Introduction
Cell death is an essential phenomenon for cell
homeostasis, as well as for cell growth. It has been well
documented during embryonic and postembryonic development (1,2).
The control of cell division by programmed cell death (apoptosis)
is indispensable for the preservation of the normal growth and
specialization of an organism. Several proteins that inhibit
apoptosis, such as p53 and Bcl-2 family, are involved in the
regulation of normal cellular homeostasis and the promotion of
tissue tumorgenesis (3–5). The physiological apoptotic pathways
are often altered in malignant cells, resulting in a significant
advantage in survival. Tumor cells that are resistant to apoptosis
can survive despite immune system tumor surveillance, and often
fail to respond to anticancer treatment.
Human survivin is a cytoplasmic protein with a
molecular weight of 16.5 kDa. As a member of the inhibitor of
apoptosis protein (IAP) family, it has been identified in
baculovirus (6). It consists of
142 amino acids, and its gene spans 14.7 kb on the telomeric
position on chromosome 17, to band q25 (7). The human survivin gene consists of 4
exons and 3 introns, and its coding strand contains an open reading
frame (ORF) of 426 nucleotides complementary to EPR-1 (7). There is a TATA-less promoter and
there are GC-rich regions of canonical CpG islands 25 upstream of
this ORF (7–9). Survivin has recently been identified
as a novel IAP. Unlike other members of the IAP family, survivin is
characterized by a unique structure that contains a single
baculovirus IAP repeat and no really interesting new gene (RING)
finger motifs (10).
Survivin is expressed during embryonal development
and in many common types of human cancers including stomach
(11), colorectal (12), lung (13), breast (14), pancreatic (15) and prostate cancers (16) and high-grade non-Hodgkin’s
lymphomas in vivo (7,17).
Nevertheless, its expression is absent in terminally differentiated
adult tissues (7). It is
expressed fetally but not in adult differentiated tissues. Recent
results suggest that survivin may counteract a default induction of
apoptosis in the G2/M phase (18)
in proliferating cells. Survivin is expressed in a cell
cycle-regulated manner with high levels in G2/M and rapid
downregulation following cell cycle arrest. At the beginning of
mitosis, survivin associates with the mitotic spindle and
disruption of this interaction results in a loss of its
anti-apoptotic function. Overexpression of survivin in cancer may
thus overcome this apoptosis-related cell cycle checkpoint and
favor aberrant progression of transformed cells through
mitosis.
Two major apoptosis pathways, the mitochondrial
pathway and the death receptor pathway, are known. Bcl-2 which
blocks release of mitochondrial cytochrome c into the cytosol has
been shown to inhibit the first of these two pathways (19,20). The apoptosis pathway is blocked by
survivin. Survivin inhibits apoptosis by directly inhibiting
downstream effectors caspase-3 and -7 through baculovirus IAP
repeat (BIR)-dependent recognition (14), and by interfering with caspase-9
activity processing. Survivin blocks a common downstream part of
both the mitochondrial apoptosis pathway and the death receptor
pathway (21,22).
The RNA interference (RNAi) phenomenon is a recently
observed process in which the introduction of a double-stranded RNA
(dsRNA) into a cell causes the specific degradation of an mRNA with
the same sequence. The 21–23 nt guide RNAs, small RNAs generated by
RNase III cleavage from longer dsRNAs, are associated with
sequence-specific mRNA degradation. Small RNAs have been proposed
as gene expression repressors with great potential for use in gene
therapy (10,23,24). This technique known as RNA
interference has been successfully adapted to mammalian cells so
that it is now possible to decrease the expression of cellular
genes specifically after transfection of annealed small interfering
21-mer RNAs. In the present study, we aimed to ascertain whether we
could specifically reduce the levels of the survivin protein in
follicular thyroid cancer cell line FTC-133, which overexpresses
survivin protein. For this analysis, RNAi using designed small
interfering RNAs (siRNAs) directed against survivin was carried out
(25,26).
We performed this retrospective study of thyroid
carcinoma patients for the purpose of investigating whether
survivin expression is signficantly associated with poor prognosis
and whether it may be used as a potential therapeutic target for
thyroid tumors.
Materials and methods
Tissue specimens
Tissue specimens from 75 patients with thyroid
carcinoma (33 with papillary thyroid cancer (PTC), 24 with
follicular thyroid cancer (FTC), 18 with undifferentiated thyroid
cancer (UTC) and 15 patients with benign thyroid goiter, 35 males
and 52 females, were studied. This study was approved by the local
committee of medical ethics and all patients provided written
consent. Tissues of all patients were obtained following surgery
performed between 2001 and 2006 at the Department of General,
Visceral and Vascular Surgery, Martin Luther University
Halle-Wittenberg, Halle/Saale, Germany. The mean age of the
patients was 58 years, with a range of 15–89 years. For
immunohistochemistry and RT-PCR, resected thyroid tissues were
immediately frozen in liquid nitrogen and maintained at −80°C until
they were used. Frozen sections (6 μm) were cut on a cryostat, and
control sections were stained with hematoxylin and eosin
(H&E).
mRNA preparation and RT-PCR analysis
Total-RNA from fresh thyroid carcinoma, benign
goiter tissues and human colorectal carcinoma cell line ‘Caco’ (as
positive control) was extracted using the TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. Reverse transcription was performed from 1 μg of
total-RNA by using the Superscript II kit (Gibco, Munich, Germany)
at 42°C for 30 min, followed by enzyme inactivation at 95°C for 5
min.
For PCR amplification, a 2 μl aliquot of the
reaction mixture was used. To obtain reproducible quantitative
performance of the multiplex RT-PCR assay, we titrated the amount
of starting cDNA and adjusted the number of amplification cycles.
The generated cDNA was amplified using the specific primer for
survivin and for the housekeeping gene β-actin. The primers used in
this study and the expected size from the reported cDNA sequence
are shown in Table I.
 | Table I.PCR primers and conditions. |
Table I.
PCR primers and conditions.
| Gene | Primer | Temperature
(°C) | Product size
(bp) |
|---|
| Survivin | 5′-AAC AGC CGA GAT
GAC CTC C-3′ | | |
| 5′-AAC TTC AGG TGG
ATG AGG AGA C-3′ | 60 | 398 |
| β-actin | 5′-GCT GGA AGT GGA
CAG CGA-3′ | | |
| 5′-GGC ATC GTG ATG
GAC TCC G-3′ | 60 | 608 |
All subsequent assays were carried out under
conditions that amplification of both survivin and β-actin (the
internal control) was yielded within a parallel linear range. The
PCR profile was as follows: 30 sec at 94°C, 30 sec at 60°C, 45 sec
at 72°C, 7 min at 72°C and a final step at 6°C. All PCR reactions
were carried out with AmpliTaq Gold (Amersham, USA). After 38 PCR
amplification cycles, 20 μl of PCR-amplified cDNA had migrated on a
1% agarose gel and bands were visualized with ethidium bromide,
photographed with Kodak Image System 440 cf and electronically
evaluated with Kodak Digital Science 1D software (Eastman Kodak,
New Heaven, CT, USA).
The human colorectal carcinoma cell line ‘Caco’
exhibited strong survivin-mRNA expression. Its expression level was
set as 100%. The expression levels of all investigated specimens
were classified in comparison to the positive control grey scale as
follows: 0–20%, negative (−); 20–50%, decreased (+), 50–75%
moderate expression (++), 75%, strongly positive (+++).
Immunohistochemistry
To confirm the results of survivin gene expression
obtained by RT-PCR, immunohistochemistry was performed using Dako
coverplates (Dako, Germany). Cryo-embedded serial sections (6 μm)
of all tissues were freshly cut and then incubated in a mixture of
3% H2O2 and ice cold 90% methanol (1:4) for
20 min. After twice washing with PBS solution, sections were
incubated in PBS solution for 10 min. Enzymatic activity and
non-specific binding sites were blocked with normal swine serum
(1:4 diluted) in 1% PBS-BSA for 10 min to suppress non-specific
binding. Subsequently, replicate sections were incubated at 4°C
overnight with the specific mouse monoclonal antibody against human
survivin (clone D8; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at a dilution of 1:200. Negative control sections were exposed
to the secondary antibody only and processed as described above.
After the sections were washed 3 times in PBS, they were incubated
for 30 min at a 1:1,000 dilution of biotinylated goat anti-mouse
secondary antibody (Dako anti-IgG kit). Detection of
antibody-antibody-antigene reaction was accomplished using the
avidin-biotin-peroxidase complex method. A 15% solution of
diaminobenzidine (DAB) (Dako, Aarhus, Denmark) was used as
chromogen. Finally, sections were lightly counterstained with
Mayer’s hematoxylin. Tissue sections from a human colorectal
adenocarcinoma were used as positive controls.
Evaluation of immunostained tissues
All sections were examined by two independent
reviewers. For better quantification, planimetric measurement of
immunoreactive cell clusters and tissue parts was evaluated
semi-quantitatively, using an Axioplan light microscope (Zeiss,
Jena, Germany) by three independent investigators, blinded to the
histological diagnosis. In addition, planimetric evaluations on
immunostained specimens were performed using Zeiss KS300 software,
and the plasma-nucleus relationship of survivin-positive cells was
documented. For better quantification, planimetric measurement of
immunoreactive cell clusters and tissue parts was performed using
PalmRobo-Software (Palm Microlaser Technologies, Tutzing, Germany).
The numbers of survivin-positive cells were calculated after
circumferential allignment in relation to microscope magnification.
Amount of positive squares was set in contrast to the total section
area (TSA=100%), and the level of staining intensity was subdivided
into four groups: 0, 0–10% negative; 1, >10–50% weak; 2,
>50–80% distinct; and 3, >80% strong.
Cell lines
The human follicular thyroid carcinoma cell line
FTC-133, supplied by P. Goretzki (Düsseldorf, Germany), was
established from a 42-year-old male patient with metastatic FTC,
characterized by expressing human thyroglobulin and thyroid
peroxidase. The cell line was cultured in Dulbecco’s modified
Eagle’s medium (DMEM) and modified HAM-F12 medium 1:1 supplemented
with 10% fetal bovine serum and penicillin/streptomycin in a
humidified incubator at 37°C in 5% CO2. Media were
changed every 3–4 days.
Preparation of siRNAs
siRNAs with two thymidine residues (dTdT) at the
3-end of the sequence were designed using the designing siRNA
program of Qiagen for survivin (5′-AAGGACCACCGCATCTCTACA-3′)
(Qiagen-Xeragon, USA), which extends between 92–112 nucleotides of
the coding mRNA sequence of survivin (NCBI accession no.
NM001168.1).
Double-stranded ultrapure siRNAs (HPLC-purified
>97% pure) were generated by mixing the corresponding pair of
sense and antisense RNA oligonucleotides. These were
fluorescein-labeled as RNAs, dissolved in 1 ml of the provided
sterile buffer (100 mM potassium acetate, 30 mM HEPES-KOH, 2 mM
magnesium acetate, pH 7.4) to give a 20 μM solution. The reaction
mixture was heated to 90°C for 1 min, allowed to incubate at 37°C
for 60 min, and then aliquoted and stored at −20°C. A non-specific
(mismarch) siRNA (Qiagen-Xeragon) served as the negative
control.
Treatment of cells with
fluorescence-labeled siRNAs
One day prior to transfection, approximate
4×105 FTC-133 cells were plated per 6-well plate in 2 ml
DMEM media containing 10% fetal bovine serum and antibiotics to
achieve 40–60% confluence. Cells were incubated under normal growth
conditions (generally 37°C and 5% CO2). Transfection of
the siRNAs was performed using TransMessenger™ transfection reagent
(Qiagen, Hilden, Germany). Two types of siRNA concentrations (135
pM, code no. sis-100 and 270 pM, code no. sis-248) were used in
this study. According to the manipulation, siRNAs were incubated
with Enhancer R, TransMessenger transfection reagent and cell
growth medium without serum and antibodies. The cells were then
washed once with pre-warmed (37°C) PBS. After washing, 1 ml
transfection mix was added to each well. The cells were incubated
for 4 h at 37°C, in 5% CO2. Following the incubation,
the cells were washed once again with pre-warmed (37°C) PBS.
Subsequently, the transfection medium was replaced by normal growth
medium DMEM containing 10% FCS and antibodies.
Survivin mRNA and protein levels were compared in
the untreated and mock-transfected cells using RT-PCR and
immunocytochemistry at 24 and 72 h and 7 days post-transfection.
The results were confirmed in three independent experiments.
Measurement of the intensity of survivin
staining by immunocytochemistry
For RT-PCR and the immunofluorescence assay, the
cells were harvested at different time points 24 and 72 h and 1
week post-transfection. Total-RNA from fresh cells was extracted
using the TRIzol reagent (Invitrogen) according to the
manufacturer’s protocol.
To confirm the effect of siRNA directed against
survivin, immunocytochemistry was performed using Dako coverplates
(Dako). At 24 and 72 h and 7 days after siRNA-transfection, cells
were immediately frozen in liquid frozen medium (42.8 g saccharose
+ 0.33 g MgCl2 in 250 ml PBS + 250 ml glycerol) and
maintained at −20°C until use. The manipulation followed the same
steps as the immunohistochemistry assay.
Statistical analysis
All experimental and clinical-pathological
parameters were calculated for statistical significance using
SigmaPlot 8.0 software (SPSS Inc., Chicago, IL, USA), and the
2-tailed unpaired t-test was used to compare the statistical
significance of the differences in data from two groups, where
appropriate. P-values of <0.05 were considered to indicate
statistical significance.
Results
mRNA expression in thyroid carcinoma and
benign goiter tissues analyzed by RT-PCR
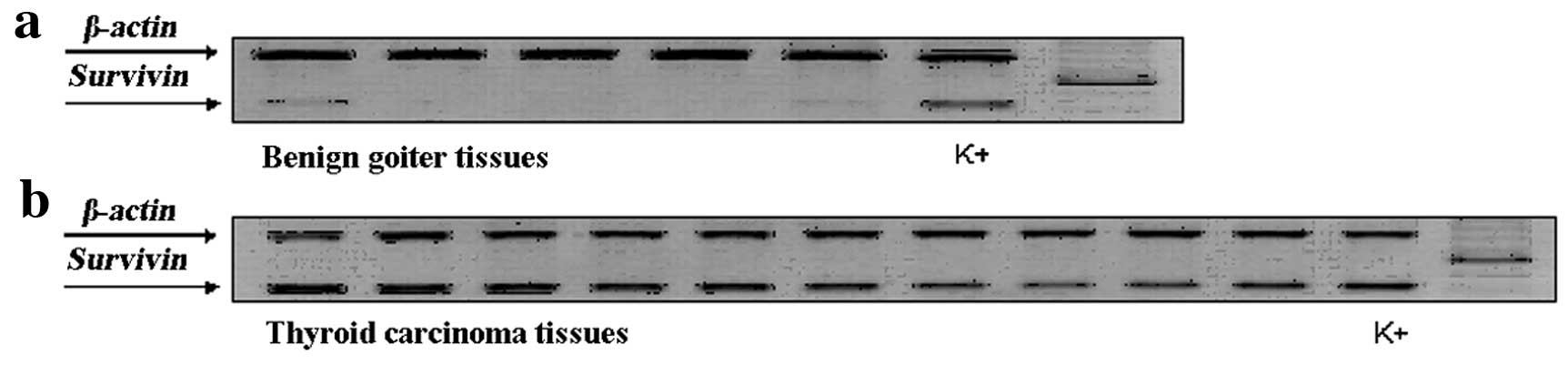
Transcripts of survivin in all of the goiter tissues
were evaluated as weak or negative, in contrast, moderate or strong
expression of survivin was observed in the carcinoma tissues
(Fig. 1). In general, RT-PCR
identified the survivin transcript in 67/75 (89.3%) of the tumors.
However, extremely weak expression of survivin mRNA was noted in
4/15 of the benign goiter samples. Expression of survivin mRNA was
significantly higher in the thyroid carcinoma tissues than that in
the benign goiter tissues. Moderate or strong positive survivin
expression was noted in 24/57 (42.1%) differentiated carcinomas and
in 12/18 (66.7%) undifferentiated carcinomas. The expression level
of survivin in undifferentiated carcinomas was generally higher
than that in differentiated carcinomas (P>0.05). The
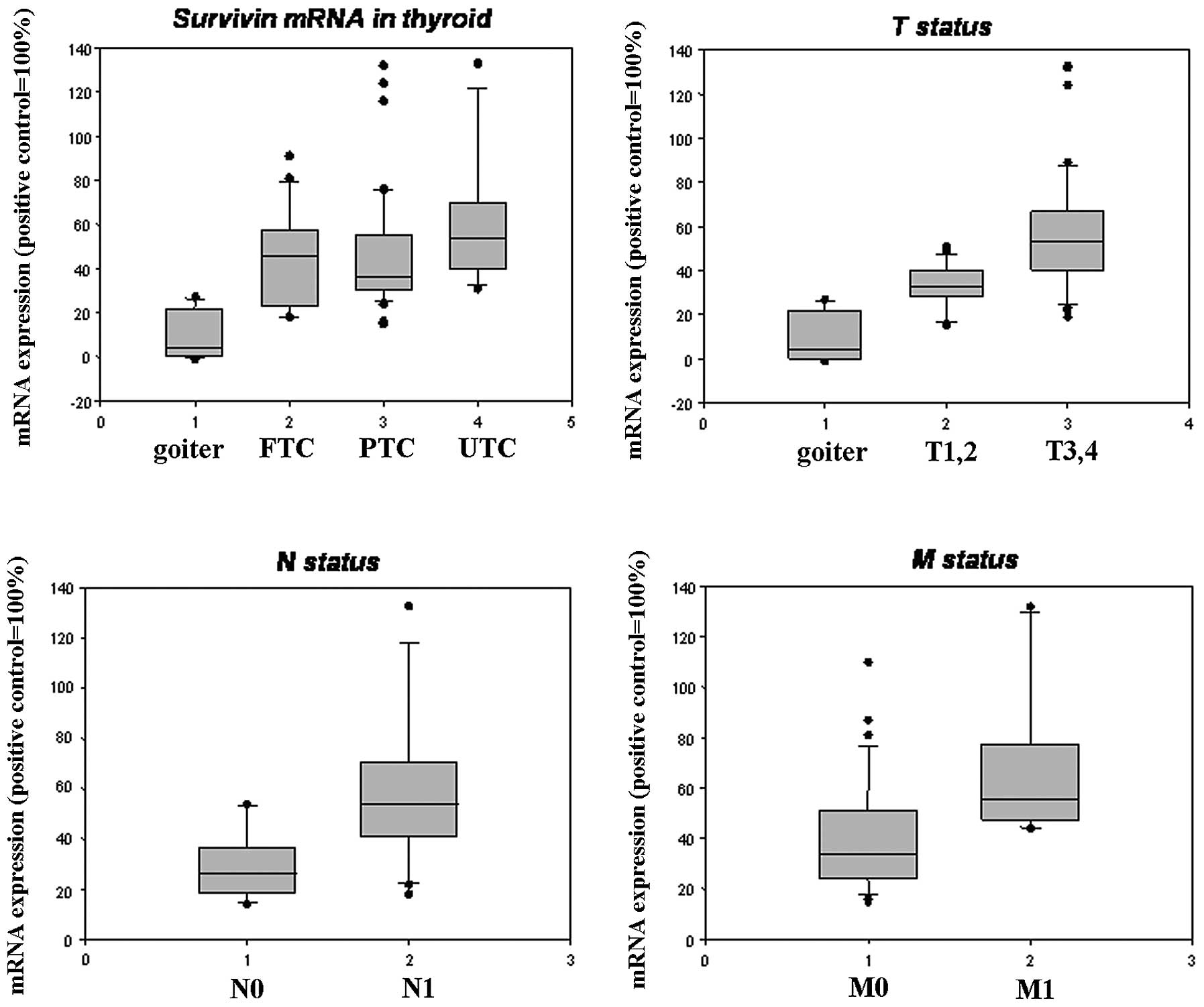
relationship between survivin mRNA expression and various
prognostic factors are documented in Table II and Fig. 2. A correlation was found between
various pT stages (pT1/2-pT3/4, P=0.009), the presence of lymph
node metastases (N0-N1, P=0.035) and distant metastasis (M0-M1,
P=0.08) in the thyroid carcinoma cases, while no significant
correlation was found in relation to age, gender and pathological
subtype.
 | Table II.Relationship of survivin expression
and various prognostic factors in 75 patients with thyroid
carcinoma. |
Table II.
Relationship of survivin expression
and various prognostic factors in 75 patients with thyroid
carcinoma.
|
Clinico-pathological characteristics | No. of patients
(total) | Survivin mRNA
positive (++, +++) | Survivin mRNA
decreased (−, +) | P-value | Survivin protein
positive (2, 3) | Survivin protein
decreased (0, 1) | P-value |
|---|
| Age (years) | | | | | | | |
| ≤45 | 17 | 5 | 12 | | 6 | 11 | |
| >45 | 58 | 32 | 26 | NS | 28 | 30 | NS |
| Gender | | | | | | | |
| Male | 29 | 18 | 11 | | 15 | 14 | |
| Female | 46 | 23 | 23 | NS | 20 | 26 | NS |
| Tumor status | | | | | | | |
| pT1, pT2 | 16 | 9 | 7 | | 6 | 10 | |
| pT3, pT4 | 59 | 42 | 17 | 0.009 | 38 | 21 | <0.001 |
| Nodal status | | | | | | | |
| N0 | 40 | 22 | 18 | | 23 | 17 | |
| N1 | 35 | 25 | 10 | 0.035 | 5 | 30 | 0.011 |
| Metastatic
status | | | | | | | |
| M0 | 64 | 38 | 26 | | 35 | 29 | |
| M1 | 11 | 10 | 1 | 0.008 | 9 | 2 | NS |
| Pathological
subtype | | | | | | | |
| FTC | 24 | 14 | 10 | NS | 12 | 9 | NS |
| PTC | 33 | 10 | 23 | NS | 10 | 19 | NS |
| UTC | 18 | 12 | 6 | NS | 2 | 9 | NS |
Protein expression in thyroid carcinoma
and benign goiter tissues analyzed by immunohistochemistry
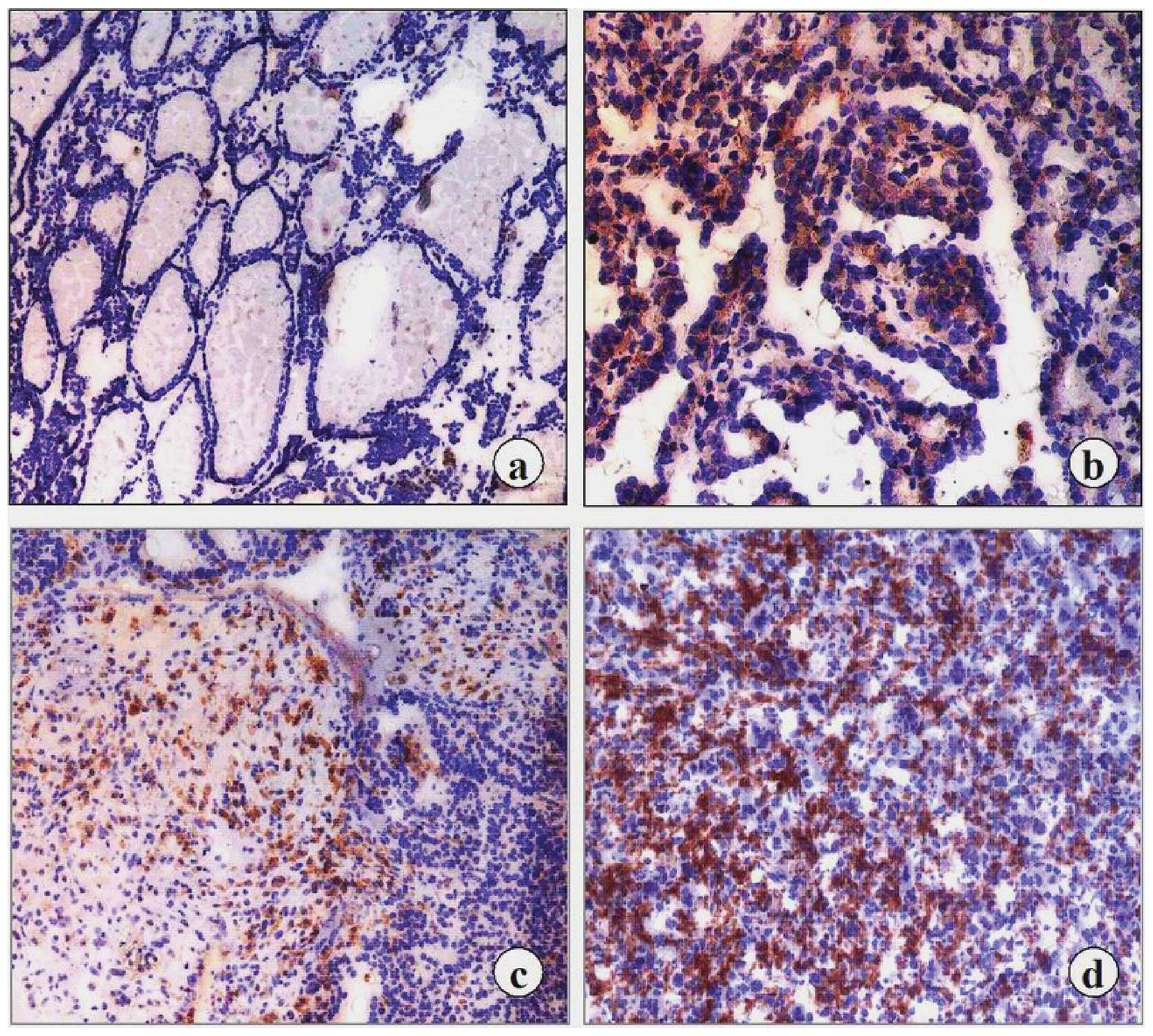
Examples of survivin immunostaining are shown in
Fig. 3. The results of the
immunohistochemical study demonstrated good correlation with that
of the RT-PCR analysis. Among the tumors examined, 65/75 (86.7%)
carcinomas revealed survivin immunoreactivity in the cytoplasm of
the tumor cells, whereas no expession was found in all of the 15
normal and benign goiter tissues. A uniformly intense survivin
protein expression was detected in the cellular cytoplasm of the
differentiated thyroid carcinoma tissues, and the staining often
appeared granular. A similar but stronger pattern was observed in
the UTC tissues. Following comparison of the different pathological
features of the carcinoma cases, the UTC cases exhibited the
strongest positive survivin immunoreactivity while the PTC and FTC
cases displayed high or moderate levels of survivin. Among the 40
examined primary tumors without metastasis, 17 cases (∼42.5%)
showed weak or moderate expression. In 25/35 (∼71.4%) primary
tumors with regional lymph node infiltration and distant
metastasis, moderate or high survivin immunostaining was noted. We
found a similar correlation with mRNA expression between the
different pT stages (pT1/2–pT3/4, P<0.001), N status (N0–N1,
P=0,011), M status (M0–M1, P<0.05) in differentiated and
undifferentiated thyroid carcinoma. However, there was no
statistically significant difference between survivin expression
and the other clinicopathologic features. The relationship between
survivin protein expression and various prognostic factors are
shown in Table II and Fig. 4. Therefore, dedifferentiation of
thyroid carcinoma cells may cause an increase in the expression of
survivin transcripts and immunoreactive protein.
Downregulating effects of siRNA on
survivin
Fluorescein signal was detected by fluorescence
microscopy as a granular pattern in the cytoplasm surrounding
nuclei (Fig. 5). Green
fluorescein-positive cells and total cells were counted in 10
randomly selected fields. The transfection efficiency was
calculated to be ∼45–55%.
The effects of survivin siRNA treatment on the cells
were visualized by immunocytochemistry. Survivin immunostaining was
undetectable in the cells which were treated with survivin siRNA
(Fig. 6c and d) on day 1, 3 and 7
following transfection. In contrast, all cells from untreated and
control experiments retained strong staining (Fig. 6a and b).
For a quantitative evaluation of endogenous survivin
protein expression in the FTC-133 cells, immunoblotting (western
blot analysis) was performed according to the protocol described
above. Total proteins in the cells were extracted from a 6-well
cell plate after 24 and 72 h and 1 week of transfection.
Twenty-four hours after transfection, a maximum downregulation to
46% of the initial protein level was achieved at 24 h in sis-110
treated cells (∼50% in comparison with untreated cells) and sis-248
(∼70%) (Fig. 7). Decreased
survivin levels were still observed after 72 h. Additionally, this
silencing was still noted 7 days following siRNA treatment (60%).
Different siRNAs had no influence on endogenous β-actin expression.
Its expression was equal in each experimental sample.
Discussion
Several studies have shown a prominent correlation
between survivin expression and tumor aggressiveness (7,11–14,16). Thus, we postulated prior to this
study that the level of survivin expression may be a significant
indicator for the progression of thyroid carcinoma. The results of
the present study suggest that decreased apoptosis integrated
partly by survivin expression is a predictive indicator of poorer
prognosis in patients with thyroid carcinoma.
In the present study, we extended initial
observations and clearly demonstrated that survivin mRNA and
protein were expressed consistently and highly in thyroid carcinoma
tissues, while all goiter specimens exhibited the absence or
significant downregulation of survivin expression and were
considered to be survivin-negative, with the exception of weak
expression in four tissue specimens. At present, it is unclear why
several normal and benign cells exhibited survivin expression. A
possible explanation is that the mRNA sample containing survivin
mRNA may have been obtained from mitotically active cells, as
survivin has been shown to regulate the cell cycle in the G2-M
phase. The presence of an invasive phenotype such as lymph node
infiltration or distant metastasis coincided with strong expression
of survivin. By contrast, cases exhibiting weak expression of
survivin were devoid of metastases (N0/M0), suggesting a role for
survivin in the tissue invasiveness of thyroid carcinoma. We found
no correlation between the expression of survivin in regards to age
and gender. However, the expression of survivin appears to be
correlated with tumor size, regional lymph node metastasis, distant
metastasis and different pathological subtypes.
To our knowledge, the present study represents the
first investigation of the expression of survivin in thyroid
carcinoma. In conclusion, the present results suggest that survivin
is upregulated during thyroid carcinoma progression. Our data
demonstrated that survivin has an increased expression profile in
advanced stages of thyroid carcinoma such as pT3/T4. Furthermore,
the diminished expression of survivin is associated with metastasis
and increased expression of survivin may represent a potentially
useful prognostic marker for the classification, staging and
subtyping of human thyroid carcinomas. Elucidation of the
mechanisms of survivin and prediction of whether its expression
proves useful for the clinical treatment of thyroid cancer patients
warrants further investigation.
The possible role of survivin as a target for cancer
vaccines in different types of cancers has been discussed (25,27–29). Therefore, in the present study,
the siRNA transfection experiment using a thyroid carcinoma cell
line was performed in our laboratory. The RNAi effect was
demonstrated by transfecting siRNA into FTC-133 cells where it
significantly reduced expression of the survivin gene. Expression
was not suppressed in cells that were untransfected, or transfected
with a nonspecific control siRNA. We confirmed the downregulation
of survivin expression by use of siRNA to block survivin mRNA and
protein expression. Notably, siRNA survivin does not induce death
in a normal cell population due to the absence of survivin
expression in normal cells. Our data suggest that the use of siRNA
survivin warrants further investigation as a novel approach to
selective cancer therapy.
Acknowledgements
We thank Mrs. Kathrin Hammje and Ms.
Anja Winkler for their excellent technical assistance and support
in collecting patient samples and clinical data. We deeply thank
Ms. Ying Li for her helpful corrections and suggestions in regards
to this manuscript. This study was supported in part by the DFG and
Deutsche Krebshilfe.
References
|
1.
|
AH WyllieGlucocorticoid-induced thymocyte
apoptosis is associated with endogenous endonuclease
activationNature284555556198010.1038/284555a06245367
|
|
2.
|
S NagataApoptosis by death
factorCell88355365199710.1016/S0092-8674(00)81874-79039262
|
|
3.
|
CB ThompsonApoptosis in the pathogenesis
and treatment of
diseaseScience26714561462199510.1126/science.78784647878464
|
|
4.
|
L SachsJ LotermControl of programmed cell
death in normal and leukemic cells: new implications for
therapyBlood82152119938324219
|
|
5.
|
E YangSJ KorsmeyerMolecular thanatopsis: a
discourse on the bcl-2 family and cell
deathBlood881456146219968695785
|
|
6.
|
NE CrookRJ ClemLK MillerAn
apoptosis-inhibiting baculovirus gene with a zinc finger-like
motifJ Virol672168217419938445726
|
|
7.
|
G AmbrosiniC AdidaDC AltieriA novel
anti-apoptosis gene, survivin, expressed in cancer and lymphomaNat
Med3917921199710.1038/nm0897-9179256286
|
|
8.
|
F LiDC AltieriThe cancer anti-apoptosis
mouse survivin gene: characterization of locus and transcriptional
requirements of basal and cell cycle-dependent expressionCancer
Res5931433151199910397257
|
|
9.
|
F LiDC AltieriTranscriptional analysis of
human survivin gene expressionBiochem
J344305311199910.1042/0264-6021:3440305
|
|
10.
|
R TakahashiQL DeverauxI TammK WelshN
Assa-MuntGS SalvesenJC ReedA single BIR domain of XIAP sufficient
for inhibiting caspasesJ Biol
Chem27377877790199810.1074/jbc.273.14.77879525868
|
|
11.
|
CD LuDC AltieriN TanigawaExpression of a
novel anti-apoptosis gene, survivin, correlated with tumor cell
apoptosis and p53 accumulation in gastric carcinomasCancer
Res581808181219989581817
|
|
12.
|
H KawasakiDC AltieriC-D LuM ToyodaT TenjoN
TanigawaInhibition of apoptosis by survivin predict shorter
survival rates in colorectal cancerCancer
Res585071507419989823313
|
|
13.
|
M MonzóR RosellE FelipA Novel
anti-apoptosis gene: Re-expression of survivin messenger RNA as a
prognosis marker in non-small-cell lung cancersJ Clin
Oncol1721002104199910561264
|
|
14.
|
K TanakaS IwamotoG GonT NoharaM IwamotoN
TanigawaExpression of survivin and its relationship to loss of
apoptosis in breast carcinomasClin Cancer
Res6127134200010656440
|
|
15.
|
C LigginsDJ OrlickyLA BloomquistR
GiananiDevelopmentally regulated expression of survivin in human
pancreatic isletsPediatr Dev
Pathol6392397200310.1007/s10024-003-2014-014708732
|
|
16.
|
M KrajewskaS KrajewskiS BanaresElevated
expression of inhibitor of apoptosis proteins in prostate
cancerClin Cancer Res949144925200314581366
|
|
17.
|
C AdidaPL CrottyJ McGrathD BerrebiJ
DieboldDC AltieriDevelopmentally regulated expression of the novel
cancer anti-apoptosis gene survivin in human and mouse
differentiationAm J Pathol152434919989422522
|
|
18.
|
F LiG AmbrosiniEY ChuJ PlesciaS TogninPC
MarchisioDC AltieriControl of apoptosis and mitotic spindle
checkpoint by survivinNature396580584199810.1038/251419859993
|
|
19.
|
E YangSJ KorsmeyerMolecular thanatosis: a
discourse on the BCL2 family and cell
deathBlood8838640119968695785
|
|
20.
|
JC ReedDouble identity for proteins of the
Bcl-2 familyNature387773776199710.1038/428679194558
|
|
21.
|
Y WeiX ZhaoY KariyaK TeshigawaraA
UchidaInhibition of proliferation and induction of apoptosis by
abrogation of heat-shock protein (HSP) expression in tumor
cellsCancer Immunol
Immunother407378199510.1007/BF015202877882385
|
|
22.
|
N MairesseS HormanR MosselmansP
GalandAntisense inhibition of the 27 kDa heat shock protein
production affects growth rate and cytoskeletal organization in
MCF-7 cellsCell Biol Int20205212199610.1006/cbir.1996.0025
|
|
23.
|
G RandallA GrakouiCM RiceClearance of
replicating hepatitis C virus replicon RNAs in cell culture by
small interfering RNAsProc Natl Acad Sci
USA100235240200310.1073/pnas.023552410012518066
|
|
24.
|
G YangJA ThompsonB FangJ LiuSilencing of
H-ras gene expression by retrovirus-mediated siRNA decreases
transformation efficiency and tumorgrowth in a model of human
ovarian cancerOncogene2256945701200310.1038/sj.onc.1206858
|
|
25.
|
G AmbrosiniC AdidaG SirugoDC
AltieriInduction of apoptosis and inhibition of cell proliferation
by survivin gene targetingJ Biol
Chem2731117711182199810.1074/jbc.273.18.111779556606
|
|
26.
|
JC ReedPromise and problems of Bcl-2
antisense therapyJ Natl Cancer
Inst89988990199710.1093/jnci/89.14.9889230876
|
|
27.
|
Q FeiH ZhangL FuExperimental cancer gene
therapy by multiple anti-survivin hammerhead ribozymesActa Biochim
Biophys Sin
(Shangai)40466477200810.1111/j.1745-7270.2008.00430.x18535745
|
|
28.
|
WY ZhengYY KangLF LiYX XuXY MaLevels of
effectiveness of gene therapies targeting survivin and its splice
variants in human breast cancer cellsDrug Discov
Ther5293298201110.5582/ddt.2011.v5.6.29322466440
|
|
29.
|
K YamanakaM NakataN KanekoYM155, a
selective survivin suppressant, inhibits tumor spread and prolongs
survival in a spontaneous metastatic model of human triple negative
breast cancerInt J Oncol395695752011
|