Introduction
Anaplastic thyroid cancer (ATC) is one of the most
aggressive types of malignant tumor, characterized by invasion into
surrounding tissues and metastasis that contribute to a poor
prognosis for patients with this disease (1–3).
The acquisition of invasive and migratory properties is associated
with epithelialmesenchymal transition (EMT) and is a prerequisite
for cancer invasion into surrounding tissues, the first stage of
metastatic disease (4–6).
The epidermal growth factor receptor (EGFR), a
transmembrane cell-surface glycoprotein with intrinsic tyrosine
kinase activity, is overexpressed in most ATCs (7). EGFR activation by its ligand, the
epidermal growth factor (EGF), initiates a signaling cascade that
results in changes in gene expression. EGF is the prototype of a
large family of structurally related peptides that possess an
EGF-like domain, consisting of 6 cysteine residues capable of
forming 3 intramolecular loops stabilized by disulfide bonds. EGF
is synthesized by the thyroid gland and can induce thyroid cell
proliferation in a number of species. In addition, EGF enhances the
migration and invasiveness of thyroid cancer (3,8–11).
The Rho/Rho kinase pathway may be important for
cancer invasion, growth and metastasis. In vitro studies and
animal experiments have suggested that the inhibition of the
Rho/Rho kinase pathway inhibits tumor invasion and metastasis
(12–16). Zhong et al (3) demonstrated that lovastatin
suppresses the invasive capability of ATC cells by inhibiting Rho
geranylgeranylation and RhoA/ROCK signaling. Rho/ROCK signaling is
required for EGF-induced invasion of ARO human ATC cells.
The expression of miR-200s is associated with tumor
invasion and regulates EMT in cancer cells (17–20). However, nothing is known about
interrelationships between EGF/EGFR, miR-200s and the EMT or
mesenchymal-epithelial transition (MET) processes. In the current
study, we first show that the activation of EGF/EGFR signaling by
EGF treatment of Nthy-ori 3-1 thyroid follicular cells
downregulates E-cadherin with a concomitant upregulation of
vimentin. Conversely, the downregulation of EGF/EGFR signaling by
EGFR silencing in SW1736 cells results in E-cadherin upregulation
and vimentin downregulation. Secondly, EGF treatment inversely
correlates with the expression of miR-200 family members. Thirdly,
re-expression of miR-200s in EGF-induced Nthy-ori 3-1 cells
restores an epithelial phenotype, whereas the silencing of miR-200s
in SW1736 cells reverses siEGFR-mediated changes. Finally, miR-200s
are shown to play a key role in EGF/EGFR-mediated thyroid cell
invasion in vitro and EMT in vivo, suggesting that
EGF/EGFR signaling regulates the aggressiveness of SW1736 cells by
modulating miR-200 expression.
Materials and methods
Cell lines and reagents
The Nthy-ori 3-1 normal thyroid follicular
epithelial cell line and the SW1736 human and ARO human ATC cell
lines were obtained from the State Key Laboratory of Molecular
Oncology, Chinese Academy of Medical Sciences (Beijing, China). All
cells were cultured in RPMI-1640 medium supplemented with 5% FBS,
100 U/ml penicillin and 100 mg/ml streptomycin in a humidified
atmosphere with 5% CO2 at 37°C. All cell lines were
tested and authenticated by short tandem repeat profiling using the
PowerPlex 16 System (Promega). Antibodies were obtained from the
following suppliers: vimentin (Abcam); E-cadherin, RhoA and β-actin
(Sigma).
Real-time reverse transcription PCR
(RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions.
Real-time PCR was used to quantify mRNA expression. Primer
sequences for E-cadherin, vimentin and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) were as described previously (21), and specific gene expression was
normalized to GAPDH. For miRNA analysis, total RNA was isolated
using the mirVana miRNA isolation kit (Ambion) and the levels of
miRNAs were determined using miRNA-specific TaqMan MGB probes from
the TaqMan MicroRNA assay kit (Applied Biosystems). miRNA
expression was normalized to RNU6B (Sigma).
Transfection experiments
Thyroid cells were seeded into 12-well plates and
subjected to transfection with specific siRNAs targeting EGFR,
pre-miR-200s (miR-200a, miR-200b and miR-200c), anti-miR-200s
(Sigma), or scrambled controls by the liposome method, as described
previously (8).
Western blot analysis
Western blot analyses were performed as previously
described (9). Briefly, proteins
were electrophoresed on 12% polyacrylamide gels and transferred to
Hybond-P polyvinylidene difluoride (PVDF) membranes (Amersham).
Western blot analysis was carried out with specific primary
antibodies diluted in 1% bovine serum albumin (BSA) in TBST,
followed by peroxidase-conjugated secondary antibody. Target
proteins were observed using the enhanced chemiluminescence (ECL)
detection system (GE Healthcare) and autoradiography on Fuji super
RX film (Fuji, Tokyo, Japan), with 1–2 min exposure.
Cell invasion assay
Cell invasion through a reconstituted Matrigel
basement membrane was assayed as previously described (22). Briefly, polycarbonate membranes
(8.0 mm pore size) in the base of the upper compartment of
Transwell culture chambers were coated with 10% Matrigel (50
μl/insert), and the lower compartment was filled with 600
μl serum-free medium containing 0.1% BSA. Cells were
suspended in serum-free medium and seeded into the Transwell
inserts. After 24 h, cells that had invaded the Matrigel membrane
were stained with 4 mg/ml calcein AM (Invitrogen) in PBS at 37°C
for 1 h and then photographed under a fluorescent microscope.
Invading cells were then trypsinized to remove them from the
inserts. The images were recorded and analyzed using a Zeiss
confocal photomicroscope LSM510 (Zeiss).
Immunohistochemistry
Immunohistochemistry analysis was performed as
previously described (23).
Paraffin-embedded tissues were cut into 4 μm thick sections.
Endogenous peroxidase activity was then blocked with 3% hydrogen
peroxide, and then endogenous biotin was blocked with 0.01% avidin.
Following blocking for 1 h with 3% BSA, the sections were incubated
with anti-E-cadherin (1:50) or anti-vimentin (1:100) antibody for 1
h at room temperature. Immunoreactivity was detected using the
biotin-streptavidin-peroxidase complex method and visualized using
the 3,3′-diaminobenzidine (DAB; Dako) chromogen. Sections were
counterstained with hematoxylin.
A modified semi-quantitative scoring system
(23,24) was used to evaluate immunostaining
by light microscopy: 0, no cells stained in any field; 1, positive
staining of <25% of cells; 2, positive staining of 25–50% of
cells; 3, positive staining of 50–75% cells; and 4, positive
staining of >75% cells. Staining intensity was evaluated using
the following scale: 0, no cell staining; 1+, mild staining; 2+,
moderate staining; and 3+, strong staining. The total score was
generated by adding the scores for the percentage of positive cells
and staining intensity together.
Animal experiments
Six-week-old athymic nude mice (nu/nu), obtained
from the Chinese Academy of Medical Sciences, were allowed to adapt
to the laboratory environment for 1 week. Experiments were then
performed as previously described (25). Briefly, SW1736 cells transfected
with either scrambled siRNA or EGFR-specific siRNA (EGFR-silenced
cells) were injected through the tail vein of female mice. In order
to examine the effect of anti-miR-200s in vivo,
EGFR-silenced SW1736 cells were also injected into the mice. SW1736
cells (3x106 cells/animal) were immobilized in Matrigel
(300 mg/ml) and injected into the mice. Ten mice were included in
each treatment group. After 4 weeks, all mice were sacrificed and
tumor tissue was removed and processed for immunohistochemistry.
All procedures were monitored and approved by the local ethics
committee and federal authorities and were conducted in accordance
with the guidelines for the welfare of animals in experimental
neoplasia.
Statistical analysis
Differences between groups were analyzed by one-way
ANOVA using SPSS13.0 software (SPSS, Chicago, IL, USA). P-values
<0.05 were considered to indicate statistically significant
differences.
Results
EGF/EGFR signaling correlates with
vimentin, E-cadherin and RhoA expression
The induction of the EGF/EGFR pathway by the EGF
treatment of Nthy-ori 3-1 cells resulted in the upregulation of the
mesenchymal marker, vimentin, accompanied by the reduced expression
of the epithelial marker, E-cadherin, suggesting the induction of
an EMT phenotype. By contrast, the downregulation of EGF/EGFR
signaling by siRNA-mediated EGFR silencing in SW1736 cells resulted
in increased E-cadherin mRNA expression, concomitant with the
reduced vimentin expression. To rule out cell line-specific
effects, we carried out similar studies using the aggressive
thyroid cancer cell line, ARO, and also found that EGFR silencing
resulted in a significant increase in E-cadherin mRNA levels and
decreased vimentin expression (Fig.
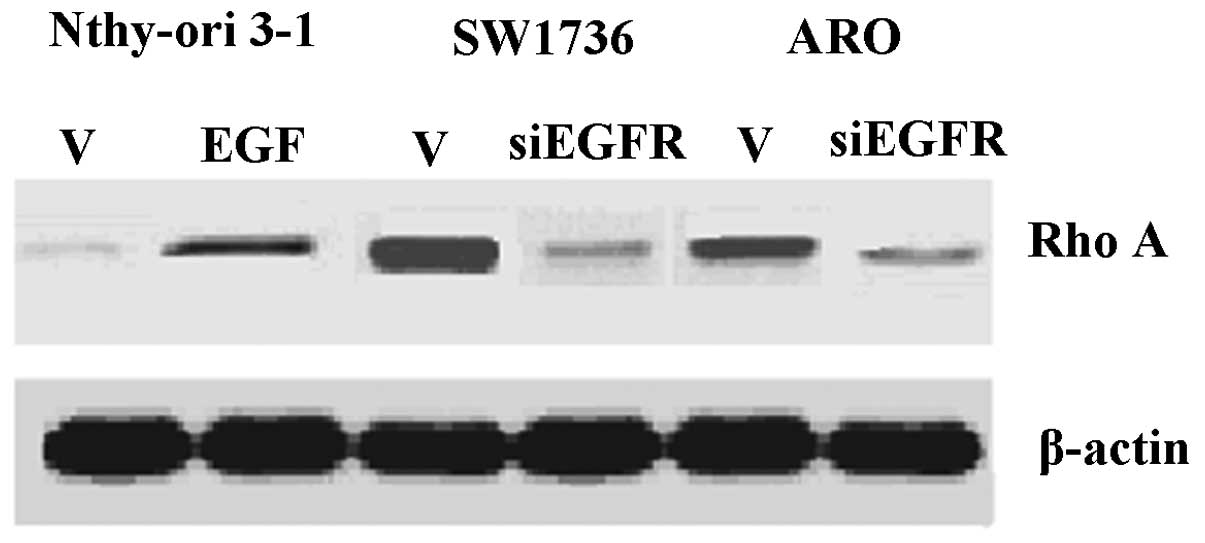
1). We evaluated RhoA expression by western blot analysis, and
found that the EGF treatment of Nthy-ori 3-1 cells led to increased
RhoA expression, whereas EGFR silencing in SW1736 and ARO cells led
to the downregulation of RhoA (Fig.
2). As EGFR silencing in both cell lines had similar effects on
EMT markers and Rho/ROCK signaling, we chose the more
experimentally tractable SW1736 cells as a model thyroid cancer
cell line for further experiments.
EGF induces downregulation of miR-200s
and EMT is reversed by re-expression of miR-200s in Nthy-ori 3-1
cells
In our study, we observed that EGF treatment
correlated with reduced miR-200 levels and EMT in Nthy-ori 3-1
cells (Fig. 3A). To assess
whether the miR-200 family regulates EMT, we transfected
EGF-induced Nthy-ori 3-1cells with pre-miR-200a/c to restore
miR-200 function. Transfection with pre-miRNAs is a standard
technique for inducing the expression of miRNAs (21). The levels of miR-200s in
premiR-200a/c-transfected cells approached the levels of the
untreated cells, indicating that miR-200s can be efficiently
re-expressed following EGF-mediated downregulation (Fig. 3A). Furthermore, miR-200a/c
re-expression resulted in increased E-cadherin expression (Fig. 3B) and decreased vimentin
expression (Fig. 3C), thus
reversing the mesenchymal phenotype of EGF-treated cells.
EGFR siRNA-mediated MET induction is
reversed by miR-200 family inhibition
We found that EGFR silencing leads to increased
miR-200 expression in SW1736 cells. Following the suppression of
upregulated miR-200s using anti-miR-200 oligonucleotides, miR-200
levels in SW1736 cells were reduced to the levels of the untreated
cells, showing an efficient downregulation of miR-200s (Fig. 4A). Moreover, vimentin
hypo-expression in EGFR-silenced cells was restored to basal levels
following anti-miR-200 treatment (Fig. 4B). Similarly, E-cadherin
hyper-expression in EGFR-silenced cells was inhibited by
anti-miR-200s treatment (Fig.
4C).
EGF/EGFR regulation of thyroid cell EMT
and invasion is reversed by miR-200s
To analyze the miR-200 modulation of EGF-mediated
EMT in thyroid cells, western blot analysis was performed to
determine the expression of EMT protein markers. The re-expression
of miR-200s in EGF-induced Nthy-ori 3-1 cells resulted in
re-expression of E-cadherin and suppression of vimentin, whereas
the suppression of miR-200s caused the downregulation of E-cadherin
and the upregulation of vimentin in EGFR-silenced SW1736 cells
(Fig. 5). We investigated whether
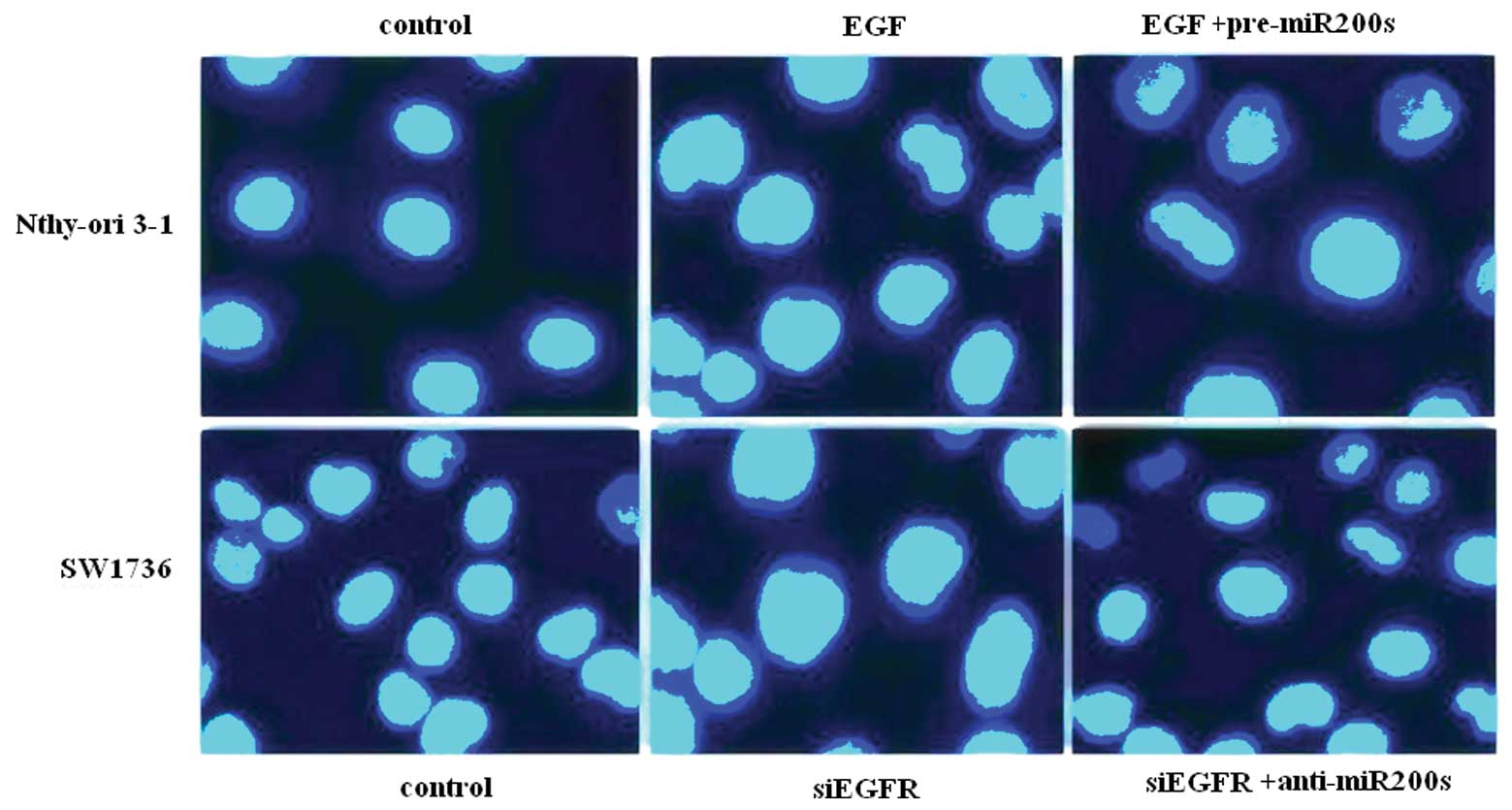
the EGF/EGFR regulation of the EMT process correlates with thyroid
cancer cell invasion using a Matrigel invasion assay. In Nthy-ori
3-1 cells, EGF treatment resulted in increased cell invasion, which
was inhibited by the re-expression of the miR-200s. Conversely,
EGFR silencing inhibited SW1736 cell invasion, which was restored
by the oligonucleotide suppression of miR-200s (Fig. 6).
In vivo evaluation of miR-200 effects on
MET in SW1736 xenografts
We analyzed the expression of E-cadherin and
vimentin in SW1736 xenografts by immunohistochemistry. Mouse
xenografts derived from SW1736 cells transfected with EGFR siRNA
targeting showed strong membranous staining of E-cadherin and
significantly lower vimentin expression (P<0.01, respectively).
By contrast, xenografts derived from SW1736 cells co-transfected
with anti-miR-200s and EGFR siRNA had E-cadherin and vimentin
expression profiles similar to the xenografts from untransfected
cells, indicating that the downregulation of miR-200s reverses the
inhibitory effect of EGFR siRNA on EMT. Therefore, miR-200s play a
crucial role in EGF/EGFR-mediated EMT in vivo (Fig. 7).
Discussion
We report that EGF induction in thyroid cancer cells
leads to the loss of miR-200 expression and increased Rho/ROCK
activity, resulting in increased EMT and subsequent cancer
invasion. EGF treatment leads to increased vimentin expression in
Nthy-ori 3-1 cells, whereas EGFR silencing results in vimentin
downregulation in SW1736 cells. Thus, EGF induces EMT in Nthy-ori
3-1 cells and EGFR silencing in SW1736 cells reverses EMT. These
observations, combined with reports that the miR-200 family
regulates EMT (19,21), led us to hypothesize that the
miR-200 family plays a key role in EGF/EGFR-induced EMT in thyroid
cells. We found that EGF treatment downregulates miR-200s in
Nthy-ori 3-1 cells, whereas EGFR silencing upregulates miR-200s in
SW1736 cells. These results show that EGF/EGFR signaling modulates
the expression of miR-200s in thyroid cancer cells, thus providing
a possible link between EGF/EGFR-mediated EMT and miR-200 family
expression.
Rho/ROCK is constitutively active in cancer cells
and is therefore an attractive therapeutic target (22,26). Rho/ROCK activation is necessary
for TGFβ-induced EMT, and inhibition of the Rho effector, ROCK,
inhibits TGFβ-induced EMT in vitro and in vivo
(27,28). In the current study, we observed
increased Rho/ROCK activity in EGF-treated Nthy-ori 3-1 cells, and
reduced Rho/ROCK activity in EGFR-silenced SW1736 cells.
Previous studies have indicated that EMT induction
is regulated by the miR-200 family (18–21). TGFβ negatively regulates the
expression of the miR-200 family and thereby promotes expression of
zinc-finger enhancer binding (ZEB) transcription factors, leading
to EMT in vitro (27). In
this study, we show that the re-expression of miR-200a/c by
transfection of pre-miR-200 inhibits cell invasion in vitro,
thus providing direct evidence in support of the involvement of
miR-200s in EGF/EGFR-mediated effects on thyroid cancer cell
aggressiveness. Using a reciprocal model in Nthy-ori 3-1 cells, we
show that EGF-mediated cell invasion is effectively blocked by the
re-expression of the miR-200s. Our in vitro and in
vivo results indicate a key regulatory role for miR-200s in the
modulation of EMT by EGF/EGFR.
Our current findings, together with existing
literature, provide a model for EMT regulation by EGF/EGFR,
miR-200s, Rho/ROCK and EMT markers (Fig. 8). We therefore provide evidence
for a mechanism linking the miR-200 family with EGF/EGFR signaling,
suggesting that miR-200 upregulation may serve as a novel
therapeutic strategy for highly invasive thyroid cancer.
Acknowledgements
The present study was supported by a
grant from the Shanghai Municipal Health Bureau Scientific
Foundation of China (2010–51).
References
|
1.
|
G Riesco-EizaguirreP SantistebanNew
insights in thyroid follicular cell biology and its impact in
thyroid cancer therapyEndocr Relat
Cancer14957977200710.1677/ERC-07-008518045949
|
|
2.
|
KN PatelAR ShahaPoorly differentiated and
anaplastic thyroid cancerCancer Control13119128200616735986
|
|
3.
|
WB ZhongYC LiangCY WangTC ChangWS
LeeLovastatin suppresses invasiveness of anaplastic thyroid cancer
cells by inhibiting Rho geranylgeranylation and RhoA/ROCK
signalingEndocr Relat
Cancer12615629200510.1677/erc.1.0101216172195
|
|
4.
|
L LarueA BellacosaEpithelial-mesenchymal
transition in development and cancer: role of phosphatidylinositol
30 kinase/AKT
pathwaysOncogene2474437454200510.1038/sj.onc.120909116288291
|
|
5.
|
JP ThieryH AcloqueRY HuangMA
NietoEpithelialmesenchymal transitions in development and
diseaseCell139871890200910.1016/j.cell.2009.11.00719945376
|
|
6.
|
JJ ChristiansenAK RajasekaranReassessing
epithelial to mesenchymal transition as a prerequisite for
carcinoma invasion and metastasisCancer
Res6683198326200610.1158/0008-5472.CAN-06-041016951136
|
|
7.
|
JD BergstromB WestermarkNE HeldinEpidermal
growth factor receptor signaling activates met in human anaplastic
thyroid carcinoma cellsExp Cell
Res259293299200010.1006/excr.2000.4967
|
|
8.
|
CT KuanCJ WikstrandDD BignerEGF mutant
receptor vIII as a molecular target in cancer therapyEndocr Relat
Cancer88396200110.1677/erc.0.008008311397666
|
|
9.
|
AK LarsenD OuaretK El OuadraniA
PetitprezTargeting EGFR and VEGF(R) pathway cross-talk in tumor
survival and angiogenesisPharmacol
Ther1318090201110.1016/j.pharmthera.2011.03.01221439312
|
|
10.
|
T CasconeMH HerynkL XuZ DuH KadaraMB
NilssonCJ ObornYY ParkB ErezJJ JacobyUpregulated stromal EGFR and
vascular remodeling in mouse xenograft models of angiogenesis
inhibitor-resistant human lung adenocarcinomaJ Clin
Invest12113131328201110.1172/JCI4240521436589
|
|
11.
|
L Ferrer-SolerA Vazquez-MartinJ BrunetJA
MenendezRD LlorensR ColomerAn update of the mechanisms of
resistance to EGFR-tyrosine kinase inhibitors in breast cancer:
Gefitinib (Iressa)-induced changes in the expression and
nucleocytoplasmic trafficking of HER-ligands (Review)Int J Mol
Med203102007
|
|
12.
|
V Sanz-MorenoC GaggioliM YeoJ AlbrenguesF
WallbergA VirosS HooperR MitterCC FéralM CookROCK and JAK1
signaling cooperate to control actomyosin contractility in tumor
cells and stromaCancer
Cell20229245201110.1016/j.ccr.2011.06.01821840487
|
|
13.
|
Z ZhangJH RenZY LiL NongG WuFasudil
inhibits lung carcinoma-conditioned endothelial cell viability and
migrationOncol Rep2715611566201222344855
|
|
14.
|
G MavriaY VercoulenM YeoH PatersonM
KarasaridesR MaraisD BirdCJ MarshallERK-MAPK signaling opposes
Rho-kinase to promote endothelial cell survival and sprouting
during angiogenesisCancer
Cell93344200610.1016/j.ccr.2005.12.02116413470
|
|
15.
|
WB ZhongSP HsuPY HoYC LiangTC ChangWS
LeeLovastatin inhibits proliferation of anaplastic thyroid cancer
cells through up-regulation of p27 by interfering with the
Rho/ROCK-mediated pathwayBiochem
Pharmacol8216631672201110.1016/j.bcp.2011.08.02121907187
|
|
16.
|
Y KideraM TsubakiY YamazoeK ShojiH
NakamuraM OgakiT SatouT ItohM IsozakiJ KanekoReduction of lung
metastasis, cell invasion, and adhesion in mouse melanoma by
statin-induced blockade of the Rho/Rho- associated coiled-coil
containing protein kinase pathwayJ Exp Clin Cancer
Res29127201010.1186/1756-9966-29-12720843370
|
|
17.
|
JA FoekensAM SieuwertsM SmidMP LookV de
WeerdAW BoersmaJG KlijnEA WiemerJW MartensFour miRNAs associated
with aggressiveness of lymph node-negative estrogen
receptor-positive human breast cancerProc Natl Acad Sci
USA1051302113026200810.1073/pnas.080330410518755890
|
|
18.
|
L MaJ Teruya-FeldsteinRA WeinbergTumour
invasion and metastasis initiated by microRNA-10b in breast
cancerNature449682688200710.1038/nature0617417898713
|
|
19.
|
PA GregoryAG BertEL PatersonSC BarryA
TsykinG FarshidMA VadasY Khew-GoodallGJ GoodallThe miR-200 family
and miR-205 regulate epithelial to mesenchymal transition by
targeting ZEB1 and SIP1Nat Cell
Biol10593601200810.1038/ncb172218376396
|
|
20.
|
SM ParkAB GaurE LengyelME PeterThe miR-200
family determines the epithelial phenotype of cancer cells by
targeting the E-cadherin repressors ZEB1 and ZEB2Genes
Dev22894907200810.1101/gad.164060818381893
|
|
21.
|
D KongY LiZ WangS BanerjeeA AhmadHR KimFH
SarkarmiR-200 regulates PDGF-D-mediated epithelialmesenchymal
transition, adhesion, and invasion of prostate cancer cellsStem
Cells2717121721200910.1002/stem.10119544444
|
|
22.
|
L YinK MorishigeT TakahashiK HashimotoS
OgataS TsutsumiK TakataT OhtaJ KawagoeK TakahashiH KurachiFasudil
inhibits vascular endothelial growth factor-induced angiogenesis in
vitro and in vivoMol Cancer
Ther615171525200710.1158/1535-7163.MCT-06-068917513600
|
|
23.
|
JM HarveyGM ClarkCK OsborneDC
AllredEstrogen receptor status by immunohistochemistry is superior
to the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancerJ Clin
Oncol1714741481199910334533
|
|
24.
|
S UmemuraJ ItohH ItohA SerizawaY SaitoY
SuzukiY TokudaT TajimaRY OsamuraImmunohistochemical evaluation of
hormone receptors in breast cancer: which scoring system is
suitable for highly sensitive procedures?Appl Immunohistochem Mol
Morphol12813200410.1097/00129039-200403000-00002
|
|
25.
|
A WunderlichM FischerT SchlosshauerA
RamaswamyBH GreeneC BrendelD DollD BartschS HoffmannEvaluation of
Aurora kinase inhibition as a new therapeutic strategy in
anaplastic and poorly differentiated follicular thyroid
cancerCancer Sci102762768201110.1111/j.1349-7006.2011.01853.x
|
|
26.
|
H YingSL BirocWW LiB AlickeJA XuanR
PagilaY OhashiT OkadaY KamataH DinterThe Rho kinase inhibitor
fasudil inhibits tumor progression in human and rat tumor modelsMol
Cancer Ther521582164200610.1158/1535-7163.MCT-05-044016985048
|
|
27.
|
HJ ChoJ YooRho activation is required for
transforming growth factor-beta-induced epithelial-mesenchymal
transition in lens epithelial cellsCell Biol
Int3112251230200710.1016/j.cellbi.2007.04.00617537651
|
|
28.
|
GP SunM KohnoP GuoY NagaiK MiyataYY FanS
KimuraH KiyomotoK OhmoriDT LiY AbeA NishiyamaInvolvements of
Rho-kinase and TGF-beta pathways in aldosterone-induced renal
injuryJ Am Soc
Nephrol1721932201200610.1681/ASN.200512137516790507
|