Introduction
The pathogenesis of atherosclerosis is complex and
involves the impact of many well-documented traditional risk
factors (1). Among these, the
accumulation of macrophage foam cells in the intima is a hallmark
of early-stage atherosclerosis (2). It is well established that foam
cells are mainly derived from macrophages due to the continuous
uptake of modified low-density lipoprotein (LDL), leading to
excessive lipoprotein-derived cholesterol accumulation inside the
cells (3,4). Once foam cells are formed, the
development of atherosclerosis can be accelerated through plaque
disruption (2). Hence, reducing
the formation of foam cells may be an efficient strategy for the
treatment and prevention of atherosclerosis. The cholesterol
homeostasis in macrophages has been reported to be mainly regulated
by cholesterol internalization and cholesterol efflux through the
so-called scavenger receptors and reverse cholesterol transporters.
Of the many cell surface proteins, scavenger receptor A (SR-A) and
cluster of differentiation 36 (CD36) have been shown to be
responsible for the uptake of modified lipoproteins by human blood
monocyte-derived macrophages (HMDMs) (5). By contrast, the efflux of
cholesterol is regulated by reverse cholesterol transporters (RCTs)
including scavenger receptor class B type I (SR-BI), ATP-binding
cassette (ABC) transporter A1 and ABCG1 (6,7).
Therefore, a therapeutic strategy involving the upregulation of the
expression of RCTs or the downregulation SR expression, resulting
in the reduction of cholesterol accumulation in macrophages would
be highly desirable for the treatment of atherosclerosis.
Flavonoids are normal constituents of the human diet
and are known to have anti-inflammatory potential, as well as
antioxidant properties both in animal and human models (8,9).
Kaempferol, a flavonoid present in various natural sources
including tea, cabbage, beans, tomatoes, onions, leeks and apples,
has been shown to exert several beneficial effects in
cardiovascular and nerve systems, such as the reduction of hydroxyl
radicals (10), and inflammatory
response in macrophage cells (11). Current research indicates that
kaempferol ameliorates the development of atherosclerosis in
apolipoprotein E-deficient mice (12); however, the mechanisms responsible
for the anti-atherogenic effects of kaempferol are not yet fully
defined. As mentioned above, SRs and RCTs are potential molecular
targets for the selective delivery of anti-atherosclerosis agents
to foam cells. Therefore, we hypothesized that the anti-atherogenic
effects of kaempferol are related to the suppression of foam cell
formation by regulating the expression of SRs and RCTs.
Furthermore, a number of studies have reported that heme
oxygenase-1 (HO-1), the critical enzyme in heme catabolism, or
activator protein-1 (AP-1) mediates the antioxidative or
anti-inflammatory properties of kaempferol, respectively (13,14). However, whether HO-1 or AP-1
contributes to the suppression of the formation of foam cells by
kaempferol warrants further investigation.
The present study was designed to investigate
whether kaempferol inhibits the formation of foam cells and explore
the molecular mechanisms involved. Our results suggest that
kaempferol suppresses macrophage-derived foam cell formation via
the HO-1-dependent upregulation of RCT expression and the c-Jun (a
subunit of AP-1)-dependent downregulation of CD36 expression.
Materials and methods
Cell culture and transfection
The THP-1 human monocyte-derived cell line was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA), and cultured in RPMI-1640 medium containing 10%
fetal bovine serum (FBS), 20 g/ml streptomycin and 20 IU/ml
penicillin at 37°C in 5% CO2. Differentiation into
macrophages was achieved by treating the cells with phorbol
12-myristate 13-acetate (PMA, 200 ng/ml) for 48 h. Reagents for
cell culture were purchased from Invitrogen (Carlsbad, CA, USA).
Cell transfections were performed with the SuperFect fragment
(Qiagen, Valencia, CA, USA) according to the manufacturer’s
instructions using scrambled or HO-1 small hairpin RNA (shRNA) in a
6-well plate or 50-ml flask. Cells were incubated for 24 h after
transfection and used for the indicated experiments.
Reagents
PMA and kaempferol (purity, 98.0%) (Fig. 1) were obtained from Sigma (St.
Louis, MO, USA). Mouse anti-ABCA1, rabbit anti-ABCG1, rabbit
anti-CD36 and rabbit anti-SR-BI antibodies were from Abcam
(Cambridge, MA, USA), and goat anti-SR-A antibody was from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-HO-1,
rabbit anti-c-Fos, rabbit anti-c-Jun antibodies were purchased from
Cell Signaling Technology (Beverly, MA, USA). Scrambled and HO-1
shRNAs were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). 3-Dodecanoyl-NBD-cholesterol was obtained from Cayman
Chemical Co. (Ann Arbor, MI, USA).
Cell viability assay with methyl
thiazoyltetrazolium (MTT)
Macrophages were seeded at density of
7.5×104 cells/well in 96-well plates and cell viability
was determined by MTT assay. MTT assay was performed as described
in our previous study (15).
Briefly, following treatment with or without kaempferol for 24 h,
the culture supernatant was removed. The cells were washed with PBS
and incubated with 100 μl of MTT (1 mg/ml) in culture medium
at 37°C for 4 h. The culture medium with the dye was then removed
and 150 μl of DMSO/well were added for formazan
solubilization. The absorbance of the converted dye was measured at
a wavelength of 490 nm using a Sunrise Remote Microplate Reader
(Tecan Austria GmbH, Grödig, Austria). The viability of the
macrophages in each well was presented as a percentage of the
control cells.
shRNA
Mouse HO-1 (NM_010442.2)-specific oligonucleotides,
including 2 complementary 21-nucleotide sequences corresponding to
positions 792–812 downstream of the transcription start site
(GCTGACAGAGGAACACAAAGA) and separated by a 9-nucleotide
non-complementary spacer (TTCAAGAGA), were selected for the
targeted suppression of HO-1. A negative control shRNA vector
expressing an oligo-nucleotide containing a 20-nucleotide sequence
not targeting HO-1 and separated by a 9-nucleotide
non-complementary spacer (CAAGAGATT) from the reverse complement of
the same 20-nucleotide sequence was used.
Oil red O staining
Foam cells derived from macrophages were identified
by Oil red O staining. Oil red O is a fat-soluble diazo dye used
for staining neutral triglycerides and lipids and some lipoproteins
(16). After the culture of
THP-1-derived macrophages with 100 μg/ml oxidized LDL
(oxLDL) in the presence or absence of kaempferol for 24 h, cells
were washed once with PBS, fixed in 10% paraformaldehyde-PBS for 30
min, and stained for 20 min in 1% Oil red O (in 60% isopropanol).
Hematoxylin was used as the counterstain. After washing with PBS
for 3 times, macrophages were photographed under a microscope at
×400 magnification.
Cholesterol efflux assay
Cholesterol efflux experiments were performed as
previously described (17). After
24 h of treatment with various concentrations of kaempferol, the
THP-1-derived macrophages were incubated with the equilibration of
NBD-cholesterol (1 μg/ml) for an additional 6 h in the
presence of kaempferol. The NBD-cholesterol-labeled cells were
incubated in RPMI-1640 medium for 6 h after washing with PBS. A
multilabel counter (Perkin-Elmer Life Sciences, Waltham, MA, USA)
was used to detect the fluorescence-labeled cholesterol released
from the cells into the medium. Cholesterol efflux was calculated
as a percentage of fluorescence in the medium relative to the total
amount of fluorescence.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Tatol RNA was isolated using RNAiso Plus and was
converted into complementary DNA by the PrimeScript RT reagent kit
(Perfect Real Time; Takara). Primers used in the qRT-PCR analysis
were as follows: mouse SR-BI forward, 5′-ACCCT AACCCAAAGGAGCAT-3′
and reverse, 5′-CACAGCAA CGGCAGAACTAC-3′; mouse ABCA1 forward,
5′-CAA TGTCAAGGTGTGGTTCAAT-3′ and reverse, 5′-GCTGCTG
TTTAGTGAGGTTCAA-3′; mouse CD36 forward, 5′-TCGCT
TCCACATTTCCTACAT-3′ and reverse, 5′-CCCAGTCTCAT TTAGCCACAG-3′;
mouse SR-A forward, 5′-TCCTTGATTT CGTCAGTCCAG-3′ and reverse,
5′-CCTCCTGTTGCTTTG CTGTAG-3′; ABCG1 forward, 5′-GCCTACTACCTGGCAA
AGACC-3′ and reverse, 5′-GAACAGCACAAAACGCACAG-3′; and GAPDH
forward, 5′-GGTGAAGGTCGGTGTGAACG-3′ and reverse,
5′-CTCGCTCCTGGAAGATGGTG-3′. The reaction of qRT-PCR was performed
by the iQ™ SYBR-Green Supermix (Bio-Rad, Hercules, CA, USA) under
the following conditions: 3 min at 95°C for 1 cycle, 10 sec at
95°C, 30 sec at 60°C for 39 cycles, 95°C for 5 sec.
Western blot analysis
Nuclear extracts were prepared as described in our
previous study (15). Total cell
proteins were extracted as follows: cells were scraped and
suspended in ice-cold PBS, then centrifuged at 1,000 × g for 10
min. Cells were lysed with 180 μl RIPA lysis buffer
(Beyotime, Jiangsu, China) and vortexed every 5 min at 4°C for 30
min. The supernatant as total cell extracts was collected after
centrifuging at 12,000 × g for 15 min at 4°C. All protein
concentrations were determined by bicinchoninic acid protein assay
kit (Biomed Biotech Co., Ltd., Beijing, China). Aliquots (50
μg) of total or nuclear extracts separated on 8–12%
SDS-polyacrylamide minigels, and transferred onto nitrocellulose
membranes (Amersham, Buckinghamshire, England). The membranes were
incubated with 1.5% BSA and then incubated with anti-ABCA1,
anti-ABCG1, anti-SR-BI, anti-SR-A, anti-CD36, anti-Fos, anti-c-Jun
or anti-β-actin antibodies overnight at 4°C. The protein expression
was detected by an enhanced chemiluminescence kit (ECL;
Perkin-Elmer Life Sciences) and densitometric analysis was
performed using the 720 BK/01837 System (Bio-Rad).
Statistical analysis
Data are presented as the means ± SEM and analyzed
using one-way analysis of variance (ANOVA) and the Newman-Keuls
test was used to locate any significant differences identified by
ANOVA. Differences were considered statistically significant when
P<0.05. All experiments were performed at least 3 times.
Results
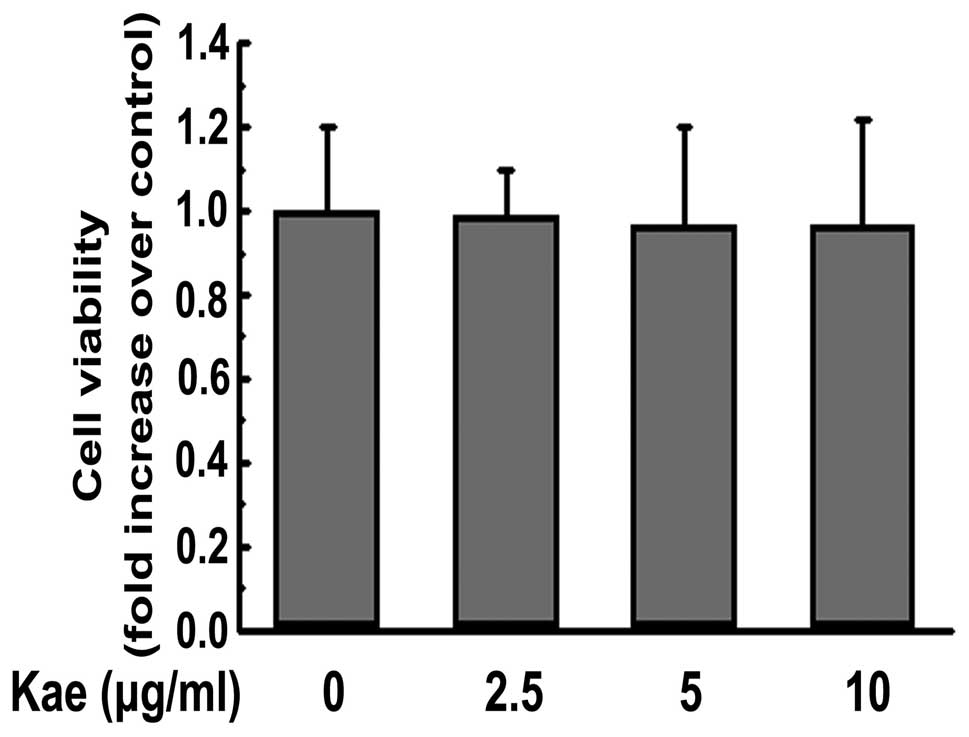
Viability of macrophages is not affected
by kaempferol at the chosen concentrations
The cytotoxicity of kaempferol at various
concentrations (2.5, 5 and 10 μg/ml) was not obvious after
24 h of incubation (Fig. 2).
Therefore, a concentration range of 2.5–10 μg/ml was chosen
for subsequent experiments.
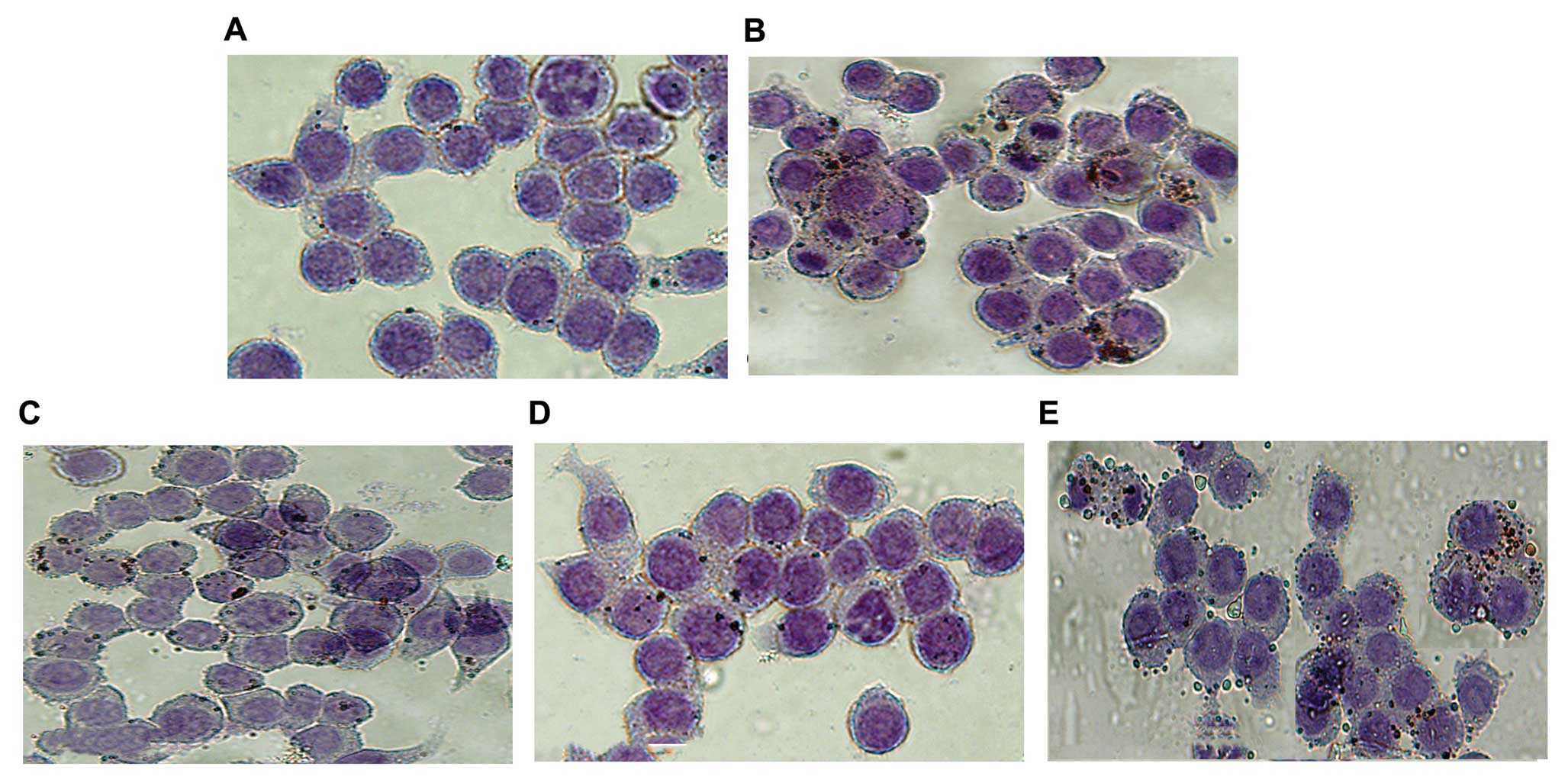
Kaempferol inhibits lipid accumulation
and promotes cholesterol efflux from THP-1-derived macrophages
Lipid accumulation, a hallmark of foam cell
formation, was detected in the cells treated with oxLDL in the
presence or absence of kaempferol. Co-incubation with kaempferol
and oxLDL significantly ameliorated intracellular lipid
accumulation in the macrophages compared with the oxLDL-treated
group, as revealed by Oil red O staining (Fig. 7). These data suggest that
kaempferol suppresses oxLDL uptake and foam cell formation in
macrophages. Moreover, kaempferol promoted cholesterol efflux from
macrophages in a dose-dependent manner, as demonstrated by the
NBD-labeled cholesterol that was used (Fig. 3). This is likely to contribute to
the protective effect of kaempferol on macrophage foam cell
formation.
Kaempferol increases the expression of
SR-BI, ABCA1 and ABCG1, but decreases the expression of CD36 in
macrophages
To investigate the mechanisms underlying the
suppression of foam cell formation by kaempferol, ABCA1, ABCG1,
SR-BI, SR-A and CD36 expression, which are reported to play
critical roles during lipid accumulation in macrophages (18), were determined following the
incubation of THP-1-derived macrophages with kaempferol. Treatment
with kaempferol at various concentrations (2.5, 5 and 10
μg/ml) for 24 h dose-dependently decreased the mRNA and
protein expression of CD36 without affecting the expression of SR-A
(Fig. 4). Additionally, the
expression of ABCA1, ABCG1 and SR-BI was significantly enhanced at
the protein and mRNA levels in response to kaempferol treatment
(Fig. 4).
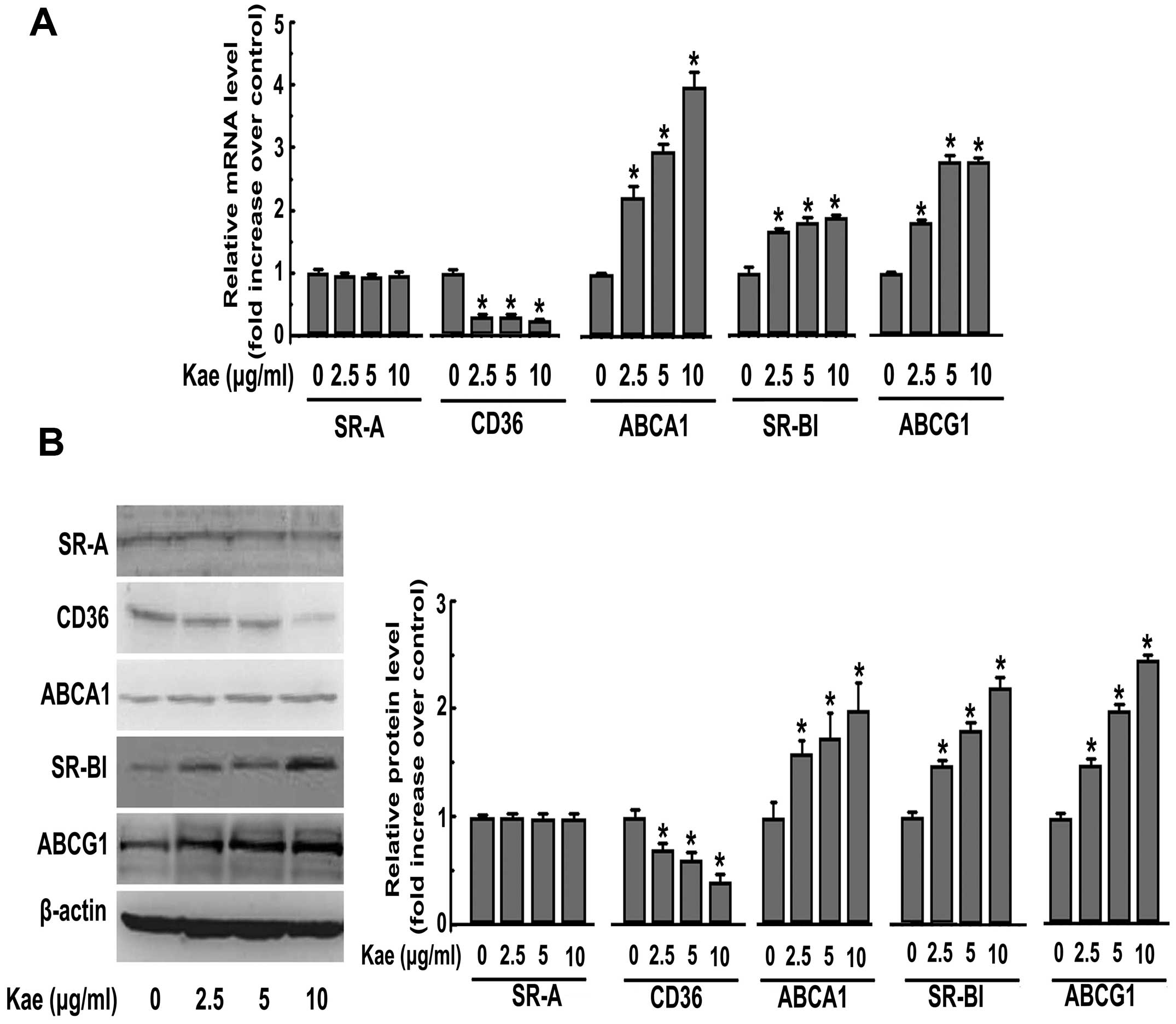 | Figure 4.Kaempferol (Kae) increases the
expression of SR-BI, ABCA1, ABCG1, but decreases the expression of
CD36 in macrophages. (A) THP-1-derived macrophages were treated
with kaempferol (2.5, 5 and 10 μg/ml) for 24 h. After
treatment, total RNA was extracted and then subjected to qRT-PCR to
detect the mRNA expression of SR-A, CD36, ABCA1, ABCG1 and SR-BI.
(B) Macrophages were incubated with various concentrations (2.5, 5
and 10 μg/ml) of kaempferol for 24 h. Western blot analysis
was used to detect the protein expression of SR-A, CD36, ABCA1,
ABCG1 and SR-BI after treatment. The data are representative of 3
independent experiments (means ± SEM). *P<0.05
compared with the control. |
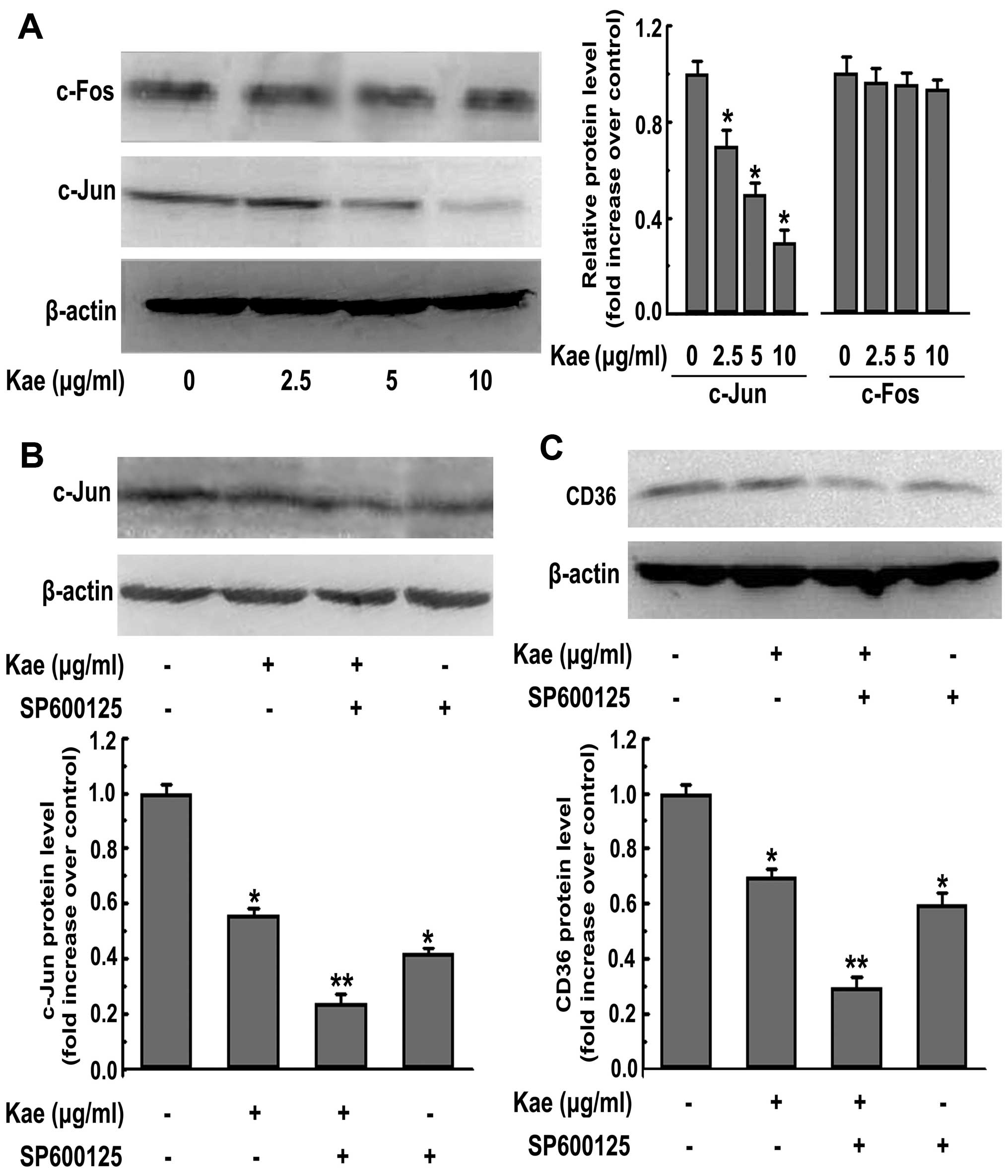
Kaempferol-induced CD36 inhibition is
mediated by the c-Jun-AP-1 pathway
It has recently been reported that in macrophages,
that c-Jun and c-Fos, 2 key subunits of AP-1, are required for the
gene expression of SR-A (18). In
addition, AP-1 activity contributes to the fate of the cell after
kaempferol treatment (19).
Therefore, in this study, we examined the possibility that the
kaempferol-mediated downregulation of CD36 expression in
macrophages is regulated by the AP-1 pathway. The nuclear
expression of c-Fos was not significantly altered following the
kaempferol treatment of THP-1-derived macrophages (Fig. 5A). However, the kaempferol
treatment of macrophages was associated with dose-dependent
decreases in nuclear c-Jun expression levels (Fig. 5A). Therefore, we subsequently
investigated whether the inhibition of the nuclear translocation of
c-Jun is critical for foam cell formation. To this end, we used the
c-Jun N-terminal kinase (JNK) inhibitor, SP600125, which is a
potent, cell-permeable selective and reversible inhibitor of JNK1,
JNK2 and JNK3. We found that the addition of the inhibitor
augmented the kaempferol-mediated effect on the downregulation of
c-Jun nuclear expression (Fig.
5B), CD36 expression (Fig.
5C) and lipid accumulation in macrophages (Fig. 7). Collectively, these results
suggest that the suppression of CD36 expression and subsequent
alleviation of foam cell formation by kaempferol are partly
c-Jun-AP-1-dependent.
Kaempferol-induced SR-BI, ABCA1 and ABCG1
upregulation is mediated by HO-1
We determined the role of HO-1 in the
kaempferol-mediated upregulation of SR-BI, ABCA1 and ABCG1. The
protein expression of HO-1 in macrophages was dose-dependently
elevated following kaempferol treatment as revealed by western blot
analysis (Fig. 6A). Additional
experiments were then performed to determine whether HO-1
participates in the suppressive effect of kaempferol on foam cell
formation. Our results showed that the transfection of HO-1 shRNA
at the concentration of 400 ng/ml completely abrogated
kaempferol-induced HO-1 protein expression (Fig. 6B), while the transfection of
corresponding scrambled shRNA failed to do so. In addition, we
found that the HO-1 shRNA transfection significantly reversed the
kaempferol-induced inhibition of foam cell formation (Fig. 7). Of note, the kaempferol-mediated
effects on the upregulation of SR-BI, ABCA1 and ABCG1 (Fig. 6C) and cholesterol efflux (Fig. 6D) were also reversed in the
presence of HO-1 shRNA transfection. Taken together, these data
indicate the involvement of HO-1 in the kaempferol-mediated
protective effects on macrophage foam cells.
Discussion
A number of previous studies have demonstrated that
kaempferol exerts protective effects against vascular diseases in
humans as well as animals (10,12). The anti-atherogenic function of
kaempferol has been demonstrated. For instance, kaempferol has been
shown to inhibit oxLDL-mediated apoptosis (19) and attenuate the cytokine-induced
expression of vascular cell adhesion molecule-1 (VCAM-1),
intercellular adhesion molecule-1 (ICAM-1) in endothelial cells
(20). Kaempferol also modulates
vascular dysfunction in the spontaneously hypertensive rat
(21). In addition, it is well
known that kaempferol alleviates the inflammatory response in
macrophages (22). However, the
effects of kaempferol on macrophage foam cell formation are not yet
well understood. Uncontrolled oxLDL uptake and impaired cholesterol
efflux are reported to be the major contributors of foam cell
formation (17). Of note, our
data suggest that kaempferol ameliorates oxLDL-induced foam cell
formation by reducing lipid accumulation and promoting cholesterol
efflux from macrophage, confirming the findings reported in the
study by Tu et al (23).
Kaempferol suppressed oxLDL uptake by THP-1-derived macrophages, as
revealed by flow cytometry analysis. We thus performed experiments
to further examine the molecular mechanisms underlying the
beneficial function of kaempferol during foam cell formation.
Macrophage SRs, such as CD36 and SR-A, have
previously been implicated in foam cell formation and
atherogenesis, during which these receptors participate in the
uptake of oxLDL (24). However,
it is not yet well known whether similar mechanisms are involved in
the kaempferol-mediated protection in macrophage foam cell
formation. In the present study, we showed that kaempferol
significantly suppressed the mRNA and protein expression of CD36
without affecting the expression of SR-A. The genetic inactivation
of CD36 has previously been shown to reduce oxLDL uptake in
vitro and in atherosclerotic lesions in mice (25), strongly supporting a
pro-atherogenic role for CD36. In view of CD36 function, it may be
concluded that the decrease in CD36 expression followed by
kaempferol treatment may contribute to the reduced lipid
accumulation and subsequent inhibition of foam cell formation.
To our knowledge, our study, for the first time
identifies c-Jun-AP-1 as the key transcriptional factor for the
kaempferol-induced downregulation of CD36 expression. c-Fos and
c-Jun are 2 dimers of AP-1. We demonstrated that the nuclear
translocation of c-Fos was not affected by kaempferol. However,
kaempferol significantly attenuated the nuclear translocation of
c-Jun, which is involved in the induction of CD36 (26). This notion is also supported by
the results that the inhibition of AP-1 increases the effects of
kaempferol on the downregulation of CD36 expression and the
inhibition of foam cell formation. This finding is consistent with
previous observations in a study of the downregulation of CD36 by
1,25(OH)2 vitamin D (27).
In addition to its effect on CD36 expression, we
further investigated the effect of kaempferol on the expression of
ABCA1, ABCG1 and SR-BI, 3 major transporters for cholesterol efflux
from macrophage foam cells. Duh et al reported that
kaempferol increases ABCA1 and SR-BI expression in RAW264.7
macrophages (28), and similar
results were obtained in THP-1-derived macrophages in this study.
Moreover, we found that ABCG1 expression was also increased in
response to kaempferol treatment. It is well established that
ABCA1, ABCG1 and SR-BI play a critical role in the cholesterol
homeostasis in macrophages (29).
Studies using individual transporter-deficient animals showed that
atherosclerotic lesions and foam cell accumulation were markedly
promoted (17). Recent studies
have indicated that the elevated function of RCTs in macrophages,
leading to reduced deposition of cholesterol in macrophages, by
several dietary flavonoids with anti-atherogenic properties, such
as quercetin, EGb 761 and daidzein and resveratrol (18,30,31). In view of their function, the
upregulation of ABCA1, ABCG1 and SR-BI expression following
kaempferol treatment observed in the current study is likely to
promote the cholesterol efflux and subsequent inhibits foam cell
formation. More importantly, our results suggest that the induced
expression of ABCA1, SR-BI and ABCG1 by kaempferol is accompanied
by increased HO-1 expression. This finding was further supported by
the results from western blot analysis, in which the
kaempferol-mediated upregulation of ABCA1, SR-BI and ABCG1 was
reversed by the knockdown of the HO-1 gene using shRNA. In
addition, the inhibition of HO-1 attenuated the kaempferol effects
on the upregulation of cholesterol efflux and the downregulation of
lipid accumulation in macrophages. These results suggest that the
expression of HO-1 is required for the induction of ABCA1, SR-BI
and ABCG1 expression by kaempferol. Our findings are in agreement
with reports that the exogenous overexpression of HO-1 using
agonist or adenovirus retards the progression of atherosclerosis in
hyperlipidaemic mice (32,33).
Although the detailed mechanisms of how kaempferol affects the
functions of HO-1 remain to be further investigated, in this study,
we discovered a unique pathway for the kaempferol-mediated
upregulation of ABCA1, SR-BI and ABCG1. A number of studies have
reported that bilirubin or carbon monoxide regulate the
antioxidative or anti-inflammatory action of HO-1 (32,34). The involvement of this pathway in
the kaempferol-elicited induction of ABCA1, SR-BI and ABCG1
expression remains to be investigated in the future.
In conclusion, our study provides new insights into
the atheroprotective nature of kaempferol in THP-1-derived
macrophages, which promotes cholesterol efflux and reduces lipid
accumulation in foam cells via the regulation of ABCA1, SR-BI,
ABCG1 and CD36 expression. CD36 is downregulated by kaempferol by
inhibiting c-Jun nuclear translocation, whereas ABCA1, SR-BI, ABCG1
expression is upregulated following kaempferol treatment through
the enhanced protein expression of HO-1. The findings of this study
provide a novel explanation for the anti-atherogenic properties of
kaempferol, as well as possible molecular targets which may be used
for the development of therapeutic strategies for the treatment of
atherosclerosis.
Acknowledgements
This study was supported by a grant
from the Public Health Bureau of Chongqing, China (no.
2009-1-6).
References
|
1.
|
Tsimikas S, Miyanohara A, Hartvigsen K, et
al: Human oxidation-specific antibodies reduce foam cell formation
and atherosclerosis progression. J Am Coll Cardiol. 58:1715–1727.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sung HJ, Kim J, Kim Y, Jang SW and Ko J:
N-acetyl cysteine suppresses the foam cell formation that is
induced by oxidized low density lipoprotein via regulation of gene
expression. Mol Biol Rep. 39:3001–3007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kleemann R, Zadelaar S and Kooistra T:
Cytokines and atherosclerosis: a comprehensive review of studies in
mice. Cardiovasc Res. 79:360–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hrboticky N, Draude G, Hapfelmeier G,
Lorenz R and Weber PC: Lovastatin decreases the receptor-mediated
degradation of acetylated and oxidized LDLs in human blood
monocytes during the early stage of differentiation into
macrophages. Arterioscler Thromb Vasc Biol. 19:1267–1275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang X and Rader DJ: Molecular regulation
of macrophage reverse cholesterol transport. Curr Opin Cardiol.
22:368–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cuchel M and Rader DJ: Macrophage reverse
cholesterol transport: key to the regression of atherosclerosis?
Circulation. 113:2548–2555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chirumbolo S: The role of quercetin,
flavonols and flavones in modulating inflammatory cell function.
Inflamm Allergy Drug Targets. 9:263–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
González-Gallego J, Sánchez-Campos S and
Tuñón MJ: Anti-inflammatory properties of dietary flavonoids. Nutr
Hosp. 22:287–293. 2007.
|
|
10.
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344.
2011.PubMed/NCBI
|
|
11.
|
Park MY, Ji GE and Sung MK: Dietary
kaempferol suppresses inflammation of dextran sulfate
sodium-induced colitis in mice. Dig Dis Sci. 57:355–363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Xiao HB, Lu XY, Sun ZL and Zhang HB:
Kaempferol regulates OPN-CD44 pathway to inhibit the atherogenesis
of apolipoprotein E deficient mice. Toxicol Appl Pharmacol.
257:405–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hong JT, Yen JH, Wang L, Lo YH, Chen ZT
and Wu MJ: Regulation of heme oxygenase-1 expression and MAPK
pathways in response to kaempferol and rhamnocitrin in PC12 cells.
Toxicol Appl Pharmacol. 237:59–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chen CC, Chow MP, Huang WC, Lin YC and
Chang YJ: Flavonoids inhibit tumor necrosis factor-alpha-induced
upregulation of intercellular adhesion molecule-1 (ICAM-1) in
respiratory epithelial cells through activator protein-1 and
nuclear factor-kappaB: structure-activity relationships. Mol
Pharmacol. 66:683–693. 2004.
|
|
15.
|
Li XY, He JL, Liu HT, Li WM and Yu C:
Tetramethylpyrazine suppresses interleukin-8 expression in
LPS-stimulated human umbilical vein endothelial cell by blocking
ERK, p38 and nuclear factor-kappaB signaling pathways. J
Ethnopharmacol. 125:83–89. 2009. View Article : Google Scholar
|
|
16.
|
Kalayoglu MV, Hoerneman B, LaVerda D,
Morrison SG, Morrison RP and Byrne GI: Cellular oxidation of
low-density lipoprotein by Chlamydia pneumoniae. J Infect
Dis. 180:780–790. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cheng LC, Su KH, Kou YR, et al: α-Lipoic
acid ameliorates foam cell formation via liver X receptor
α-dependent upregulation of ATP-binding cassette transporters A1
and G1. Free Radic Biol Med. 50:47–54. 2011.
|
|
18.
|
Tsai JY, Su KH, Shyue SK, et al: EGb761
ameliorates the formation of foam cells by regulating the
expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc
Res. 88:415–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lim H and Kim HP: Inhibition of mammalian
collagenase, matrix metalloproteinase-1, by naturally-occurring
flavonoids. Planta Med. 73:1267–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Crespo I, García-Mediavilla MV, Gutiérrez
B, Sánchez-Campos S, Tuñón MJ and González-Gallego J: A comparison
of the effects of kaempferol and quercetin on cytokine-induced
pro-inflammatory status of cultured human endothelial cells. Br J
Nutr. 100:968–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Abeywardena MY and Head RJ: Dietary
polyunsaturated fatty acid and antioxidant modulation of vascular
dysfunction in the spontaneously hypertensive rat. Prostaglandins
Leukot Essent Fatty Acids. 65:91–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mekhora C, Muangnoi C, Chingsuwanrote P,
Dawilai S, Svasti S, Chasri K and Tuntipopipat S: Eryngium foetidum
suppresses inflammatory mediators produced by macrophages. Asian
Pac J Cancer Prev. 13:653–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tu YC, Lian TW, Yen JH, Chen ZT and Wu MJ:
Antiatherogenic effects of kaempferol and rhamnocitrin. J Agric
Food Chem. 28:9969–9976. 2007.
|
|
24.
|
Witztum JL: You are right too! J Clin
Invest. 115:2072–2075. 2005.
|
|
25.
|
Kunjathoor VV, Febbraio M, Podrez EA, et
al: Scavenger receptors class A-I/II and CD36 are the principal
receptors responsible for the uptake of modified low density
lipoprotein leading to lipid loading in macrophages. J Biol Chem.
277:49982–49988. 2002. View Article : Google Scholar
|
|
26.
|
Katayama I, Hotokezaka Y, Matsuyama T,
Sumi T and Nakamura T: Ionizing radiation induces macrophage foam
cell formation and aggregation through JNK-dependent activation of
CD36 scavenger receptors. Int J Radiat Oncol Biol Phys. 70:835–846.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Oh J, Weng S, Felton SK, et al: 1,25(OH)2
vitamin d inhibits foam cell formation and suppresses macrophage
cholesterol uptake in patients with type 2 diabetes mellitus.
Circulation. 120:687–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Duh PD, Hsiao WC and Wang BS: An aqueous
extract of Welsh onion green leaves increase ABCA1 and SR-BI
expression in macrophage RAW 264.7 cells. Food Chemistry.
107:1029–1038. 2008. View Article : Google Scholar
|
|
29.
|
Lu KY, Ching LC, Su KH, et al:
Erythropoietin suppresses the formation of macrophage foam cells:
role of liver X receptor alpha. Circulation. 121:1828–1837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chang YC, Lee TS and Chiang AN: Quercetin
enhances ABCA1 expression and cholesterol efflux through a
p38-dependent pathway in macrophages. J Lipid Res. 53:1840–1850.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gao J, Xu Y, Yang Y, et al: Identification
of upregulators of human ATP-binding cassette transporter A1 via
high-throughput screening of a synthetic and natural compound
library. J Biomol Screen. 13:648–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Ishikawa K, Sugawara D, Wang Xp, Suzuki K,
Itabe H, Maruyama Y and Lusis AJ: Heme oxygenase-1 inhibits
atherosclerotic lesion formation in ldl-receptor knockout mice.
Circ Res. 88:506–512. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Juan SH, Lee TS, Tseng KW, Liou JY, Shyue
SK, Wu KK and Chau LY: J Adenovirus-mediated heme oxygenase-1 gene
transfer inhibits the development of atherosclerosis in
apolipoprotein E-deficient mice. Circulation. 104:1519–1525. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Idriss NK, Blann AD and Lip GY:
Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol.
52:971–978. 2008. View Article : Google Scholar : PubMed/NCBI
|