Introduction
Mature insulin is a protein of 51 residues (21 in A
chain and 30 in B chain) produced in specialized beta cell islet of
the Langerhans in the pancreas. Insulin binds on transmembrane
tyrosine kinase receptor (insulin receptor) present in liver,
muscle and cells in the fat tissues and stimulates increase glucose
uptake from blood and converts it into glycogen to store in the
liver and muscles. Insulin regulates carbohydrate and fat
metabolism in the body. Failure to control insulin level leads to
diabetes mellitus type 1 or 2. Patients with type 1 and ∼40% of
type 2 diabetic patients need insulin to control their blood
glucose level. Type 2 diabetes is the most common and results from
insulin resistance, a condition in which cells fail to use insulin
properly.
The number of people diagnosed with type 2 diabetes
has risen steeply in last decades severely exhausting the ability
of health care systems to deal with the epidemic. Over 300 million
people worldwide have diabetes and this most likely will rise to
500 million within the next 20 years. Seventy-five percent of
people with diabetes live in low- and middle-income countries and
according to prognostics Africa will experience a largest increase
in the next generation. The highest incidence of this disease is in
the Arabic Middle East, but the largest populations of diabetics
are in China and India, with many of those people living in extreme
poverty (1–5). According to a 2005 World Bank
estimate, >40% of the total Indian population falls below the
international poverty line defined as an income less than US$1.25 a
day (Wikipedia, 2011). Combined forces of governmental health care,
charities, and donation of pharmaceutical companies would not be
able to cope with the financial demands needed for medicaments and
treatments for these people. Therefore it is worth looking into
traditional folk remedies to investigate if there is any scientific
merit to justify their claims for alleviating symptoms of
diabetes.
The traditional belief in the Middle East is that
regular consumption of camel milk helps in prevention and control
of diabetes, it has also been reported that camel milk can have
such properties (6–8). This is a tempting hypothesis since
over a few generations the Arab population has drastically changed
its diet including drastic reduction of camel milk consumption.
This was accompanied by a robust rise of incidence of diabetes. Two
independent groups studied influence of regular consumption of
camel milk on diabetes and have reported a substantial reduction in
the mean dose of insulin needed to obtain glycemic control
(6,8,9)
and improvement of fasting blood sugar (227.2±17.7 vs. 98.9±16.2
mg/dl), HbA1c (glucosylated hemoglobin) (9.59±2.05% vs.
7.16±1.84%), serum anti-insulin antibodies (26.20±7.69 vs.
20.92±5.45 μU/ml), urinary albumin excretion (25.17±5.43 vs.
14.54±5.62 mg/dl/24 h), reduction of daily insulin dose (48.1±6.95
vs. 23±4.05 units), and body mass index (18.43±3.59 vs. 24.3±2.95
kg/m2) in randomized human study (9). No mechanism was provided to explain
this phenomenon.
In different studies it was found that regular
consumption of camel milk for a few months significantly improved
the condition of diabetic patients and experimental animals
(6–8,10).
Zero prevalence of diabetes in camel milk drinking population and
the results of use of camel milk in controlled clinical trials on
diabetic humans and animals are highly encouraging to use it as
natural therapy for the prevention and treatment of diabetes
(6–8,10,11). Such beneficial effects of camel
milk might be due to presence of insulin in the milk or some other
substance(s) able to modulate glucose level. It contains higher
level of insulin than milk from other animals (12) but to be effective it would have to
be absorbed directly in the buccal cavity or completely
proteolytically protected during passage through stomach and
absorbed in the intestine. Camel milk is unique in the sense that
it does not respond to acidic agents like other animal milk,
possesses different casein content and much larger lipid micelles
(13).
Literature review suggests following possibilities:
i) insulin in camel milk possesses special properties that make
absorption into circulation easier than insulin from other sources
or cause resistance to proteolysis; ii) camel insulin is
encapsulated in nanoparticles (lipid vesicles) that make possible
its passage through stomach and entry into circulation; iii) some
other elements of camel milk make it anti-diabetic.
In this study we are trying to understand the role
of insulin in camel milk using bioinformatic tools. Sequence,
structure similarity and literature review suggest that camel
insulin similar to water buffalo and bovine does not possess any
properties that should make it more resistant to proteolysis and
easier to be absorbed into the circulation. There is no evidence
that cow milk has any anti-diabetic properties albeit it does
include insulin at lower level (12). However, it cannot be excluded that
insulin if encapsulated in nanoparticles can cross digestive track
walls. Lastly it is possible also that camel milk contains
unidentified small molecules of ‘insulin-like’ regulatory value or
of protease inhibitory properties to prevent proteolysis.
Materials and methods
Insulin sequences from different organisms were
obtained from UniProt web search engine (http://www.uniprot.org/). Camel insulin (UniProt id:
P01320) was used as a template for sequence in PSI-BLAST. The
homologous insulin sequences from animals and plants were selected
and subjected to multiple sequence alignment performed by Jalview
(http://www.jalview.org/). The Multiple Sequence
Alignment was color coded according to conservancy. The amino acid
sequences of insulin were used to construct phylogenetic tree using
BLOSUM62 from MAFFT Multiple Sequence Alignment (http://www.jalview.org/). The alignment quality of the
amino acid sequences is based on BLOSUM62. Conservation among
insulin sequences were calculated according to Livingstone and
Barton. After multiple sequence alignment, consensus sequence
represents the most common residues at a particular position.
Quality measures the inverse likelihood of unfavorable mutations in
the multiple aligned insulin sequences.
Digestive pattern of different insulin was performed
by online software, peptide cutter (http://web.expasy.org/peptide_cutter/). The number of
the cut sites for pepsin at pH 1.3 and 2.0, trypsin and
chymotrypsin with high and low specificity were recorded.
Protein structure modeling
An internet service I-TASSER server was used for
protein structure and function predictions. It allows to
automatically generate high-quality predictions of 3D structure
based on amino acids sequence (14,15).
Results and Discussion
Proteolysis sites of digestive proteases
in different types of insulin of different species
Models of human and camel insulin are essentially
the same as predicted by I-TASSER (Fig. 1) (14,15). We hypothesized that camel insulin
is protected from digestive enzymes in the stomach and thus
absorbed in the intestine. The numbers of calculated cut sites for
different types of insulin were the same for camel, human, bovine,
goat, buffalo, sheep and pig insulin (Table I). The preferred cut sites for
pepsin are Phe, Tyr, Trp and Leu. Trypsin prefers Arg and Lys at P1
while chymotrypsin preferentially cleaves at Trp, Tyr and Phe in
position P1 (high specificity) and to a lesser extent at Leu, Met
and His (low specificity). Camel insulin differs from human insulin
by four mutations and from bovine and buffalo by just one mutation.
None of the mutations affect specificity toward digestive enzymes.
Therefore, camel insulin should be identical to human, bovine,
buffalo, goat, sheep and pig insulin in terms of susceptibility
toward proteolysis. Thus, when camel insulin comes in contact with
the proteases of digestive track it should be digested like other
mammalian insulin unless otherwise protected.
 | Table I.Proteolysis sites of digestive
proteases in different types of insulin. |
Table I.
Proteolysis sites of digestive
proteases in different types of insulin.
| | No. of cleavages
|
|---|
| Insulin | Uniport accession
no. | Pepsin pH 1.3 | Pepsin pH
>2.0 | Trypsin | Chymotrypsin high
specificity | Chymotrypsin low
specificity |
|---|
| Human (Homo
sapiens) | P01308 | 22 | 15 | 2 | 7 | 15 |
| Camel (Camelus
dromedaries) | P01320 | 22 | 15 | 2 | 7 | 15 |
| Bovine (Bos
taurus) | P01317 | 22 | 15 | 2 | 7 | 15 |
| Water buffalo
(Bubalus bubalis) | Q25C78 | 22 | 15 | 2 | 7 | 15 |
| Domestic goat
(Capra hircus) | P01319 | 22 | 15 | 2 | 7 | 15 |
| Elephant
(Elephas maximus) | P01318 | 22 | 15 | 2 | 7 | 15 |
| Sheep (Ovis
aries) | P01316 | 22 | 15 | 2 | 7 | 15 |
| Whale (Physeter
macrocephalus) | P67974 | 22 | 15 | 2 | 7 | 15 |
| Chimpanzee (Pan
troglodytes) | P30410 | 22 | 15 | 2 | 7 | 15 |
| Hamster
(Cricetidae sp.) | Q7M0G1 | 22 | 15 | 2 | 7 | 15 |
| Pig (Sus
scrofa) | P01315 | 22 | 15 | 2 | 7 | 15 |
| Rabbit
(Oryctolagus cuniculus) | P01311 | 22 | 15 | 2 | 7 | 15 |
| Dog (Canis
familiaris) | P01321 | 22 | 15 | 2 | 7 | 15 |
| Cat (Felis
catus) | P06306 | 22 | 15 | 2 | 7 | 16 |
| Horse (Equus
caballus) | P01310 | 22 | 15 | 2 | 7 | 15 |
| Muscovy duck
(Cairina moschata) | P68243 | 21 | 14 | 2 | 6 | 14 |
| Goose
(Anser) | P68245 | 21 | 14 | 2 | 6 | 14 |
| Turtle
(Trachemys scripta) | P69048 | 21 | 14 | 2 | 6 | 15 |
| Ostrich
(Struthio camelus) | P67969 | 21 | 14 | 2 | 6 | 15 |
| Turkey
(Meleagris gallopavo) | P67968 | 21 | 14 | 2 | 6 | 15 |
| Alligator
(Alligator mississippiensis) | P12703 | 20 | 13 | 3 | 6 | 14 |
| Opossum
(Didelphis marsupialis virginiana) | P18109 | 22 | 15 | 2 | 6 | 15 |
| Chinchilla
(Chinchilla) | Q5BVF6 | 21 | 14 | 2 | 7 | 16 |
| Viscacha
(Lagidium viscacia) | Q5BVF4 | 18 | 11 | 3 | 6 | 15 |
| Mouse (Mus
musculus) | E0CXX7 | 21 | 14 | 2 | 7 | 16 |
| Bat (Rhinolophus
ferrumequinum) | B2KIN7 | 22 | 15 | 2 | 7 | 15 |
| Crab-eating macaque
(Macaca fascicularis) | P30406 | 22 | 15 | 2 | 7 | 15 |
| Guinea pig
(Cavia porcellus) | P01329 | 15 | 10 | 3 | 6 | 12 |
| Jack-bean
(Canavalia ensiformis) | Q7M217 | 22 | 15 | 2 | 7 | 15 |
| Camel’s foot tree
(Bauhinia purpurea) | 721138A | 22 | 15 | 2 | 7 | 15 |
| Cowpea (Vigna
unguiculata) | P83770 | 22 | 15 | 2 | 7 | 15 |
Insulin sequences
It is commonly believed that insulin sequences of
different types of species are highly conserved (16–18). However, as shown in Figs. 2 and 3 some species may differ from human by
as many as 18 amino acids (out of 51) in mature form of insulin.
Camel insulin is identical to bovine and water buffalo, varying
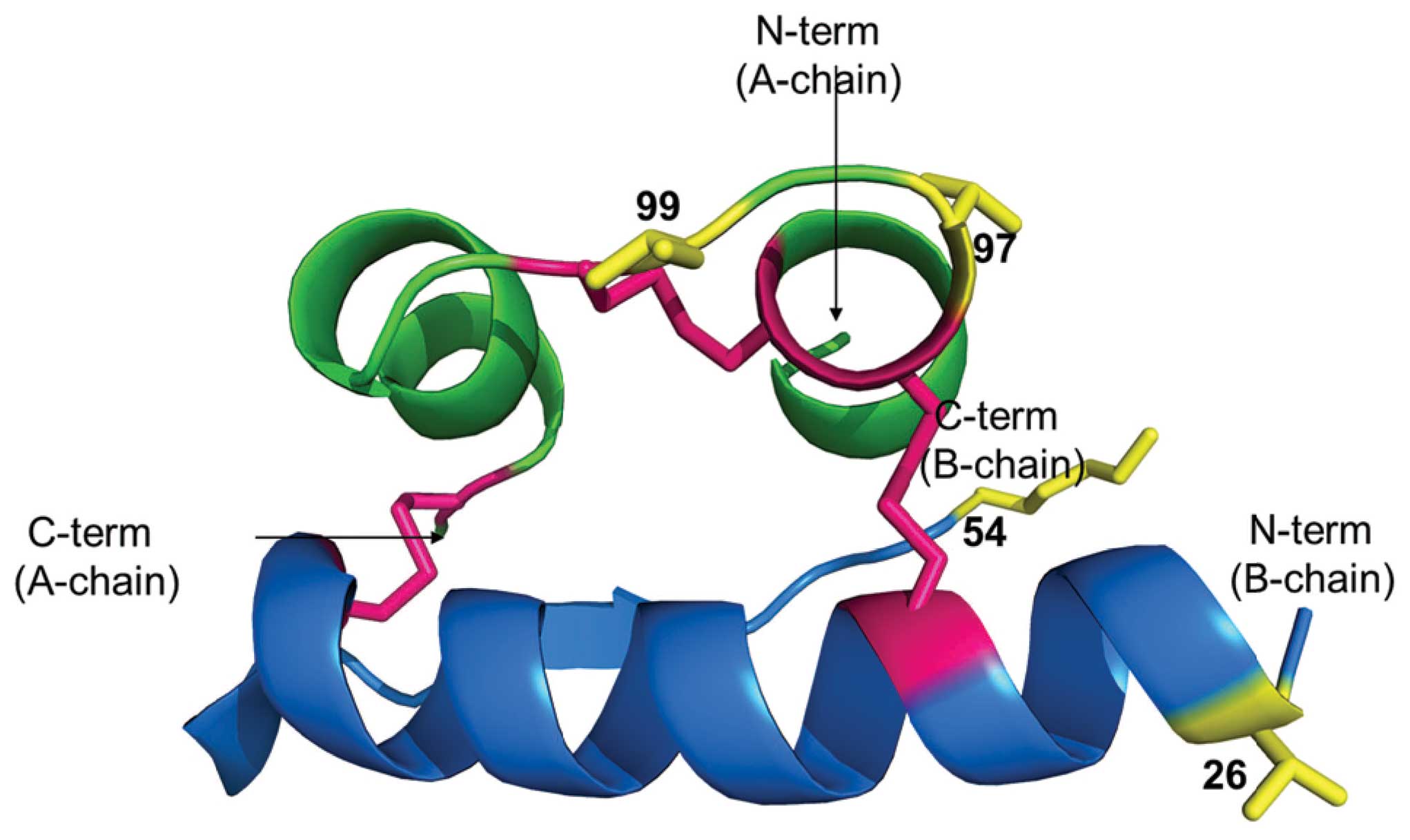
from human in Thr54Ala, Thr97Ala, Ile99Val. There is a
contradiction on additional variation in camel insulin sequence at
Val26Ala as reported byUniProt (19), while Al-Swailem et al
reports only Thr54Ala, Thr97Ala, Ile99Val (20).
According to early studies by Pullen et al a
number of conserved surface residues, forming the ‘classical
binding surface’, were most likely involved in the insulin receptor
binding (Gly90, Gln95, Tyr108, Asn110, Val37, Tyr41, Gly48, Phe49,
Phe50, Tyr51) (21). The subset
of this binding surface (Asn110, Phe49, Phe50, Tyr51), was later
proposed to be essential for negative cooperativityin receptor
binding by De Meyts et al (22) and confirmed by Xu et al
(23). Two insulin mutations
known to cause insulinopathy resulting in mild symptoms similar to
diabetes type 2 are in this region (insulin Los Angeles:
Phe-49-Ser) (24), (insulin
Chicago: Phe-50-Leu) (25), see
Fig. 4. Although insulin is such
a small protein itself it forms dimers that further associate into
hexamers important for this enzyme stability. The other residues
such as Leu42 and Leu102 that are involved in hexamer-forming
surfaces, are engaged also in receptor binding (26). In addition to the original surface
residues shown to be important in receptor binding, a cluster of
residues (Ser101, Leu102, Glu106, His35, Glu38, and Leu42) known as
the primary binding surface disrupt binding to receptor if mutated
(27,28). It is worth noting that His10 (35
in Fig. 2) is involved in Zn
coordination necessary for the hormone activity.
We analyzed the effect of mutations on the specific
activity of insulin. Three relevant human insulin mutants are known
(B: Val26Ala, Thr54Ala and A: Thr97Ala). Two mutations (B: Val26Ala
and Thr54Ala) in the human insulin increase its specific activity
by 110 and 102±21%, respectively, while the third mutation (A:
Thr97Ala) decreases specific activity to 87±13% (29). Only one residue (Thr98 of A chain)
out of three mentioned above interacts with insulin receptor
(27). The mutations in the B
chain terminals might have an impact on the conformational changes
and stability of the hexamer and their conversion into active
monomer.
All these amino acids are conserved in camel, water
buffalo and bovine insulin. All three types of insulin are quite
unique if compared with others that are identical in primates and
vary by one or more amino acid in other species. There is no
evidence of anti-diabetic properties of water buffalo and cow milk
(30–32). Literature search on insulin from
sheep and goat varying form, Thr54Ala, Thr97Ala, Ile99Val, by
additional mutation Ser98Gly do not provide any evidence on
anti-diabetic properties. We conclude that the camel insulin itself
is most likely not responsible for anti-diabetic properties of
camel milk.
Nano particles
Mucosal surfaces are frequent routes for delivering
drugs to the body. Unfortunately, drugs such as peptides and
proteins are unable to overcome the mucosal barriers and are
degraded (by digestive enzymes if delivered orally) before reaching
the blood stream. It provokes the question how insulin in the camel
milk could be protected in the stomach to reach the target.
Possible explanation may be hidden in the uniqueness of camel milk.
Camel milk does not easily coagulate at low pH, it has good
buffering capacity, has different proportions of caseines and fatty
acids and makes larger lipid micelles than observed in milk of
other mammals. It may be possible that insulin in the camel milk is
encapsulated in the micelles and passes through the stomach to the
intestine. For example when compared with the cow’s milk: i) Kappa
caseine micellar fraction reacting with clotting enzymes has
different electro-potential and lower electrophoretic mobility and
accounts for only ∼5% of total casein vs. ∼13.6% in cow, ii) The
micellar size show the mean diameter of 280÷325 μm vs. 160 μm for
cow (13), iii) Raw cow milk
contains less insulin than camel and it loses even more in
processing before reaching a diary store (12). There is evidence that size of
lipid micelles becomes larger in milk of cows exposed to hot
weather and water deprivation (33). In the desert climate camels are
well adjusted to both such conditions, which might explain unique
properties of their milk even during drought (34). If due to unique features of the
camel milk, insulin is able to cross stomach and get absorbed
efficiently into blood stream then ‘camel milk-like features’ could
be used for the formulation of insulin for oral delivery in humans.
We do not have evidence of insulin presence in micelles, although
nanoparticles were used for oral delivery of proteins (35–37). Nafissi-Varcheh et al
investigated biodegradable polyester polymers with different
molecular weights and lactic/glycolic acids ratios in simulated
gastrointestinal fluids. They intend to use microparticles for oral
protein delivery. They reported that nanoparticles could be
suitable for the preparation of protein-loaded microspheres
(35).
Prego et al used the mucoadhesive
polysaccharide chitosan nanoparticles, chitosan-coated oil
nanoparticles and chitosan-coated lipid nanoparticles showing
significant capacity for the association of proteins such as
insulin, salmon calcitonin and other proteins. They showed that
chitosan-coated nanoparticles exhibited capacity to enhance the
intestinal absorption of the model peptide, salmon calcitonin, and
long-lasting decrease in the calcemia levels in animals (36). Vila et al developed new
biodegradable polymer nanoparticles: poly(ethylene glycol)
(PEG)-coated poly(lactic acid) (PLA) nanoparticles, chitosan
(CS)-coated poly(lactic acid-glycolic acid (PLGA) nanoparticles and
chitosan (CS) nanoparticles. These were tested successfully to load
proteins, and to deliver them in an active form to transport them
across intestinal mucosae (37).
Insulin-like small molecules
He et al developed an in vitro
screening assay searching for insulin mimetics. Screening the small
molecule chemical libraries, they found a compound
(5,8-diacetyloxy-2,3-dichloro-1,4-naphthoquinone, Fig. 5a) that activates insulin receptor
directly binding to the receptor kinase domain, to trigger its
kinase activity sensitizing insulin’s action. Drug was delivered
orally to wild-type C57BL/6J mice and db/db (diabetic) and ob/ob
(obese) mice and it was shown to elevate glucose uptake in
adipocytes (38).
Mozaffarian et al investigated over 3700
adults in the Cardiovascular Health Study to determine if
trans-palmitoleate (trans-16:1n-7, Fig. 5b) was related to new-onset
diabetes. An endogenous cis-palmitoleic acid (Fig. 5c) (of adipose or hepatic source),
could be beneficial protecting against insulin resistance but also
harmful causing cardiovascular risk in humans. Contrary,
trans-palmitoleic was associated with lower incidence of diabetes.
The individuals taking it had a much lower risk of developing
diabetes; ∼60% lower risk among participants in the highest
quintile (39).
Trans-palmitoleate is strictly exogenous and naturally, occurring
in dairy/ruminant trans-fats. It is worth noting that among long
chain fatty acids present in camel milk C16 and C18 dominate with
C16 on par to cow milk in saturated category but ∼3 times higher
for unsaturated C16:1 (39). This
might further support the anti-diabetic benefits of drinking camel
milk. Additionally, substantial work has been carried out in
plants, as reviewed below.
Insulin and insulin-like molecules in
plants
Traditional holistic practitioners in many different
parts of the world recommend the consumption of plants variety for
regulation of glycaemia (40–46). There are also more systematic
approaches to evaluate anti-diabetic activity of food. Broadhurst
et al examined the possible effects of 49 herbs, spices, and
medicinal plant extracts on the insulin-dependent utilization of
glucose using a rat epididymal adipocyte assay. They found that
cinnamon was the most bioactive product followed by witch hazel,
green and black teas, allspice, bay leaves, nutmeg, cloves,
mushrooms, and brewer’s yeast (43). However, no particular active
chemicals were identified.
Varieties of legumes were reported to have
anti-diabetic properties (47–50). Bean pods (Phaseolus vulgaris) are
among the most used traditional remedies with anti-diabetic
activity. To be effective, fairly high doses of aqueous extracts
need to be given. There is no clear evidence what the active
ingredient is. However, authors suggest that by α-amylase
inhibitory effect, beans might be effective in preventing or
ameliorating type 2 diabetes (48). Nevertheless, beans similarly like
camel milk contain insulin or insulin-like protein sequences. Soon
after discovery of pancreatic insulin in early 1920s, insulin-like
protenaceous material was found in many plants (bean, lettuce,
onion and beat). In the 1970s and 80s, several research groups have
isolated and well characterized insulin-like protenaceous material
and found that it exhibits same hypoglycemic activity, identical
molecular weight, chromatographic and immunological properties. In
2003, high level (50 mg insulin/100 g protein = ∼1000 units
insulin/100 g protein) of insulin-like substance from legume
Vigna unguicultata (cowpea) was detected.
Of note, sequence of cowpea insulin-like material
was identical to bovine insulin and similar to human insulin (three
mutations at Thr54Ala in the B chain and Thr97Ala and Ile99Val in
the A chain as shown in Fig. 2)
and camel insulin (just one mutation at Val26Ala in the B chain).
Presence of insulin in plants is disputed by biologists despite the
fact that insulin was proven to be present in beans and beans
supplemented with insulin/glucose were able to accelerate
Canavalia ensiformis (Jack bean) seedling development
(51,52). Additionally, Xavier-FilhoI et
al reported that proteins associated with insulin signaling
pathways in vertebrates are also present with insulin-like
molecules in plants (52). This
raises question if consumption of insulin containing beans can
alleviate symptoms of diabetes. It seems to be very unlikely due to
the fact that most beans are consumed boiled that would denature
proteins.
The other possibility is presence of other molecules
that can have drug-like properties. For example α-amylase is an
enzyme that hydrolyses α-bonds of large polysaccharides, such as
starch and glycogen, yielding glucose and maltose (53,54). Inhibitors of α-amylase are oral
anti-diabetic drugs that reduce the impact of carbohydrates on
blood sugar.
Reducing excessive intake of refined carbohydrates
plays an important role in prevention of obesity and type 2
diabetes mellitus. Tormo et al, studied purified pancreatic
α-amylase inhibitor from white beans (Phaseolus vulgaris)
that was administered orally for 22 days to non-diabetic and type 2
diabetic Wistar rats. α-Amylase inhibitor from that bean
significantly reduced glycaemia in the ND and diabetic animals
(55). Two other reports strongly
support these findings about anti-diabetic effects of α-amylase
inhibitors from beans (47,56).
Controlled clinical experiment of camel insulin on
diabetic patients showed that regular consumption of camel milk
lowered blood glucose level and in 25% of patients additional
insulin requirement was reduced. It is contrary to the results of
insulin therapy on the diabetic patients. Once insulin therapy
starts, patient has to take insulin lifelong and generally insulin
dose keeps on increasing with time. It seems that camel milk
delivers insulin in a different form (than in other mammals) and/or
provides some other compound in addition to insulin that improve
the health of diabetic patients.
Sequence of camel insulin and its predicted
digestion pattern do not suggest differentiability to overcome the
mucosal barriers before been degraded and reaching the blood
stream. However we cannot exclude the possibility that insulin in
camel milk is present in nanoparticles capable of transporting this
hormone into the blood stream. Although, much more probable is that
camel milk contains ‘insulin-like’ small molecular substances that
mimic insulin interaction with its receptor.
Acknowledgements
This work was supported by grant from
Stranahan Endowment Fund for Oncological Research. The authors
(A.M. and A.A.-S.) extend their appreciation to the Deanship of
Scientific Research at King Saud University for funding the work
through the research group project no RGP-VPP-151.
References
|
1.
|
NA Al-BaghliAJ Al-GhamdiKA Al-TurkiAH Al
ElqAG El-ZubaierA BahnassyPrevalence of diabetes mellitus and
impaired fasting glucose levels in the Eastern Province of Saudi
Arabia: results of a screening campaignSingapore Med
J51923930201121221496
|
|
2.
|
KA AlqurashiKS AljabriSA BokhariPrevalence
of diabetes mellitus in a Saudi communityAnn Saudi
Med311923201110.4103/0256-4947.7577321245594
|
|
3.
|
Diabetes - a global
threatLancet3731735200910.1016/S0140-6736(09)60954-5
|
|
4.
|
E GinterV SimkoDiabetes type 2 pandemic in
21st centuryBratisl Lek Listy111134137201020437822
|
|
5.
|
C SetacciG de DonatoF SetacciE
ChisciDiabetic patients: epidemiology and global impactJ Cardiovasc
Surg50263273200919543188
|
|
6.
|
RP AgrawalR DograN MohtaR TiwariS SinghalS
SultaniaBeneficial effect of camel milk in diabetic nephropathyActa
Biomed80131134200919848050
|
|
7.
|
RP AgrawalS JainS ShahA ChopraV
AgarwalEffect of camel milk on glycemic control and insulin
requirement in patients with type 1 diabetes: 2-years randomized
controlled trialEur J Clin Nutr6510481052201121629270
|
|
8.
|
RH MohamadZK ZekryHA Al-MehdarCamel milk
as an adjuvant therapy for the treatment of type 1 diabetes:
verification of a traditional ethnomedical practiceJ Med
Food12461465200910.1089/jmf.2008.000919459752
|
|
9.
|
RP AgrawalR BeniwalDK KocharCamel milk as
an adjunct to insulin therapy improves long-term glycemic control
and reduction in doses of insulin in patients with type-1 diabetes
A 1 year randomized controlled trialDiabetes Res Clin
Pract681761772005
|
|
10.
|
A SbouiT KhorchaniM DjeghamA AgrebiH
ElhatmiO BelhadjAnti-diabetic effect of camel milk in
alloxan-induced diabetic dogs: a dose-response experimentJ Anim
Physiol Anim Nutr94540546201019906135
|
|
11.
|
OU BegH von Bahr-LindstromZH ZaidiH
JornvallA camel milk whey protein rich in half-cystine. Primary
structure, assessment of variations, internal repeat patterns, and
relationships with neurophysin and other active polypeptidesEur J
Biochem159195201198610.1111/j.1432-1033.1986.tb09852.x
|
|
12.
|
O ZagòrskiA MamanA YafeeA MeislesC van
CreveldR YagilInsulin in milk - a comparative studyInt J Anim
Sci1324124419983530717
|
|
13.
|
Food and Agriculture Organization of the
United NationsCamel milk and cheese makingThe Technology of Making
Cheese from Camel Milk (Camelus dromedarius)FAO Animal
Production and Health Paper 113Rome2011
|
|
14.
|
A RoyA KucukuralY ZhangI-TASSER: a unified
platform for automated protein structure and function predictionNat
Protoc5725738201010.1038/nprot.2010.520360767
|
|
15.
|
Y ZhangTemplate-based modeling and free
modeling by I-TASSER in CASP7Proteins69Suppl
8108117200710.1002/prot.2170217894355
|
|
16.
|
GI BellMM StempienNM FongLB RallSequences
of liver cDNAs encoding two different mouse insulin-like growth
factor I precursorsNucleic Acids
Res1478737882198610.1093/nar/14.20.78733774549
|
|
17.
|
I ShikataY MaeharaT FujiyoshiH
EndoIsolation and characterization of a conserved sequence highly
expressed in tumors and growing
cellsOncology44192198198710.1159/0002264752439968
|
|
18.
|
CR SnellDG SmythProinsulin: a proposed
three-dimensional structureJ Biol Chem250629162951975808541
|
|
19.
|
WO DanhoThe isolation and characterization
of insulin of camel (Camelus dromedarius)J Fac Med
Baghdad1416281972
|
|
20.
|
AM Al-SwailemMB Al-FageehEJ AlyamaniMM
ShehataTA Al-ShammariCharacterization of recombinant Arabian camel
(Camelus dromedarius) insulinAfr J
Biotechnol7338933942008
|
|
21.
|
RA PullenDG LindsaySP WoodReceptor-binding
region of insulinNature259369373197610.1038/259369a0175286
|
|
22.
|
P De MeytsE Van ObberghenJ RothMapping of
the residues responsible for the negative cooperativity of the
receptor-binding region of insulinNature2735045091978661960
|
|
23.
|
J XuV ChangSB JosephPeroxisomal
proliferator-activated receptor alpha deficiency diminishes
insulin-responsiveness of gluconeogenic/glycolytic/pentose gene
expression and substrate cycle
fluxEndocrinology14510871095200410.1210/en.2003-1173
|
|
24.
|
S ShoelsonM FickovaM HanedaIdentification
of a mutant human insulin predicted to contain a
serine-for-phenylalanine substitutionProc Natl Acad Sci
USA8073907394198310.1073/pnas.80.24.73906424111
|
|
25.
|
DF SteinerHS TagerSJ ChanK NanjoT SankeAH
RubensteinLessons learned from molecular biology of insulin-gene
mutationsDiabetes
Care13600609199010.2337/diacare.13.6.6002192846
|
|
26.
|
L SchafferA model for insulin binding to
the insulin receptorEur J
Biochem22111271132199410.1111/j.1432-1033.1994.tb18833.x8181471
|
|
27.
|
CC YipP OttensmeyerThree-dimensional
structural interactions of insulin and its receptorJ Biol
Chem2782732927332200310.1074/jbc.R30002120012764141
|
|
28.
|
AM JorgensenHB OlsenP BalschmidtJJ
LedSolution structure of the superactive monomeric des-[Phe(B25)]
human insulin mutant: elucidation of the structural basis for the
monomerization of des-[Phe(B25)] insulin and the dimerization of
native insulinJ Mol Biol25768469919968648633
|
|
29.
|
C KristensenT KjeldsenFC WibergAlanine
scanning mutagenesis of insulinJ Biol
Chem2721297812983199710.1074/jbc.272.20.129789148904
|
|
30.
|
E MedhammarR Wijesinha-BettoniB StadlmayrE
NilssonUR CharrondiereB BurlingameComposition of milk from minor
dairy animals and buffalo breeds: a biodiversity perspectiveJ Sci
Food AgricNov142011(Epub ahead of print)
|
|
31.
|
VN MichelizziMV DodsonZ PanWater buffalo
genome science comes of ageInt J Biol
Sci6333349201010.7150/ijbs.6.33320582226
|
|
32.
|
GP PizzutiGC SalvatoriSome blood
parameters of water buffalo in different physiological
conditionsBoll Soc Ital Biol Sper6964965419938198807
|
|
33.
|
J ScherContribution à l’étude de
l’influence de la composition des micelles sur la coagulation
enzymatique Inst. National Polytech., Vandéuvre-lès-Nancy, 1988 (In
French)
|
|
34.
|
T BekeleN LundeheimK DahlbornMilk
production and feeding behavior in the camel (Camelus
dromedarius) during 4 watering regimensJ Dairy
Sci9413101317201110.3168/jds.2010-365421338796
|
|
35.
|
N Nafissi-VarchehM ErfanR AboofazeliAn
approach to the design of a particulate system for oral protein
delivery. I In vitro stability of various poly (alpha-hydroxy
acids)-microspheres in simulated gastrointestinal fluidsJ
Microencapsul25584592200810.1080/02652040802485485
|
|
36.
|
C PregoM GarciaD TorresMJ
AlonsoTransmucosal macromolecular drug deliveryJ Control
Release101151162200510.1016/j.jconrel.2004.07.03015588901
|
|
37.
|
A VilaA SanchezM TobioP CalvoMJ
AlonsoDesign of biodegradable particles for protein deliveryJ
Control Release781524200210.1016/S0168-3659(01)00486-211772445
|
|
38.
|
K HeCB ChanX LiuIdentification of a
molecular activator for insulin receptor with potent anti-diabetic
effectsJ Biol
Chem2863737937388201110.1074/jbc.M111.24738721908618
|
|
39.
|
D MozaffarianH CaoIB KingTrans-palmitoleic
acid, metabolic risk factors, and new-onset diabetes in U.S.
adults: a cohort studyAnn Intern
Med153790799201010.7326/0003-4819-153-12-201012210-0000521173413
|
|
40.
|
KR AbeywickramaWD RatnasooriyaAM
AmarakoonOral hypoglycaemic, antihyperglycaemic and antidiabetic
activities of Sri Lankan Broken Orange Pekoe Fannings (BOPF) grade
black tea (Camellia sinensis L.) in ratsJ
Ethnopharmacol135278286201110.1016/j.jep.2011.02.03521397000
|
|
41.
|
MR ArdalanMK TarzamniMM ShojaBlack tea
improves endothelial function in renal transplant
recipientsTransplant
Proc3911391142200710.1016/j.transproceed.2007.04.01017524915
|
|
42.
|
N BoonHealth potential for functional
green teas?Int J Vitam Nutr
Res78275281200810.1024/0300-9831.78.6.27519685436
|
|
43.
|
CL BroadhurstMM PolanskyRA
AndersonInsulin-like biological activity of culinary and medicinal
plant aqueous extracts in vitroJ Agric Food
Chem48849852200010.1021/jf990451710725162
|
|
44.
|
A BuyukbalciSN ElDetermination of in vitro
antidiabetic effects, antioxidant activities and phenol contents of
some herbal teasPlant Foods Hum
Nutr632733200810.1007/s11130-007-0065-518183488
|
|
45.
|
A CrozierIB JaganathMN CliffordDietary
phenolics: chemistry, bioavailability and effects on healthNat Prod
Rep2610011043200910.1039/b802662a19636448
|
|
46.
|
PL OwenLC MartineauD CavesPS HaddadT
MatainahoT JohnsConsumption of guava (Psidium guajava L) and
noni (Morinda citrifolia L) may protect betel quid-chewing
Papua New Guineans against diabetesAsia Pac J Clin
Nutr176356432008
|
|
47.
|
DG BlackTM TaylorHJ KerrS PadhiTJ
MontvillePM DavidsonDecontamination of fluid milk containing
Bacillus spores using commercial household productsJ Food
Prot71473478200818389688
|
|
48.
|
E SkrodenieneD MarciulionyteZ PadaigaE
JasinskieneV Sadauskaite-KuehneJ LudvigssonEnvironmental risk
factors in prediction of childhood
prediabetesMedicina445663200818277090
|
|
49.
|
HB XuKX HuangYS ZhuHypoglycaemic effect of
a novel insulin buccal formulation on rabbitsPharmacol
Res46459467200210.1016/S104366180200204912419651
|
|
50.
|
GG BriggsPJ AmbroseMP NageotteG
PadillaHigh-dose carisoprodol during pregnancy and lactationAnn
Pharmacother42898901200810.1345/aph.1L04218460586
|
|
51.
|
O CalderonC PadillaC ChavesL VillalobosML
AriasEvaluation of the effect of Lactobacillus rhamnosus probiotic
culture added to yogurt over Staphylococcus aureus,
Escherichia coli O157:H7, Listeria monocytogenes and
Salmonella enteritidis populationsArch Latinoam
Nutr5751552007(In Spanish)
|
|
52.
|
P PadraoN LunetAC SantosH BarrosSmoking,
alcohol, and dietary choices: evidence from the Portuguese National
Health SurveyBMC Public
Health7138200710.1186/1471-2458-7-13817608935
|
|
53.
|
R PadhiFeedback linearization based
computer controlled medication design for automatic treatment of
parturient paresis of cowsComput Methods Programs
Biomed841926200610.1016/j.cmpb.2006.07.009
|
|
54.
|
R LoriniL MinicucciF NapoliScreening for
type 1 diabetes genetic risk in newborns of continental Italy.
Primary prevention (Prevefin Italy) - preliminary dataActa
Biomed76Suppl 33135200516915793
|
|
55.
|
CF GomesEM TrezzaEC MuradeCR
PadovaniSurface electromyography of facial muscles during natural
and artificial feeding of infantsJ
Pediatr82103109200610.2223/JPED.145616614763
|
|
56.
|
A WaldmannJ KurykinU JaakmaThe effects of
ovarian function on estrus synchronization with PGF in dairy
cowsTheriogenology6613641374200610.1016/j.theriogenology.2006.04.03016815540
|