear
staining was performed using 4′, 6-diamidino-2-phenylindole (DAPI).
The cells were visualized using confocal laser scanning microscopy
(Zeiss, LSM 710, Wetzlar, Germany).
Invasion and migration assay
The invasion assay was performed using a BD BioCoat
Matrigel invasion chamber kit (BD Biosciences, Franklin Lakes, NJ,
USA). BxPC-3 cells were separated using cell dissociation solution
(Sigma) and then (5x104 cells/600 μl) were added to the top chamber
of a cell culture insert in a 24-well companion plate. After
overnight incubation, the cells that had invaded the lower surface
of the membrane were fixed with methanol and subjected to Giemsa
staining. The number of cells that had migrated was quantified by
counting them in ten distinct randomly chosen fields using a light
microscope. For the migration assay, BxPC-3 cells were seeded in a
4-chamber slide glass and an artificial ‘wound’ was carefully
created at 0 h by scratching the confluent cell monolayer with the
tip of a P-200 pipette. Microphotographs were taken at 0, 24 and 48
h.
Human pancreatic tissues
We performed an immunohistochemical analysis of 17
surgically resected pancreatic tumors, which had been stored at
Hirosaki University Hospital, Japan (Table I). All of the 17 tumors had been
diagnosed as invasive ductal carcinoma of the pancreas. The
histological specimens were retrieved from the archives of our
hospital according to the guidelines produced by the Japanese
Society of Pathology. We examined the immunohistochemical
expression of DEC2 protein in the cancer tissues and the adjacent
non-cancerous tissues.
 | Table I.Immunohistochemical expression of
DEC2 proteins in human pancreatic cancer tissues. |
Table I.
Immunohistochemical expression of
DEC2 proteins in human pancreatic cancer tissues.
| | | DEC2
|
|---|
| C | A/G | D | T | N |
|---|
| 1 | 66/F | Moderately | Weak | Strong |
| 2 | 62/M | Moderately | Weak | Strong |
| 3 | 66/F | Moderately | Weak | Strong |
| 4 | 66/M | Moderately | Weak | Strong |
| 5 | 67/F | Moderately | Weak | Strong |
| 6 | 62/F | Moderately | Weak | Strong |
| 7 | 75/M | Moderately | Weak | Strong |
| 8 | 58/M | Moderately | Weak | Strong |
| 9 | 65/M | Moderately | Weak | Strong |
| 10 | 72/F | Mell | Weak | Strong |
| 11 | 67/M | Poorly | Weak | Strong |
| 12 | 71/F | Well | Weak | Weak |
| 13 | 74/M | Moderately | Weak | Strong |
| 14 | 72/M | Poorly | Weak | Strong |
| 15 | 55/M | Moderately | Weak | Strong |
| 16 | 61/F | Moderately | Weak | Strong |
| 17 | 50/F | Moderately | Weak | Strong |
Immunohistochemistry
The expression of DEC2 in the pancreatic cancer
tissues was examined in serial deparaffinized sections and was
detected using the Dako EnVision kit/HRP (DAB) (DakoCytomation,
Kyoto, Japan). Sections were pretreated with LAB solution
(Polysciences, Eppelheim, Germany) for 6 min for antigen retrieval
and were incubated overnight at 4°C with anti-DEC2 (1:100)
(20) antibody diluted in Can Get
Signal Immunostain Solution. The sections were then treated with
the HRP-conjugated secondary antibody. Finally, the sections were
counterstained with Mayer’s hematoxylin.
Results
DEC2 expression is induced in BxPC-3
cells by TGF-β
In this study, we used human pancreatic cancer
BxPC-3 cells to functionally analyze DEC2 and investigate the
protein expression of endogenous DEC2 and various EMT-related
factors during TGF-β treatment. In these cells, TGF-β treatment
induced Smad3 phosphorylation and upregulated the expression of
DEC1, DEC2, slug and vimentin and downregulated the expression of
claudin-1 (Fig. 1A). The highest
DEC2 expression level was observed in the cells treated with 10
ng/ml of TGF-β for 24 h. TGF-β had little effect on the expression
of Smad3, snail, E-cadherin, claudin-4 and N-cadherin. Next, we
investigated the endogenous mRNA expression of DEC1 and DEC2 in the
presence of TGF-β using real-time and RT-PCR. The mRNA expression
levels of DEC1 and DEC2 were upregulated by TGF-β (Fig. 1B).
 | Figure 1.TGF-β-induced upregulation of DEC2
expression in human pancreatic cancer BxPC-3 cells. (A) BxPC-3
cells were treated with various concentrations of TGF-β for 24 h.
The cells were then lysed and their lysates were subjected to
western blot analyses of pSmad3, Smad3, DEC1, DEC2, snail, slug,
E-cadherin, claudin-1, claudin-4, N-cadherin, vimentin and actin.
One representative of at least three independent experiments with
similar results is shown. (B) BxPC-3 cells were treated as above
and total-RNA was prepared and subjected to real-time and RT-PCR
for DEC1 and DEC2. Each value represents the mean ± SE (bars) of
three independent experiments. *p<0.05, according to
the t-test. (C) A ChIP assay was performed using BxPC-3 cells that
had been treated with or without TGF-β (10 ng/ml) for 24 h. The
eluted DNA fragments were subjected to PCR. Anti-rabbit IgG was
used as an immunoprecipitation control. |
Since DEC2 has five CAGAC Smad3 binding elements
(SBE) in its promoter, we investigated whether Smad3 binds to the
SBE in the DEC2 gene. We selected a Smad3 binding target sequence
containing a single SBE, which was located near to the
transcription starting point of the DEC2 gene (Fig. 1C). The binding of Smad3 to the SBE
in the DEC2 promoter was significantly increased in the TGF-β
treated cells compared with the non-TGF-β treated cells.
DEC2 negatively regulates slug
expression
To clarify the biological functions of DEC2 in
TGF-β-induced tumor progression, we examined whether the
transfection of DEC2 siRNA altered the expression of EMT-related
factors. In the presence of TGF-β, DEC2 siRNA upregulated the
expression of DEC1 and slug, whereas it had little effect on the
Smad3 phosphorylation and the expression of Smad3, claudin-1 and
vimentin (Fig. 2A and B). The
inhibitory effect of DEC2 knockdown by siRNA in the absence or
presence of TGF-β was about 60%. In the absence of TGF-β, DEC2
siRNA transfection had little effect on the expression of DEC1,
Smad3, slug, claudin-1 and vimentin and Smad3 phosphorylation. We
further examined whether DEC2 overexpression affects the expression
of slug by transiently transfecting the cells with a DEC2
expressing plasmid. As a result, we found that in the presence of
TGF-β, the addition of the DEC2 pcDNA decreased the expression of
slug compared with the control pcDNA (Fig. 2C).
 | Figure 2.DEC2 negatively regulates slug. (A)
BxPC-3 cells were transfected with control siRNA or siRNA against
DEC2. At 24 h post-transfection, the cells were treated with TGF-β
(10 ng/ml) and incubated for 24 h and their lysates were subjected
to western blot analyses of DEC1, pSmad3, Smad3, slug, claudin-1,
vimentin and actin. One representative of at least three
independent experiments with similar results is shown. (B) BxPC-3
cells were treated as above and total-RNA was prepared and
subjected to real-time and RT-PCR for DEC2, slug and claudin-1.
Each value represents the mean ± SE (bars) of three independent
experiments. *p<0.05, according to the t-test. (C)
BxPC-3 cells were transfected with pcDNA or DEC2 pcDNA. At 24 h
post-transfection, the cells were treated with or without TGF-β (10
ng/ml) for 24 h and their lysates were subjected to western blot
analyses of DEC2, slug and actin. (D) BxPC-3 cells were treated as
above and the cells were fixed, incubated with anti-slug antibody
and visualized using Alexa488-conjugated secondary antibody
(green). The cells were also counterstained with DAPI (blue) in
order to detect their nuclei. A merged image that is representative
of at least two independent experiments with similar results is
shown. The arrows indicate strong colocalized amounts. (E) BxPC-3
cells were transfected with control siRNA or siRNA against DEC2. At
24 h post-transfection, a ChIP assay was performed using BxPC-3
cells that had been treated with or without TGF-β (10 ng/ml) for 24
h. PCR was performed as above. |
Using immunofluorescence analysis, we examined
whether DEC2 siRNA affects the nuclear/cytoplasmic amounts of slug.
As shown in Fig. 2D, DEC2 siRNA
significantly increased the nuclear concentration of slug in the
presence of TGF-β. Since the slug gene has one CACGTG E-box element
in its promoter, we investigated whether DEC2 binds to this E-box.
In the presence of TGF-β, the addition of control siRNA
significantly increased the binding of DEC2 to the slug promoter
CACGTG E-box compared with that observed after DEC2 siRNA
transfection, whereas in the absence of TGF-β, control siRNA only
slightly increased the binding of DEC2 to the slug promoter CACGTG
E-box compared with DEC2 siRNA (Fig.
2E).
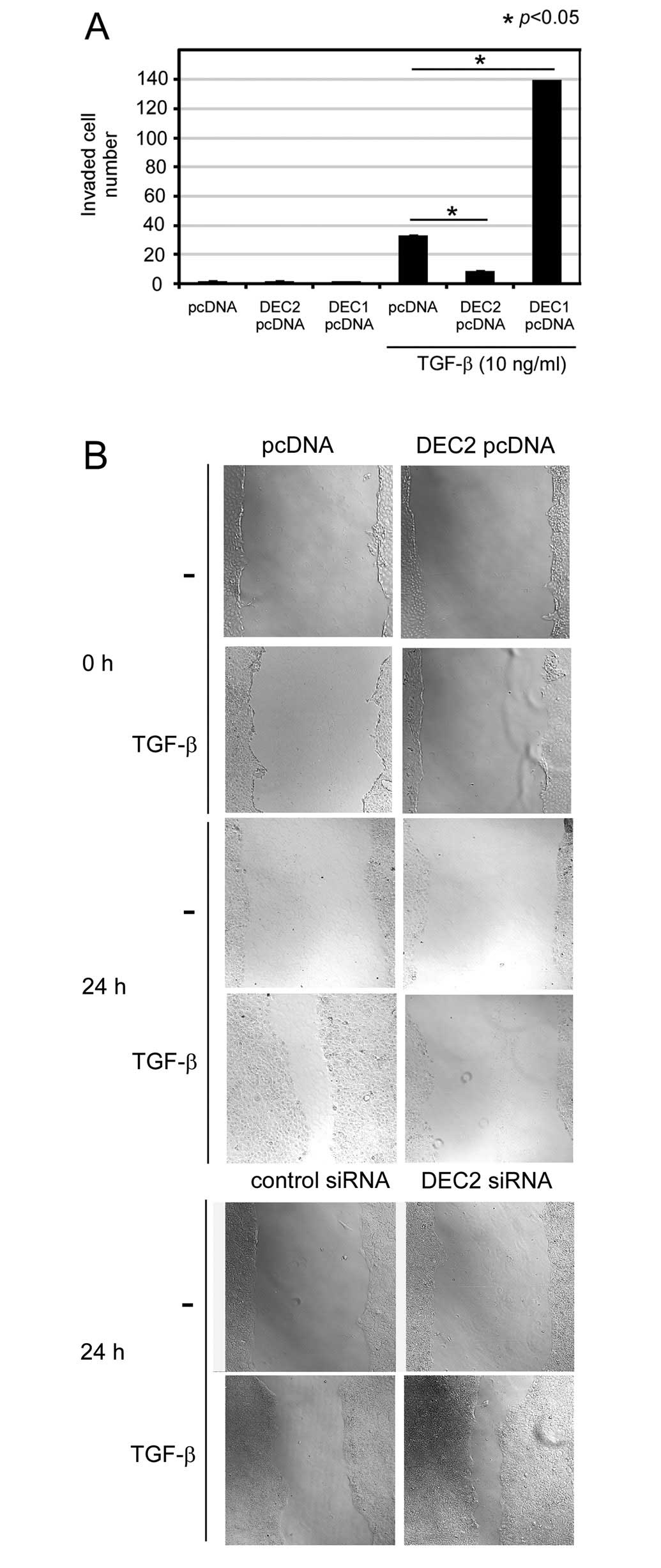
DEC2 overexpression inhibits the invasion
and migration induced by TGF-β
Migration and invasion are important phenomena in
tumor progression. Thus we examined whether DEC2 is involved in
invasion and migration. We performed an invasion assay in which we
transiently transfected the cells with a DEC1- or DEC2-expressing
plasmid. As a result, we found that in the presence of TGF-β, the
number of pcDNA-transfected invasive BxPC-3 cells was increased
∼32-fold compared with the number of pcDNA-transfected cells
observed in the absence of TGF-β (Fig. 3A). In the presence of TGF-β, the
number of invasive DEC1 pcDNA-transfected cells was increased about
4-fold compared with the number of pcDNA-transfected cells and the
number of invasive DEC2 pcDNA-transfected cells was decreased about
4-fold compared with the number of invasive pcDNA-transfected
control cells. We also found that in the presence of TGF-β, DEC2
pcDNA delayed cell migration for 24 h compared with that observed
in the cells transfected with the pcDNA (Fig. 3B). On the other hand, in the
presence of TGF-β, DEC2 siRNA increased the amount of migration
detected at 24 h compared with that observed in the cells
transfected with control siRNA.
DEC2 protein expression in human
pancreatic cancer tissues and the adjacent non-cancerous pancreatic
tissues
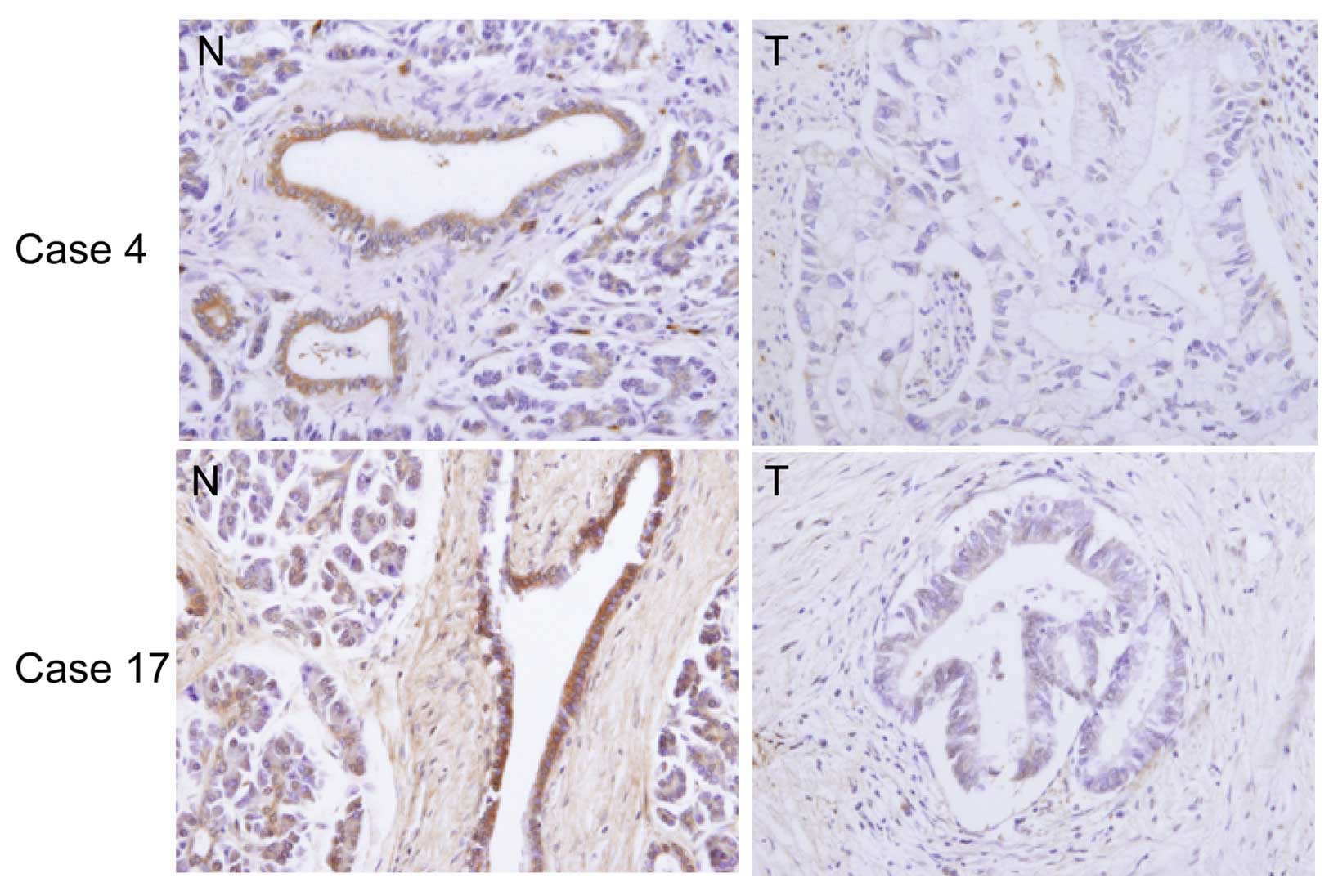
We examined the immunohistochemical expression of
DEC2 in human pancreatic cancer tissues. Photographs of the DEC2
expression in representative cases are shown in Fig. 4. Significant DEC2 immunoreactivity
was detected in the non-cancerous pancreatic tissues (94%; 16/17
cases) compared with the adjacent cancer tissues and it was
predominantly localized within the cytoplasm of the non-cancerous
pancreatic cells, although very weak DEC2 immunoreactivity was
found in the adjacent cancer cells in all cases.
Discussion
DEC2 regulates apoptosis, the response to hypoxia,
differentiation and circadian rhythms (16–18,20,23). However, the
role of DEC2 in tumor progression is poorly understood. Epithelial
mesenchymal transition (EMT) is characterized by the loss of
epithelial factors and upregulated mesenchymal marker expression
and results in a spindle cell morphology and invasive phenotype
(3,8). We showed that TGF-β increased the
migration and invasion of BxPC-3 cells and upregulated their DEC2
expression. In addition, TGF-β affected the levels of some
EMT-related molecules, such as p-Smad3, slug, claudin-1 and
vimentin, although it had little effect on the expression of snail,
claudin-4, E-cadherin and N-cadherin and the cell morphology.
Nishioka et al (24) also
found that TGF-β had little effect on the expression of E-cadherin
and the cell morphology in BxPC-3 cells. These results suggest that
TGF-β upregulates the malignancy of BxPC-3 cells by increasing
their migration and invasion and upregulating their slug
expression, but does not affect the EMT.
We found that Smad3 binds to the SBE in the DEC2
promoter. These findings suggest that TGF-β and Smad3 are upstream
factors of DEC2. It was reported that TGF-β increases the
expression and promoter activities of snail and slug in various
cancer cells (25–27). Transcription factors snail and
slug play important roles in TGF-β-induced tumor progression and
have E-boxes in their promoters (28,29). However, snail was not upregulated
by TGF-β in the BxPC-3 cells. Thus, we focused on the effects of
DEC2 on slug. Slug enhances invasion in various cancer cells,
involving upregulation of metalloproteinase-9 and downregulation of
E-cadherin (30,31). Slug also enhances proliferation
and migration in prostate and ovarian cancer cells (32,33). We showed that DEC2 knockdown or
overexpression in the presence of TGF-β affected both the
expression and the nuclear concentration of slug. In addition, DEC2
bound to the E-box in the slug promoter. These findings suggest
that DEC2 negatively regulates slug through its E-box. We also
showed that DEC2 inhibits the expression of DEC1, as previously
described (34), as well as the
migration and invasion induced by TGF-β. DEC1 is highly expressed
in various tumors (21,35–37), whereas DEC2 is highly expressed in
non-cancerous pancreatic ducts compared with cancer tissues. DEC2
also negatively regulates DEC1 through its E-box (34). Based on the above findings, DEC2
may inhibit tumor progression by suppressing slug or DEC1. Further
studies are needed to clarify the details of the mechanism by which
DEC1 regulates slug.
In the present study, we demonstrated that DEC2
inhibits TGF-β-induced tumor progression through slug in BxPC-3
cells and DEC2 is expressed at relatively low levels in cancer
tissues compared with its levels in non-cancerous tissues. Further
studies are needed to clarify the role of DEC2 in the effects
induced by TGF-β and to elucidate the detailed molecular mechanisms
by which DEC2 inhibits tumor progression.
Abbreviations:
|
DEC2
|
differentiated embryo chondrocyte
2;
|
|
TGF-β
|
transforming growth factor-β;
|
|
PBS
|
phosphate-buffered saline;
|
|
siRNA
|
short interference RNA
|
Acknowledgements
This study was supported by
Grants-in-Aid for Science from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan; a Grant for Hirosaki
University Institutional Research; and the Fund for the Promotion
of International Scientific Research.
References
|
1.
|
A JemalR SiegelE WardT MurrayJ XuC
SmigalMJ ThunCancer statistics, 2006CA Cancer J
Clin56106130200610.3322/canjclin.56.2.106
|
|
2.
|
TP YeoRH HrubanSD LeachRE WilentzTA SohnSE
KernCA Iacobuzio-DonahueA MaitraM GogginsMI CantoPancreatic
cancerCurr Probl Cancer
Rev26176275200210.1067/mcn.2002.12957912399802
|
|
3.
|
J ZavadilEP BöttingerTGF-beta and
epithelial-to-mesenchymal
transitionsOncogene2457645774200510.1038/sj.onc.120892716123809
|
|
4.
|
PJ MiettinenR EbnerAR LopezR
DerynckTGF-beta induced transdifferentiation of mammary epithelial
cells to mesenchymal cells: involvement of type I receptorsJ Cell
Biol12720212036199410.1083/jcb.127.6.20217806579
|
|
5.
|
J KleeffH FriessP SimonS SusmallianP
BüchlerA ZimmermannMW BüchlerM KorcOverexpression of Smad2 and
colocalization with TGF-beta1 in human pancreatic cancerDig Dis
Sci4417931802199910.1023/A:101888232050010505717
|
|
6.
|
H FriessY YamanakaM BüchlerM EbertHG
BegerLI GoldM KorcEnhanced expression of transforming growth factor
beta isoforms in pancreatic cancer correlates with decreased
survivalGastroenterology1051846185619938253361
|
|
7.
|
MA HuberN KrautH BeugMolecular
requirements for epithelial-mesenchymal transition during tumor
progressionCurr Opin Cell
Biol17548558200510.1016/j.ceb.2005.08.00116098727
|
|
8.
|
JP ThieryJP SleemanComplex networks
orchestrate epithelial-mesenchymal transitionsNat Rev Mol Cell
Biol7131142200610.1038/nrm183516493418
|
|
9.
|
D MediciED HayBR OlsenSnail and Slug
promote epithelial-mesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta 3Mol Biol
Cell1948754887200810.1091/mbc.E08-05-050618799618
|
|
10.
|
SA HahnM SchutteAT HoqueCA MoskalukLT da
CostaE RozenblumCL WeinsteinA FischerCJ YeoRH HrubanSE KernDPC4, a
candidate tumor suppressor gene at human chromosome
18q21.1Science271350353199610.1126/science.271.5247.3508553070
|
|
11.
|
D BartschSA HahnKD DanichevskiA RamaswamyD
BastianH GalehdariP BarthW SchmiegelB SimonM RothmundMutations of
the DPC4/Smad4 gene in neuroendocrine pancreatic
tumorsOncogene1823672371199910.1038/sj.onc.120258510327057
|
|
12.
|
K MiyazawaM ShinozakiT HaraT FuruyaK
MiyazonoTwo major Smad pathways in TGF-beta superfamily
signallingGenes
Cells711911204200210.1046/j.1365-2443.2002.00599.x12485160
|
|
13.
|
J MassaguéHow cells read TGF-beta
signalsNat Rev Mol Cell Biol1169178200011252892
|
|
14.
|
L ZawelJL DaiP BuckhaultsS ZhouKW KinzlerB
VogelsteinSE KernHuman Smad3 and Smad4 are sequence-specific
transcription activatorsMol
Cell1611617199810.1016/S1097-2765(00)80061-19660945
|
|
15.
|
F SatoT KawamotoK FujimotoM NoshiroKK
HondaS HonmaK HonmaY KatoFunctional analysis of the basic
helix-loop-helix transcription factor DEC1 in circadian regulation.
Interaction with BMAL1Eur J
Biochem27144094419200410.1111/j.1432-1033.2004.04379.x15560782
|
|
16.
|
F SatoUK BhawalT KawamotoK FujimotoT
ImaizumiT ImanakaJ KondoS KoyanagiM NoshiroH
YoshidaBasic-helix-loop-helix (bHLH) transcription factor DEC2
negatively regulates vascular endothelial growth factor
expressionGenes
Cells13131144200810.1111/j.1365-2443.2007.01153.x18233956
|
|
17.
|
Y WuF SatoUK BhawalT KawamotoK FujimotoM
NoshiroS MorohashiY KatoH KijimaBasic helix-loop-helix
transcription factors DEC1 and DEC2 regulate the paclitaxel-induced
apoptotic pathway of MCF-7 human breast cancer cellsInt J Mol
Med274914952011
|
|
18.
|
Y WuF SatoUK BhawalT KawamotoK FujimotoM
NoshiroH SeinoS MorohashiY KatoH KijimaBHLH transcription factor
DEC2 regulates pro-apoptotic factor Bim in human oral cancer HSC-3
cellsBiomed Res337582201210.2220/biomedres.33.7522572381
|
|
19.
|
T ImaizumiF SatoH TanakaT MatsumiyaH
YoshidaT Yashiro-AizawaK TsurugaR HayakariH KijimaK
SatohBasic-helix-loop-helix transcription factor DEC2 constitutes
negative feedback loop in IFN-β-mediated inflammatory responses in
human mesangial cellsImmunol Lett1363743201121129405
|
|
20.
|
Y LiuF SatoT KawamotoK FujimotoS
MorohashiH AkasakaJ KondoY WuM NoshiroY KatoH KijimaAnti-apoptotic
effect of the basic helix-loop-helix (bHLH) transcription factor
DEC2 in human breast cancer cellsGenes
Cells15315325201010.1111/j.1365-2443.2010.01381.x20236182
|
|
21.
|
UK BhawalF SatoY ArakawaK FujimotoT
KawamotoK TanimotoY ItoT SasahiraT SakuraiM KobayashiBasic
helix-loop-helix transcription factor DEC1 negatively regulates
cyclin D1J Pathol224420429201110.1002/path.287821506129
|
|
22.
|
F SatoY WuUK BhawalY LiuT ImaizumiS
MorohashiY KatoH KijimaPERIOD1 (PER1) has anti-apoptotic effects,
and PER3 has pro-apoptotic effects during cisplatin (CDDP)
treatment in human gingival cancer CA9-22 cellsEur J
Cancer4717471758201110.1016/j.ejca.2011.02.02521459569
|
|
23.
|
SM ChoiHJ ChoH ChoKH KimJB KimH
ParkStra13/DEC1 and DEC2 inhibit sterol regulatory element binding
protein-1c in a hypoxia-inducible factor-dependent mechanismNucleic
Acids Res3663726385200810.1093/nar/gkn62018838394
|
|
24.
|
R NishiokaS ItohT GuiZ GaiK OikawaM KawaiM
TaniH YamaueY MuragakiSNAIL induces epithelial-to-mesenchymal
transition in a human pancreatic cancer cell line (BxPC3) and
promotes distant metastasis and invasiveness in vivoExp Mol
Pathol89149157201010.1016/j.yexmp.2010.05.008
|
|
25.
|
PP ShahSS KakarPituitary tumor
transforming gene induces epithelial to mesenchymal transition by
regulation of twist, snail, slug, and E-cadherinCancer
Lett3116676201110.1016/j.canlet.2011.06.03321839581
|
|
26.
|
S TakanoF KanaiA JazagH IjichiJ YaoH
OgawaN EnomotoM OmataA NakaoSmad4 is essential for down-regulation
of E-cadherin induced by TGF-beta in pancreatic cancer cell line
PANC-1J Biochem141345351200710.1093/jb/mvm03917301079
|
|
27.
|
S ThuaultU ValcourtM PetersenG
ManfiolettiCH HeldinA MoustakasTransforming growth factor-beta
employs HMGA2 to elicit epithelial-mesenchymal transitionJ Cell
Biol174175183200610.1083/jcb.20051211016831886
|
|
28.
|
M Sánchez-MartínA Rodríguez-GarcíaJ
Pérez-LosadaA SagreraAP ReadI Sánchez-GarcíaSLUG (SNAI2) deletions
in patients with Waardenburg diseaseHum Mol
Genet1132313236200212444107
|
|
29.
|
S GrotegutD von SchweinitzG ChristoforiF
LehembreHepatocyte growth factor induces cell scattering through
MAPK/Egr-1-mediated upregulation of SnailEMBO
J2535343545200610.1038/sj.emboj.760121316858414
|
|
30.
|
K ZhangD ChenX JiaoS ZhangX LiuJ CaoL WuD
WangSlug enhances invasion ability of pancreatic cancer cells
through upregulation of matrix metalloproteinase-9 and actin
cytoskeleton remodelingLab
Invest91426438201110.1038/labinvest.2010.201
|
|
31.
|
P TangZ YuK ZhangY WangZ MaS ZhangD ChenY
ZhouSlug down-regulation by RNA interference inhibits invasion
growth in human esophageal squamous cell carcinomaBMC
Gastroenterol1160201110.1186/1471-230X-11-6021599940
|
|
32.
|
M Emadi BaygiZS SoheiliF EssmannA DeezagiR
EngersW GoeringWA SchulzSlug/SNAI2 regulates cell proliferation and
invasiveness of metastatic prostate cancer cell linesTumour
Biol31297307201020506051
|
|
33.
|
NK KurreyK AmitSA BapatSnail and Slug are
major determinants of ovarian cancer invasiveness at the
transcription levelGynecol
Oncol97155165200510.1016/j.ygyno.2004.12.04315790452
|
|
34.
|
T KawamotoM NoshiroF SatoK MaemuraN
TakedaR NagaiT IwataK FujimotoM FurukawaK MiyazakiA novel
autofeedback loop of Dec1 transcription involved in circadian
rhythm regulationBiochem Biophys Res
Commun2117124200410.1016/j.bbrc.2003.11.09914672706
|
|
35.
|
J ChakrabartiH TurleyL CampoC HanAL
HarrisKC GatterSB FoxThe transcription factor DEC1 (stra13, SHARP2)
is associated with the hypoxic response and high tumour grade in
human breast cancersBr J
Cancer91954958200410.1038/sj.bjc.660205915328513
|
|
36.
|
A GiatromanolakiMI KoukourakisE SivridisH
TurleyCC WykoffKC GatterAL HarrisDEC1 (STRA13) protein expression
relates to hypoxia-inducible factor 1-alpha and carbonic
anhydrase-9 overexpression in non-small cell lung cancerJ
Pathol200222228200310.1002/path.133012754744
|
|
37.
|
Y LiH ZhangM XieM HuS GeD YangY WanB
YanAbundant expression of Dec1/stra13/sharp2 in colon carcinoma:
its antagonizing role in serum deprivation-induced apoptosis and
selective inhibition of procaspase activationBiochem
J367413422200210.1042/BJ2002051412119049
|