Introduction
Evaluating the toxicity of combinations
endocrine-disrupting chemicals (EDCs) is one of the most important
toxicological issues. A previous study on the multiplicity of
xenobiotics found in the environment have demonstrated the need to
develop methods for evaluating the risk associated with different
chemical combinations (1). The
synergistic effects of EDCs have been previously reported (2); however, the mechanisms responsible
for these interactions remain elusive. An assessment of the hazards
associated with each of these chemicals alone has indicated that
they have negligible risks (3).
Nevertheless, the evaluation of each chemical individually does not
clarify its effect when used in combination. Understanding the
combined effects of EDCs may lead to an estimation of the hazards
that exist under physiological conditions (4).
Many environmental chemicals classified as EDCs have
been found to potentially disturb the endocrine system and organs
that respond to endocrine signals (5). Among these EDCs, bisphenol A (BPA),
4-nonylphenol (NP), 4-tert octylphenol (OP) and isobutylparaben
(IBP) are being produced and utilized at high levels, and are also
found in high levels in the environment. Furthermore, only very
limited data are available on the additive or synergistic
estrogenic effects of these compounds. BPA is widely used for the
manufacture of polycarbonate plastics and resins that are used as
linings for food and beverage containers, and as dental sealants.
Alkylphenolic compounds, including NP and OP, are components of
soaps, paints, herbicides and pesticides, and are also used as
additives in plastics (6).
Parabens are widely utilized as preservatives in food, cosmetics
and pharmaceutical products (7).
Although the estrogenic activity of EDCs alone is
weak, the combined exposure to EDCs or industrial chemicals can
induce additional burden to the body (8). Some of these compounds may have the
ability to generate genomic instability rather than estrogenicity
via non-classical pathways of the estrogen receptor (ER). For
example, BPA, NP and OP alter cell cycle kinetics, induce DNA
damage and produce telomeric associations (9). Previously, Roy et al
(8) indicated that alkylphenols,
including BPA are capable of producing mutations in the
mitochondrial or nuclear genome by inhibiting DNA replication. When
fish are exposed to alkylphenolic compounds, the expression of
R-ras genes (R-ras1, R-ras2 and R-ras3)
is induced (10). In addition,
the upregulation of N-ras in the gonads, intestine and liver
has been observed in hermaphrodite fish (K. marmoratus)
following exposure to BP, NP and OP (11). Other studies have found that BPA
and NP affect the central nervous system during embryonic
development (12,13). Moreover, exposure to BPA and NP
during the developmental stage results in a marked influence on
neuronal vulnerability and synaptogenesis through mechanisms other
than the ER-mediated pathway (14). Similar to many other
xenoestrogens, parabens can mimic the effects of endogenous
estrogen. Parabens have been shown to bind to ERs in the rodent
uterus and induce estrogen-regulated gene expression in yeast cells
(15). Furthermore, an
association between the use of cosmetics containing parabens and an
increased incidence of breast cancer in humans and animals has been
reported (16,17).
The additive or synergistic burden of estrogenicity
and genomic instability can produce more detrimental effects
compared to estrogenic action alone. The estrogenic effect of the
combination of 17β-estradiol (E2) and BPA has been shown to result
in higher vitellogenin contents than treatment with a single
treatment at identical concentrations in a time- and dose-dependent
manner in male Chinese loaches (18). The synergistic effect of
triiodothyronine plus BPA on growth hormone release is due to
post-translational regulation, and BPA can disrupt thyroid hormone
function in GH3 cells (19). In
our previous studies, the synergistic effects of BPA and OP
(20) or paraben (21) were evaluated. The results
demonstrated that the combined exposure to these chemicals
increases their synergistic estrogenic activities in vitro.
In addition, the presence of combinations of industrial chemicals
and parabens (e.g., Methyparaben and propylparaben in combination
with phthalates) was observed in the majority of body care
cosmetics (22). However, there
are very few reports on the additive or synergistic effects of BPA,
NP, OP and IBP in vitro or in vivo.
The gene that we used to measure the effects of EDCs
in the present study, encoding the cytosolic calcium-binding
protein, calbindin-D9k (CaBP-9k), has been extensively utilized for
detecting estrogenic compounds in vitro and in vivo
(23). CaBP-9k expression is
rapidly and highly induced by estrogenic compounds (e.g., parabens,
BPA, OP, NP, phthalates, methoxychlor, diethylstilbestrol and
genistein) both in vivo and in vitro, possibly
through an ER-mediated pathway (24–29). In the present study, we developed
an assay for evaluating the synergistic impact of the exposure to
combinations of EDCs on GH3 rat pituitary cells using the
CaBP-9k gene as an estrogenic biomarker.
Materials and methods
Reagents and chemicals
E2, BPA and NP were obtained from Sigma-Aldrich (St.
Louis, MO). OP was purchased from Fluka Chemie (Seoul, Korea). IBP
was obtained from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) and
fulvestrant was purchased from Tocris (Ellisville, MO). A 1 M stock
solution of each compound was prepared using 100% dimethyl
sulfoxide (DMSO; Sigma-Aldrich, Ayrshire, UK) as a solvent and
stored at −20°C to avoid contamination. All antibodies, including
ones specific for CaBP-9k (P-18), ERα (MC-20), progesterone
receptor (PR) (C-19), glyceraldehyde-3-phosphate dehydrogenase
(GAPDH; A-3), goat anti-rabbit IgG (sc-362292), and goat anti-mouse
IgG (sc-2005) were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA).
Cell culture and treatment
GH3 cells were purchased from the Korean Cell Line
Bank (Seoul, Korea) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco-BRL, Grand Island, NY) with 10% fetal bovine
serum (FBS; Gibco-BRL) plus penicillin/streptomycin (Gibco-BRL).
The cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2 and were passaged every 7 days using
0.25% trypsin with 1 mM EDTA. In order to mitigate the effect of
endogenous steroids, cells were cultured in phenol red-free medium
containing 5% (v/v) charcoal dextran-stripped serum (DMEM-5% CD)
for 7 days as previously described (30). The experimental treatments (DMSO
as the negative control; 10−9 and 10−8 M E2
as the positive control; 10−7, 10−6 and
10−5 M of BPA, NP, OP and IBP alone or in various
combinations with equivalent concentrations of each compound) were
performed in triplicate. The cells were harvested at a single
end-point (24 h after treatment) to measure mRNA and protein
levels.
To examine the mechanism of CaBP-9k induction by
these EDCs, the cells were pre-treated with 10−7 M
fulvestrant for 30 min prior to EDC exposure as previously
described (30). Following
treatment with fulvestrant, the cells were exposed to a high dose
(10−5 M) of BPA, NP, OP and IBP alone or combinations of
these compounds (BPA + NP, BPA + NP + OP and BPA + NP + IBP).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
For RT-PCR analysis, GH3 cells were grown in 6-well
plates (Nunc, Roskilde, Denmark), and stimulated with the chemicals
(alone or in combination) 24 h later. The cells were harvested by
trypsinization and washed twice in Dulbecco’s phosphate-buffered
saline (DPBS; Gibco-BRL). Total RNA was isolated using TRI reagent
(Ambion, Austin, TX) according to the manufacturer’s instructions.
Complementary DNA (cDNA) was then generated with M-MLV reverse
transcriptase (Invitrogen, Carlsbad, CA) and 9-mer random primers
(Takara Bio, Inc., Shiga, Japan). cDNA (1 μl) was used for PCR at
standard conditions: denaturation at 95°C for 30 sec, annealing at
55°C for 30 sec, and extension at 72°C for 1 min. The following
primers were used: cytochrome c oxidase subunit 1
(1A) forward, 5′-CCA GGG TTT GGA ATT ATT TC-3′ and reverse,
5′-GAA GAT AAA CCC TAA GGC TC-3′; CaBP-9k forward, 5′-AAG
AGC ATT TTT CAA AAA TA-3′ and reverse, 5′-GTC TCA GAA TTT GCT TTA
TT-3′; PR forward, 5′-CAC AGG AGT TTG TCA AGG TC-3′ and
reverse, 5′-GGG ATT GGA TGA ACG TAT TC-3′; and ERα forward,
5′-GAC TTG AAT CTC CAC GAT CA-3′ and reverse, 5′-CTT CAA GGT GCT
GGA TAG AA-3′. The PCR products (8 μl) were separated on a 2%
agarose gel and stained with ethidium bromide. The gel was
photographed, scanned and analyzed using the Quantity One program
(Gel Doc EQ; Bio-Rad, Hercules, CA). The housekeeping 1A
gene was used for normalization. Data are shown as the average ±
SEM of 3 independent experiments.
Western blot analysis
Following treatment with the EDCs, the cells were
rinsed twice with DPBS solution. Protein samples were extracted
with Pro-prep solution (Intron Biotechnology, Seoul, Korea)
following the manufacturer’s instructions, and the total protein
concentration of the supernatant was determined by a BCA protein
assay (Pierce Chemical Co., Rockford, IL). Equal amounts of
proteins were used for western blot analysis. Briefly, 30 μg of
cytosolic protein were subjected to electrophoresis on 7.5 and 12%
SDS-PAGE gels. The separated proteins were transferred onto
nitrocellulose membranes (Millipore, Bedford, MA). The membranes
were washed twice with PBS containing 0.05% Tween-20 (PBS-T) and
then incubated in blocking buffer (5% non-fat milk; Difco, Franklin
Lakes, NJ) for 2 h at room temperature. The membranes were then
incubated with primary antibodies (anti-CaBP-9k, 1:500; anti-ERα,
1:500; anti-PR, 1:500; and anti-GAPDH, 1:2,000) overnight at 4°C.
Incubation with secondary antibodies (diluted 1:3,000) was
performed at room temperature for 1 h. Antibody-bound proteins were
visualized with the ECL chemiluminescent system (Amersham Pharmacia
Biotech, Arlington, VA) and exposed to CP-BU NEW X-ray film (Agfa
HealthCare NV, Mortsel, Belgium). Band intensities were measured
using a molecular analysis program (Gel Doc 1000, version 1.5;
Bio-Rad) and normalized to GAPDH levels as previously described
(30).
Statistical analyses
Data were calculated as a percentage of the vehicle
control (DMSO) and expressed as the means ± standard error of the
mean (SEM) of a single experiment performed in triplicate.
Statistical significance was determined using one-way ANOVA
followed by post hoc analysis (Tukey’s range test). P-values
<0.05 were considered to indicate statistically significant
differences.
Results
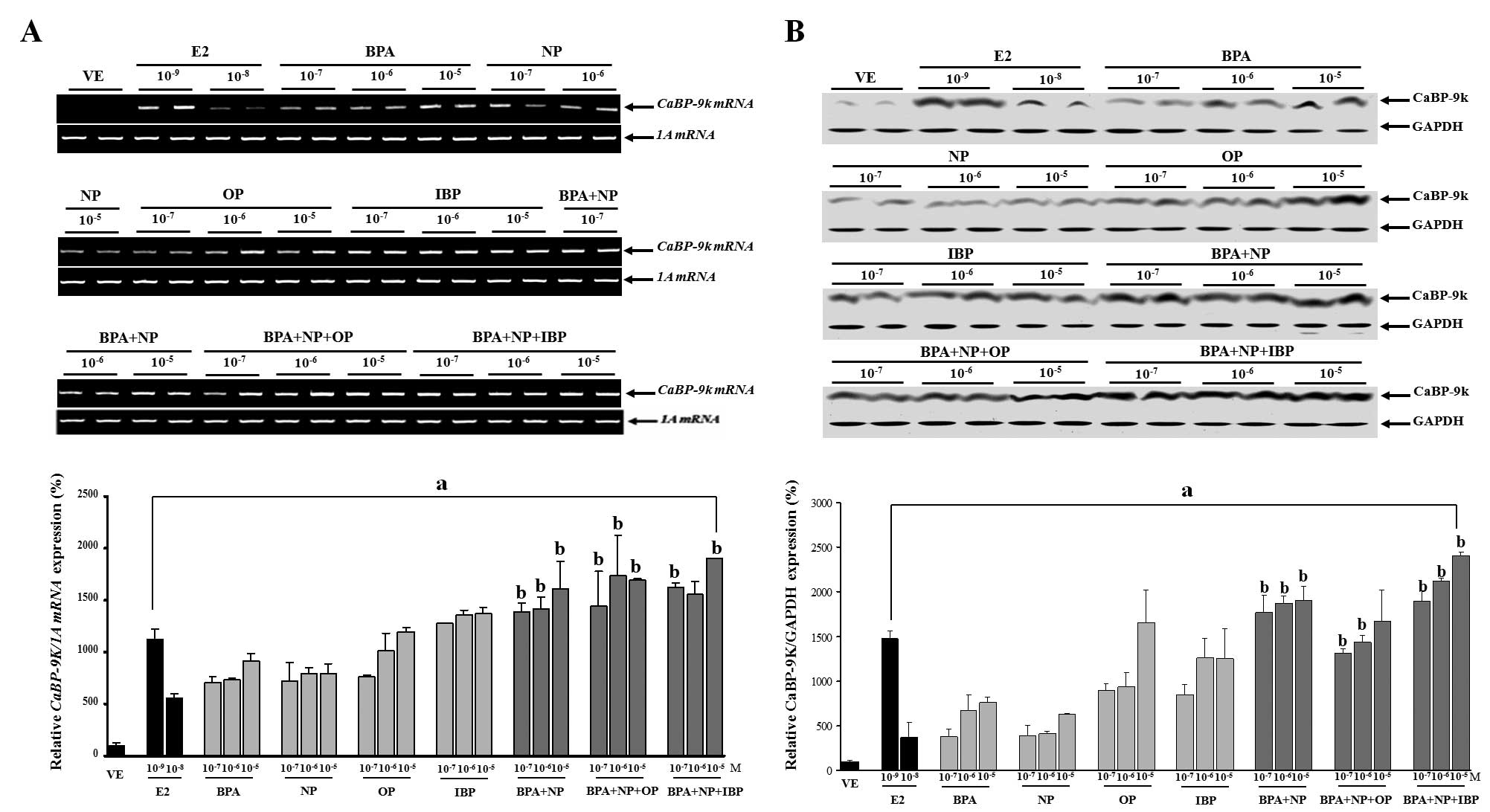
Combined effects of BPA, NP, OP and IBP
on CaBP-9k mRNA and protein expression in GH3 cells
EDCs can interfere with hormone signaling through
various mechanisms. Some of these mechanisms are interrelated in a
manner that may result in synergistic interactions. In this study,
we examined the hypothesis that combined exposure to chemicals
which function as hormone receptor agonists may result in greater
additive toxicity. We performed experiments by assessing the
effects of the chemicals (BPA, NP, OP and IBP) alone or in
combination on CaBP-9k mRNA and protein expression in GH3 cells.
Exposure of the cells to BPA, NP, OP and IBP alone or in
combination, at concentrations which were equivalent to those of
each chemical alone, significantly increased both CaBP-9k mRNA and
protein expression in a dose-dependent manner. In particular, doses
of the combined chemicals from 10−7 to 10−5 M
(BPA + NP), 10−7 and 10−6 M (BPA + NP + OP),
or 10−7 and 10−5 M (BPA + NP + IBP),
significantly increased CaBP-9k gene expression compared to
identical doses of each chemical alone.
These findings implied that these EDCs had a
synergistic impact on estrogenic activity in the cells. The results
from the experiments in which the cells were exposed to the single
or combined treatment of BPA, NP, OP and IBP are presented in
Fig. 1.
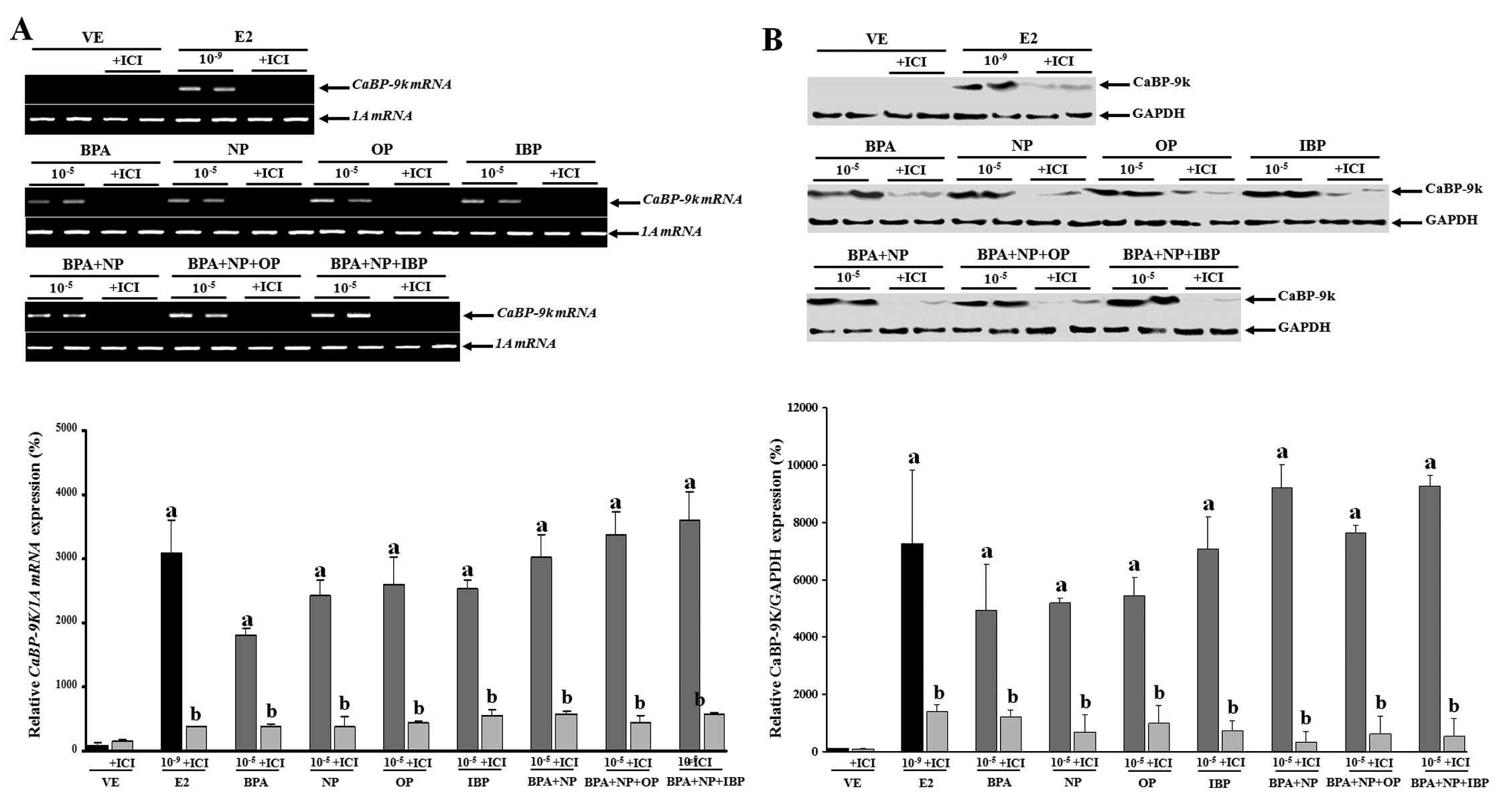
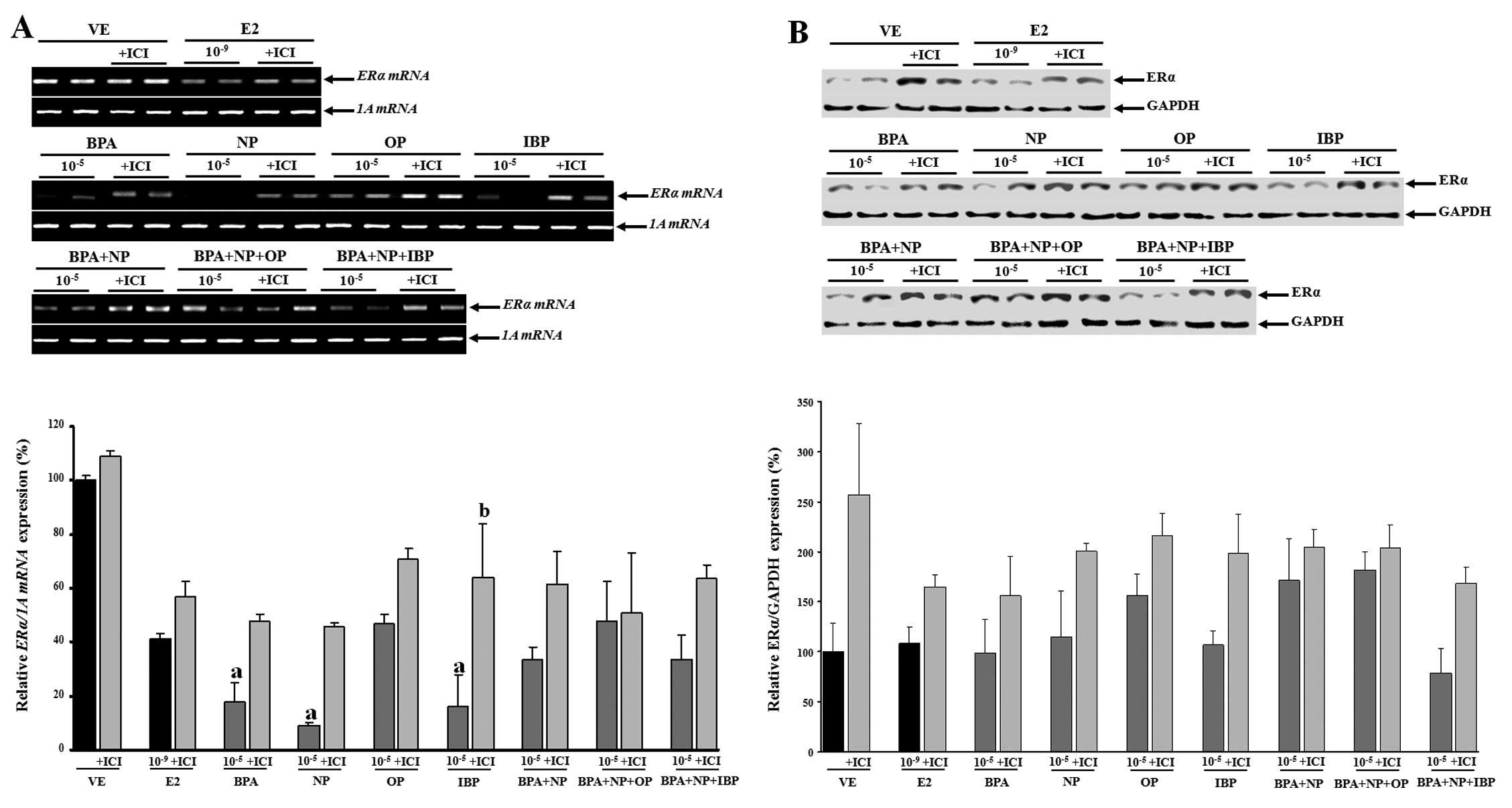
Based on these findings, the cells were then treated
with a high concentration (10−5 M) of the chemicals
alone or in combination with or without fulvestrant (an
anti-estrogen compound) to examine the mechanism underlying the
synergistic effects of the examined chemicals (Fig. 2). The inhibition of CaBP-9k mRNA
and protein expression following treatment with fulvestrant prior
to treatment with the compounds (alone or in combination) was
observed. These data suggest that the biological effects of BPA,
NP, OP and IBP (alone or in combination) on CaBP-9k gene
expression may involve an ER-mediated pathway in the GH3 cells.
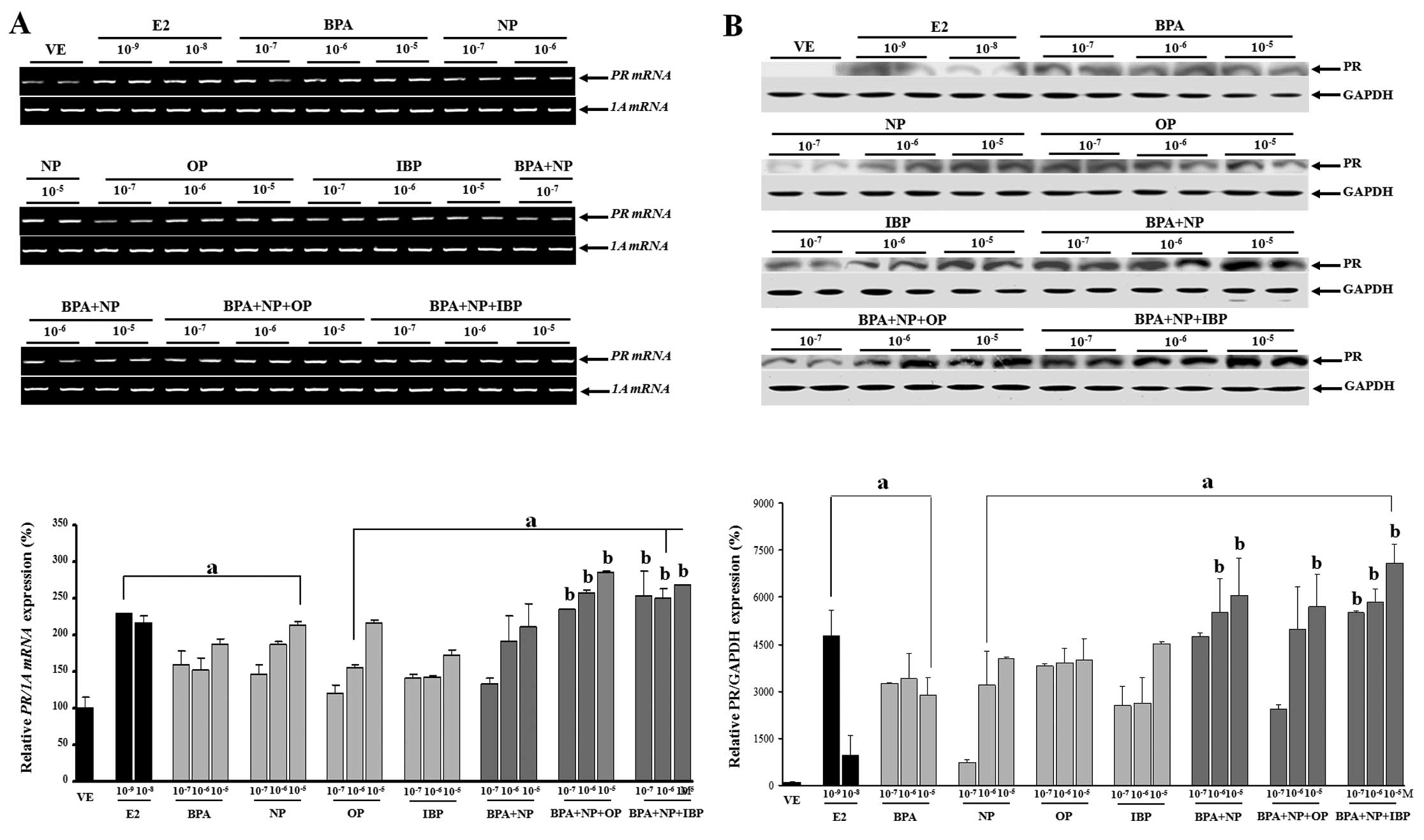
Combined effects of BPA, NP, OP and IBP
on PR mRNA and protein expression in GH3 cells
The effects of the single or combination treatment
with EDCs (BPA, NP, OP and IBP) on PR mRNA and protein expression
in the GH3 cells were evaluated. Four compounds elicited
significant effects on both PR transcription and translation.
Analysis of these results clearly showed that the EDC combinations
at all doses, from 10−7 to 10−5 M of BPA + NP
+ OP or BPA + NP + IBP increased PR mRNA expression compared to
each chemical alone. High doses of BPA + NP (10−6 and
10−5 M), BPA + NP + OP (10−5 M) and BPA + NP
+ IBP (10−7, 10−6 and 10−5 M) also
increased PR protein levels (P<0.05) compared to the exposure to
each chemical alone (Fig. 3). The
discrepancy observed between PR transcriptional and translational
regulation may be explained by mRNA stability and/or protein
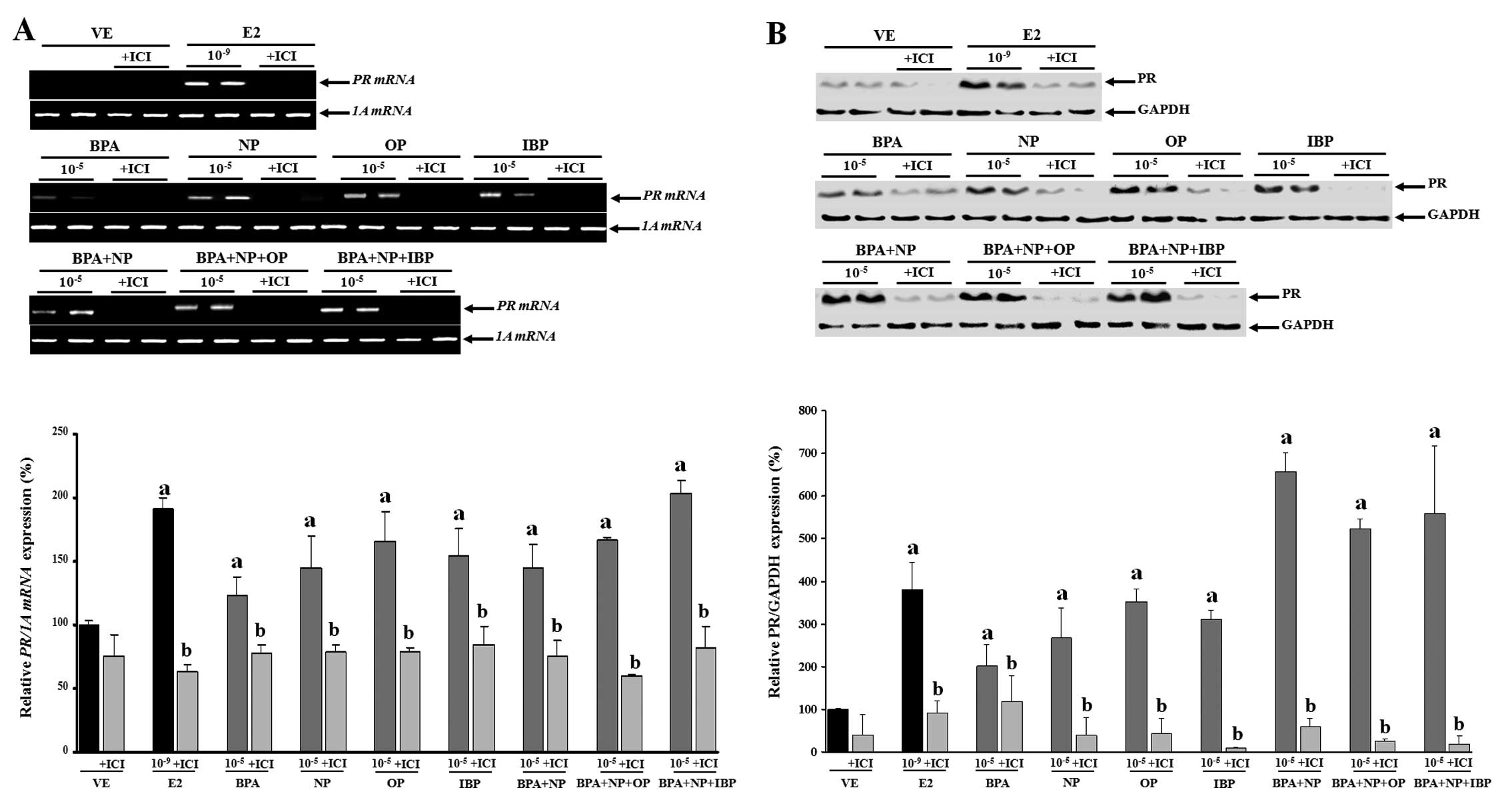
accumulation. On the contrary, pre-treatment with fulvestrant
completely blocked PR mRNA and protein expression (Fig. 4). These results were concomitant
with those observed for CaBP-9k, suggesting that the expression of
CaBP-9k and PR genes was regulated in response to the
combined effect of the estrogenic chemicals. In addition, the
biological effects of the single or combined administration of BPA,
NP, OP and IBP on CaBP-9k and PR expression may involve an
ER-mediated pathway in GH3 cells.
Combined effects of BPA, NP, OP and IBP
on ER mRNA and protein expression in GH3 cells
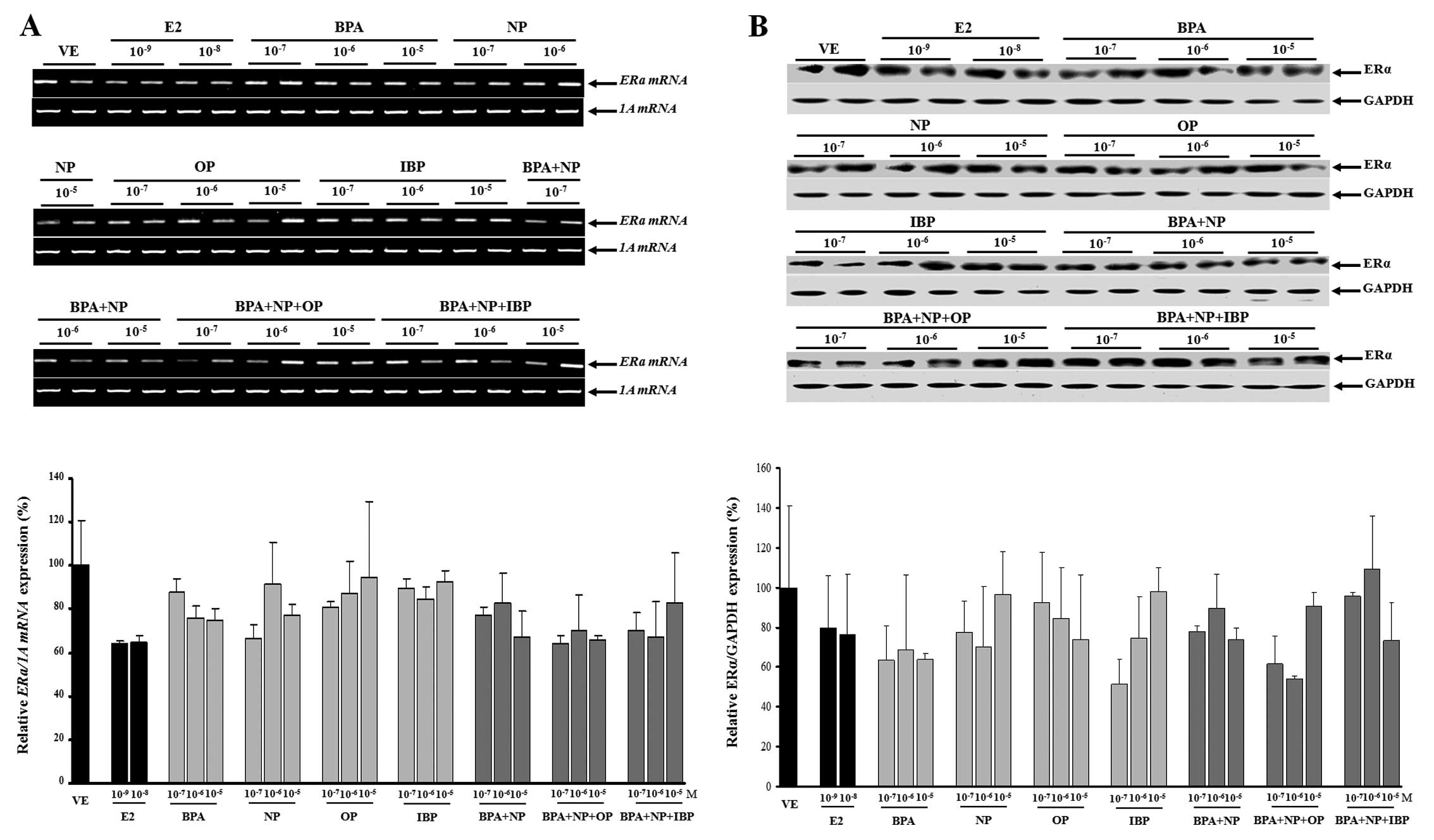
In this study, no evidence of a synergistic effect
on ERα mRNA and protein expression was observed when the
EDCs were combined (Fig. 5). The
expression levels of the ERα gene were upregulated when the
cells were treated with fulvestrant prior to being treated with
single or combined doses of EDCs (Fig. 6); however, these changes were not
statistically significant (except for treatment with
10−5 M IBP).
Discussion
There are a number of studies describing the potent
estrogenic effects of BPA, NP, OP and IBP in vitro and in
vivo (24,30,31). The relative levels of the
estrogenic activity of these chemicals have been reported as
E2>NP>OP>BPA (32). The
ability of BPA to bind ERs is estimated to be 1/1,000 of E2
(33) and IBP showed an
approximately 1,000-fold lower affinity than E2 (34). However, combinations of these EDCs
at low concentrations used in consumer products have still shown
estrogenic activities, and may be harmful to humans and animals
(35). In a previous study, the
combined effects of estrogens and xenoestrogens were shown to
participate in non-genomic responses. The available experimental
evidence showed that the effect of low-dose combinations may result
in biological perturbation (36).
Rajapakse et al (2)
examined a combination of 11 xenoestrogens at levels close to their
individual no observed effect concentrations (NOECs) and suggested
doubling the dose of E2. Combinations of weak xenoestrogens
(hydroxylated PCBs, benzophenones, parabenes, BPA and genistein) at
low levels that individually have undetectable effects are able to
create an impact on the actions of endogenous estrogens in body
fluids and tissues (35).
Furthermore, the combined estrogenic effects of EDCs are a risk
factor for breast cancer in women (37).
Humans are exposed to a variety and combinations of
natural and synthetic compounds that may influence various
endocrine processes. From a hazard and risk assessment point of
view, it is important to know whether exposure to combinations of
these compounds results in additive, antagonistic, or synergistic
actions (38). Very few studies
have addressed the estrogenic effects of simultaneous exposure to 2
or 3 different EDCs. Our previous studies indicated that combined
exposure to OP and IBP (20) or
multiple parabens (21) at low
concentrations synergistically affects the induction of
CaBP-9k gene expression via an ER- or PR-dependent pathway.
In the present study, we expanded the scope of our research to
examine the synergistic effects of BPA, NP, OP and IBP on
estrogenic activity in a rat pituitary cell line. The estrogenic
effects of BPA + NP, BPA + NP + OP and BPA + NP + IBP combinations
on CaBP-9k mRNA and protein expression were dose-dependent and more
potent than those of the individual compounds administered at the
equivalent concentrations. An endogenous gene expression assay that
measures estrogen-induced alterations has been created and has been
used extensively to detect the estrogenic activity of EDCs in
vitro and in vivo (39). In previous studies, CaBP-9k was
found to be expressed in a variety of mammalian tissues including
the uterus, placenta, intestine, kidney, pituitary gland and bone
(23,25,28,40). It has also been shown that CaBP-9k gene
expression is highly modulated by E2 in rats, mice and dogs
(24–26,30,31), suggesting that this gene may be used as a biomarker
for evaluating the estrogenicity of putative estrogenic compounds
(23). In the present study, our
results showed that there was a synergistic effect when 2 or 3
estrogenic chemicals (BPA, NP, OP and IBP) were administered in
combination. Based on our findings, we also suggest that the
CaBP-9k gene is a suitable biomarker to assess the variation
in the estrogenic activity of combined EDCs.
A previous study has demonstrated that a broad range
of EDCs is capable of interacting with ERs and inducing ER-mediated
responses (41). Cellular levels
of estrogens regulate development and growth by inducing
differentiation and cell proliferation classically through ERs by
stimulating the transcription of target genes. The disruption of ER
signaling pathways can contribute to adverse health effects, such
as developmental reproduction abnormalities and endocrine-related
cancers (42). To better
understand the molecular events promoted by the administration of a
combination of EDCs in this study, fulvestrant was used to examine
the potential involvement of ER signaling in the individual or
combined action of the compounds. The levels of CaBP-9k mRNA and
protein were significantly increased in a synergistic manner,
whereas pre-treatment with fulvestrant completely attenuated the
single or combined effects on CaBP-9k gene expression. Of
note, we found that the patterns of PR gene expression were
similar to those of CaBP-9k in response to individual and
combined chemical exposure. Pre-treatment with fulvestrant
completely blocked PR expression. These results suggest that
the single and combined effects of BPA, NP, OP and IBP require an
ER-mediated signaling pathway. Although there were fluctuations in
ERα mRNA and protein expression levels after treatment with these
EDCs, no synergistic effect compared to the vehicle or each
individual chemical was observed. In addition, the levels of
ERα gene expression were not altered when the cells were
pre-treated with fulvestrant.
In a previous study, treatment with industrial
compounds (BPA, NP and OP) increased the expression of PR
mRNA in the frontal cortex of adult ovariectomized rats (43). In CD-1 mouse uterus, exposure to
25 and 250 ng BPA/kg body weight/ day resulted in increased PR
expression in the endometrium and subepithelial stroma (44). Okubo et al (45) found that MCF-7 cells treated with
IBP exhibited a decrease in ERα gene expression while PR
expression was enhanced. In addition, observing the involvement of
ERs in paraben-induced responses has increased the understanding of
the mechanism(s) underlying molecular events promoted by estrogenic
compounds (25). Other studies
have indicated that CaBP-9k expression is responsive to E2 via ER
signaling in the rat uterus, ovary, and GH3 cells in which ERα, ERβ
and PR are expressed (21,25,46).
In conclusion, the results of the present study
provide evidence that combinations of BPA, NP, OP and IBP have
synergistic effects on estrogenic activity in GH3 cells. In
addition, this synergistic activity of the EDCs is induced via an
ER-mediated signaling pathway. We also suggest that the
CaBP-9k gene is an appropriate biomarker for evaluating the
synergistic actions of EDCs in vitro. However, studies with
wider concentration ranges and multiple combinations of EDCs in
vivo and in vitro are warranted to determine the actual
profile of xenoestrogenic activities.
Acknowledgements
This study was supported by a grant
(12182KFDA638) from the Korea Food and Drug Administration.
T.T.B.V. received funding from the Vietnam National Foundation for
Science and Technology Development (NAFOSTED) no.
106.06-2011.33.
References
|
1.
|
L SehulsterRY ChinnCDC; HICPACGuidelines
for environmental infection control in health-care facilities.
Recommendations of CDC and the Healthcare Infection Control
Practices Advisory Committee (HICPAC)MMWR Recomm Rep521422003
|
|
2.
|
N RajapakseE SilvaA KortenkampCombining
xenoestrogens at levels below individual no-observed-effect
concentrations dramatically enhances steroid hormone actionEnviron
Health Perspect110917921200210.1289/ehp.02110917
|
|
3.
|
S JinF YangT LiaoY HuiS WenY XuEnhanced
effects by mixtures of three estrogenic compounds at
environmentally relevant levels on development of Chinese rare
minnow (Gobiocypris rarus)Environ Toxicol
Pharmacol33277283201110.1016/j.etap.2011.12.01622240186
|
|
4.
|
JV BrianCA HarrisM ScholzeAccurate
prediction of the response of freshwater fish to a mixture of
estrogenic chemicalsEnviron Health
Perspect113721728200510.1289/ehp.759815929895
|
|
5.
|
S PoongothaiR RavikrishnanPB
MurthyEndocrine disruption and perspective human health
implications: a reviewInternet J Toxicol42200810.5580/263
|
|
6.
|
N OleaJP ArrebolaJ TaoufikiR
Fernández-ValadesR PradaN NaveaJM Molina-MolinaMF
FernandezAlkylphenols and bisphenol-A and its chlorinated
derivatives in adipose tissue of childrenEnviron
Toxicol1103692008
|
|
7.
|
MG SoniIG CarabinGA BurdockSafety
assessment of esters of p-hydroxybenzoic acid (parabens)Food Chem
Toxicol439851015200510.1016/j.fct.2005.01.02015833376
|
|
8.
|
D RoyJB ColerangleKP SinghIs exposure to
environmental or industrial endocrine disrupting estrogen-like
chemicals able to cause genomic instability?Front
Biosci3d913d92119989696883
|
|
9.
|
S TayamaY NakagawaK TayamaGenotoxic
effects of environmental estrogen-like compounds in CHO-K1
cellsMutat
Res649114125200810.1016/j.mrgentox.2007.08.00617913570
|
|
10.
|
JS RheeYM LeeS RaisuddinJS LeeExpression
of R-ras oncogenes in the hermaphroditic fish Kryptolebias
marmoratus, exposed to endocrine disrupting chemicalsComp
Biochem Physiol C Toxicol
Pharmacol149433439200910.1016/j.cbpc.2008.10.10219000778
|
|
11.
|
YM LeeS RaisuddinJS RheeJS KiIC KimJS
LeeModulatory effect of environmental endocrine disruptors on N-ras
oncogene expression in the hermaphroditic fish, Kryptolebias
marmoratusComp Biochem Physiol C Toxicol
Pharmacol147299305200810.1016/j.cbpc.2007.11.00618248853
|
|
12.
|
K BabaK OkadaT KinoshitaS ImaokaBisphenol
A disrupts Notch signaling by inhibiting gamma-secretase activity
and causes eye dysplasia of Xenopus laevisToxicol
Sci108344355200910.1093/toxsci/kfp02519218331
|
|
13.
|
C KudoK WadaT MasudaNonylphenol induces
the death of neural stem cells due to activation of the caspase
cascade and regulation of the cell cycleJ
Neurochem8814161423200410.1046/j.1471-4159.2003.02270.x15009642
|
|
14.
|
K SatoN MatsukiY OhnoK NakazawaEffects of
17beta-estradiol and xenoestrogens on the neuronal survival in an
organotypic hippocampal
cultureNeuroendocrinology76223234200210.1159/00006594812411739
|
|
15.
|
RM BlairH FangWS BranhamThe estrogen
receptor relative binding affinities of 188 natural and
xenochemicals: structural diversity of ligandsToxicol
Sci54138153200010.1093/toxsci/54.1.13810746941
|
|
16.
|
PW HarveyParabens, oestrogenicity,
underarm cosmetics and breast cancer: a perspective on a
hypothesisJ Appl Toxicol23285288200310.1002/jat.94612975767
|
|
17.
|
PD DarbreJR ByfordLE ShawRA HortonGS
PopeMJ SauerOestrogenic activity of isobutylparaben in vitro and in
vivoJ Appl Toxicol22219226200210.1002/jat.86012210538
|
|
18.
|
X LvQ ZhouM SongG JiangJ ShaoVitellogenic
responses of 17beta-estradiol and bisphenol A in male Chinese loach
(Misgurnus anguillicaudatus)Environ Toxicol
Pharmacol24155159200710.1016/j.etap.2007.04.00721783804
|
|
19.
|
M KanekoR OkadaK YamamotoBisphenol A acts
differently from and independently of thyroid hormone in
suppressing thyrotropin release from the bullfrog pituitaryGen Comp
Endocrinol155574580200810.1016/j.ygcen.2007.09.00917959175
|
|
20.
|
YR KimEM JungKC ChoiEB JeungSynergistic
effects of octylphenol and isobutyl paraben on the expression of
calbindin-D9k in GH3 rat pituitary cellsInt J Mol
Med29294302201222076563
|
|
21.
|
H YangTT NguyenBS AnKC ChoiEB
JeungSynergistic effects of parabens on the induction of
calbindin-D9k gene expression act via a progesterone
receptor-mediated pathway in GH3 cellsHum Exp
Toxicol31134144201210.1177/096032711142240222027501
|
|
22.
|
HY ShenHL JiangHL MaoG PanL ZhouYF
CaoSimultaneous determination of seven phthalates and four parabens
in cosmetic products using HPLC-DAD and GC-MS methodsJ Sep
Sci304854200710.1002/jssc.20060021517313141
|
|
23.
|
KC ChoiEB JeungMolecular mechanism of
regulation of the calcium-binding protein calbindin-D9k,
and its physiological role(s) in mammals: a review of current
researchJ Cell Mol
Med12409420200810.1111/j.1582-4934.2007.00209.x18182065
|
|
24.
|
BS AnSK KangJH ShinEB JeungStimulation of
calbindin-D(9k) mRNA expression in the rat uterus by octyl-phenol,
nonylphenol and bisphenolMol Cell
Endocrinol191177186200210.1016/S0303-7207(02)00042-412062901
|
|
25.
|
TT VoEB JeungAn evaluation of estrogenic
activity of parabens using uterine calbindin-d9k gene in an
immature rat modelToxicol
Sci1126877200910.1093/toxsci/kfp17619654335
|
|
26.
|
YK JiGS LeeKC ChoiEB
JeungAnti-progestogenic effect of flutamide on uterine expression
of calbindin-D9k mRNA and protein in immature miceReprod
Toxicol22694701200610.1016/j.reprotox.2006.04.01516777378
|
|
27.
|
JH ShinHJ MoonIH KangCalbindin-D9k mRNA
expression in the rat uterus following exposure to methoxychlor: a
comparison of oral and subcutaneous exposureJ Reprod
Dev53179188200710.1262/jrd.1805417077578
|
|
28.
|
GS LeeKC ChoiEB JeungGlucocorticoids
differentially regulate expression of duodenal and renal
calbindin-D9k through glucocorticoid receptor-mediated pathway in
mouse modelAm J Physiol Endocrinol Metab290E299E3072006
|
|
29.
|
GS LeeKC ChoiHJ KimEB JeungEffect of
genistein as a selective estrogen receptor beta agonist on the
expression of Calbindin-D9k in the uterus of immature ratsToxicol
Sci82451457200410.1093/toxsci/kfh29615456916
|
|
30.
|
TT VoEM JungKC ChoiFH YuEB JeungEstrogen
receptor alpha is involved in the induction of Calbindin-D(9k) and
progesterone receptor by parabens in GH3 cells: a biomarker gene
for screening
xenoestrogensSteroids76675681201110.1016/j.steroids.2011.03.00621473877
|
|
31.
|
VH DangTH NguyenKC ChoiEB JeungA
calcium-binding protein, calbindin-D9k, is regulated through an
estrogen-receptor mediated mechanism following xenoestrogen
exposure in the GH3 cell lineToxicol
Sci98408415200710.1093/toxsci/kfm120
|
|
32.
|
M SongY XuQ JiangMeasurement of estrogenic
activity in sediments from Haihe and Dagu River, ChinaEnviron
Int32676681200610.1016/j.envint.2006.03.00216624408
|
|
33.
|
S TakayanagiT TokunagaX LiuH OkadaA
MatsushimaY ShimohigashiEndocrine disruptor bisphenol A strongly
binds to human estrogen-related receptor gamma (ERRgamma) with high
constitutive activityToxicol
Lett16795105200610.1016/j.toxlet.2006.08.012
|
|
34.
|
TT VoYM YooKC ChoiEB JeungPotential
estrogenic effect(s) of parabens at the prepubertal stage of a
postnatal female rat modelReprod
Toxicol29306316201010.1016/j.reprotox.2010.01.01320132880
|
|
35.
|
E SilvaN RajapakseA KortenkampSomething
from ‘nothing’ - eight weak estrogenic chemicals combined at
concentrations below NOECs produce significant mixture
effectsEnviron Sci Technol36175117562002
|
|
36.
|
A KortenkampLow dose mixture effects of
endocrine disrupters: implications for risk assessment and
epidemiologyInt J
Androl31233240200810.1111/j.1365-2605.2007.00862.x18248400
|
|
37.
|
JM IbarluzeaMF FernándezL Santa-MarinaMF
Olea-SerranoAM RivasJJ AurrekoetxeaJ ExpósitoM LorenzoP TornéM
VillalobosV PedrazaAJ SascoN OleaBreast cancer risk and the
combined effect of environmental estrogensCancer Causes
Control15591600200410.1023/B:CACO.0000036167.51236.8615280638
|
|
38.
|
AG StewartJ CarterTowards the development
of a multidisciplinary understanding of the effects of toxic
chemical mixtures on healthEnviron Geochem
Health31239251200910.1007/s10653-008-9210-919023667
|
|
39.
|
BS AnKC ChoiSK KangWS HwangEB JeungNovel
Calbindin-D(9k) protein as a useful biomarker for environmental
estrogenic compounds in the uterus of immature ratsReprod
Toxicol17311319200310.1016/S0890-6238(03)00003-012759100
|
|
40.
|
P TinnanooruVH DangTH NguyenGS LeeKC
ChoiEB JeungEstrogen regulates the localization and expression of
calbindin-D9k in the pituitary gland of immature male rats via the
ERalpha–pathwayMol Cell Endocrinol2852633200818313836
|
|
41.
|
EC Bonefeld-JorgensenM LongMV HofmeisterAM
VinggaardEndocrine-disrupting potential of bisphenol A, bisphenol A
dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new
data and a brief reviewEnviron Health Perspect115Suppl
1S69S76200710.1289/ehp.936818174953
|
|
42.
|
M GhisariEC Bonefeld-JorgensenEffects of
plasticizers and their mixtures on estrogen receptor and thyroid
hormone functionsToxicol
Lett1896777200910.1016/j.toxlet.2009.05.00419463926
|
|
43.
|
T FunabashiTJ NakamuraF
Kimurap-Nonylphenol, 4-tert-octylphenol and bisphenol A increase
the expression of progesterone receptor mRNA in the frontal cortex
of adult ovariectomized ratsJ
Neuroendocrinol1699104200410.1111/j.0953-8194.2004.01136.x14763995
|
|
44.
|
CM MarkeyPR WadiaBS RubinC SonnenscheinAM
SotoLong-term effects of fetal exposure to low doses of the
xenoestrogen bisphenol-A in the female mouse genital tractBiol
Reprod7213441351200510.1095/biolreprod.104.03630115689538
|
|
45.
|
T OkuboY YokoyamaK KanoI KanoER-dependent
estrogenic activity of parabens assessed by proliferation of human
breast cancer MCF-7 cells and expression of ERalpha and PRFood Chem
Toxicol3912251232200110.1016/S0278-6915(01)00073-411696396
|
|
46.
|
GS LeeHJ KimYW JungKC ChoiEB JeungEstrogen
receptor alpha pathway is involved in the regulation of
Calbindin-D9k in the uterus of immature ratsToxicol
Sci84270277200510.1093/toxsci/kfi07215635152
|