Introduction
Endometrial cancer is the most common gynecological
malignancy in western countries, and its incidence has recently
increased (1). The risk factors
of endometrial adenocarcinoma include nulliparity (2), late menopause onset (3) and use of estrogen-only hormone
replacement therapy (HRT) (4). An
exposure to high estrogen and low progesterone levels increases
proliferation of endometrial cells, and therefore the risk of
cancer development of endometrial adenocarcinoma (5).
Among various risk factors, estrogens are well
recognized to play a significant role in endometrial cancer
development and growth (6).
Estradiol has been shown to exert its proliferative and
anti-apoptotic effects through the Akt activation in human
endometrial cells (7). Estrogen
acts via the estrogen receptor, which hormone induce the estradiol
activation of the estrogen signal members and their mutual
communication (8).
Estrogen receptor and progesterone receptors belong
to the steroid hormone nuclear receptor superfamily and their
upregulation in Ishikawa cell is induced by estradiol treatment
(9). They are ligand-activated
transcription factors involved in hormone-mediated signaling,
hormone-mediated gene expression, and cellular proliferation and
differentiation.
MAPK pathway is involved in the control of many
fundamental cellular functions that include cell proliferation,
survival, differentiation, apoptosis and metabolism (10).
Green tea has been studied extensively for its
health benefits, including anticancer and cancer chemopreventive
properties (11). Green tea
contains a variety of polyphenols known as catechins.
(−)-epigallocathechin-3-gallate (EGCG) is a major component of
polyphenols in green tea (12).
EGCG interacts with various molecules such as proteins,
transcription factors, and enzymes, which block multiple stages of
carcinogenesis via the regulation of intracellular signaling
transduction pathways. EGCG possesses pharmacological and
physiological properties including induction of phase II enzymes,
mediation of anti-inflammation response, regulation of cell
proliferation and apoptosis effects and prevention of tumor
angiogenesis, invasion and metastasis (13). However, the EGCG amounts used in
previous studies seem too high to explain the anticancer effect
associated with green tea, compared with the levels measured in
human blood and serum after oral consumption (14). Several mechanisms of cancer
inhibition by EGCG in vivo have been proposed. It is
reported that EGCG inhibited angiogenesis and matrix
metalloproteinase in vivo (15). It is also reported that EGCG
prevented the carcinogenesis of cervical cancer, induced apoptosis
and inhibited telomerase activity (16).
The present study was undertaken to evaluate
antiproliferative effects on human endometrial adenocarcinoma cell
line, Ishikawa cell under the influence of EGCG. The
anti-proliferative mechanism of action of EGCG was determined by
studying the ER-mediated signaling and modulation of downstream
genes involved in cell proliferation and apoptosis. We report that
EGCG inhibits proliferation via modulating ER-dependent signaling
mechanisms, interfere with Akt activation and induce apoptosis via
intrinsic and extrinsic pathway in human endometrial cancer
cells.
Materials and methods
Reagents and cell culture
Antibodies for caspase-3, -9, Akt, ERK, P38 and each
phospho-form were purchased from Cell Signaling Technology.
Enhanced chemiluminescence (ECL) was obtained from ELPIs
Biotechnology, and other reagents were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). SYBR green was purchased from
Agilent Technologies, and Tri reagent was purchased from Invitrogen
(Carlsbad, CA). Human endometrial adenocarcinoma (Ishikawa) cells,
which were kindly gifted by Dr Y.H. Kim at Asan Medical Center,
Seoul, Korea, were grown in DMEM-F12 (Gibco, Invitrogen)
supplemented with 10% FBS at 37°C in 5% CO2. Passages
4–8 of cultures were used in all experiments.
Cell proliferation assay
The effects of EGCG on Ishikawa cells were assessed
by MTT assay. Ishikawa cells (1×104 cells/ml) were
incubated in a 96-well. The cells were incubated with EGCG, and
cultured at 37°C under humidified atmosphere with 5% CO2
for 24 h. Subsequently, 20 μl MTT solution (5 mg/ml in PBS) was
added to each well and placed at room temperature for 4 h. The
absorbance was measured on an ELISA reader at a wavelength of 495
nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was used to analyze the expression of mRNA
for cell cycle factors, apoptosis factors and β-actin (internal
control) in estrogen-stimulated Ishikawa cells. The Ishikawa cells
were treated with 100 μM EGCG and 1 μM E2 for 24 h, and
total RNA was isolated using Tri-reagent. cDNA was generated from
0.2 μg of total RNA using RevertAid First strand cDNA Synthesis kit
(Fermentas, St. Leon-Rot, Germany). The primers used for
amplification of each gene were as shown in Table I. Real-time PCR was performed on
Applied Biosystems 7000 real-time PCR system (Life Technologies,
Carlsbad, CA, USA) with SYBR green premix. The expression levels of
genes were normalized to that of β-actin gene.
 | Table I.Primers for gene expression. |
Table I.
Primers for gene expression.
| Gene | | Primers |
|---|
| Estrogen
receptor | S: | 5′-AAG AGC TGC CAG
GCC TGC-3′ |
| AS: | 5′-TTG GCA GCT CTC
ATG TCT CC-3′ |
| Progesterone
receptor | S: | 5′-AAC ACA AAA CCT
GAC ACC TC-3′ |
| AS: | 5′-CGT GTT TGT AGG
ATC TCC AT-3′ |
| Cyclin D1 | S: | 5′-TCG CCA CCT GGA
TGC TGG AG-3′ |
| AS: | 5′-CAC CAG GAG CAG
CTC CAT TTG-3′ |
| Cyclin D3 | S: | 5′-CTG CCT CCA GGA
ACC ACA-3′ |
| AS: | 5′-GCT TGA CTA GCC
ACC GAA AT-3′ |
| Cdk 2 | S: | 5′-ATG GAG AAC TTC
CAA AAG GTG-3′ |
| AS: | 5′-CAG GCG GAT TTT
CTT AAG CG-3′ |
| Cdk4 | S: | 5′-ACA AGT GGT GGA
ACA GTC AAG-3′ |
| AS: | 5′-GCA TAT GTG GAC
TGC AGA AGA -3′ |
| CCNA2 | S: | 5′-TGG GCA CTG CTG
CTA TGC T-3′ |
| AS: | 5′-GCA ATA ACT GAT
GGC AAA TAC TTG A-3′ |
| CCNB2 | S: | 5′-CCA CAC CTG AGG
ATG TCT CCA T-3′ |
| AS: | 5′-ATG CCA ACG CAC
ATG TAC AGA-3′ |
| CCNB1 | S: | 5′-TTT CTG CTG GGT
GTA GGT CCT T-3′ |
| AS: | 5′-GCC ATG TTG ATC
TTC GCC TTA-3′ |
| Cdk 1 | S: | 5′-AAA ATT GGA GAA
GGT ACC TAT-3′ |
| AS: | 5′-CCC TTC CTC TTC
ACT TTC TAG T-3′ |
| Caspase-6 | S: | 5′-ACT GGC TTG TTC
AAA GG-3′ |
| AS: | 5′-CAG CGT GTA AAC
GGA G-3′ |
| Caspase-8 | S: | 5′-ATG CAA ACT GGA
TGA TGA CA-3′ |
| AS: | 5′-GAT TAT CTT CAG
CAG GCT CTT-3′ |
| Caspase-10 | S: | 5′-AAT CTG ACA TGC
CTG GAG-3′ |
| AS: | 5′-ACT CGG CTT CCT
TGT CTA C-3′ |
| Bcl-2 | S: | 5′-GCT CTA AAA TCC
ATC CAG-3′ |
| AS: | 5′-CCT CTC CAT CAT
CAA CTT-3′ |
| Bax | S: | 5′-CCC GAG AGG TCT
TTT TCC-3′ |
| AS: | 5′-GCC TTG AGC ACC
AGT TTG-3′ |
| Bcl-XL | S: | 5′-TTA CCT GAA TGA
CCA CCT A-3′ |
| AS: | 5′-ATT TCC GAC TGA
AGA GTG A-3′ |
| GAPDH | S: | 5′-TGA ACG GGA AGC
TCA CTG G-3′ |
| AS: | 5′-TCC ACC ACC CTG
TTGCTG TA-3′ |
Western blot analyses
For immunodetection, cells were harvested and lysed
in lysis buffer consisting of 20 mM Tris, 137 mM NaCl, 2 mM EDTA,
10% glycerol, 1% Triton X-100, Protease inhibitor (Sigma Chemical
Co., MO, USA), and phosphatase inhibitor cocktail III (Calbiochem
Co., USA). The cleared protein lysates were separated by 12%
SDS-PAGE and transferred electrophoretically onto nitrocellulose
membrane. Blots were blocked with 3% non-fat dry milk in TBS, them
incubated with primary antibody. The Akt, ERK, JNK, caspase-3 and-9
were assayed using anti Akt, ERK, JNK, caspase-3 and -9 antibody
(Cell Signaling Technoligy, MA, USA). After washing, blots were
incubated with anti-goat horseradish peroxidase-conjugated
secondary antibodies for 1 h. Immunodetection was carried out using
an enhanced chemiluminescence peroxidase substrate solution (ELPIs
Biotechnology, Taejeon, Korea). Each band was analyzed by
densitometry for statistical comparisons.
Cell cycle analysis
Cells were seeded in 6-well plates and treated with
EGCG and E2 for 24 h. After treatment, cells were washed
with PBS, fixed in 70% cold ethanol for 72 h, and then stained with
propidium iodide (PI) solution (50 μg/ml). Cell cycle distribution
was analyzed with flow cytometry (Beckman Coulter Inc., Brea, CA,
USA). The percentage of DNA content at different phases of the cell
cycle was analyzed with Multicycle-software.
Annexin-V/propidium iodide labeling and
flow cytometry assay
Annexin-V binding is indicative of early apoptosis
(17). The cells were culture in
6-well plates and treated with EGCG for 24 h. Adherent and
non-adherent cells were probed with FITC-conjugated Annexin-V and
PI (BD Bioscience, NJ, USA) for 15 min. The staining profiles were
determined with FACScan. Stained cells were observed by
fluoromicros-copy (Zeiss, Oberkochen, Germany). The experiments
were performed three times with three replicates in each.
Statistical analysis
The data are expressed as the mean ± SD. The
statistical significance of the difference in mean values was
tested using one-way analysis of variance (ANOVA) and Student’s
t-test. Significance was defined as P<0.05.
Results
EGCG inhibits Ishikawa cell
proliferation
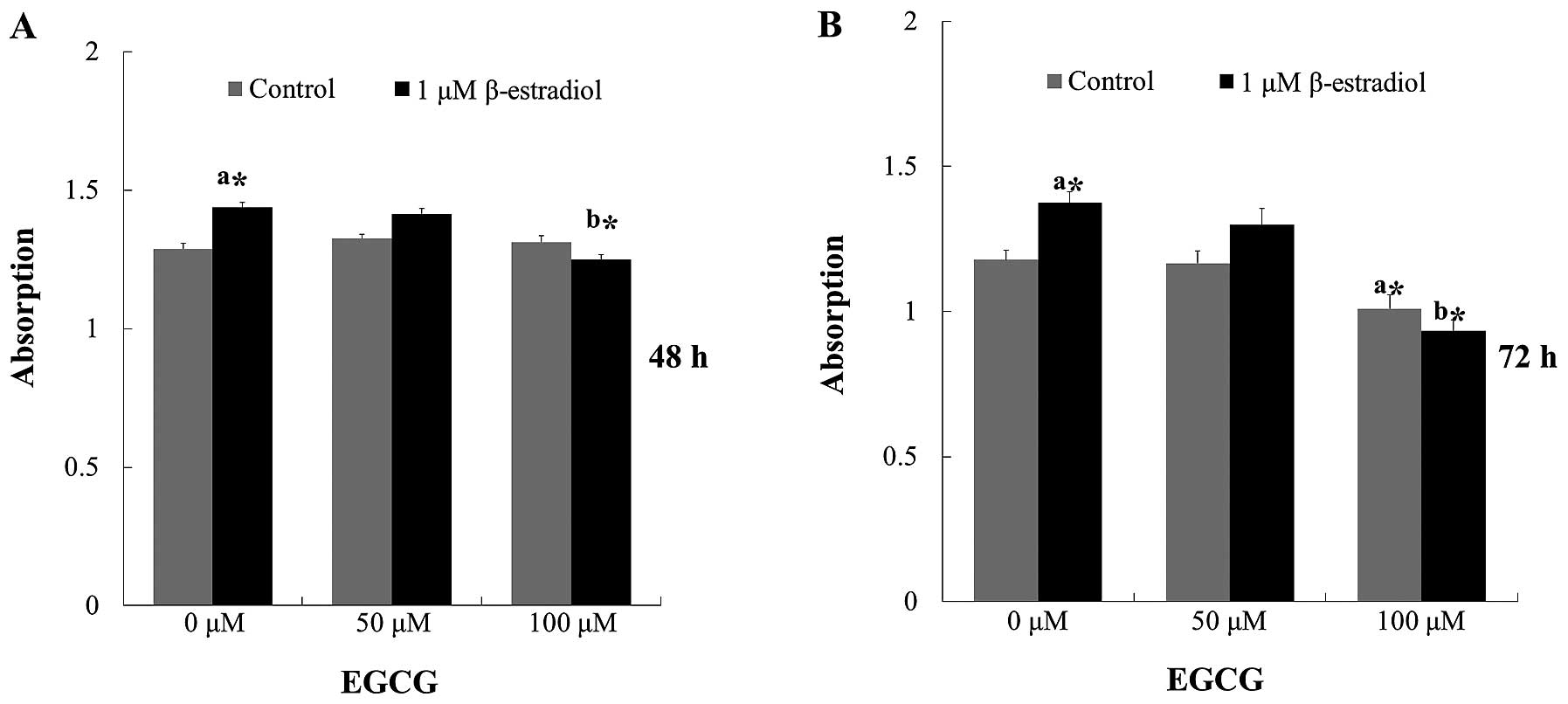
To investigate the effect of EGCG on Ishikawa cell
stimulated with E2, we applied EGCG and E2
for 24, 48, and 72 h. Inhibitory effects were not found at 24 h,
treated with 50 and 100 μM of EGCG (data not shown). Comparable
results were found for E2-stimulated cells which were
significantly increased at 48 and 72 h, this effect could be
inhibited by treatment with EGCG, and high concentration of EGCG
also inhibited cell proliferation without E2 treatment
(Fig. 1). Therefore, 100 μM EGCG
treatment was selected for further experiments.
Effect of EGCG on the expression of
estrogen and progesterone receptor
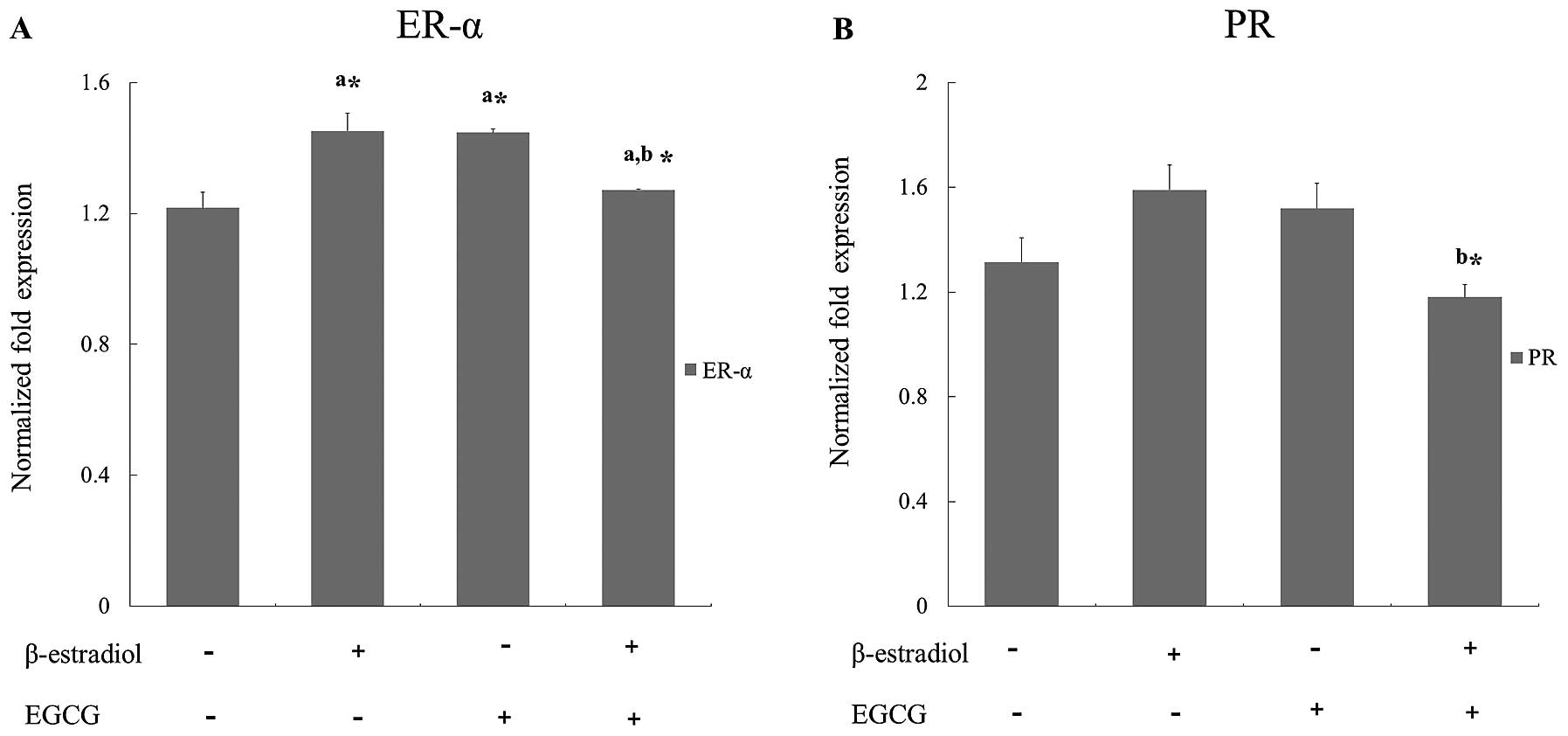
For the analysis of ER-α and PR expression
differences, cells treated with EGCG were incubated with or without
1 µM E2 for 24 h. E2 treatment induces
association of estrogen receptor α (8). As shown on Fig. 2, E2 treated cells
showed increased expressions of ER-α and PR. However, the increased
expressions were strongly inhibited by EGCG with statistical
significance.
EGCG induces cell cycle arrest in
Iishikawa cells
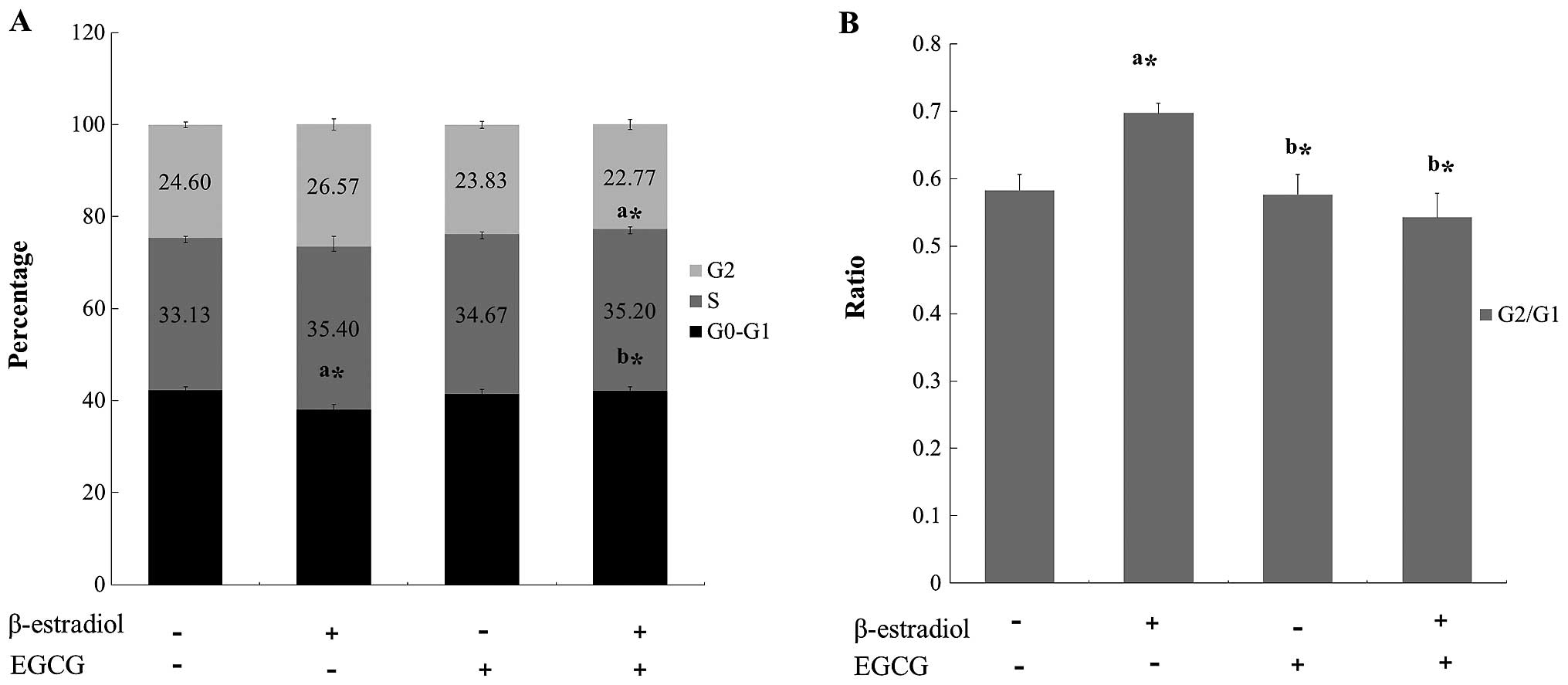
To examine the mechanism responsible for EGCG
mediated cell proliferation inhibition, cell cycle distribution was
evaluated using flow cytometric analysis. The results showed that
EGCG treated cells caused a significant inhibition of cell cycle
progression in Ishikawa cells (Fig.
3). Cyclin D and Cdk 4 genes are responsible for the transition
of cells from G1 phase. Cyclin A and Cdk 2 genes are from S phase,
and cyclin B and Cdk 1 genes are from M phase. Real-time PCR
analysis showed that mRNA expression level of G1-Cdk, M-Cdk, and
S-Cdk genes were decreased after EGCG treatment (Table II). These results clearly showed
that EGCG induced cell cycle arrest through the control of
cyclin-Cdk complexes.
 | Table II.Effect of EGCG on E2
stimulated gene expression of the cell cycle from Ishikawa
cells. |
Table II.
Effect of EGCG on E2
stimulated gene expression of the cell cycle from Ishikawa
cells.
| Treatment
|
|---|
| Gene | None | β-estradiol | EGCG |
β-estradiol+EGCG |
|---|
| Cdk 1 | 1.061±0.242 | 3.502±0.871 | 1.028±0.134 |
1.157±0.200b,c |
| Cyclin B1 | 3.126±0.686 | 3.055±0.564 |
1.694±0.046a,c | 1.946±0.417 |
| Cdk 2 | 1.460±0.244 | 3.752±0.712 | 2.264±0.512 |
0.933±0.063b,c |
| Cyclin A2 | 1.692±0.505 | 2.196±0.446 |
1.252±0.072a,c | 1.177±0.291 |
| Cyclin D1 | 1.035±0.112 | 0.741±0.267 | 1.472±0.620 | 1.595±0.193 |
| Cyclin D3 | 1.149±0.047 | 2.515±0.924 | 1.200±0.284 |
1.414±0.094b,c |
| Cdk 4 | 1.922±0.569 | 2.319±0.418 | 1.327±0.140 | 1.131±0.246 |
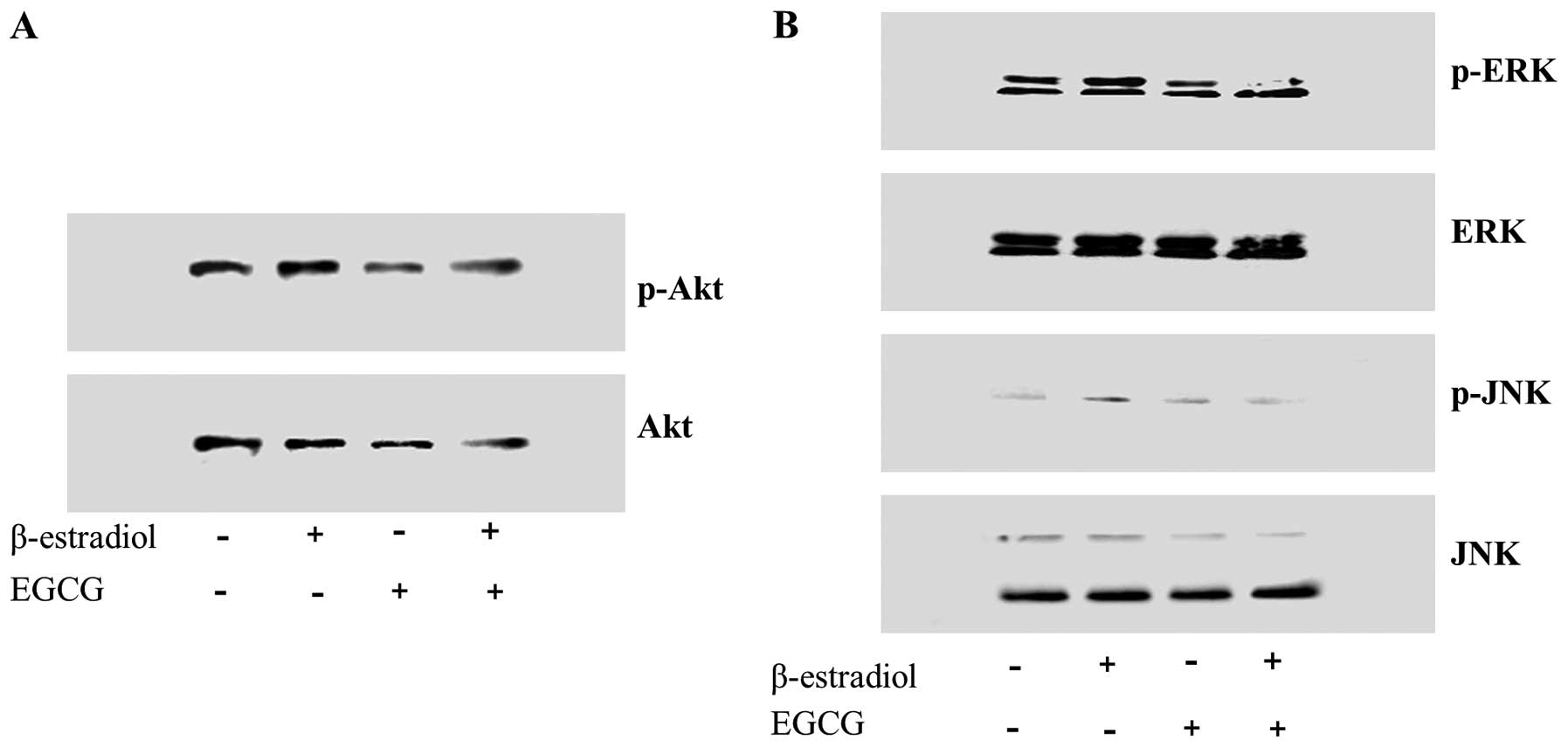
EGCG disturbs the Akt pathway in Ishikawa
cell proliferation treated with E2
In case of estrogen-dependent cells, estrogen
induces cell proliferation and metabolic activity via PI3K-Akt
signal and its downstream mitogen activated protein kinase (MAPK)
(8). To study the effect of EGCG
on Akt cell survival pathway, phosphorylation status of Akt and
expression of its downstream signal MAPK was studied in Ishikawa
cells (Fig. 4). Immunoblots
showed that EGCG decreased the intracellular levels of
phosphorylated Akt (Fig. 4A), and
Akt downstream MAPK, ERK and JNK also decreased. Estrogen receptor
belongs to steroid hormone nuclear receptor super-family, and they
are ligand-activated transcription factors involved in cellular
proliferation and differentiation. They are translocated from the
plasma membrane to the nucleus and are mediated by MAPK activation
(1). This suggests EGCG regulate
cell proliferation by the Akt signal and MAP kinase activation.
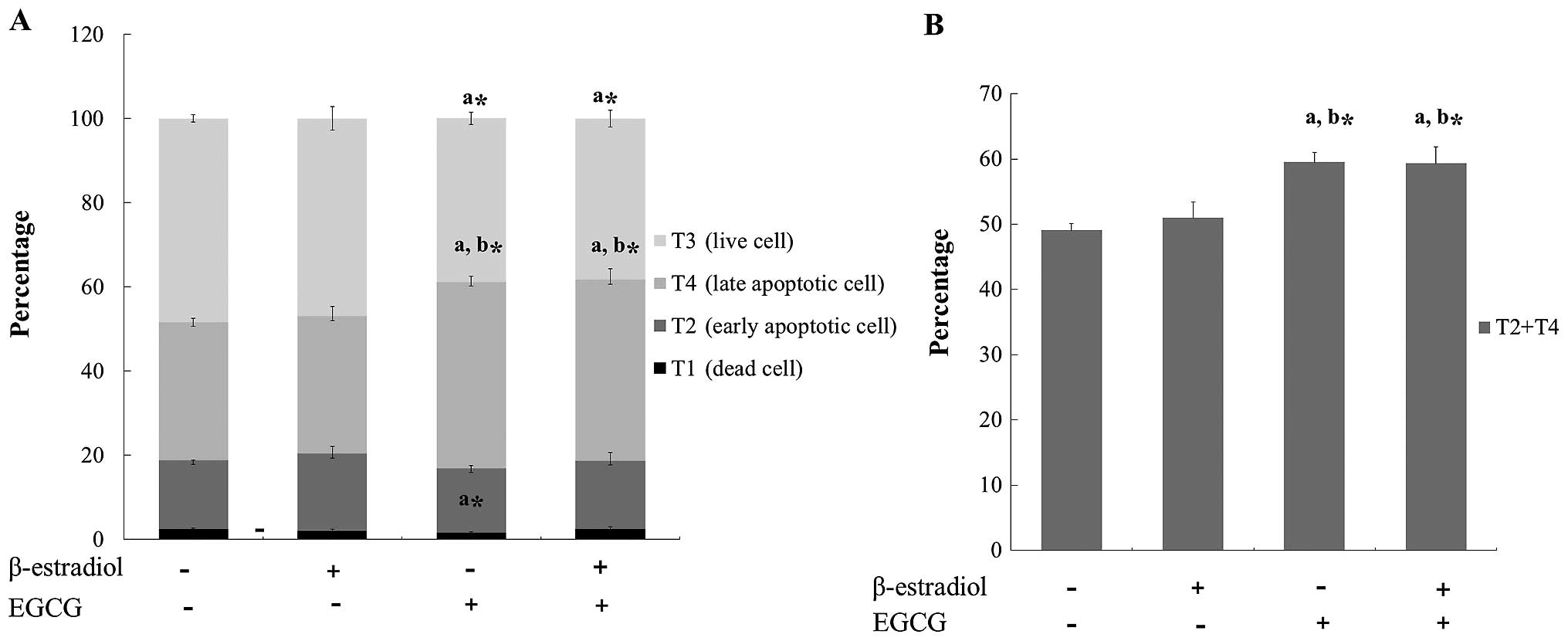
EGCG induces apoptosis in Ishikawa
cells
We next assessed the effect of EGCG on the induction
of apoptosis in Ishikawa cells by FACS analysis. To analyze the
apoptosis quantitatively, Ishikawa cells treated with EGCG were
examined by flow cytometric analysis to determine the total DNA
content of each cell (Fig. 5A).
In the present study, we observed that EGCG treatment increased the
apoptotic regions of T2 and T4 through the accumulation of sub-G1
cells (Fig. 5B).
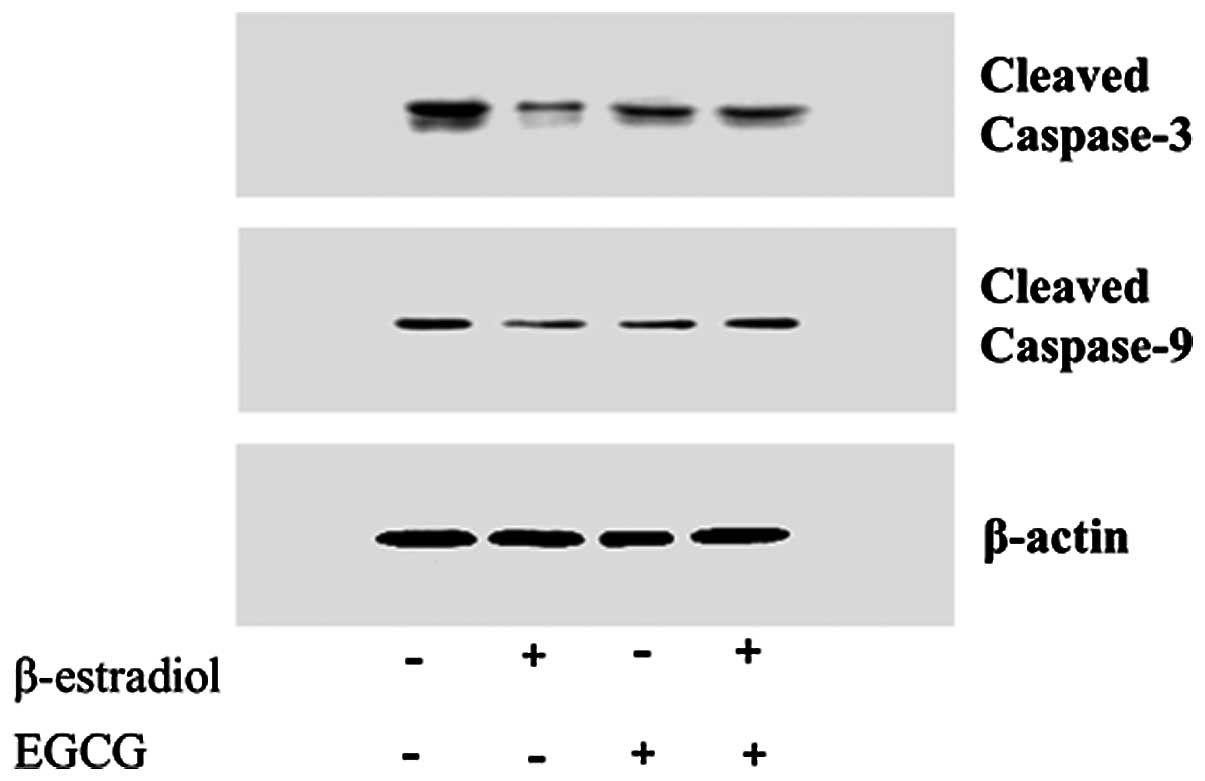
EGCG induces apoptotic genes in Ishikawa
cells
After EGCG treatment of Ishikawa cells,
apoptosis-related proteins, such as caspase-6, 8, 10, Bcl-2, and
Bax, were upregulated in E2 treated cells. The results
revealed that EGCG causes significant downregulation of the
anti-apoptotic gene, Bcl-XL (Table
III). By quantification of Bax and Bcl family mRNA, it was
found that EGCG increase the Bax/Bcl family ratio. EGCG may induce
apoptosis by altering the Bax/Bcl family ratio and controlling the
initiating caspases. Western blot analysis showed that EGCG
increased the level of cleaved caspase-3 and -9 (Fig. 6).
 | Table III.Effect of EGCG on E2
stimulated gene expression of apoptosis from Ishikawa cells. |
Table III.
Effect of EGCG on E2
stimulated gene expression of apoptosis from Ishikawa cells.
| Treatment
|
|---|
| Gene | None | β-estradiol | EGCG |
β-estradiol+EGCG |
|---|
| Caspase-6 | 0.965±0.376 | 0.933±0.279 | 1.571±0.955 |
1.608±0.102b,c |
| Caspase-8 | 1.268±0.120 | 0.823±0.348 | 1.718±0.344 | 1.132±0.392 |
| Caspase-10 | 1.022±0.088 | 0.735±0.197 | 1.592±0.408 |
0.871±0.131b,c |
| Bcl-XL | 1.275±0.076 | 1.183±0.094 |
0.592±0.054a,c |
0.454±0.013b,c |
| Bcl-2 | 1.453±0.143 | 1.060±0.257 | 1.77±0.316 | 1.183±0.490 |
| Bax | 1.266±0.126 | 0.970±0.218 | 1.694±0.324 |
1.198±0.114b,c |
Discussion
In agreement with previous experimental studies
demonstrating the anticancer effect of EGCG using various cell
lines, we also found that EGCG had anticancer effect on an
endometrial cancer cell line in vitro. Endometrial cancer is
one of the most significant gynecological malignancies in the world
and accounts for almost 50,000 deaths worldwide per year (18). It is well known that estrogen
plays an important role in carcino-genesis of endometrial cancer.
There have been many studies demonstrating the relationship between
unopposed estrogen exposure and endometrial cancer occurrence
(4,19). EGCG, one of the major catechins in
green tea, is found in 50–80% of green tea and shows anticancer
properties through blocking multiple signaling pathways, thereby
causing strong cancer chemopreventive effects (23). Although the anticancer effects of
EGCG has been reported in many studies, we could not find the
molecular mechanisms on endometrial cancer cells. Thus, this study
is the first trial to verify the anticancer effect of EGCG on an
endometrial cancer cell line in vitro.
Ishikawa cells are one of the best characterized of
human endometrial cancer cell line derived from a well
differentiated adenocarcinoma and expresses functional steroid
receptors of estrogen, progesterone, and androgen (20). In the present study, we used 1 μM
E2 to induce estrogen receptor upregulation. It would be
considered that E2 treatment provides more physiologic
environment to the cell culture because the endometrial tissue is
grown by estrogen secreted from ovary during reproductive life.
Estrogen receptors are upregulated maximally in the late
proliferative phase when the serum estrogen level is the highest
during the menstrual cycle (21).
We found that estrogen and progesterone receptor mRNAs were
downregulated in the cells treated with both E2 and
EGCG, but not in EGCG only treated cells (Fig. 2). In a previous study on EGCG
effect on endometriosis, EGCG inhibited vascular endothelial growth
factor (VEGF) expression in endometrial glandular cells and stromal
cells stimulated with E2. It was considered that the
decreased VEGF expressions might be due to the ability of EGCG
competing with E2 for binding to ER-α (21). Considering the lower expressions
of ER-α and progesterone receptor mRNA from this study, EGCG might
disturb the process of estrogen and progesterone receptor synthesis
in the cells stimulated by estrogen. However, more studies would be
necessary to obtain the exact mechanism of EGCG on estrogen
receptor. Irrespectively, EGCG might be applied as an alternative
therapeutic trial to the disorders characterized by
estrogen-dependent growth, such as endometriosis, endometrial
hyperplasia and breast cancer.
The extracellular signal regulated protein kinases
(ERK), the c-NH2-terminal kinases (JNK), and the p38
MAPKs have been considered as three major MAPKs and activated Akt
plays a key role in signaling for cell growth, cell survival
(anti-apoptotic) and cell cycle progression. We could find that
ERK, JNK and Akt phosphorylations were reduced more in the EGCG
treated cells than control or E2 only treated cell
(Fig. 4). These results suggest
that EGCG have inhibitory effects on cell proliferation and
differentiation through MAPK and Akt pathway. These findings are
consistent with other studies on human colon cancer cells
demonstrating that EGCG inhibited the activation of ERK and Akt
(22,23).
We analyzed the enzymes involved in apoptosis
processes, cleaved caspase-3 and -9. Increased expressions were
observed in the cells co-treated with E2 and EGCG, when
we compared with E2 only treated cells (Fig. 6). Quantitative real-time PCR was
used to compare the expression levels of apoptosis related enzyme
and proteins implicated in the cancer cell survival. Caspase-6 and
-10 expressions in cells co-treated with E2 and EGCG
were increased. Bcl-XL, an anti-apoptotic protein, was decreased,
but bcl-2 level was not changed (Table III). Proportions of apoptotic
cells treated with EGCG were higher than the control cells
regardless of E2 treatment (Fig. 5). These results suggest that EGCG
induces apoptosis on Ishikawa cells stimulated with or without
E2. Since the apoptotic effect of EGCG on cancer cells
published in 1997, subsequent studies verified the effect in
various cell types, such as lung, colon, pancreas, skin, and
prostate (24,25), but, there have been no report on
the apoptotic effect of EGCG on endometrial cancer cells.
Cell cycle dysregulation by cylin-dependent kinase
(CDK) activity is generally observed in human malignant cells. In
the present study, we found that Cdk1, Cdk2 and cyclin D3 mRNA
expressions in ECGC and E2 co-treated cells were
decreased, comparing with the cells treated with E2 only
(Table II). In flow cytometric
analysis, we observed that the percentage of G0/G1-phase cell cycle
arrest in EGCG treated cells was more than that of E2
only treated cells (Fig. 3). This
fact implies that EGCG inhibits cell cycle progression by
decreasing Cdk 1, Cdk 2 and cyclin D3 of cells treated with
E2. Therefore, it is suggested that EGCG shows an
inhibitory effect on Ishikawa cell cycle progressions as previous
studies have been reported (26,27).
Clinical trials to apply EGCG to the cancer patients
have been performed. A few studies have reported the effectiveness
in human papilloma virus-infected cervical lesion and androgen
independent prostate cancer (28,29). In the case of endometrial cancer
or estrogen-dependent disease, the present study would provide the
beginning of clinical application.
Conclusively, the present study demonstrates for the
first time that EGCG inhibits proliferation and induces apoptosis
of the endometrial cancer Ishikawa cells in vitro. Estrogen
and progesterone receptor expressions are also decreased among the
cells co-treated with E2 and EGCG. Thus, EGCG, major
component of green tea, might have therapeutic or chemopreventive
effect on endometrial cancer or estrogen-related disorders.
Acknowledgements
This study was supported by the Dong-A
University Research Fund.
References
|
1.
|
A DollM AbalM RigauNovel molecular
profiles of endometrial cancer-new light through old windowsJ
Steroid Biochem Mol
Biol108221229200810.1016/j.jsbmb.2007.09.02018061438
|
|
2.
|
G AlbrektsenI HeuchS TretliG KvaleIs the
risk of cancer of the corpus uteri reduced by a recent pregnancy? A
prospective study of 765,756 Norwegian womenInt J
Cancer61485490199510.1002/ijc.29106104107759154
|
|
3.
|
A KalandidiA TzonouL LipworthI GamatsiD
FilippaD TrichopoulosA case-control study of endometrial cancer in
relation to reproductive, somatometric, and life-style
variablesOncology53354359199610.1159/0002275878784467
|
|
4.
|
D GradyT GebretsadikK KerlikowskeV
ErnsterD PetittiHormone replacement therapy and endometrial cancer
risk: a meta-analysisObstet
Gynecol85304313199510.1016/0029-7844(94)00383-O7824251
|
|
5.
|
A AkhmedkhanovA Zeleniuch-JacquotteP
TonioloRole of exogenous and endogenous hormones in endometrial
cancer: review of the evidence and research perspectivesAnn NY Acad
Sci943296315200110.1111/j.1749-6632.2001.tb03811.x11594550
|
|
6.
|
CF HolinkaY AnzaiH HataN KimmelH KuramotoE
GurpideProliferation and responsiveness to estrogen of human
endometrial cancer cells under serum-free culture conditionsCancer
Res493297330119892720684
|
|
7.
|
A BouskineM NeboutB MograbiF
Brucker-DavisC RogerP FenichelEstrogens promote human testicular
germ cell cancer through a membrane-mediated activation of
extracellular regulated kinase and protein kinase
AEndocrinology149565573200810.1210/en.2007-1318
|
|
8.
|
G CastoriaA MigliaccioA BilancioPI3-kinase
in concert with Src promotes the S-phase entry of
oestradiol-stimulated MCF-7 cellsEMBO
J2060506059200110.1093/emboj/20.21.605011689445
|
|
9.
|
JA RobertsonY FarnellLS LindahlNH
IngEstradiol up-regulates estrogen receptor messenger ribonucleic
acid in endometrial carcinoma (Ishikawa) cells by stabilizing the
messageJ Mol
Endocrinol29125135200210.1677/jme.0.029012512200234
|
|
10.
|
BJ CheskisJ GregerN CoochMNAR plays an
important role in ERa activation of Src/MAPK and PI3K/Akt signaling
pathwaysSteroids73901905200810.1016/j.steroids.2007.12.02818261753
|
|
11.
|
BM WolpinRJ MayerSystemic treatment of
colorectal
cancerGastroenterology13412961310200810.1053/j.gastro.2008.02.09818471507
|
|
12.
|
H FujikiM SuganumaS OkabeJapanese green
tea as a cancer preventive in humansNutr
Rev54S67S70199610.1111/j.1753-4887.1996.tb03821.x9110578
|
|
13.
|
MH PanYS ChiouYJ WangCT HoJK LinMultistage
carcinogenesis process as molecular targets in cancer
chemoprevention by epicatechin-3-gallateFood
Funct2101110201110.1039/c0fo00174k21779554
|
|
14.
|
T WebbGreen tea experiments in lab, clinic
yield mixed resultsJ Natl Cancer
Inst9210381039200010.1093/jnci/92.13.103810880545
|
|
15.
|
Y CaoR CaoAngiogenesis inhibited by
drinking teaNature398381199910.1038/1879310201368
|
|
16.
|
M YokoyamaM NoguchiY NakaoA PaterT
IwasakaThe tea polyphenol, (−)-epigallocatechin gallate effects on
growth, apoptosis, and telomerase activity in cervical cell
linesGynecol Oncol921972042004
|
|
17.
|
G ZhangV GurtuSR KainG YanEarly detection
of apoptosis using a fluorescent conjugate of annexin
VBiotechniques2352553119979298227
|
|
18.
|
DM ParkinF BrayJ FerlayP PisaniGlobal
cancer statistics, 2002CA Cancer J
Clin5574108200510.3322/canjclin.55.2.74
|
|
19.
|
V BeralD BullG ReevesEndometrial cancer
and hormone-replacement therapy in the Million Women
StudyLancet36515431551200510.1016/S0140-6736(05)66455-015866308
|
|
20.
|
LP LovelyKB Appa RaoY GuiBA
LesseyCharacterization of androgen receptors in a
well-differentiated endometrial adenocarcinoma cell line
(Ishikawa)J Steroid Biochem Mol
Biol74235241200010.1016/S0960-0760(00)00127-811162929
|
|
21.
|
MP SnijdersAF de GoeijMJ Debets-Te
BaertsMJ RouschJ KoudstaalFT BosmanImmunocytochemical analysis of
oestrogen receptors and progesterone receptors in the human uterus
throughout the menstrual cycle and after the menopauseJ Reprod
Fertil94363371199210.1530/jrf.0.0940363
|
|
22.
|
M ShimizuA DeguchiJT LimH MoriwakiL
KopelovichIB Weinstein(−)-Epigallocatechin gallate and polyphenon E
inhibit growth and activation of the epidermal growth factor
receptor and human epidermal growth factor receptor-2 signaling
pathways in human colon cancer cellsClin Cancer
Res11273527462005
|
|
23.
|
N KhanF AfaqM SaleemN AhmadH
MukhtarTargeting multiple signaling pathways by green tea
polyphenol (−)-epigallocatechin-3-gallateCancer
Res66250025052006
|
|
24.
|
N AhmadDK FeyesAL NieminenR AgarwalH
MukhtarGreen tea constituent epigallocatechin-3-gallate and
induction of apoptosis and cell cycle arrest in human carcinoma
cellsJ Natl Cancer
Inst8918811886199710.1093/jnci/89.24.18819414176
|
|
25.
|
CS YangP MaliakalX MengInhibition of
carcinogenesis by teaAnnu Rev Pharmacol
Toxicol422554200210.1146/annurev.pharmtox.42.082101.15430911807163
|
|
26.
|
N AhmadP ChengH MukhtarCell cycle
dysregulation by green tea polyphenol
epigallocatechin-3-gallateBiochem Biophys Res
Commun275328334200010.1006/bbrc.2000.329710964666
|
|
27.
|
X LiuDY ZhangW ZhangX ZhaoC YuanF YeThe
effect of green tea extract and EGCG on the signaling network in
squamous cell carcinomaNutr
Cancer63466475201110.1080/01635581.2011.53290121391127
|
|
28.
|
WS AhnJ YooSW HuhProtective effects of
green tea extracts (polyphenon E and EGCG) on human cervical
lesionsEur J Cancer
Prev12383390200310.1097/00008469-200310000-0000714512803
|
|
29.
|
A JatoiN EllisonPA BurchA phase II trial
of green tea in the treatment of patients with androgen independent
metastatic prostate
carcinomaCancer9714421446200310.1002/cncr.1120012627508
|