Introduction
Oligodendrocyte precursor cells (OPCs) play an
important role not only as precursor cells that give rise to
myelinating cells in the central nervous system (CNS), but also as
active participants in the neural network. Generation of OPCs from
multi-potent neural stem cells (NSCs) is considered to be induced
by factors produced by the notochord and/or floor plate of the
neural tube (1). These OPCs
express specific markers A2B5, NG2 and PDGFRα and can differentiate
into oligodendrocytes in serum-free medium or type-2 astrocytes in
serum-containing medium (2).
The B104 neuroblastoma cell is a neuronal cell line.
It is well known that the conditioned medium prepared from B104
neuroblastoma cells (B104CM) expands the OPCs in vitro
(3). However, the
OPC-proliferative factors present in B104CM have yet to be
identified.
It has been reported the purified OPCs from
postnatal rat optic nerve, cultured in serum-free medium containing
platelet-derived growth factor AA (PDGF-AA) and basic fibroblast
growth factor (bFGF), are able to undergo continuous self-renewal
in the absence of differentiation (4,5).
Moreover, a previous study showed that insulin-like growth factor-1
(IGF-1) also induced OPC proliferation in vitro (6). However, whether PDGF-AA, bFGF and/or
IGF-1 are key factors in B104CM-induced OPC proliferation remains
unknown. Thus, in the present study, we examined the expressions of
PDGF-AA, bFGF and IGF-1 in the B104 cell line and observed the
effects of their inhibitors on B104CM-induced OPC
proliferation.
Materials and methods
Isolation and culture of spinal
cord-derived OPCs
OPCs were immunopanned from embryonic day (E) 15
Wistar rat spinal cords using an A2B5 antibody and a protocol
modified from previous studies (7–9).
Immunopanned OPCs were plated onto PDL/fibronectin-coated 10×12 cm
culture dishes and growth medium was added and changed every other
day. The growth medium contained DMEM/Ham’s F12, 1×N2 and 1×B27
supplements, fibroblast growth factor 2 (FGF-2, 20 ng/ml)
(Invitrogen, Carlsbad, CA, USA), and PDGF-AA, (10 ng/ml; R&D
Systems, Minneapolis, MN, USA). In all cases, an aliquot of cells
was analyzed the following day to determine the efficiency of
immunopanning. Only preparations in which >90% of the bound
cells expressed A2B5 were used. After 5–7 days, the cells were
passaged. In all experiments, cells at passage 2 (P2) were
used.
Animals
All embryonic rats were obtained from female
pregnant SD rats bred in the Animal Care Facility at Bengbu Medical
College. All animal care was carried out in accordance with the
National Institute of Health Guide for the Care and Use of
Laboratory Animals (NIH publication no. 80–23; revised 1996) and
was approved by the Bengbu Medical College Animal Care Committee of
the Use of Laboratory Animals.
Preparation of B104CM
B104 neuroblastoma cells were a generous gift from
Dr Ian Duncan (University of Wisconsin) and B104CM was prepared
according to the method of Louis et al (3). Cultures of B104 neuroblastoma cells
were maintained in logarithmic phase of growth in DMEM (Invitrogen)
supplemented with 10% FCS. For conditioned medium (B104CM)
production, cultures (100–150 cells/mm2) were washed 2
times with Hank’s salt solution and incubated in serum-free DMEM.
Three days later, the medium was removed, filtered (Nalgene
filters, 0.45 μm) and stored at −20°C.
Immunocytochemistry
OPCs were plated onto PDL/fibronectin-coated
coverslips in 35-mm dishes at a density of 5×104
cells/coverslip (12 mm). Oligodendrocytes (OLs) and type-2
astrocytes were induced from OPCs as previously described (10). To identify the purity of OPCs, to
detect the expression of several receptors in OPCs and to confirm
the differentiation potential of OPCs, OPCs as well as the induced
OLs and type-2 astrocytes cultured on coverslips were rinsed in PBS
and fixed with 4% paraformaldehyde (PFA) in PBS for 20 min at room
temperature (RT). After 3 rinses in PBS (10 min each), the cells
were incubated with 10% normal goat serum (NGS) in PBS in the
presence (for intracellular antigens) or absence (for surface
markers) of 0.3% Triton X-100 for 1 h at RT and then with one of
the monoclonal primary antibodies against A2B5 (1:100), and NG2
(1:100), the markers for OPCs, Rip (1:100) (Millipore, Billerica,
MA, USA), the markers for oligodendrocytes, glial fibrillary acidic
protein (GFAP; 1:200; Sigma-Aldrich, St. Louis, MO, USA), the
marker for type-2 astrocytes, PDGFR (1:500; Abcam, Cambridge, MA,
USA), FGFR2 (1:500; Abcam) or IGF-1R (1:100; Millipore) overnight
at 4°C. On the second day, the cultures were incubated with
rhodamine- or fluorescein isothiocyanate (FITC)-conjugated
secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) for 1 h at 37°C. After staining, the coverslips
were rinsed and mounted with Gel/Mount aqueous mounting media
(Biomeda Corp., Foster City, CA, USA) containing Hoechst 33342, a
fluorescent nuclear dye (1 μg/ml;Sigma-Aldrich). The coverslips
were examined using an Olympus BX60 microscope. For cell counts, at
least 5 randomly selected fields with a total of >500 cells were
counted. In all experiments, primary antibody omission controls
were used to confirm the specificity of the immunofluorescence
labeling.
5-bromo-2′-deoxyuridine (BrdU)
incorporation assay
To assess proliferation of OPCs cultured under
B104CM, we used in situ BrdU incorporation assay. Briefly,
the cultured OPCs at passage 2 were seeded onto poly-L-lysine (200
μg/ml)-coated coverslips at a density of 3×104
cells/coverslip and cultured in growth medium with FGF and PDGF-AA.
After 24 h, both PDGF and bFGF were withdrawn from the OPC-medium
and the cells were cultured another 24 h. Then, different
concentrations of B104CM (0, 10, 30, 50 and 100%) were added into
the culture medium. The OPCs were allowed to grow for 24 h, BrdU
(10 μM; Sigma-Aldrich) was added to the medium for 16 h, and cells
were fixed in 4% PFA for 15 min and washed twice with PBS. For
anti-BrdU and A2B5 immunofluorescence double-labeling, the cells
were treated with 1 N HCl for 40 min at 37°C to denature the DNA
prior to the use of primary (mouse anti-BrdU; 1:80; Dako, Santa
Barbara, CA, USA; mouse anti-A2B5 IgM mAb) and secondary antibody
(rhodamine-conjugated goat anti-mouse IgG, 1:50, Sigma-Aldrich;
FITC-conjugated donkey anti-mouse IgM, 1:200, Jackson
ImmunoResearch Lab, Inc.). The coverslips were examined and
photographed using an Olympus BX60 microscope.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was used to detect the expression of PDGF-AA
mRNA in B104 cells. Briefly, total-RNA from B104 cells was
extracted with the TRIzol (Invitrogen) according to the
manufacturer’s instructions. Two micrograms of total-RNA was first
reverse transcribed into cDNA, and then PCR was performed by a
routine method (11). PCR
products were analyzed on 1% agarose gel. β-actin was used as an
internal control. The sequences of specific primers for RT-PCR are
given in Table I.
 | Table I.Sequences of primers and PCR product
sizes used in RT-PCR. |
Table I.
Sequences of primers and PCR product
sizes used in RT-PCR.
| Genes | Primer
sequences | Size (bp) |
|---|
| PDGF-AA | Sense |
5′-TGTGCCCATCCGCAGGAAGAG-3′ | 225 |
| Antisense |
5′-TTGGCCACCTTGACACTGCG-3′ |
| bFGF | Sense |
5′-GGCTTCTTCCTGCGCATCCA-3′ | 353 |
| Antisense |
5′-GCTCTTAGCAGACATTGGAAGA-3′ |
| IGF-1 | Sense | 5′-GGGCA
TTGTGGATGAGTG-3′ | 246 |
| Antisense |
5′-CAAAGGATCTTGCGGTGA-3′ |
| β-actin | Sense |
5′-ATTGTAACCAACTGGGACG-3′ | 533 |
| Antisense |
5′-TTGCCGATAGTGATGACCT-3′ |
Measurement of PDGF-AA, bFGF and IGF-1
proteins in B104CM by enzyme-linked immunosorbent assay
(ELISA)
The levels of PDGF-AA, bFGF and IGF-1 proteins in
B104CM were quantified using commercially available ELISA kits
(R&D Systems). ELISA assay was performed according to the
manufacturer’s instructions. Briefly, assay diluents (100 μl) were
added to each well that had been pre-coated with a monoclonal
antibody specific for PDGF-AA, bFGF or IGF-1. This was followed by
the addition of 50 μl of control standard or sample/well and
incubation for 2 h at RT on a horizontal orbital microplate shaker
at 500 rpm. Each well was aspirated and subsequently washed with
wash buffer using an autowasher; this was repeated 3 times for a
total of 4 washes. After removing any remaining wash buffer, 200 μl
of PDGF-AA, bFGF or IGF-1 conjugate was added to each well and
plates were incubated for 2 h at RT on a shaker. Aspiration and
washes were repeated as described above. Substrate solution (200
μl) was added to each well and the plates were incubated for 30 min
at RT, protected from light. Stop solution (50 μl) was added to
each well. The optical density of each well was determined using a
microplate reader at 450 nm. PDGF-AA, bFGF or IGF-1 concentrations
of samples were determined from the optical densities in relation
to standard experimental curves. No interference and no cross
reactivity was expected based on the manufacturer’s instructions.
Each sample was measured three times and the mean level of each
measurement was used for analysis.
Treatment of OPCs with the inhibitors of
growth factor signals
To identify the roles of PDGF-AA, bFGF and IGF-1 in
B104CM-induced OPC proliferation, the OPC cultures were pretreated
with AG1295 (a specific inhibitor of the PDGFR signal pathway; 5
μM; R&D Systems), PD173074 (a specific inhibitor of the bFGFR
signal pathway; 2 μM; Sigma-Aldrich) or AG1204 (a specific
inhibitor of the IGFR signal pathway; 5 μM; Calbiochem, La Jolla,
CA, USA) for 45 min before adding of B104CM-contained OPC-growth
medium each time.
Statistical analysis
Data are presented as the mean ± standard deviation
of the mean (SD). One-way analysis of variance (ANOVA) with post
hoc Tukey’s t-test was used to determine statistical significance.
P-value <0.05 was considered to indicate statistically
significant differences.
Results
Cultivation and identification of
OPCs
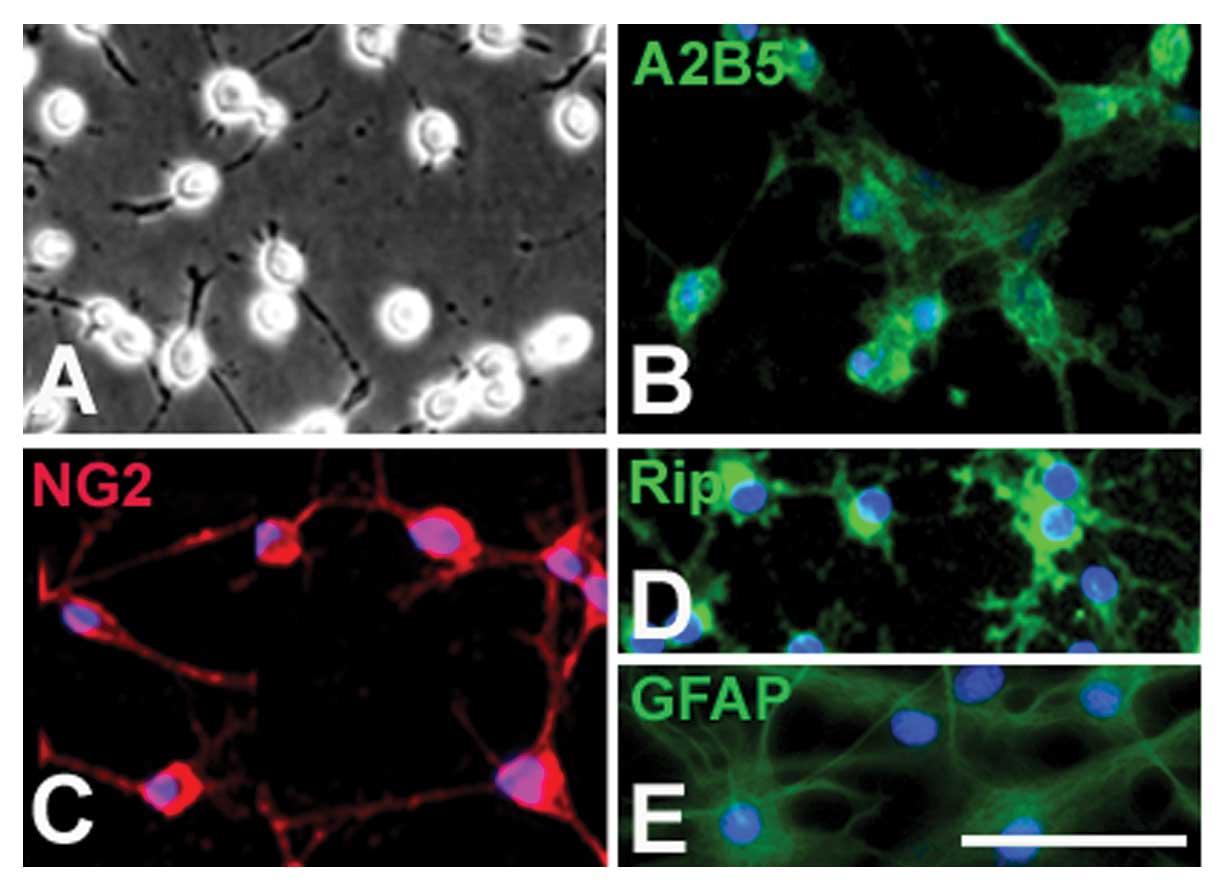
The OPCs displayed typical bipolar or tri-polar
morphology (Fig. 1A) and
expressed A2B5 (Fig. 1B) and
PDGFR (Fig. 1C), the markers of
OPCs. When cultured in the differentiation medium (without PDGF-AA
and bFGF) for 5 days, these cells displayed a multi-polar
morphology and the majority expressed oligodendrocyte-specific
marker RIP (Fig. 1D). However,
when OPCs were cultured in the presence of 10% FBS, almost all
displayed the typical process-bearing morphology of astrocytes and
expressed GFAP (Fig. 1E).
Proliferation of OPCs induced by
B104CM
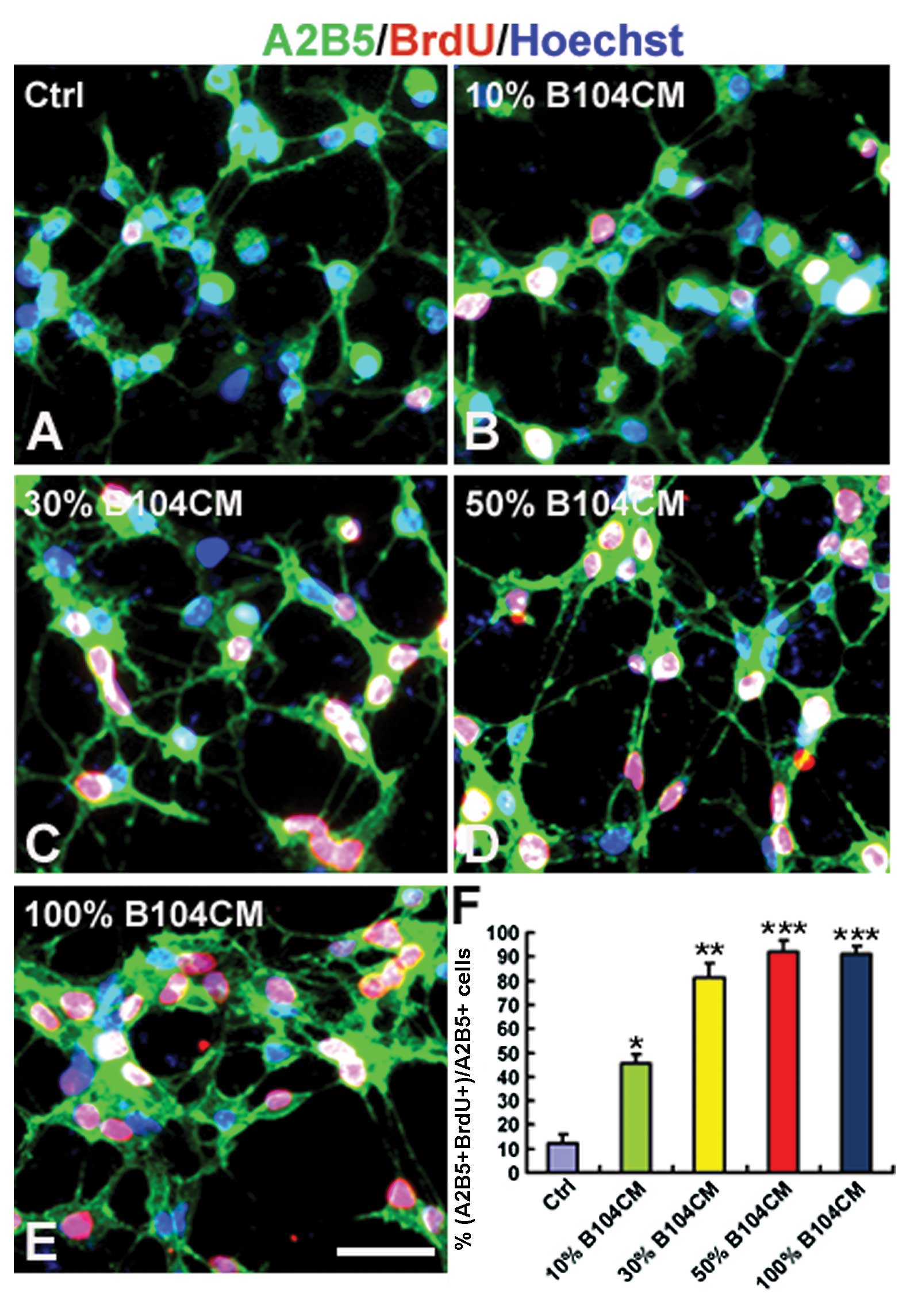
The effect of B104CM on the proliferation of OPCs
was determined using BrdU incorporation assay. We counted the
number of BrdU-positive OPCs cultured in the control medium and
different concentrations of B104CM (Fig. 2A–F). The result showed that the
percentage of BrdU-positive cells was significantly increased in
A2B5-positive OPCs cultured in B104CM of 10% (45.28±3.91%,
P<0.05), 30% (81.45±5.69%, P<0.01) and 50% (91.92±4.52%,
P<0.001) and 100% (91.78±3.22%, P<0.001) compared to the
control group (12.24±3.76%). The proliferation of OPCs reached peak
values when cultured in 50% B104CM (Fig. 2D and F). This result strongly
suggests that B104CM is a strong promoter of OPC proliferation.
mRNA expression and protein
concentrations of PDGF-AA, bFGF and IGF-1 in B104 cells and B104CM,
respectively
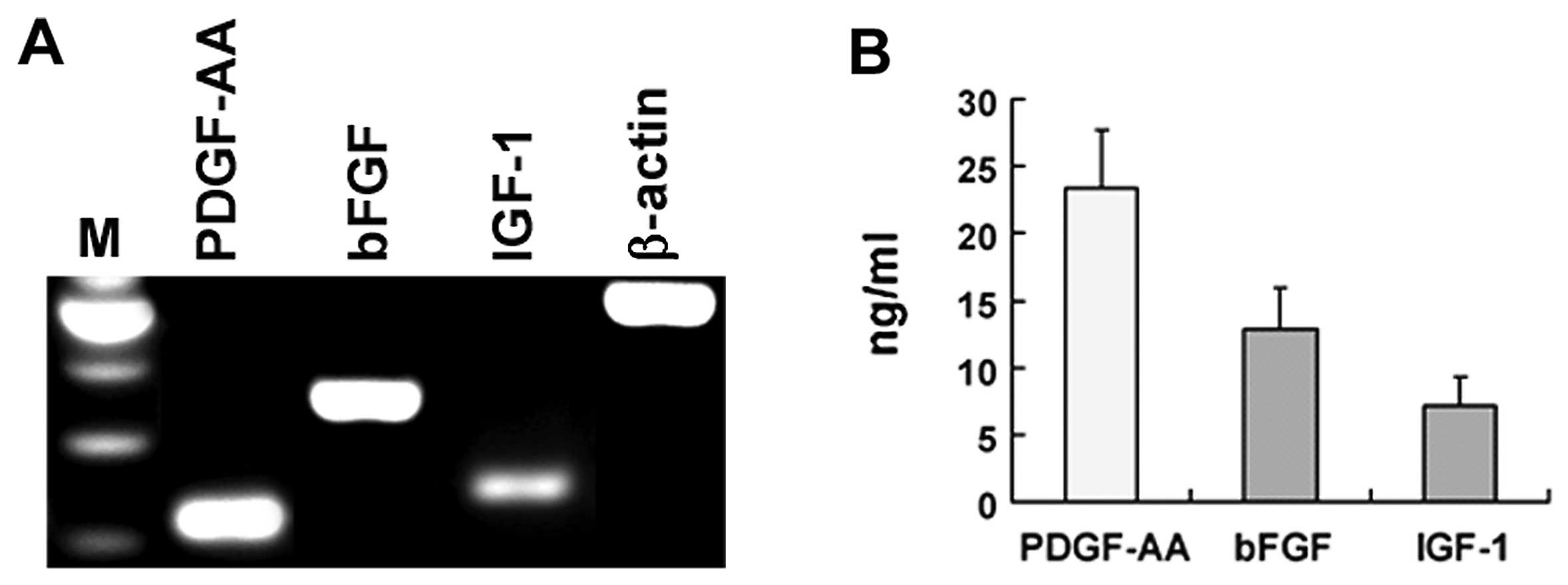
To determine the possibility that PDGF-AA, bFGF and
IGF-1 mediate B104CM-induced proliferation of OPC, we firstly
confirmed whether B104 cells express these 3 growth factors. Using
RT-PCR, we showed that the mRNA of PDGFAA, bFGF and IGF-1 was
expressed substantially in B104 cells (Fig. 3A). More importantly, we detected
these growth factors in protein level in B104CM by ELISA. The
concentration of PDGF-AA, bFGF and IGF-1 in B104CM (without being
concentrated) reached 23.42±4.28, 12.94±3.05 and 7.21±2.12 ng/ml,
respectively (Fig. 3B).
Expression of growth factor receptors in
OPCs
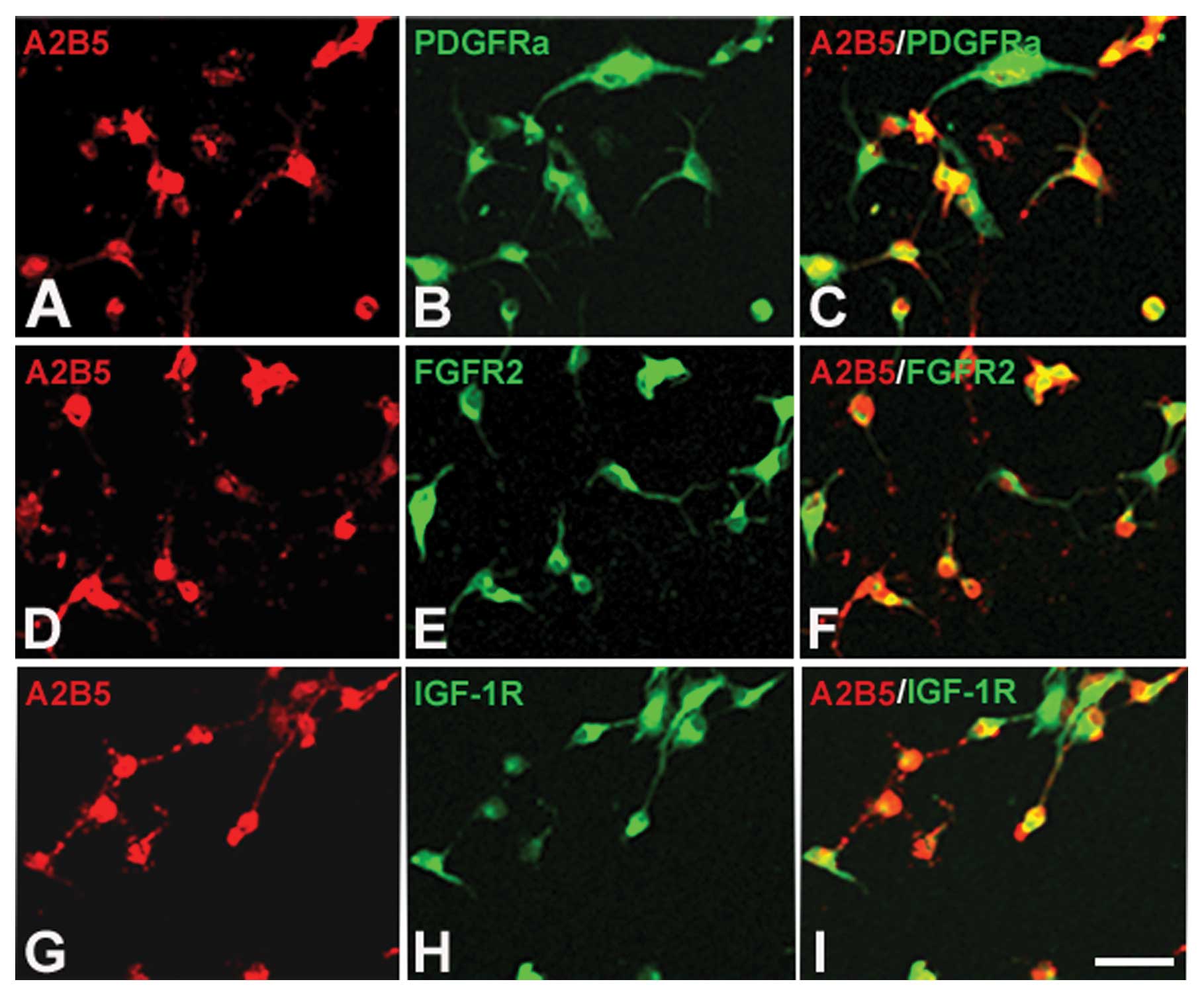
The functions of growth factors rely on binding to
their receptors. If they can induce OPCs to proliferate, the OPCs
should express their receptors. To determine whether the OPCs
cultured in our system express the receptors of PDGF-AA, bFGF and
IGF-1, we performed immunofluorescence staining. The results showed
that all 3 receptors, PDGFR, FGFR2 and IGF-1R, could be detected in
OPCs (Fig. 4A–I). This finding,
together with the result that these growth factors exist in B104CM,
indicates that these three growth factors contained in B104CM may
be factors which induce OPC proliferation.
PDGF-AA and bFGF are 2 key factors for
B104CM-induced OPC proliferation
To determine whether these three growth factors are
key factors for B104CM-induced OPC proliferation, we next used
AG1295 (a specific inhibitor of the PDGFR signal pathway), PD173074
(a specific inhibitor of the bFGFR signal pathway) and AG1204 (a
specific inhibitor of the IGFR signal pathway) to observe whether
OPC proliferation can be blocked or decreased following addition of
these inhibitors. The result showed that administration of both
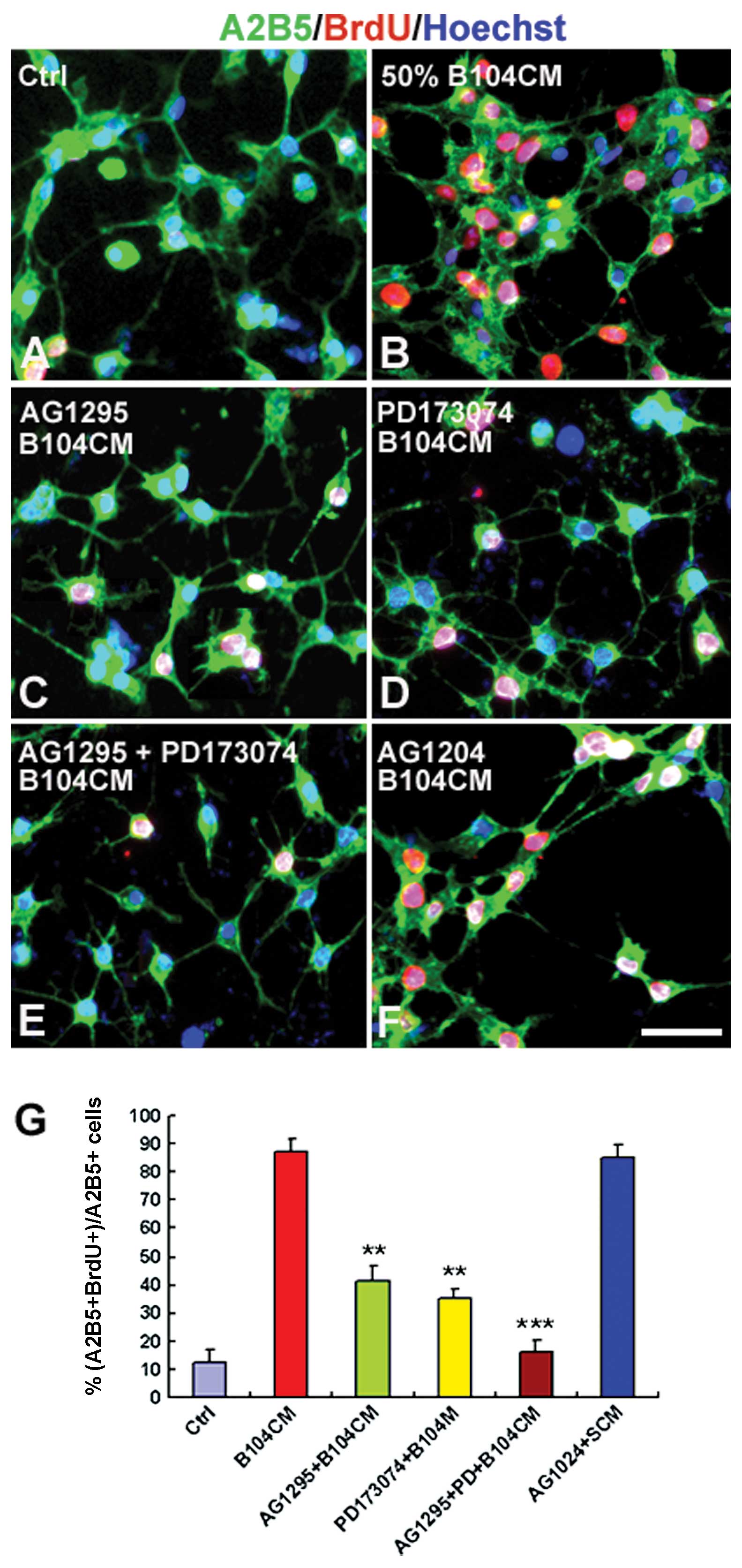
AG1295 and PD173074 prior to the addition of B104CM decreased
B104CM-induced OPC proliferation from 88.05±4.76% (Fig. 5B) to 41.62±5.29% (Fig. 5C and G) (P<0.01) and
35.25±3.28% (Fig. 5D and G)
(P<0.01), respectively. Moreover, the combination of AG1295 and
PD173074 mostly blocked all proliferation of OPCs induced by B104CM
(Fig. 5E and G) (P<0.001).
However, we did not observe a significant effect of AG1204 on OPC
proliferation (Fig. 5F and G)
(P>0.05). These results suggest that PDGF-AA and bFGF in B104CM
are 2 key factors that induce OPCs to proliferate.
Discussion
Previous reports have confirmed that B104CM can
induce OPCs to expand when cultured in vitro (3), indicating that certain factors which
exist in B104CM induce OPC proliferation. However, which factors
within B104CM are key factors responsible for this induction has
yet to be clarified. To understand the mechanism by which B104CM
induces OPC proliferation, in the present study we investigated the
possible factors involved in B104CM-induced OPC proliferation.
Firstly, we isolated and cultured E15 rat spinal
cord-derived OPCs and confirmed that almost all were A2B5- and
NG2-postive cells and were therefore highly pure. The majority
differentiated into oligodendrocytes in the absence of serum and
nearly all differentiated into the type II astrocytes in the
presence of 10% serum when both PDGF-AA and bFGF were withdrawn.
These results demonstrated that these OPCs were characteristic.
Next, we examined the effect of B104CM on the proliferation of OPCs
using BrdU incorporation assay. Our results confirmed that B104CM
at several different concentrations significantly promoted
proliferation of OPCs and reached their peak values in the presence
of 50% B104CM.
It has been well established that PDGF-AA and bFGF
are important mitogens for the proliferation of OPCs (2,12).
Moreover, a previous study found that IGF-I also induces
oligodendrocyte progenitor proliferation in vitro (6). These findings raise the possibility
that PDGF-AA, bFGF and IGF-I could be potent candidates that
mediate B104CM-induced OPC proliferation. To clarify this
possibility, we firstly explored the mRNA expressions and protein
contents of these 3 proteins in B104 cells and B104CM,
respectively. As expected, we observed the mRNA expressions of all
in B104 cells and their protein contents in B104CM prepared from
B104 cells. The functions of growth factors rely on binding to
their receptors (13). Our
results demonstrated that OPCs expressed PDGFR, FGFR2 and IGF-IR,
which further increases the possibility that these growth factors
are key factors in B104CM-induced OPC proliferation.
To investigate whether these 3 growth factors
mediate B104CM-induced OPC proliferation, we blocked the functions
of these 3 factors by treatment of the specific inhibitors. AG1295
is a specific inhibitor of the PDGFR signal pathway (14–16). Our results showed that AG1295
markedly decreased OPC proliferation induced by B104CM. PD173074 is
a selective inhibitor of the bFGFR signal pathway (17). We also confirmed that PD173074
significantly reduced OPC proliferation. Markedly, the combination
of AG1295 and PD173074 mostly blocked all proliferation of
B104CM-induced OPC proliferation, suggesting that PDGF-AA and bFGF
in B104CM are key factors that induce OPCs to proliferate. However,
we did not observe evident change following administration of
AG1204, a specific inhibitor of the IGFR signal pathway (18), although it has been reported that
IGF-I also induces oligodendrocyte progenitor proliferation in
vitro (6). We consider the
reason behind this may be a low concentration of IGF-I in B104CM,
which is not enough to induce OPC proliferation. Although PDGF-AA
and bFGF were confirmed to mediate B104CM-induced OPC
proliferation, it must be noted that there may still be other
components which are also involved in instructing OPC proliferation
within B104CM.
In conclusion, the present study has provided
convincing evidence to suggest that PDGF-AA and bFGF contained in
B104CM serve as the key inducing factors that instruct OPC
proliferation in vitro. Identifying these molecules
contributes to understanding the mechanism of B104CM-induced OPC
proliferation.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 81071268 and
81171465), the Science and Technological Fund of Anhui Province for
Outstanding Youth (no. 10040606Y13), the Key Project of Chinese
Ministry of Education (no. 210103), and a grant from the Advanced
Programs of Anhui province academic and technical leader and
candidates.
References
|
1.
|
NP PringleWP YuS GuthrieDetermination of
neuroepithelial cell fate: induction of the oligodendrocyte lineage
by ventral midline cells and sonic hedgehogDev
Biol1773042199610.1006/dbio.1996.01428660874
|
|
2.
|
MC RaffRH MillerM NobleA glial progenitor
cell that develops in vitro into an astrocyte or an oligodendrocyte
depending on culture
mediumNature303390396198310.1038/303390a06304520
|
|
3.
|
JC LouisE MagalD MuirCG-4, a new
bipotential glial cell line from rat brain, is capable of
differentiating in vitro into either mature oligodendrocytes or
type-2 astrocytesJ Neurosci
Res31193204199210.1002/jnr.4903101251613821
|
|
4.
|
M NobleSC BarnettO BoglerControl of
division and differentiation in oligodendrocyte-type-2 astrocyte
progenitor cellsCiba Found Symp15022724319902373025
|
|
5.
|
DG TangYM TokumotoMC RaffLong-term culture
of purified postnatal oligodendrocyte precursor cells. Evidence for
an intrinsic maturation program that plays out over monthsJ Cell
Biol148971984200010.1083/jcb.148.5.971
|
|
6.
|
QL CuiG AlmazanIGF-I-induced
oligodendrocyte progenitor proliferation requires PI3K/Akt,
MEK/ERK, and Src-like tyrosine kinasesJ
Neurochem100148014932007
|
|
7.
|
M Mayer-ProschelAJ KalyaniT MujtabaMS
RaoIsolation of lineage-restricted neuronal precursors from
multipotent neuroepithelial stem
cellsNeuron19773785199710.1016/S0896-6273(00)80960-59354325
|
|
8.
|
T MujtabaDR PiperA
KalyaniLineage-restricted neural precursors can be isolated from
both the mouse neural tube and cultured ES cellsDev
Biol214113127199910.1006/dbio.1999.941810491261
|
|
9.
|
Q CaoXM XuWH DevriesFunctional recovery in
traumatic spinal cord injury after transplantation of
multineurotrophin-expressing glial-restricted precursor cellsJ
Neurosci2569476957200510.1523/JNEUROSCI.1065-05.2005
|
|
10.
|
J HuL DengX WangXM XuEffects of
extracellular matrix molecules on the growth properties of
oligodendrocyte progenitor cells in vitroJ Neurosci
Res8728542862200910.1002/jnr.2211119472225
|
|
11.
|
JG HuSL FuKH ZhangDifferential gene
expression in neural stem cells and oligodendrocyte precursor
cells: a cDNA microarray analysisJ Neurosci
Res78637646200410.1002/jnr.2031715499592
|
|
12.
|
K AsakuraSF HunterM RodriguezEffects of
transforming growth factor-beta and platelet-derived growth factor
on oligodendrocyte precursors: insights gained from a neuronal cell
lineJ
Neurochem6822812290199710.1046/j.1471-4159.1997.68062281.x9166720
|
|
13.
|
U McDermottRY AmesAJ
IafrateLigand-dependent platelet-derived growth factor receptor
(PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma
cells to PDGFR kinase inhibitorsCancer
Res6939373946200910.1158/0008-5472.CAN-08-4327
|
|
14.
|
S BanaiY WolfG GolombPDGF-receptor
tyrosine kinase blocker AG1295 selectively attenuates smooth muscle
cell growth in vitro and reduces neointimal formation after balloon
angioplasty in
swineCirculation9719601969199810.1161/01.CIR.97.19.1960
|
|
15.
|
JG HuYX WangHJ WangMS BaoZh WangX GeFC
WangJS ZhouHZ LüPDGF-AA mediates B104CM-induced oligodendrocyte
precursor cell differentiation of embryonic neural stem cells
through Erk, PI3K, and p38 signalingJ Mol
Neurosci46644653201121953009
|
|
16.
|
H HeA LevitzkiHJ ZhuPlatelet-derived
growth factor requires epidermal growth factor receptor to activate
p21-activated kinase family kinasesJ Biol
Chem2762674126744200110.1074/jbc.C10022920011356824
|
|
17.
|
OE PardoJ LatigoRE JefferyThe fibroblast
growth factor receptor inhibitor PD173074 blocks small cell lung
cancer growth in vitro and in vivoCancer
Res6986458651200910.1158/0008-5472.CAN-09-157619903855
|
|
18.
|
F LukY YuWR WalshJL YangIGF1R-targeted
therapy and its enhancement of doxorubicin chemosensitivity in
human osteosarcoma cell linesCancer
Invest29521532201110.3109/07357907.2011.60625221843050
|