Introduction
Breast cancer is the most common type of cancer
among women in most parts of the world and accounts for 23% of all
female cancers (1). It is the
second most common type of cancer overall, second to lung cancer.
Annually, over one million women are diagnosed with breast cancer
across the globe, with approximately 400,000 deaths (2). However, during the past few decades,
breast cancer mortality appears to be declining in the United
States and many other Western countries (3–5),
suggesting a benefit from early detection and more effective
treatment. Radiation therapy is a key strategy for the treatment of
many epithelial carcinomas. In breast cancer, post-operative
radiotherapy (RT) is one of the most commonly used and efective
strategies for local control.
Berberine, an isoquinoline derivative alkaloid, is
isolated from many medicinal herbs, such as Hydrastis
canadensis, Cortex phellodendri and Rhizoma
coptidis. Berberine has a wide range of pharmacological and
biochemical effects. It has been reported that berberine can be
used as an anti-diarrhea, anti-arrhythmia and anti-inflammatory
agent (6–8). Additionally, berberine has also been
shown to have antitumor effects on many cancer cell lines,
including leucocytes, liver, lung, stomach, colon, skin, oral,
esophageal, brain, bone, breast and genital cancer cells (9–14).
The cytotoxic effects of berberine on cancer cells may be mediated
through DNA topoisomerase I inhibition and cell cycle arrest which
eventually induces apoptosis via the caspase-3 or Fas/FasL signal
pathways (15). In addition to
the direct induction of apoptosis, the pro-inflammatory or nuclear
factor (NF)-κB pathway, antioxidant defense system and the
anti-metastatic pathway have been reported to play a role in the
anticancer effects of berberine (16–18).
A number of studies have reported that berberine has
synergistic effects against cancer in combination with irradiation.
Berberine has been shown to radiosensitize lung cancer cells by
inducing autophagy (19), and
esophageal cancer cells by the downregulation of the homologous
recombination repair protein, RAD51 (20). In addition to the synergistic
effects against cancer, berberine also has protective effects
against radiation-induced injury. It has been reported that
berberine significantly reduces the incidence of radiation-induced
lung injury and intestinal injury (21,22). Berberine may be a useful
therapeutic agent for breast cancer therapy. In the present study,
we evaluated the direct effects of berberine on irradiated MCF-7
and MDA-MB-468 human breast cancer cells in vitro, and
explored the mechanisms of interaction.
Materials and methods
Cell culture
The human breast cancer cell lines, MCF-7 and
MDA-MB-468, were obtained from the American Type Culture Collection
(ATCC; Rockville, MA, USA) and maintained in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Rockville, IN, USA) supplemented
with 10% fetal bovine serum (FBS; Haoyang Biological Manufacture
Co., Ltd., Tianjin, China), 100 U/ml penicillin and 100 μg/ml
streptomycin. All cell cultures were maintained at 37°C in a
humidified atmosphere of 5% CO2. All the cells used were
passaged for >4 months. The identities of these cell lines were
validated by short tandem repeat (STR) profiling generated by using
the Promega PowerPlex® 1.2 system. The STR profiles for
these cell lines matched their known ATCC fingerprints.
Reagents
Antibodies against Ku70, Ku86 and RAD51 were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Mouse monoclonal antibody against β-actin was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Mouse anti-γ-H2AX antibody was
purchased from Millipore (Billerica, MA, USA). HRP-labeled
secondary antibodies and rhodamine-labeled secondary antibody were
purchased from KPL (Gaithersburg, MD, USA). Berberine was purchased
from Sigma-Aldrich and dissolved in 100% dimethyl sulfoxide (DMSO)
to obtain stock concentrations.
Combined effect of berberine with
radiation investigated by clonogenic assay
Clonogenic assays were used to assess the survival
and proliferation potential of the cells treated with berberine
and/or ionizing radiation (IR). The cells were treated with the
vehicle control (DMSO) or 15 μM berberine for 24 h. The cells were
then irradiated using a Faxitron Cabinet X-ray System (Faxitron
X-ray Corp., Wheeling, IL, USA) to deliver the indicated doses (0,
1, 2, 3 and 4 Gy) at room temperature. The X-rays were filtered
through a 0.5-mm aluminum filter resulting in a dose rate of 0.4
Gy/min. The cells were trypsinized, suspended in complete medium,
counted and replated in 60-mm tissue culture dishes. After
irradiation, serial dilutions of irradiated cells were plated
immediately. After incubation at 37°C in a humidified atmosphere of
5% CO2 for 14–21 days to allow the formation of
macroscopic colonies, the plates were fixed with methanol and
stained with Giemsa. Colonies containing at least 50 cells in size
were counted. The fraction surviving a given X-ray dose was
calculated based on the survival of non-irradiated cells treated
with the vehicle or berberine. Survival (S) data after a radiation
dose (D) were fit by a weighted, stratified, linear regression
according to the linear-quadratic formula S(D) = S(0) exp(-αD -
βD2). The α and β terms in this equation and their
ratios were used to describe survival curve characteristics and to
classify the cellular response to radiation (23).
Cell cycle analysis by flow
cytometry
Cells were harvested with trypsin, washed with
phosphate-buffered saline (PBS) and then stained with buffer
including 50 μg/ml propidium iodide (Sigma-Aldrich) for 30 min at
room temperature. For fluorescence-activated cell sorting (FACS)
analysis, data were collected using a FACSCalibur (BD Bioscience,
San Jose, CA, USA) flow cytometer and analyzed by ModFit (Verity,
Topsham, ME, USA). The cell-cycle distribution was evaluated by
counting >20,000 cells for each sample.
Immunofluorescence staining for
γ-H2AX
Cells were grown on coverslips in 6-well plates and
treated with berberine 15 μM and/or X-ray. At specific times, the
medium was aspirated, washed with PBS 3 times and the cells were
fixed in 4% paraformaldehyde for 15 min at room temperature,
followed by treatment with 0.2% Triton X-100 for 5 min. The cells
were then washed with PBS twice and then blocked with 10% normal
goat serum in PBS for 50 min, following which mouse anti-γ-H2AX
antibody (Millipore) was added at a dilution of 1:200 and incubated
overnight at 4°C. The cells were then washed 3 times with PBS
before being incubated in the dark with a rhodamine-labeled
secondary antibody (KPL) at a dilution of 1:100 in 1% goat serum
albumin in PBS for 60 min. The secondary antibody solution was then
aspirated and the cells were washed 4 times with PBS. The cells
were then incubated in the dark with 4′,6-diamidino-2-phenylindole
(1 μg/ml) in PBS for 5 min and coverslips were mounted with an
antifade solution (Molecular Probes, Eugene, OR, USA). The slides
were then examined on a Leica fluorescent microscope. Images were
captured by a charge coupled device camera. For each treatment
condition, γ-H2AX foci were counted in at least 100 cells from
randomly captured images.
Western blot analysis
The cells were washed twice with cold PBS and lysed
on ice in RIPA buffer [1X PBS, 1% NP 40, 0.1% sodium dodecyl
sulfate (SDS), 5 mM EDTA, 0.5% sodium deoxycholate and 1 mM sodium
orthovanadate] with protease inhibitors and quantified by the BCA
method (24). Nuclear and
cytosolic extracts were prepared with a nuclear/cytosol
fractionation system according to the manufacturer’s instructions.
Equal amounts of protein (30–50 μg) were separated by SDS
polyacrylamide gel electrophoresis, electrotransferred onto
polyvinylidene fluoride membranes (Immobilon-P; Millipore) and
blocked with 5% non-fat dry milk in Tris-buffered saline, pH 7.5
(100 mM NaCl, 50 mM Tris and 0.1% Tween-20). The membranes were
immunoblotted overnight at 4°C with anti-Ku70, anti-Ku86 and
anti-RAD51 monoclonal antibodies (1:200; Santa Cruz Biotechnology,
Inc.), and anti-β-actin monoclonal antibody (1:5,000;
Sigma-Aldrich), followed by their respective horseradish
peroxidase-conjugated secondary antibodies. Signals were detected
by enhanced chemiluminescence. β-actin was used as the endogenous
control.
Statistical analysis
The data are presented as the means ± SD and
analyzed with Microsoft Excel analysis tools and SPSS statistics
17.0 software. All experiments were repeated independently 3 times.
The radiation dose survival curves were analyzed by weighted,
stratified, linear regression. Differences between individual
groups were analyzed by a paired t-test. P-values of <0.05 were
considered to indicate statistically significant differences.
Results
Berberine sensitizes breast cancer cells
to IR
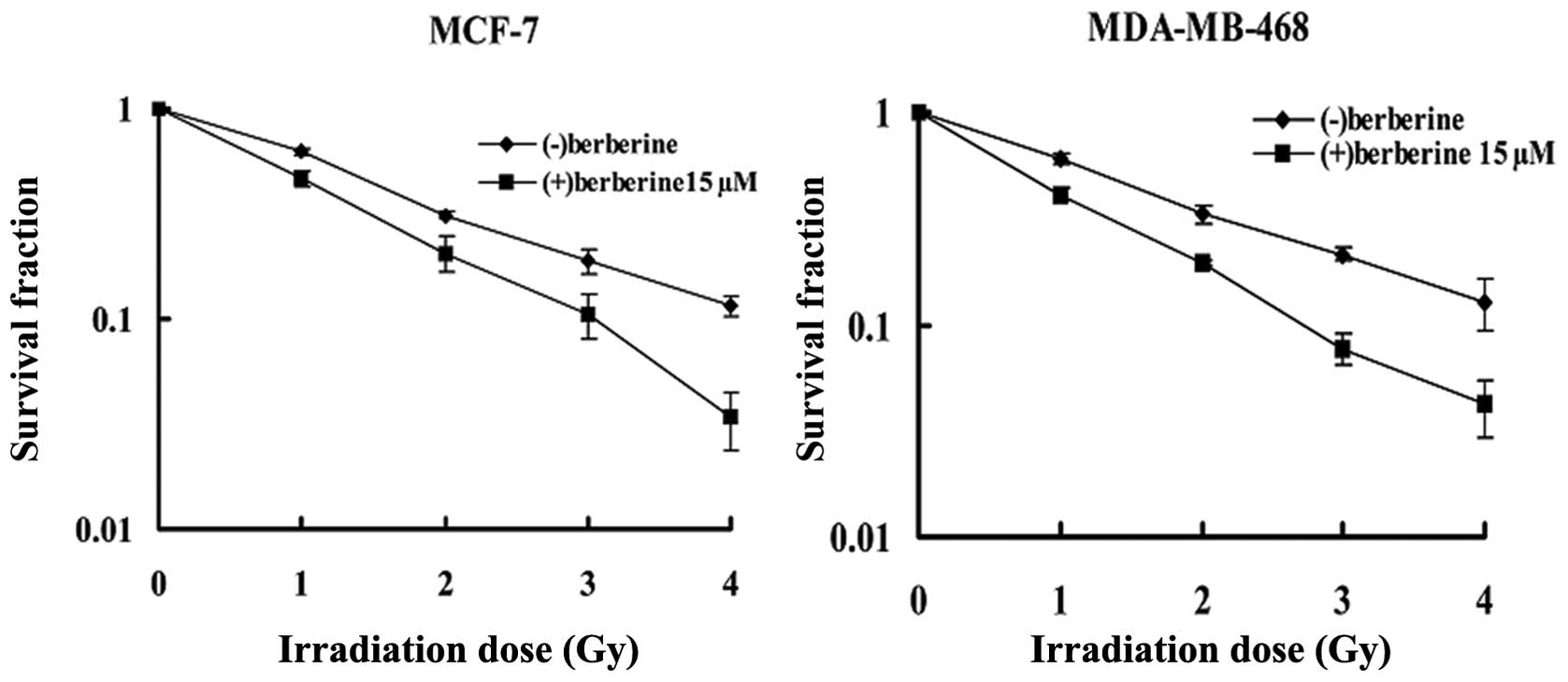
In the MCF-7 cell line (Fig. 1), clonogenic assay revealed that
berberine pre-treatment (15 μM, 24 h) reduced the surviving
fraction at 2 Gy (SF2) of the irradiated cells from 31.2±0.8 to
20.5±3.9% in the cells treated with radiation alone. The
combination treatment caused a reduction of approximately 30% in
the SF2 (P=0.002). The data were further analyzed according to the
linear quadratic model; the α and β components were 0.552±0.050/Gy
and −0.002±0.014/Gy2 for the cells treated radiation
alone, and 0.758±0.104/Gy and −0.007±0.033/Gy2 for the
cells treated with the combination treatment, respectively, leading
to survival curves which were significantly different (P<0.001)
as tested with the linear regression analysis. A similar response
was observed in the other human breast cancer cell line,
MDA-MB-468, with the SF2 being reduced to 20.1±0.6% when the
irradiated cells were pre-treated with berberine at 15 μM for 24 h,
in comparison with 33.1±3.1% for the cells treated with radiation
alone (P<0.01). The α and β components were 0.538±0.058/Gy and
−0.009±0.016/Gy2 for the radiation group, and
0.902±0.07/Gy and −0.034±0.021/Gy2 for the combination
treatment group, respectively. According to the linear regression
analysis, a statistical difference between 2 groups was obtained
(P<0.001).
Berberine treatment causes cell cycle
arrest
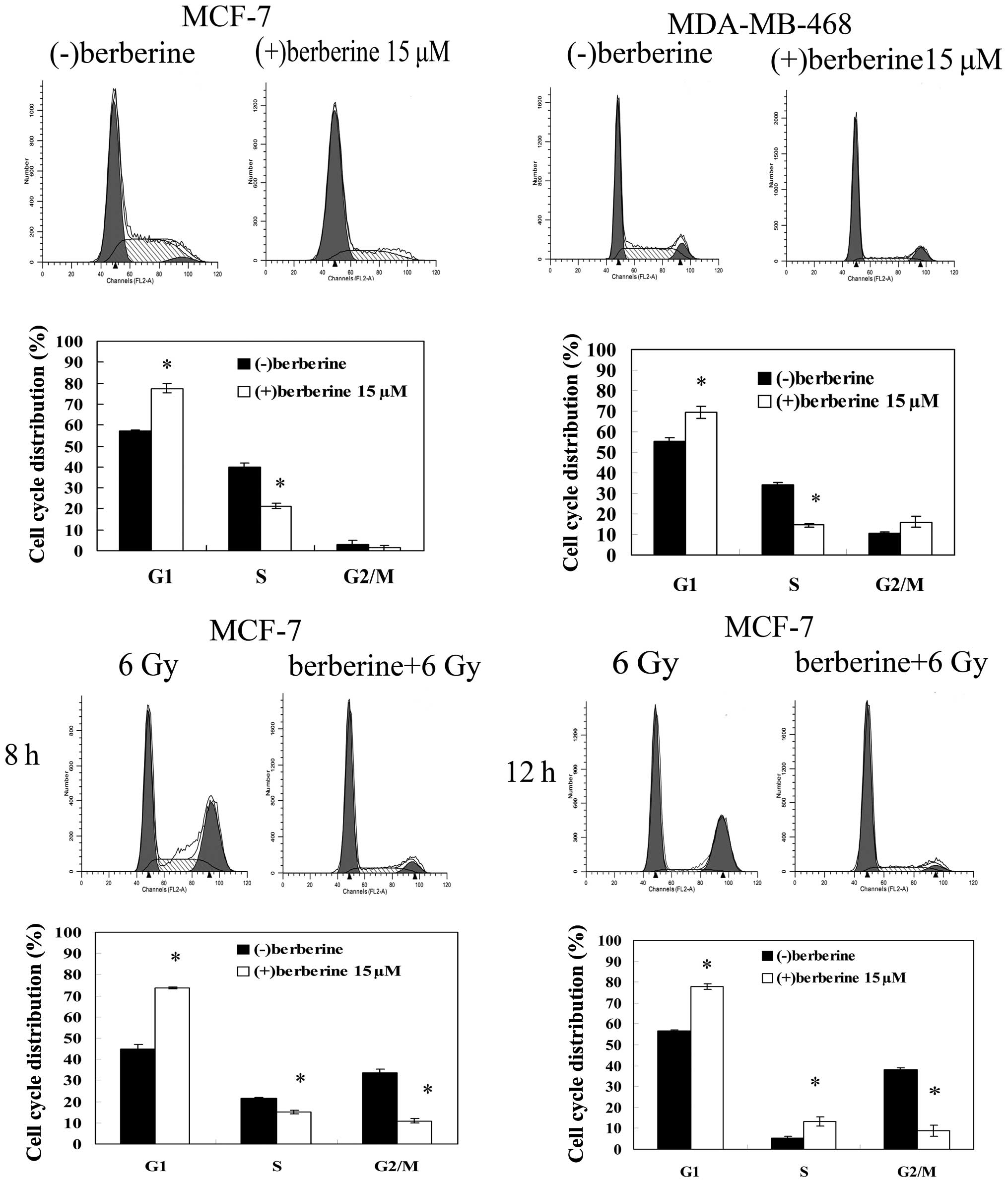
We performed flow cytometry analysis of the cells
treated with 15 μM berberine or DMSO for 24 h by propidium iodide
staining to evaluate the effect of berberine treatment on the cell
cycle progression of human breast cancer cells. Berberine treatment
induced an increase in the proportion of cells in the G1 phase
(77.5±2.1 vs. 57.1±0.5%) and a marked decrease in the proportion of
cells in the S phase (21.3±1.2 vs. 40.0±1.9%) in comparison with
the control MCF-7 cells (Fig. 2).
The same results were observed in the MDA-MB-468 cells. The G0/G1
fraction of the cells treated with berberine increased from
55.4±1.5 to 69.5±2.9%, compared to the control cells, and the
fraction of the cells treated with berberine in the S phase
decreased to 14.4±1.0% in comparison with the control cells
(34.1±0.9%). When the MCF-7 cells were exposed to 6 Gy irradiation
alone for 8–24 h, a significant cell cycle arrest in the G2/M phase
was observed, with a decrease in the percentage of cells in the
G0/G1 phase and S phase. Following pre-treatment with berberine,
the radiation-induced G2/M phase arrest did not occur within 24 h
after irradiation, with an increase in the percentage of cells in
the G0/G1 phase compared with irradiation alone.
Berberine pre-treatment prolongs the
persistence of DNA double-strand breaks (DSBs)
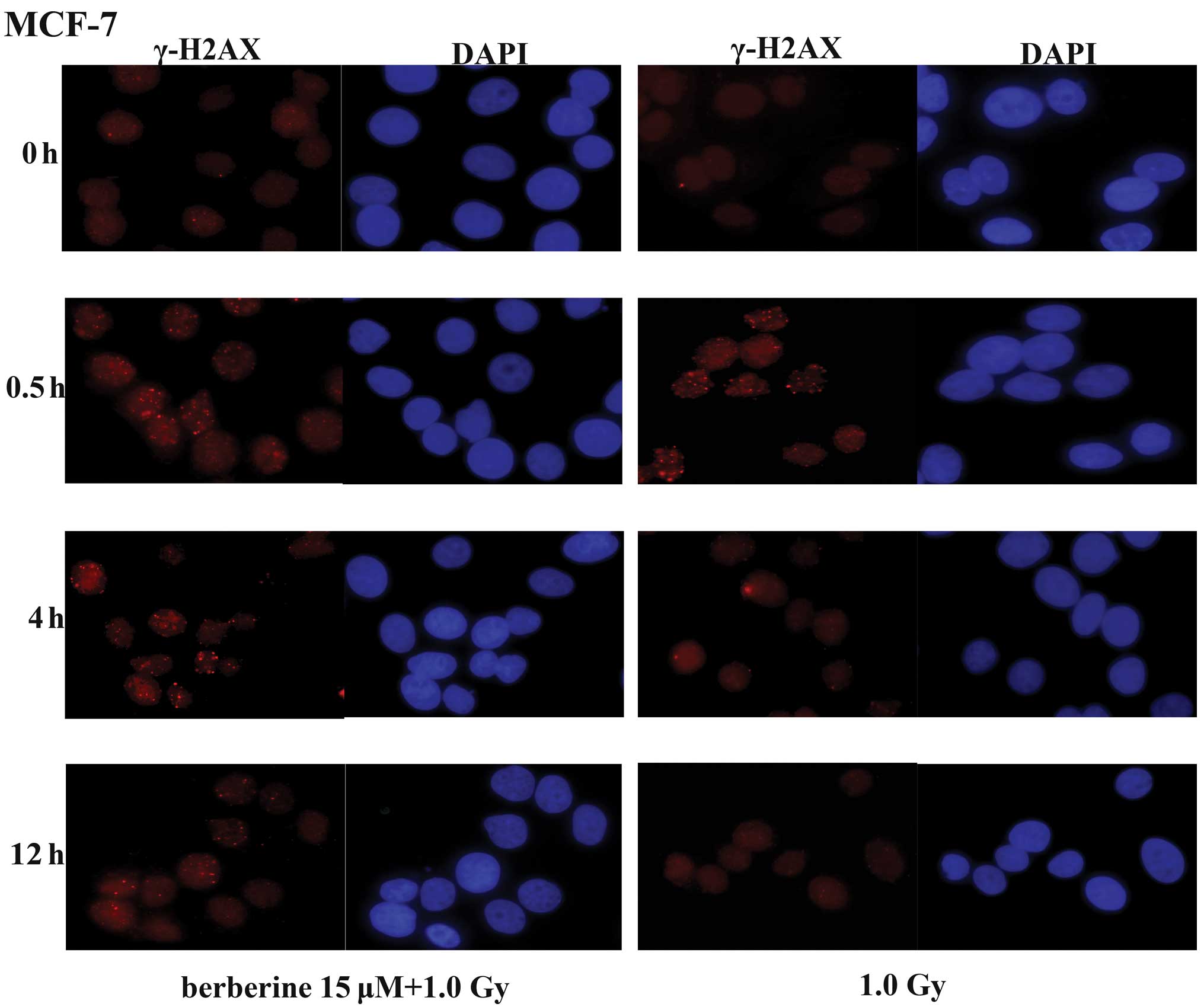
H2AX, a variant of the core histone H2A family,
contains a unique SQ motif within its C-terminal tail that is
highly conserved from plants to humans, suggesting a crucial role
of this variant throughout evolution. The phosphorylated form of
H2AX was termed γ-H2AX, as it was first observed in cells exposed
to γ-rays. The formation of γ-H2AX in response to DNA DSBs provides
the basis for a sensitive assay for DNA damage. In the MCF-7 cells,
we determined the levels of DSBs by immunofluorescence staining of
γ-H2AX foci at different time-points (0, 0.5, 4 and 12 h) after
exposure to X-rays. In the MCF-7 cells not pre-treated with
berberine, the majority of the γ-H2AX foci cleared 4 h following
exposure to 1.0 Gy of X-ray radiation (Fig. 3). By contrast, a delayed clearance
of the X-ray-induced γ-H2AX foci was observed in the MCF-7 cells
pre-treated with berberine (15 μM). These results indicate that
berberine pre-treatment radiosensitizes the cancer cells via the
impairment of the repair of X-ray-induced DSBs.
Berberine downregulates RAD51
DSBs are primarily repaired by 2 pathways. Ku70 and
Ku86 are essential for nonhomologous end joining, whereas RAD51 is
a central player in homologous recombination. We examined the
expression of Ku70, Ku86 and RAD51 by western blot analysis to
assess whether the levels of these 3 proteins were altered in human
breast cancer cells pre-treated with berberine. As shown in
Fig. 4, there were no obvious
changes in the levels of Ku70 and Ku86, but the levels of RAD51
were significantly decreased in the MCF-7 and MDA-MB-468 cells
treated with 15 μM berberine for 24–48 h. In the MCF-7 cells
treated with 15 μM berberine for 24 h prior to 6 Gy irradiation at
the indicated time-points (0, 2, 6 and 24 h), the levels of Ku70
and Ku86 protein had no obvious change, but the level of RAD51
protein had decreased significantly. However, these changes were
not observed in the cells treated with irradiation alone. In the
MDA-MB-468 cells the same results were observed.
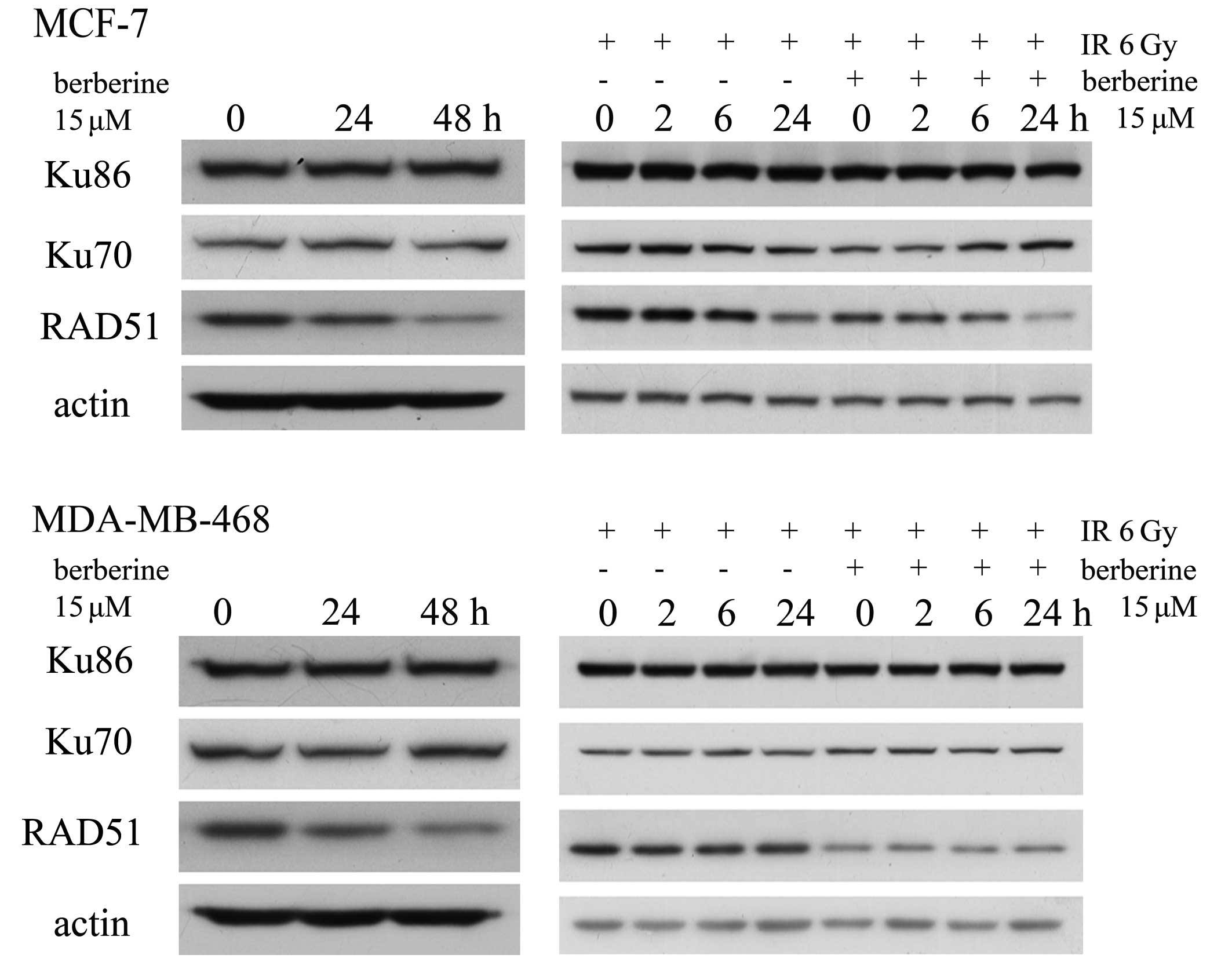 | Figure 4.Western blot analysis for the RAD51,
Ku70 and Ku86 proteins. MCF-7 and MDA-MB-468 cells were treated
with berberine for 24–48 h at 15 μM. The levels of RAD51 protein
had decreased. MCF-7 and MDA-MB-468 cells treated with 15 μM
berberine for 24 h before 6 Gy irradiation, or irradiation alone,
were harvested for the indicated times (0, 2, 6 and 24 h), then the
protein levels of RAD51, Ku70 and Ku86 were detected at the
indicated time-points (0, 2, 6 and 24 h). Immunoblot analysis was
conducted with anti-RAD51, Ku70 and Ku86 antibodies. |
Discussion
IR induces measurable arrests in the G1, S and G2
phases of the mammalian cell cycle, which allows for the repair of
DNA damage in cells prior to DNA replication or mitosis. In this
study, analyses of the treatment effects on the cell cycle revealed
a significantly increased proportion of cells in G1 arrest,
accompanied by a simultaneous decrease in the number of cells in
the S phase following berberine treatment. The arrest in G1 in
response to irradiation is thought to result from a signal
transmitted via the p53 tumor suppressor protein in response to
cellular damage, and the delay in progression through the S phase
of the cell cycle is due to a reduction of the DNA synthesis rate
(25). It has been established
that the cancer cells are more radiosensitive in the G2/M phase,
less sensitive in the G0/G1 phase, and least sensitive during the
latter part of the S phase (26).
Thus, we postulated that the decreased percentage of cells in the S
phase prior to irradiation may result in the observed decrease in
the surviving fraction that was observed in the combination
treatment group.
In addition, we observed a radiation-induced G2/M
arrest in the MCF-7 cells treated with RT alone; however, in the
cells treated with radiation and berberine, the radiation-induced
G2/M arrest did not occur at the indicated time-points. Two
molecularly distinct G2/M checkpoints were identified. The first of
these G2/M checkpoints occurs early after IR and is ATM-dependent
and dose-independent, and represents the failure of cells in the G2
phase at the time of irradiation to progress into mitosis. By
contrast, G2/M accumulation, begins to be measurable only several
hours after IR, is ATM-independent, and represents the accumulation
of cells in the earlier phases of the cell cycle at the time of
exposure to radiation (27).
Studies using caffeine have provided further evidence to support
the role of the G2 delay in irradiated cell survival. Caffeine
reduces or abolishes the radiation-induced G2 delay and renders
cells more sensitive to irradiation (28). The reduction of radiation-induced
G2/M delay may also contribute to the radiosensitizating effects of
berberine on MCF-7 and MDA-MB-468 cells observed in this study.
In mammalian cells, there are 2 major DSB repair
pathways: homologous recombination and non-homologous DNA
end-joining (29,30). Ku70 and Ku86 are essential for the
former, whereas RAD51 is a central player in the latter. As shown
by western blot analysis, the level of the RAD51 protein decreased
significantly in the MCF-7 and MDA-MB-468 cells following berberine
administration for 24 or 48 h at 15 μM; however, the levels of the
Ku70 and Ku86 proteins did not change significantly compared with
the controls when the cells were treated with berberine. In the
berberine-treated cells, the levels of RAD51 decreased continuously
compared with those in the cells treated with RT alone at 3
time-points after irradiation treatment, in the 2 breast cancer
cell lines. Inhibitors of homologous recombination proteins may be
used in combination with RT or chemotherapy to sensitize the cells
(31–34). Mao et al (29) revealed that compared with normal
mammary epithelial cells, the efficiency of homologous
recombination is significantly elevated in breast cancer cell
lines, including MCF-7 and MDA-MB-468 cells, and suggested that the
inhibition of homologous recombination has a selective effect
against breast tumor cells.
In conclusion, in this study, we demonstrate that
berberine increases the radiosensitivity of MCF-7 and MDA-MB-468
cells. Berberine sensitizes human breast cancer cells to IR by
inducing cell cycle arrest, and the downregulation of the
homologous recombination repair protein, RAD51. Berberine may be a
promising radiosensitizer for the treatment of breast cancer.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (No. 81072150
and 81172529). We thank Professor Changshun Shao (Institute of
Molecular Medicine and Genetics and Key Laboratory of Experimental
Teratology, Shandong University School of Medicine, China) for
providing a critical evaluation of our study.
References
|
1.
|
CH YipRA SmithBO AndersonGuideline
implementation for breast healthcare in low- and middle-income
countries: early detection resource
allocationCancer11322442256200818837017
|
|
2.
|
H IgeneGlobal health inequalities and
breast cancer: an impending public health problem for developing
countriesBreast
J14428434200810.1111/j.1524-4741.2008.00618.x18821930
|
|
3.
|
A JemalR SiegelJ XuE WardCancer
statistics, 2010CA Cancer J Clin60277300201010.3322/caac.20073
|
|
4.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised
trialsLancet36516871717200515894097
|
|
5.
|
A JemalMM CenterC DeSantisEM WardGlobal
patterns of cancer incidence and mortality rates and trendsCancer
Epidemiol Biomarkers
Prev1918931907201010.1158/1055-9965.EPI-10-043720647400
|
|
6.
|
K YamamotoH TakaseK AbeY SaitoA
SuzukiPharmacological studies on antidiarrheal effects of a
preparation containing berberine and geranii herbaNihon
Yakurigaku Zasshi1011691751993(In Japanese)
|
|
7.
|
WM HuangZD WuYQ GanEffects of berberine on
ischemic ventricular arrhythmiaZhonghua Xin Xue Guan Bing Za
Zhi173003013191989(In Chinese)
|
|
8.
|
H TakaseK YamamotoK ItoE
YumiokaPharmacological studies on antidiarrheal effects of
berberine and geranii herbaNihon Yakurigaku
Zasshi1021011121993(In Japanese)
|
|
9.
|
MM SandersAA LiuTK LiSelective
cytotoxicity of topoisomerase-directed protoberberines against
glioblastoma cellsBiochem
Pharmacol5611571166199810.1016/S0006-2952(98)00243-39802326
|
|
10.
|
CC LinJS YangJT ChenBerberine induces
apoptosis in human HSC-3 oral cancer cells via simultaneous
activation of the death receptor-mediated and mitochondrial
pathwayAnticancer Res2733713378200717970083
|
|
11.
|
JM HwangHC KuoTH TsengJY LiuCY
ChuBerberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cellsArch
Toxicol806273200610.1007/s00204-005-0014-816189662
|
|
12.
|
JP LinJS YangJH LeeWT HsiehJG
ChungBerberine induces cell cycle arrest and apoptosis in human
gastric carcinoma SNU-5 cell lineWorld J
Gastroenterol122128200616440412
|
|
13.
|
SK MantenaSD SharmaSK KatiyarBerberine, a
natural product, induces G1-phase cell cycle arrest and
caspase-3-dependent apoptosis in human prostate carcinoma cellsMol
Cancer Ther5296308200610.1158/1535-7163.MCT-05-044816505103
|
|
14.
|
Z LiuQ LiuB XuBerberine induces
p53-dependent cell cycle arrest and apoptosis of human osteosarcoma
cells by inflicting DNA damageMutat
Res6627583200910.1016/j.mrfmmm.2008.12.00919159633
|
|
15.
|
J TangY FengS TsaoN WangR CurtainY
WangBerberine and Coptidis rhizoma as novel antineoplastic
agents: a review of traditional use and biomedical investigationsJ
Ethnopharmacol1265172009
|
|
16.
|
CH LeeJC ChenCY HsiangSL WuHC WuTY
HoBerberine suppresses inflammatory agents-induced
interleukin-1beta and tumor necrosis factor-alpha productions via
the inhibition of IkappaB degradation in human lung cellsPharmacol
Res56193201200710.1016/j.phrs.2007.06.003
|
|
17.
|
CJ ThirupurasundariR PadminiSN
DevarajEffect of berberine on the antioxidant status,
ultrastructural modifications and protein bound carbohydrates in
azoxymethane-induced colon cancer in ratsChem Biol
Interact177190195200910.1016/j.cbi.2008.09.02718951886
|
|
18.
|
S KimJH ChoiJB KimBerberine suppresses
TNF-alpha-induced MMP-9 and cell invasion through inhibition of
AP-1 activity in MDA-MB-231 human breast cancer
cellsMolecules1329752985200810.3390/molecules1312297519052522
|
|
19.
|
PL PengWH KuoHC TsengFP ChouSynergistic
tumor-killing effect of radiation and berberine combined treatment
in lung cancer: the contribution of autophagic cell deathInt J
Radiat Oncol Biol
Phys70529542200810.1016/j.ijrobp.2007.08.03418207031
|
|
20.
|
Q LiuH JiangZ LiuBerberine radiosensitizes
human esophageal cancer cells by downregulating homologous
recombination repair protein RAD51PLoS
One6e23427201110.1371/journal.pone.0023427
|
|
21.
|
Y LiuH YuC ZhangProtective effects of
berberine on radiation-induced lung injury via intercellular
adhesion molecular-1 and transforming growth factor-beta-1 in
patients with lung cancerEur J
Cancer4424252432200810.1016/j.ejca.2008.07.04018789680
|
|
22.
|
GH LiYP ZhangJL TangEffects of berberine
against radiation-induced intestinal injury in miceInt J Radiat
Oncol Biol
Phys7715361544201010.1016/j.ijrobp.2010.02.06220637981
|
|
23.
|
NA FrankenHM RodermondJ StapJ HavemanC van
BreeClonogenic assay of cells in vitroNat
Protoc123152319200610.1038/nprot.2006.33917406473
|
|
24.
|
X LiX KongQ HuoMetadherin enhances the
invasiveness of breast cancer cells by inducing epithelial to
mesenchymal transitionCancer
Sci10211511157201110.1111/j.1349-7006.2011.01919.x21371176
|
|
25.
|
EJ BernhardA MaityRJ MuschelWG
McKennaEffects of ionizing radiation on cell cycle progressionA
review Radiat Environ
Biophys347983199510.1007/BF012752107652155
|
|
26.
|
TM PawlikK KeyomarsiRole of cell cycle in
mediating sensitivity to radiotherapyInt J Radiat Oncol Biol
Phys59928942200410.1016/j.ijrobp.2004.03.00515234026
|
|
27.
|
B XuST KimDS LimMB KastanTwo molecularly
distinct G(2)/M checkpoints are induced by ionizing irradiationMol
Cell Biol2210491059200210.1128/MCB.22.4.1049-1059.200211809797
|
|
28.
|
PM BusseSK BoseRW JonesLJ TolmachThe
action of caffeine on X-irradiated HeLa cells. III Enhancement of
X-ray-induced killing during G2 arrestRadiat
Res76292307197810.2307/3574780156382
|
|
29.
|
Z MaoY JiangX LiuA SeluanovV GorbunovaDNA
repair by homologous recombination, but not by nonhomologous end
joining, is elevated in breast cancer
cellsNeoplasia11683691200919568413
|
|
30.
|
T HelledayJ LoDC van GentBP EngelwardDNA
double-strand break repair: from mechanistic understanding to
cancer treatmentDNA Repair
(Amst)6923935200710.1016/j.dnarep.2007.02.00617363343
|
|
31.
|
SJ CollisA TigheSD ScottSA RobertsJH
HendryGP MargisonRibozyme minigene-mediated RAD51 down-regulation
increases radiosensitivity of human prostate cancer cellsNucleic
Acids Res2915341538200110.1093/nar/29.7.153411266555
|
|
32.
|
T OhnishiT TakiS HiragaN AritaT MoritaIn
vitro and in vivo potentiation of radiosensitivity of malignant
gliomas by antisense inhibition of the RAD51 geneBiochem Biophys
Res Commun245319324199810.1006/bbrc.1998.84409571148
|
|
33.
|
JS RussellK BradyWE BurganGleevec-mediated
inhibition of Rad51 expression and enhancement of tumor cell
radiosensitivityCancer Res6373777383200314612536
|
|
34.
|
MS TsaiYH KuoYF ChiuYC SuYW
LinDown-regulation of Rad51 expression overcomes drug resistance to
gemcitabine in human non-small-cell lung cancer cellsJ Pharmacol
Exp Ther335830840201010.1124/jpet.110.17314620855443
|