Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide. Among all types of lung cancers, non-small
cell lung carcinoma (NSCLC) accounts for approximately 85% of all
lung cancer cases (1). As the
first-line chemotherapeutic agent in lung cancer, cisplatin, has
encountered a plateau due to its side effects and lack of
specificity (2,3). Therefore, it is particularly urgent
to discover methods by which to enhance the chemosensitivity of
cisplatin.
Inducible nitric oxide (NO) synthase (iNOS)
is a new promising cancer target gene involved in NO-mediated
antitumor effects (4–7). It was first identified and
characterized in cytokine-activated murine macrophages (5). The relatively lower expression of
the iNOS gene was observed in several types of human
cancers, where it is difficult to maintain high levels of NO for
long periods of time (6,8,9),
while full expression of the iNOS gene may generate high
concentrations of NO for prolonged periods of time (10). Generally, cytokine stimulation, NO
donor and iNOS gene transfer have been identified as the
main methods used to upregulate the expression of iNOS and
further produce high concentrations of NO in human cancer cells
(4). Among all known methods,
however, cytokine stimulation and NO donor may cause hypotension,
drug resistant, toxic and other side effects, while iNOS
gene transfer may essentially avoid the above side effects
(11,12). iNOS gene transfer has a
unique feature of a bystander effect, which would be an absolute
requirement to the future success of cancer gene therapy as a
contribution to the high efficiency of gene transfer (13,14).
The forced high level of NO was reported to have
antitumor activities (15). In
addition, clinical studies have demonstrated that overexpression of
the iNOS gene may increase the survival of colorectal,
ovarian and NSCLC patients (9,16,17). The apparent antitumor effects of a
high NO level generated by iNOS gene transfer have been
confirmed in several types of cancers including breast, colorectal,
prostate, ovarian, melanoma, kidney, thyroid cancer in vitro
and in vivo (7,18–24). Thus, high concentrations of NO
generated from the full expression of the iNOS gene may play
an important role in cancer treatment.
Notably, studies have also demonstrated that high
concentrations of NO generated from iNOS gene transfer,
cytokine stimulation or NO donor may enhance the cytotoxicity of
the chemotherapeutic drug cisplatin in RIF-1 tumors, ovarian
cancer, leukemia, prostate cancer, colon cancer cells and lung
fibroblasts (18,25–27). It is proposed that NO enhances
cisplatin toxicity through inhibition of the repair enzymes that
act on cisplatin-induced DNA damage (18), but the exact mechanisms of the
enhanced effects of iNOS gene therapy and cisplatin in human
cancers remain unclear. The antitumor effects of high levels of NO
generated from iNOS gene transfer combined with cisplatin in
lung cancer cells have yet to be reported.
Previous studies have demonstrated that the activity
of the iNOS gene was observed in lung adenocarcinoma
compared with normal tissues (28,29) and the iNOS gene expression
levels are moderate in NSCLC cells as well as in other cancer cells
(9). As the stages developed, the
expression of iNOS protein gradually decreased in NSCLC
cells. The intense expression of NOSs including the iNOS
gene seems to be a favorable prognostic sign in NSCLC patients.
Based on this, we hypothesize that delivering a high level of the
exogenous iNOS gene by gene therapy in lung cancer may
generate a large amount of NO and thus enhance the effects of
cisplatin in lung cancer treatment.
To identify the hypothesis, in the current study, we
first evaluated the effects of cationic liposome (LP)-mediated
iNOS gene transfection on enhancing low-dose
cisplatin-mediated antitumor effects in the human lung
adenocarcinoma A549 cell line in vitro. Based on the in
vitro results, we then aimed to demonstrate that intratumoral
delivery of the LP-pVAX-iNOS complex enhanced low-dose
cisplatin-mediated suppression of tumor growth. Meanwhile, systemic
delivery of the LP-pVAX-iNOS complex enhanced low-dose
cisplatin-mediated inhibition of experimental lung metastasis and
prolonged animal survival. Apoptosis was further detected in the
subcutaneous tumor tissues. Furthermore, we primarily detected
several cellular targets involved in NO- and/or cisplatin-mediated
signaling pathways during the antitumor procedure to understand the
molecular mechanism of the enhanced effects by western blotting.
This study may provide a new method for clinically effective
treatment of lung cancer.
Materials and methods
Cell cultures, chemotherapeutic drug and
animals
Human lung adenocarcinoma cell line A549 was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA) and was grown in RPMI-1640 medium (Gibco-BRL, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) at
37°C in a humidified 5% CO2 atmosphere. Cisplatin was
obtained from the West China Hospital pharmacy. Female athymic
BALB/c nude mice were purchased from the Shanghai Laboratory Animal
Centre (SLAC, Shanghai, China). The mice were housed in laminar
flow cabinets under specific pathogen-free conditions. All animal
experiments were performed in accordance with the institutional
guidelines established for animal care and use.
Construction of iNOS gene expression
vector and preparation
According to the iNOS cDNA coding sequence
(GeneBank serial no., BC130283.1), the CDS sequence of the
iNOS gene was cloned from iNOS-pCR4-TOPO plasmid
(Open Biosystems, USA) with a PrimeSTAR™ HS PCR kit (Takara,
Dalian, China) and connected to the pVAX plasmid vector (Invitrogen
Life Technologies, Carlsbad, CA, USA). Pure pVAX and
pVAX-iNOS plasmids were prepared using an EndoFree™ Plasmid
Giga kit (Qiagen, Chatsworth, CA, USA), measured for concentration
using a spectrophotometer and diluted to 1 μg/μl of DNA.
Preparation of plasmid LP-DNA complexes
for cell transfection and animal treatment
Preparations of plasmid LP-DNA complexes were
previously described (30).
Briefly, pVAX/pVAX-iNOS plasmid and LPs diluted in equal
volumes of RPMI-1640 were mixed to form LP-DNA complexes (LP-pVAX
and LP-pVAX-iNOS) according to their molecular weight ratio
(1:6). Cells were transfected with the complexes for 4 h and
replenished with RPMI-1640 medium supplemented with 10% FBS and
iNOS gene co-factor tetrahydrobiopterin (BH4)
(Sigma, St. Louis, MO, USA) (1×10−5 M) if cells were
transfected with LP-pVAX-iNOS and incubated for another 48 h
(18,19,21,22). The transfection efficiency was
evaluated by a parallel transfection with an equal amount of
enhanced green fluorescent protein-expressing plasmid vector
p-EGFP-N1 (Clontech, Beijing, China) in each of the cell lines.
For the animal experiments, pVAX/pVAX-iNOS
plasmid and LPs diluted in equal volume of 5% glucose were mixed to
form a final concentration of 20 μg DNA-4 mM LPs in 200 μl final
volume (weight ratio 1:7). Particle size and ζ-potential of plasmid
LP-DNA complexes were measured by a Zeta Nano series (Malvern
Instruments, Herrenberg, Germany) at room temperature. The average
particle size of the complexes was limited from 200 to 350 nm and
the average ζ-potential of the complexes was limited from 20 to 35
mV.
Cell viability assay
To assess the sensitivity of the A549 cells to
cisplatin, the 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide
(MTT) (Sigma) assay was used. Cells grown in 96-well plates were
transfected with LP-DNA complex using the method described above.
The transfection media were replaced with fresh medium containing
cisplatin with serial concentrations (0.0–15.0 μM) and incubated
for another 48 h. Twenty microliters of MTT in each well together
with 180 μl RPMI-1640 were added and incubated for another 4 h.
Absorbance at 490 nm was measured with a microplate reader
(Bio-Rad, Hercules, CA, USA). Both the IC50 and
IC20 values of cisplatin were calculated using the
Origin 8 software (OriginLab Corporation, Northampton, MA, USA;
www.OriginLab.com). To detect the inhibition of A549
cell proliferation in the different treatment groups, cells seeded
in 96-well plates were treated with LPs-DNA and/or an
IC20 dose level of cisplatin. The cell viabilities after
a 48-h treatment were quantified by MTT assay as described above.
IC20 dose level of cisplatin was used in all of the
following in vitro experiments.
Cell apoptosis assay
To determine the apoptosis rates of A549 cells
following different treatments, Hoechst 33258 staining and an
Annexin V-FITC Apoptosis Detection kit (KeyGEN, Nanjing, China) and
flow cytometry [fluorescence-activated cell sorting (FACS)] were
used according to the manufacturer’s instructions. A549 cells
following different treatments were stained with Hoechst 33258 as
previously described (31).
Additionally, cells with the same treatments were rinsed with
pre-chilled PBS 3 times, trypsinized with EDTA-free trypsin and
then rinsed with PBS supplemented with 2% BSA. Cells were then
labeled by Annexin V-PI reagent and analyzed with the aid of FACS
for cell apoptosis analysis.
Cell invasion and migration assays
Evaluation of the invasion and migration abilities
was performed using cell invasion and migration assays. After
treatment with LPs-DNA and/or low-dose cisplatin for 24 h, cells
were harvested and 4×104 cells of each treatment in RPMI
plus 1% FBS were replaced in the upper chamber. To assess cell
invasion, membranes of the Boyden cell system were coated with BD
Matrigel™ (all were from BD Biosciences, Franklin Lakes, NJ, USA).
The bottom chamber was filled with RPMI containing 10% FBS as a
chemoattractant. After being incubated for another 24 h, the
attached cells in the lower section of the chamber were stained
with 0.1% crystal violet solution (Sigma). The number of invading
cells was manually counted as the sum of 3 randomly selected fields
at a ×20 magnification. The same experimental design was used for
the migration experiments except that the membranes were not coated
with BD Matrigel; RPMI plus 1% FBS were placed in the lower chamber
of Millicell systems (Millipore, Billerica, MA, USA).
Animal studies
In vivo lung metastasis nude mouse
model
Lung metastatic tumors were established via tail
vein injection of 2×106 A549 cells in a volume of 200 μl
of RPMI-1640 into female BALB/c athymic nude mice (3–4 weeks old).
On Day 12 after cell injection, animals were randomly divided into
6 treatment groups (5 mice/group) including glucose, LP-pVAX,
cisplatin, LP-pVAX plus cisplatin, LP-pVAX-iNOS and
LP-pVAX-iNOS plus cisplatin groups. Gene therapy was
administered through tail vein injection at a dose of 20 μg/mouse
of the LP-DNA complexes. Twenty-four hours later and immediately
prior to i.p. injection of low-dose cisplatin (2 mg/kg/mouse), all
mice (including the controls) were injected i.p. with 200 μl of
10−3 M BH4 as previously described (18,19,21,22). Mice were treated every 3 days and
a total of 4 injections were administered to all mice. On the 14th
day after the last treatment, all mice were anesthetized and their
lungs were filled with India ink to count the number of metastases.
In addition, the same lung metastasis mouse model (5 mice/group)
was established to analyze the effect of the combination treatment
on mice survival. After the same administration, mice were fed
until all mice in the glucose group were sacrificed. Mice survival
curves were assessed according to the Kaplan-Meier method.
In vivo subcutaneous tumor nude mouse
model
A subcutaneous tumor nude mouse model was obtained
by intradermal injection with 5×106 A549 cells in a
volume of 100 μl of RPMI-1640 in the right flank of female BALB/c
athymic nude mice (5–6 weeks old) as previously described (32). When the tumor volume reached ~80
mm3, the same administration procedures for 6 treated
groups (5 mice/group) as described above were used in this
experiment except that mice were treated by the administration of
intratumoral injection at a dose of 20μg/mouse of the LPs-DNA.
Tumor size was measured with callipers in 3 dimensions twice every
week. The tumor volume was calculated using the following formula:
Volume (mm3) = 0.52 × length (mm) × width (mm) × width
(mm). When the average tumor volume reached ~1,000 mm3
or the tumors were necrotic, all mice were sacrificed and the
tumors were peeled off and fixed in formalin. Growth curves were
plotted for each group. Efficiency of the combination treatment was
also assessed by the time required for tumors to reach 3 times
their volume from the commencement of treatment.
Analysis of subcutaneous tumor cell
apoptosis and histology
To evaluate apoptosis, the fixed subcutaneous tumors
were evaluated by in situ TUNEL (terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling)
staining using an In Situ Cell Death Detection kit (Roche
Molecular Biochemicals, Indianapolis, IN, USA) according to the
manufacturer’s instructions (33). The number of apoptotic bodies was
counted from tumor tissues at ×200 magnification in 20 randomly
selected fields. The hematoxylin and eosin (H&E) double
staining was applied to detect the histology of the tumor tissue
and organic tissue paraffin sections as previously described
(34).
Western blot analysis
Lung cancer cells following different treatments
were collected and lysed in RIPA buffer supplemented with Protease
Inhibitor Cocktail Set I (Merck KGaA, Germany). Equal amounts of
lysate proteins were separated by electrophoresis on 10%
SDS-polyacrylamide gels, electrotransferred onto PVDF membranes
(Millipore) and probed with anti-iNOS, anti-MMP2 (Abcam,
Cambridge, MA, USA), anti-phosho-p53, anti-p53, anti-phospho-mTOR,
anti-mTOR (Cell Signaling Technology, Inc., Beverly, MA, USA) and
anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Protein bands were detected using an enhanced ECL system (GE
Healthcare Life Sciences, Piscataway, NJ, USA).
Statistic analysis
SPSS13.0 software was used. Each experiment was
performed at least 3 times. The data are expressed as the means ±
SD and one-way ANOVA and an unpaired Student’s t-test were used to
determine the significant differences of all the results. P<0.05
was considered to indicate a statistically significant
difference.
Results
High expression level of iNOS protein on
the sensitivity of A549 cells to cisplatin
In this study, the expression of iNOS protein
in the A549 cell line after LP-pVAX-iNOS transfection was
first detected by western blotting. The moderate expression level
of iNOS protein was demonstrated in A549 cells, while
LP-pVAX-iNOS transfection induced high expression of the
iNOS protein in A549 cells (Fig. 1A). To evaluate whether enforced
high expression of the iNOS gene sensitizes the response of
A549 cells to cisplatin, we analyzed the cell viability of the A549
cells following cisplatin treatment alone or the combination
treatment with LP-pVAX-iNOS transfection and cisplatin by
MTT assay. The IC50 value of cisplatin in the A549 cell
line was decreased from 8.80±1.95 μM in the cisplatin alone group
to 5.08±0.73 μM in the combination treatment group. Therefore, the
results suggest that exogenously enforced high expression of the
iNOS gene significantly enhances the sensitivity of A549
cells to cisplatin.
Enhanced proliferation inhibition
following the combination treatment of LP-pVAX-iNOS and low-dose
cisplatin
To examine whether the exogenously enforced high
expression of the iNOS gene enhances cisplatin-mediated cell
proliferation inhibition, the IC20 dose level (3.05±1.25
μM) of cisplatin in A549 cells was adopted in the following
experiments. The cell viability of A549 cells following different
treatments was assessed by MTT assay. There was >30 and 15% of
an average decrease in cell viability at 48 h in the combination
treatment group compared with the low-dose cisplatin alone or
iNOS gene alone groups (P<0.01) (Fig. 1B). The results indicated that the
exogenously enforced high expression of the iNOS gene
significantly enhances the proliferation inhibition of low-dose
cisplatin in A549 cells.
Enhanced induction of apoptosis following
combination treatment of LP-pVAX-iNOS and low-dose cisplatin
To evaluate whether the enforced high expression of
the iNOS gene is implicated in the reactivity of cells to
cisplatin-induced apoptosis, both Hoechst 33258 and Annexin V-PI
staining by flow cytometry were used. Nuclear condensation,
cleavage fragments and additional apoptotic bodies appeared in the
A549 cells following the combination treatment, while these were
rarely noted in the low-dose cisplatin alone or iNOS gene
alone treated groups (Fig. 1D).
Moreover, the results of Annexin V and PI double staining
demonstrated that the early apoptosis rate in cells following
treatment of low-dose cisplatin alone or iNOS gene alone was
an average of 8.00 or 20.97%, while significantly enhanced
induction of early apoptosis was observed (an average of 31.7%) in
the combination treatment group (Fig.
1C). Taken together, these results implied that the exogenously
enforced high expression of the iNOS gene may significantly
enhance low-dose cisplatin-mediated cell apoptosis (P<0.01).
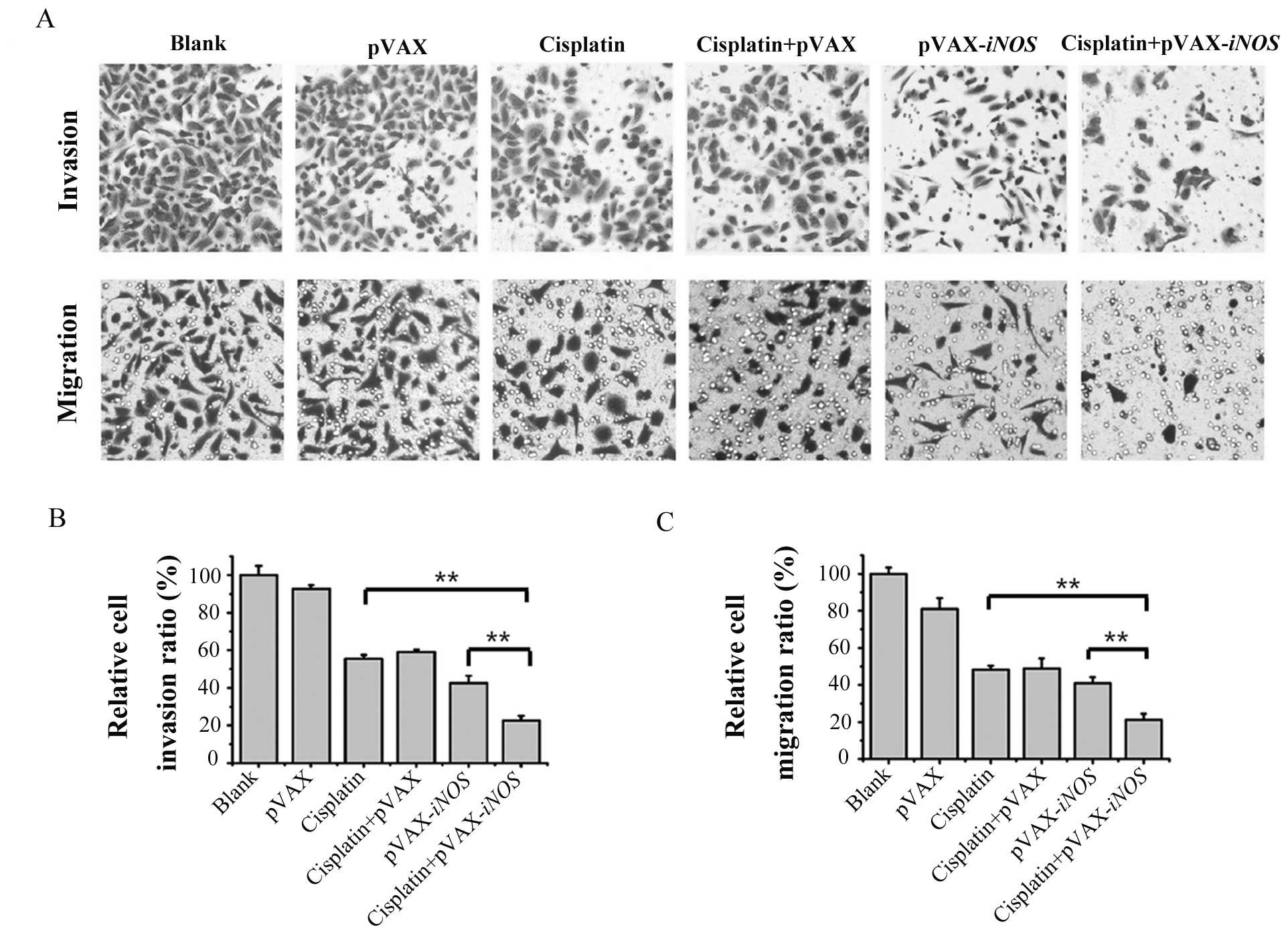
Enhanced inhibition of cell invasion and
migration abilities by the combination treatment with LP-pVAX-iNOS
and low-dose cisplatin
As distant metastasis is responsible for the failure
of lung cancer treatment, assessment of cell invasion and migration
ability is significantly important for studying cancer treatment.
Moderate inhibition (an average of 44.4 and 57.3%, respectively) of
invasion was observed in the A549 cells treated with low-dose
cisplatin or the iNOS gene alone, while the combination
treatment resulted in significant inhibition (an average of 77.4%)
of invasion in the A549 cells (P<0.05) (Fig. 2A and B). Similar results were
obtained in the Boyden Millicell assay. As shown in Fig. 2A and C, the changes in the
inhibition of migration (an average of 51.8 and 59.1% respectively)
were also considered modest in the A549 cells treated with low-dose
cisplatin or the iNOS gene alone, but more significant
suppression (~78.8%) was observed in the cells following
combination treatment with LP-pVAX-iNOS and low-dose
cisplatin (P<0.05). These results indicated that the iNOS
gene may significantly enhance the low-dose cisplatin-mediated
inhibition of cell invasion and migration in A549 lung cancer
cells.
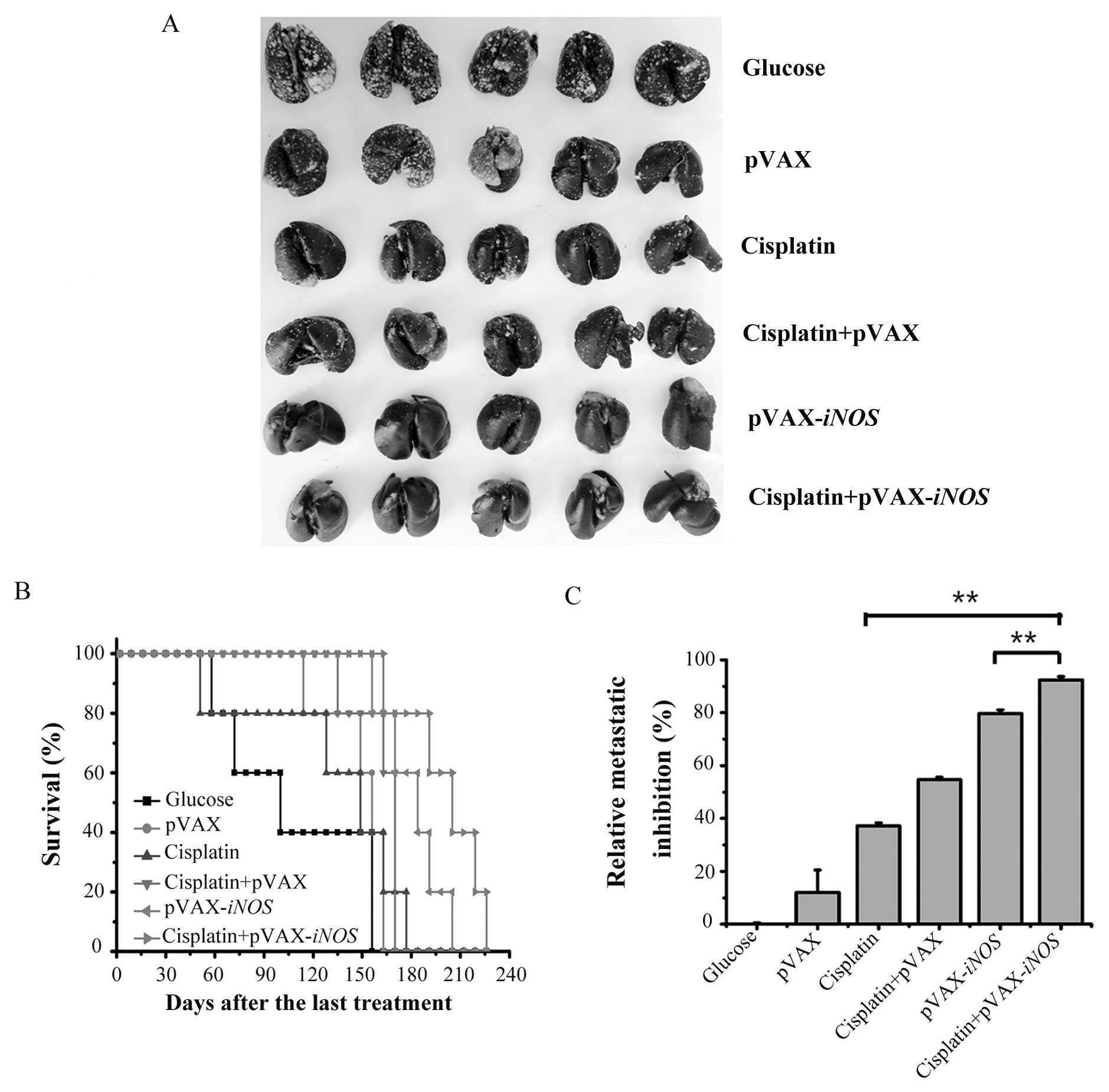
Enhanced tumor growth inhibition and
apoptosis induction by the combination treatment with LP-pVAX-iNOS
and low-dose cisplatin in vivo
As the enhanced antitumor activity in A549 cells in
the combination treatment was observed in vitro, we
hypothesized that the same effects may emerge in vivo. To
verify this assumption, we established a human A549 lung cancer
metastasis mouse model and a subcutaneous tumor xenograft mouse
model to evaluate the effects of the iNOS gene on
cisplatin-induced tumor regression. In the human A549 lung cancer
metastasis mouse model, as shown in Fig. 3A and C, consistent with the in
vitro experiment, the combination treatment significantly
inhibited tumor growth with an average reduction of 92.37% compared
with an average reduction of 37.25 and 79.67%, respectively, in
low-dose cisplatin- or iNOS gene-mediated tumor growth
inhibition (P<0.01). Meanwhile, we evaluated the combination
treatment on animal survival in the human A549 lung cancer
metastasis mouse models. The combination treatment resulted in a
significant and prolonged survival (mean survival time, 200.8±11.2
days) compared to the group treated with low-dose cisplatin alone
(mean, 133.6±22.2 days) or treated with LP-pVAX-iNOS alone
(mean, 181.2±8.5 days) (Fig.
3B).
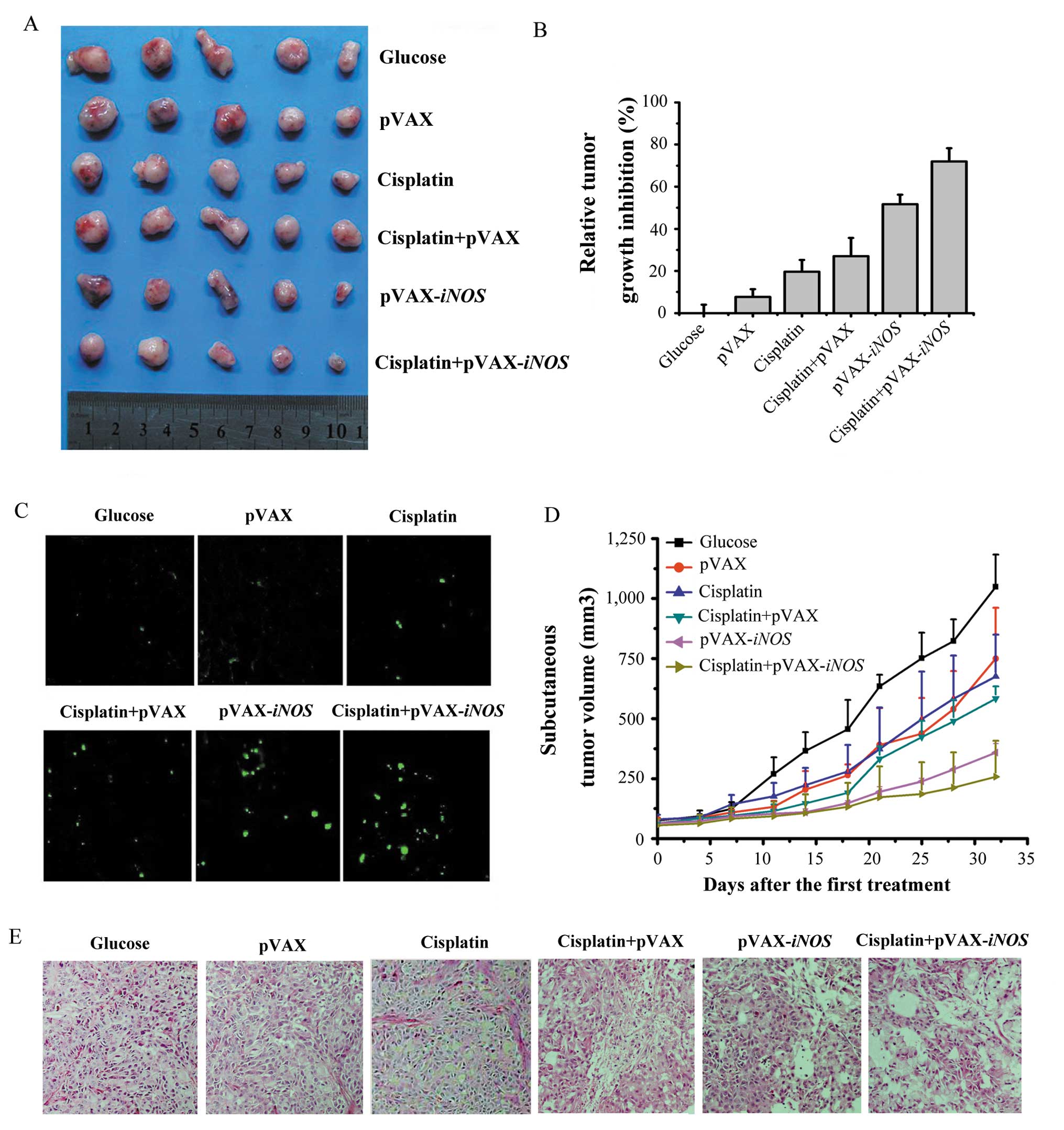
In addition, the A549 cell subcutaneous tumor
xenograft mouse model was also established to explore the effects
of the iNOS gene on enhancing the cisplatin-induced
antitumor effects. Combination treatment significantly inhibited
tumor growth with an average reduction of 71.99% compared with a
tumor growth reduction of 19.65 and 51.71%, respectively following
treatment with a low-dose cisplatin- or the iNOS gene alone
(P<0.01) (Fig. 4A, B and D).
The tumors in the glucose-treated groups increased 3 times in
volume on day 8.53±1.04 after treatment while a single injection of
2 mg/kg cisplatin or LP-pVAX-iNOS slowed this growth in the
tumor volume to 13.53±0.94 or 18.04±1.72 days, respectively
(Table I). Moreover, the
combination treatment significantly slowed tumor growth. Tumor
growth 3 times the original volume in the combination treatment
group was not reached until 20.34±0.88 days (P<0.05).
 | Table ITime (days) required for tumors to
grow to three times their volume from the day of treatment. |
Table I
Time (days) required for tumors to
grow to three times their volume from the day of treatment.
| Treatment | Glucose | pVAX | Cisplatin | Cisplatin +
pVAX |
pVAX-iNOS | Cisplatin +
pVAX-iNOS |
|---|
| Time to reach 3
times treatment volume (days) | 8.53±1.04 | 13.30±3.76 | 13.53±0.94 | 15.18±0.88 | 18.04±1.72 | 20.34±0.88 |
The peeled off subcutaneous tumor tissues were fixed
in formalin and further used by TUNEL staining to analyze the
apoptosis of tumor tissues. Histological analysis was also applied
to detect necrosis/apoptosis in the formalin- fixed tumor tissues.
As shown in Fig. 4C and E, the
tumor tissues in the combination treatment groups displayed more
TUNEL-positive nuclei and visible necrotic/apoptotic regions
compared to that in the iNOS gene or low-dose
cisplatin-treated groups. Histological analysis of the various
organs demonstrated no significant treatment-related toxicity. Our
results further demonstrated that the increased apoptosis in tumor
tissues following the combination treatment may be responsible for
the enhancement of low-dose cisplatin-induced antitumor effects
in vivo.
Induction of p-p53 overexpression and
suppression of p-mTOR and MMP2 expression by combination treatment
with LP-pVAX-iNOS and low-dose cisplatin in vitro
Phosphorylation of p53 plays important roles in both
cisplatin- and NO-induced cell apoptosis (4,35,36). To evaluate whether phosphorylation
of p53 was implied in the enhanced effects of the combination
treatment, we detected the expression levels of p-p53 protein in
the different treatment groups by western blotting. The expression
of p-p53 protein was increased in cells treated with the
iNOS gene or low-dose cisplatin alone, but a dramatic
upregulated level of p-p53 protein was detected in cells following
the combination treatment (Fig.
5).
 | Figure 5The in vitro expression of
related proteins in the various treated cell groups by western
blotting. After treatment with LP-pVAX-iNOS and/or cisplatin, A549
cells were collected and lysated. The total cellular lysates were
analyzed by western blotting with special antibodies including,
iNOS, p-mTOR, mTOR, p-p53, p53, MMP2 and β-actin (an internal
control). Lane I, untreated group; lane II, pVAX treated group;
lane III, cisplatin-treated group; lane IV, cisplatin plus
pVAX-treated group; lane V, pVAX-iNOS-treated group; lane
VI, cisplatin plus pVAX-iNOS-treated group. |
mTOR is vital in mediating cisplatin sensitivity
(37–39). However, the relationship of mTOR
and NO in antitumor processes has not been reported. To examine
whether a correlation exists between mTOR and NO, which may
participate in the reactivity of cells to low-dose cisplatin after
LP-pVAX-iNOS treatment, we analyzed the expression of
phosphorylated mTOR protein in the differently treated cells by
western blotting. Only a slight downregulation of p-mTOR was
detected in cells treated with the iNOS gene or low-dose
cisplatin alone, but a dramatic downregulation of p-mTOR was
observed in cells after the combination treatment (Fig. 5).
MMP2 is also an important kinase in the process of
cell invasion. To explore whether MMP2 protein was involved in the
enhanced effects of the combination treatment, we also detected the
expression levels of the MMP2 protein in the different treatment
groups by western blotting. The expression level of the MMP2
protein in A549 cells after the combination treatment was also
significantly downregulated compared with that in cells treated
with the iNOS gene or low-dose cisplatin alone (Fig. 5).
Discussion
Cisplatin is one of the first-line chemotherapeutic
drugs used in the clinical treatment of lung cancer patients.
However, the side effects and drug resistance restrict its wide
use. The important task of cancer therapy is to seek a suitable
method to enhance the sensitivity of existing chemotherapeutic
agents (25). Thus, refinement
for enhancing the sensitivity of cisplatin is highly required.
As previous studies reported, high NO levels
generated from iNOS gene transfer, cytokine stimulation or
NO donor may have antitumor effects and even enhance the
cytotoxicity of the chemotherapeutic drug cisplatin in RIF-1
tumors, ovarian cancer, leukemia, prostate or colon cancer cells
(18,26,27). Among all the methods used for high
concentrations of NO production, iNOS gene transfer may be
accepted as a superior way due to its marked ‘bystander’ effect and
safety (11,12,14). Only one article previously
demonstrated that iNOS gene transfer treatment may increase
the cytotoxicity and cause a delay in the growth of
cisplatin-treated RIF-1 tumors, prostate and colon cancer cells
(18). However, whether this
combined efficacy is observed in other types of tumors remains
unknown. Moreover, whether the combination treatment affects the
invasion and metastasis of cancer cells, which is important in
cancer treatment, remains unclear.
Consistent with the above-mentioned study, we also
showed that the IC50 of cisplatin was reduced in A549
cells after transfection with the iNOS gene. The combination
treatment significantly inhibited the growth of subcutaneous
tumors. Most importantly, we further aimed to clarify that
iNOS gene therapy may significantly enhance the antitumor
effects of cisplatin through the promotion of cell apoptosis, as
well as effective inhibition of proliferation, invasion and
migration abilities in vitro and in vivo. In order to
simulate the metastatic characteristics of lung cancer cells in
vivo, an A549 lung metastatic tumor- bearing mouse model was
established. The combination treatment significantly suppressed the
formation of lung metastases via systemic administration of tail
vein injection of LP-pVAX or LP-pVAX-iNOS (20 μg DNA/mouse)
and/or i.p. injection of low-dose cisplatin (2 mg/kg/mouse) and
dramatically prolonged the life spans of tumor-bearing mice with no
significant organ-related toxicity. Systemic administration of the
LP-DNA complex is a novel drug delivery method that has been shown
to deliver genes effectively to the lungs when administered
intravenously (40). More
importantly, systemic administration is more suitable for lung
cancer patients in clinical treatment procedure. To the best of our
knowledge, this is the first report demonstrating that exogenously
enforced high expression of the iNOS gene by cationic
liposome (LP)-mediated iNOS gene transfer in lung cancer
A549 cells increases the cisplatin sensitivity and significantly
enhances the antitumor effects of cisplatin in human lung cancer
A549 cells both in vitro and in vivo. The iNOS
gene significantly enhanced the cisplatin-mediated inhibition of
lung cancer cell migration and invasion. Tumor metastasis is
responsible for approximately 90% of lung cancer-related death
(41). The combination treatment
with iNOS gene therapy and low-dose cisplatin would be a
novel and potential strategy for lung cancer treatment (24).
The mechanisms of cisplatin- or NO-based antitumor
activity have been basically reported (35–37,42,43). Studies suggest that both p53 and
mTOR signaling pathways are important in cisplatin-mediated
antitumor activity (35,37,42). Meanwhile, the antitumor effects of
NO generated by iNOS gene transfer were reported to be at
least partly dependent on the phosphorylation of p53 and MMP2
(36,44). However, the associated mechanisms
of the combined effects of iNOS gene therapy and cisplatin
in human cancers have not been reported. Thus, to reveal the
molecular mechanisms of iNOS involved in enhancing low-dose
cisplatin-mediated antitumor activities, we further detected the
expression levels of these key proteins in NO-mediated and/or
cisplatin-mediated signaling pathways by western blotting.
p53 is an important marker in the process of cell
apoptosis (4), which is the main
mode of cisplatin-induced cell death. Cisplatin sensitivity is
closely related with the presence of the pro-apoptotic protein p53
(35). Meanwhile, studies have
demonstrated that a high level of NO resulting from the high
expression of the iNOS gene may promote cell apoptosis in
melanoma, renal cell cancer and their adjacent cells (7,44).
Cook et al (36) reported
that the pro-apoptotic mechanisms of NO generated by iNOS
gene transfer involved in the death signaling pathway were at least
partly dependent on the phosphorylation of p53. When p53 was
knocked out, the combination treatment of iNOS gene therapy
and radiotherapy reduced the incidence of tumor cell apoptosis and
antitumor effects in colon cancer cells. Consistent with previous
studies, we also observed that iNOS gene therapy may
significantly enhance the low-dose cisplatin-mediated apoptosis of
human lung cancer A549 cells. Similarly, the dramatic upregulation
of p-p53 protein expression was observed in the combination
treatment group, while a slight upregulation of p-p53 protein
expression was noted in the iNOS gene or cisplatin alone
treatment group. The results indicated that iNOS gene
therapy enhancing the antitumor effects of low-dose cisplatin in
lung cancer may be partly related to the upregulated expression of
p-p53 protein.
mTOR is a serine/threonine protein kinase, which
plays an important role in the regulation of cell functions
including cell proliferation, cell cycle, biosynthesis and cell
migration (42). Meanwhile, mTOR
protein is one of the widely studied kinases involved in the main
signaling pathway of cisplatin. A high degree of intracellular
phosphorylation of mTOR was often noted in several
cisplatin-resistant cancer cells, including NLCLC cells (37,38), which indicates that inhibition of
mTOR activity may enhance cancer cell sensitivity to cisplatin
(37,39). Currently, the relationship between
NO-mediated antitumor effects and p-mTOR protein expression has not
been reported. To the best of our knowledge, our study first
discovered that the expression of p-mTOR protein was significantly
diminished in the combination treatment group compared with the
iNOS gene or cisplatin alone-treated group. The results
suggested that the downregulated expression of p-mTOR protein may
be another probable reason for iNOS enhancing low-dose
cisplatin-mediated inhibition of metastasis and invasion in lung
cancer.
Metastasis is responsible for the poor effect of
clinical treatment in lung cancer. Matrix metalloproteinases (MMPs)
play a key role in the process of tumor metastasis (45). Karam et al (46) reported that cisplatin may inhibit
the invasion and migration of human ovarian cancer cells by
downregulating the expression of MMP2, TIMP1 and TIMP2. Another
study also discovered that an increased amount of cisplatin
resulted in a time- and dose-dependent decreased level of the MMP2
protein in transformed rat thyroid cancer cells (47). Meanwhile, iNOS gene
activity was inversely related to the metastasis of tumor cells
(4,7). NO may affect the invasion of mouse
mammary adenocarcinoma through breaking the balance between MMP2
and its inhibitors, including tissue inhibitor of metalloproteinase
(TIMP2 and TIMP3) (43). When the
high concentration of NO occurred, the expression levels of MMPs
gradually decreased (48). In our
study, we discovered that iNOS gene therapy significantly
reduced the low-dose cisplatin-mediated invasion and migration
capacity in lung cancer A549 cells and the expression levels of the
MMP2 protein were downregulated in the combination treatment group
compared with the iNOS gene or cisplatin alone treated
groups. The results indicated that iNOS gene therapy
enhancing the antitumor effects of low-dose cisplatin in lung
cancer may occur through the downregulation of MMP2 protein
expression.
In conclusion, our study confirmed that the
combination treatment with cationic LP-mediated iNOS gene
therapy and low-dose cisplatin may significantly enhance
cisplatin-mediated cell apoptosis and inhibition of cell
proliferation, metastasis and invasion in human lung adenocarcinoma
A549 cells in vitro and in vivo. The enhanced
antitumor effects of low-dose cisplatin by iNOS gene therapy
in lung adenocarcinoma is associated with the upregulation of p-p53
expression and the downregulation of MMP2 and p-mTOR protein
expression. Therefore, the combination treatment of iNOS
gene therapy and cisplatin is an effective strategy for the
treatment of lung cancer.
Acknowledgements
This study was partly supported by the National
Natural Science Foundation of China (no. 81071863). The authors
thank Xiang Chen and Qiaorong Huang (State Key Laboratory of
Biotherapy and Cancer Center, West China Hospital, Sichuan
University) for their technical assistance.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Jamieson ER and Lippard SJ: Structure,
recognition and processing of cisplatin-DNA adducts. Chem Rev.
99:2467–2498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Adjei AA and Jett JR: Advances
in chemotherapy of non-small cell lung cancer. Chest.
130:1211–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie K and Fidler IJ: Therapy of cancer
metastasis by activation of the inducible nitric oxide synthase.
Cancer Metastasis Rev. 17:55–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie QW, Cho HJ, Calaycay J, Mumford RA,
Swiderek KM, Lee TD, Ding A, Troso T and Nathan C: Cloning and
characterization of inducible nitric oxide synthase from mouse
macrophages. Science. 256:225–228. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heller A: Apoptosis-inducing high (.)NO
concentrations are not sustained either in nascent or in developed
cancers. ChemMedChem. 3:1493–1499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lala PK and Chakraborty C: Role of nitric
oxide in carcinogenesis and tumour progression. Lancet Oncol.
3:149–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujimoto H, Ando Y, Yamashita T, Terazaki
H, Tanaka Y, Sasaki J, Matsumoto M, Suga M and Ando M: Nitric oxide
synthase activity in human lung cancer. Jpn J Cancer Res.
88:1190–1198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puhakka A, Kinnula V, Näpänkangas U, Säily
M, Koistinen P, Pääkkö P and Soini Y: High expression of nitric
oxide synthases is a favorable prognostic sign in non-small cell
lung carcinoma. APMIS. 111:1137–1146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fitzpatrick B, Mehibel M, Cowen RL and
Stratford IJ: iNOS as a therapeutic target for treatment of human
tumors. Nitric Oxide. 19:217–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khare PD, Shao-Xi L and Kuroki M, Hirose
Y, Arakawa F, Nakamura K, Tomita Y and Kuroki M: Specifically
targeted killing of carcinoembryonic antigen (CEA)-expressing cells
by a retroviral vector displaying single-chain variable fragmented
antibody to CEA and carrying the gene for inducible nitric oxide
synthase. Cancer Res. 61:370–375. 2001.
|
|
12
|
Xu W, Liu L and Charles IG:
Microencapsulated iNOS-expressing cells cause tumor suppression in
mice. FASEB J. 16:213–215. 2002.PubMed/NCBI
|
|
13
|
Soler MN, Bobé P, Benihoud K, Lemaire G,
Roos BA and Lausson S: Gene therapy of rat medullary thyroid cancer
by naked nitric oxide synthase II DNA injection. J Gene Med.
2:344–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lumniczky K and Safrany G: Cancer gene
therapy: combination with radiation therapy and the role of
bystander cell killing in the anti-tumor effect. Pathol Oncol Res.
12:118–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ropponen KM, Kellokoski JK, Lipponen PK,
Eskelinen MJ, Alanne L, Alhava EM and Kosma VM: Expression of
inducible nitric oxide synthase in colorectal cancer and its
association with prognosis. Scand J Gastroenterol. 35:1204–1211.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raspollini MR, Amunni G, Villanucci A,
Boddi V, Baroni G, Taddei A and Taddei GL: Expression of inducible
nitric oxide synthase and cyclooxygenase-2 in ovarian cancer:
correlation with clinical outcome. Gynecol Oncol. 92:806–812. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adams C, McCarthy HO, Coulter JA,
Worthington J, Murphy C, Robson T and Hirst DG: Nitric oxide
synthase gene therapy enhances the toxicity of cisplatin in cancer
cells. J Gene Med. 11:160–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Worthington J, McCarthy HO, Barrett E,
Adams C, Robson T and Hirst DG: Use of the radiation-inducible WAF1
promoter to drive iNOS gene therapy as a novel anti-cancer
treatment. J Gene Med. 6:673–680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Worthington J, Robson T, O’Keeffe M and
Hirst DG: Tumour cell radiosensitization using constitutive (CMV)
and radiation inducible (WAF1) promoters to drive the iNOS gene: a
novel suicide gene therapy. Gene Ther. 9:263–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarthy HO, Worthington J, Barrett E,
Cosimo E, Boyd M, Mairs RJ, Ward C, McKeown SR, Hirst DG and Robson
T: p21 (WAF1)-mediated transcriptional targeting of inducible
nitric oxide synthase gene therapy sensitizes tumours to
fractionated radiotherapy. Gene Ther. 14:246–255. 2007. View Article : Google Scholar
|
|
22
|
Coulter JA, McCarthy HO, Worthington J,
Robson T, Scott S and Hirst DG: The radiation-inducible pE9
promoter driving inducible nitric oxide synthase radiosensitizes
hypoxic tumour cells to radiation. Gene Ther. 15:495–503. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Cook T, Alber S, Liu K, Kovesdi I,
Watkins SK, Vodovotz Y, Billiar TR and Blumberg D: Adenoviral gene
transfer of the human inducible nitric oxide synthase gene enhances
the radiation response of human colorectal cancer associated with
alterations in tumor vascularity. Cancer Res. 64:1386–1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evig CB, Kelley EE, Weydert CJ, Chu Y,
Buettner GR and Burns CP: Endogenous production and exogenous
exposure to nitric oxide augment doxorubicin cytotoxicity for
breast cancer cells but not cardiac myoblasts. Nitric Oxide.
10:119–129. 2004. View Article : Google Scholar
|
|
25
|
Wink DA, Cook JA, Christodoulou D, Krishna
MC, Pacelli R, Kim S, DeGraff W, Gamson J, Vodovotz Y, Russo A and
Mitchell JB: Nitric oxide and some nitric oxide donor compounds
enhance the cytotoxicity of cisplatin. Nitric Oxide. 1:88–94. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Son KK and Hall KJ: Nitric oxide-mediated
tumor cell killing of cisplatin-based interferon-gamma gene therapy
in murine ovarian carcinoma. Cancer Gene Ther. 7:1324–1328.
2000.PubMed/NCBI
|
|
27
|
Konovalova NP, Goncharova SA, Volkova LM,
Rajewskaya TA, Eremenko L and Korolev AM: Nitric oxide donor
increases the efficiency of cytostatic therapy and retards the
development of drug resistance. Nitric Oxide. 8:59–64. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JH, Lin HH, Chiang TA, Hsu JD, Ho HH,
Lee YC and Wang CJ: Gaseous nitrogen oxide promotes human lung
cancer cell line A549 migration, invasion, and metastasis via
iNOS-mediated MMP-2 production. Toxicol Sci. 106:364–375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kisley LR, Barrett BS, Bauer AK,
Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC and Malkinson AM:
Genetic ablation of inducible nitric oxide synthase decreases mouse
lung tumorigenesis. Cancer Res. 62:6850–6856. 2002.PubMed/NCBI
|
|
30
|
Chen X, Wang X, Wang Y, Yang L, Hu J, Xiao
W, Fu A, Cai L, Li X, Ye X, Liu Y, et al: Improved tumor-targeting
drug delivery and therapeutic efficacy by cationic liposome
modified with truncated bFGF peptide. J Control Release. 145:17–25.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shamimi-Noori S, Yeow W-S, Ziauddin MF,
Xin H, Tran TL, Xie J, Loehfelm A, Patel P, Yang J, Schrump DS, et
al: Cisplatin enhances the antitumor effect of tumor necrosis
factor-related apoptosis-inducing ligand gene therapy via
recruitment of the mitochondria-dependent death signaling pathway.
Cancer Gene Ther. 15:356–370. 2008. View Article : Google Scholar
|
|
32
|
Ito I, Ji L, Tanaka F, Saito Y, Gopalan B,
Branch CD, Xu K, Atkinson EN, Bekele BN, Stephens LC, et al:
Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates
potent antitumor activity against human lung cancer in vivo. Cancer
Gene Ther. 11:733–739. 2004. View Article : Google Scholar
|
|
33
|
Wei YQ, Wang QR, Zhao X, Yang L, Tian L,
Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, et al: Immunotherapy of
tumors with xenogeneic endothelial cells as a vaccine. Nat Med.
6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fraser M, Chan SL, Chan SS, Fiscus RR and
Tsang BK: Regulation of p53 and suppression of apoptosis by the
soluble guanylyl cyclase/cGMP pathway in human ovarian cancer
cells. Oncogene. 25:2203–2212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nguyen DM, Spitz FR, Yen N, Cristiano RJ
and Roth JA: Gene therapy for lung cancer: enhancement of tumor
suppression by a combination of sequential systemic cisplatin and
adenovirus-mediated p53 gene transfer. J Thorac Cardiovasc Surg.
112:1372–1377. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cook T, Wang Z, Alber S, Liu K, Watkins
SC, Vodovotz Y, Billiar TR and Blumberg D: Nitric oxide and
ionizing radiation synergistically promote apoptosis and growth
inhibition of cancer by activating p53. Cancer Res. 64:8015–8021.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wangpaichitr M, Wu C, You M, Kuo MT, Feun
L, Lampidis T and Savaraj N: Inhibition of mTOR restores cisplatin
sensitivity through down-regulation of growth and anti-apoptotic
proteins. Eur J Pharmacol. 591:124–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mabuchi S, Kawase C, Altomare DA,
Morishige K, Sawada K, Hayashi M, Tsujimoto M, Yamoto M,
Klein-Szanto AJ, Schilder RJ, et al: mTOR is a promising
therapeutic target both in cisplatin-sensitive and
cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer
Res. 15:5404–5413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Templeton NS, Lasic DD, Frederik PM, Strey
HH, Roberts DD and Pavlakis GN: Improved DNA:liposome complexes for
increased systemic delivery and gene expression. Nat Biotechnol.
15:647–652. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keshamouni V, Arenberg D and Kalemkeriam
G: Lung Cancer Metastasis: Novel Biological Mechanisms and Impact
on Clinical Practice. Springer; New York: pp. 1–395. 2009
|
|
42
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–23. 2007. View Article : Google Scholar
|
|
43
|
Orucevic A, Bechberger J, Green AM,
Shapiro RA, Billiar TR and Lala PK: Nitric-oxide production by
murine mammary adenocarcinoma cells promotes tumor-cell
invasiveness. Int J Cancer. 81:889–896. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obara H and Harasawa R: Nitric oxide
causes anoikis through attenuation of E-cadherin and activation of
caspase-3 in human gastric carcinoma AZ-521 cells infected with
Mycoplasma hyorhinis. J Vet Med Sci. 72:869–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bjorklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
46
|
Karam AK, Santiskulvong C, Fekete M, Zabih
S, Eng C and Dorigo O: Cisplatin and PI3kinase inhibition decrease
invasion and migration of human ovarian carcinoma cells and
regulate matrix-metalloproteinase expression. Cytoskeleton
(Hoboken). 67:535–544. 2010.
|
|
47
|
Urso L, Muscella A, Calabriso N, Vetrugno
C, Jiménez E, Montiel M and Marsigliante S: Effects of cisplatin on
matrix metalloproteinase-2 in transformed thyroid cells. Biochem
Pharmacol. 79:810–816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ridnour LA, Thomas DD, Switzer C,
Flores-Santana W, Isenberg JS, Ambs S, Roberts DD and Wink DA:
Molecular mechanisms for discrete nitric oxide levels in cancer.
Nitric Oxide. 19:73–76. 2008. View Article : Google Scholar : PubMed/NCBI
|