Introduction
Liver cancer, particularly primary liver cancer, is
the leading cause of cancer-related mortality in both men and women
in China (1,2). At present, hepatectomy is recognized
as the first-line therapeutic method for primary and metastatic
liver tumors (3). However, liver
resection is restricted by the need to preserve a sufficient amount
of hepatocytes to maintain normal physiological function (4,5).
Owing to improved understanding of the segmental anatomy of the
liver and developments in surgical techniques, the mortality rate
after liver resection has been reduced to 0–5% (6,7).
Postresection liver failure (PLF), which is considered to be caused
by excess inflammatory cytokines, reactive oxygen intermediates,
apoptosis and disturbances of hepatic microcirculation, is the
major cause of mortality following massive hepatectomy (8–11).
Therefore, development of agents to prevent acute liver damage,
particularly PLF, following liver resection, is of great
significance in clinical application.
Prostaglandin E1 (PGE1) exerts a broad range of
effects on cytoprotection and is widely used as a vasodilator in
clinical practice (12). It has
been shown that administration of liposome PGE1 (Lipo-PGE1) in rats
could suppress ischemia-reperfusion (I/R) injury of the liver by
improving microcirculation, reducing portal venous pressure (PVP)
and alleviating parenchyma injury (13). Matsuo et al (14) reported that PGE1 induces heat
shock proteins (HSPs) immediately after hepatic I/R. The
intraportal infusion of PGE1 prevents the accumulation of excessive
ascites and hyperbilirubinemia in patients with small-for-size
graft (15). Moreover, it has
been demonstrated that pretreatment of rats with PGE1 could improve
the survival rate after massive hepatectomy by inhibiting apoptosis
and stimulating hepatocyte proliferation as well as heat shock
protein 70 (HSP70) accumulation (16–19). However, the underlying molecular
mechanisms by which PGE1 exerts its protective effects on rats
after massive hepatectomy are not fully elucidated. Therefore, a
better understanding of such mechanisms will greatly promote the
application of PGE1 to prevent acute liver damage after liver
resection in the clinical setting.
Somatostatin (SST), a regulatory peptide hormone, is
widely distributed in the body and is involved in multiple
biological processes (20,21).
Previous reports have documented that SST was able to suppress
hepatic I/R injury (22) and
reduce PVP (23). Additionally,
it has been shown that administration of SST or its analogues,
octreotide, could exert protective effects against hepatic injury
of rats undergoing partial hepatectomy and liver transplantation
(24–28). However, it remains unknown whether
SST possesses similar effects on rats undergoing massive
hepatectomy.
In the present study, we used 90% hepatectomy of
rats as a model to investigate whether SST can potentiate the
therapeutical effects of PGE1 on rats undergoing massive liver
resection. Our results showed that co-administration of PGE1 with
SST attenuated acute liver damage after massive hepatectomy in rats
more efficiently than either drug alone. The underlying mechanisms
are associated with inhibition of inflammatory responses, apoptosis
and endoplasmic reticulum (ER) stress.
Materials and methods
Animals and drugs
Male Sprague-Dawley rats, weighing 250–350 g, were
purchased from the Experimental Animal Center of Shengjing
Hospital. They were housed in an air-conditioned room at 25°C with
a controlled 12 h dark/light cycle. The animals received human care
with unlimited access to chow and water. The surgeries were
performed between 9 a.m. and 5 p.m.. All rats were fasted for 12 h
before surgery. The animal study protocol was reviewed and approved
by the Ethics Committee of China Medical University. SST (100
μg/vial) was obtained from Sandoz (Basel, Switzerland) and
dissolved in physiological saline. PGE1 (10 μg/vial) was
purchased from Tide Pharmaceutical Co., Ltd. (Beijing, China). One
hundred micrograms PGE1 were dissolved in 20 ml physiological
saline. A dose of 0.5 μg/kg/min PGE1 and 20 μg/kg
body weight SST were used in the present study.
Experimental protocol
All rats were randomly divided into the control,
SST, PGE1 or combined treatment group, with 26 rats each group.
Rats in the SST group were injected intravenously with SST (20
μg/kg body weight, dissolved in 1 ml physiological saline)
30 min before the operation. During the surgery, physiological
saline was continuously pumped intravenously and the total volume
was the same as PGE1 treatment. Another intravenous injection of
SST (20 μg/kg body weight) was given after the hepatectomy.
Rats in the PGE1 group received 1 ml physiological saline 30 min
before the operation and continuous intravenous pumping (0.5
μg/kg/min) during the surgery. Instead of SST, 1 ml
physiological saline was given after the operation. For the
combined group, the treatment protocol was the same as the PGE1
group, except that SST (20 μg/kg body weight) was injected
before or after the operation instead of physiological saline
alone. Rats in the control group received physiological saline
during the entire process. All animals were anesthetized with
sodium pentobarbital (40 mg/kg) and the surgery was performed as
described by Togo et al (18). Briefly, the rats were placed in a
supine position and then a midline incision was made. After
detaching from surrounding tissues, the liver was pushed out. All
rats received a hypodermic injection of 10% glucose after the
hepatectomy. Animals were sacrificed (16 rats in each group) at 10
min, 6, 12 and 24 h after the operation, except for survival
observation (10 rats in each group). Liver specimens and blood
samples were collected at each time point. The residual livers were
fixed in 10% formalin or frozen in liquid nitrogen immediately, and
were then subjected to histological examination or western blot
analysis. PVP was measured by presser transducer which was placed
into the portal vein after puncturing the vein with a 23-gauge
needle. Activities of ALT and AST in serum were determined by the
automatic Biochemistry Analyzer (Abbott Laboratories, Irving, TX,
USA).
Histological examination
The remnant liver tissues were collected at 10 min,
6, 12 and 24 h after 90% hepatectomy for all groups. Liver samples
were fixed in 10% neutral buffered formalin and then frozen section
was performed. Subsequently, these sections were stained with
routine hematoxylin and eosin (H&E) to evaluate the
morphological alterations by using a standard protocol.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA kits (Senxiong Biotechnology Co., Ltd.,
Shanghai, China) were used to measure the concentration of tumor
necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in serum
according to the manufacturer’s instructions. The sample
concentrations were calculated from a standard curve and expressed
as pg/ml.
Western blot analysis
Liver tissues were obtained at 10 min, 6, 12 and 24
h after 90% hepatectomy from all groups. The samples were weighed,
sectioned and then washed with 0.9% NaCl. The homogenates were
sonicated for 5 sec, 20–30 times, and then centrifuged at 12,000 ×
g for 30 min at 4°C. Following centrifugation, supernatant was
collected and the protein concentrations were determined using a
bovine serum albumin (BSA) standard line. Equal amounts of protein
were separated on sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred electrophoretically
onto polyvinylidene difluoride (PVDF) membranes (Millipore,
Bedford, MA, USA). The blotted membranes were blocked with 5%
non-fat dry milk (w/v) in Tris-buffered saline with 0.1% Tween-20
(TBST). Subsequently, the membranes were incubated with anti-TNF-α,
IL-6, Bax, Bcl-2, caspase-3, HSP70, glucose-regulated protein 78
(GRP78) and CHOP antibodies, respectively (1:1,000 dilution; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C.
After three rinses with TBST at 10-min intervals, the membranes
were incubated with horseradish peroxidase-labeled goat anti-rabbit
IgG (1:5,000 dilution; Santa Cruz Biotechnology, Inc.,) for 2 h at
room temperature. Then, protein bands were visualized by an ECL
plus chemiluminescence kit (Millipore). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as a loading control.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) staining
Apoptosis was evaluated by the TUNEL staining which
was performed with a commercially available in situ cell
death detection kit (ZSGB-Bio Co., Ltd., Beijing, China), according
to the manufacturer’s instructions. Liver samples were embedded in
paraffin and then sectioned at a thickness of 4 μm. The
sections were dewaxed in xylene and rehydrated through a graded
series of ethanol (100-70%). Then, the sections were digested with
proteinase K (40 μg/ml) for 10 min at 37°C. After digestion,
the sections were incubated with 3% H2O2 for
10 min at room temperature to block the endogenous peroxidase
activity. The slides were covered with citric acid buffer for 5 min
and then with working solution which contained TdT enzyme overnight
at 4°C. After washing with PBS, the slides were incubated with
peroxidase for 1 h at 37°C. The color was developed with the DAB
substrate kit and the reactions were terminated by tap water. The
sections were then counterstained with hematoxylin, dehydrated and
mounted. Total hepatocytes and apoptotic cells were counted from 5
random high-power fields for each section. Apoptotic index
(apoptotic cells/100 hepatocytes) was used to evaluate the effect
of PGE1 and SST on apoptosis after 90% hepatectomy.
Statistical analysis
All data analyses were performed with SPSS 17.0
software package (SPSS Inc., Chicago, IL, USA) and are presented as
the means ± standard deviation (SD). Statistical analysis was
evaluated by one-way ANOVA followed by LSD test unless otherwise
stated. Survival rates after 90% hepatectomy were expressed using
the Kaplan-Meier curves. The log-rank test was used for the
survival statistics. P<0.05 was considered to indicate a
statistically significant difference.
Results
Co-administration of PGE1 with SST
improves survival in rats after massive hepatectomy
To investigate the protective effects of PGE1 and
SST on rats after massive hepatectomy, rats were treated as
described in Materials and methods, and the survival of these rats
was recorded for 7 days after 90% hepatectomy. The Kaplan-Meier
method was used to analyze the obtained results. We found that both
PGE1 and SST ameliorated the survival rate of rats after 90%
hepatectomy compared with the control group (P<0.05, log-rank
test). The survival rate was improved significantly by
co-administration of these two drugs (P<0.01, log-rank test)
(Fig. 1). Our data suggest that
co-administration of PGE1 with SST exerts a much stronger
protective effect on rats following massive hepatectomy than either
drug alone.
Co-administration of PGE1 with SST
attenuates liver injury in rats following massive hepatectomy
Previous reports have shown that acute portal
hypertension after hepatectomy could induce liver injury (29,30). To determine whether PGE1 and SST
can attenuate liver injury caused by acute portal hypertension in
rats after massive hepatectomy, PVP was measured at different time
points. As shown in Fig. 2A,
rapid increases in PVP were observed 10 min after 90% hepatectomy
in rats in all treatment groups. However, pretreatment with PGE1
and SST, either alone or in combination, significantly inhibited
the increase in PVP compared to the control group (P<0.01).
Next, we analyzed the releases of alanine
aminotransferase (ALT) and aspartate aminotransaminase (AST) in
serum which was used as an indicator of liver injury. The obtained
results showed that the serum levels of ALT and AST were elevated
postoperatively in all groups. In contrast to the control group,
co-administration of PGE1 with SST significantly reduced ALT and
AST in serum (P<0.001) (Fig. 2B
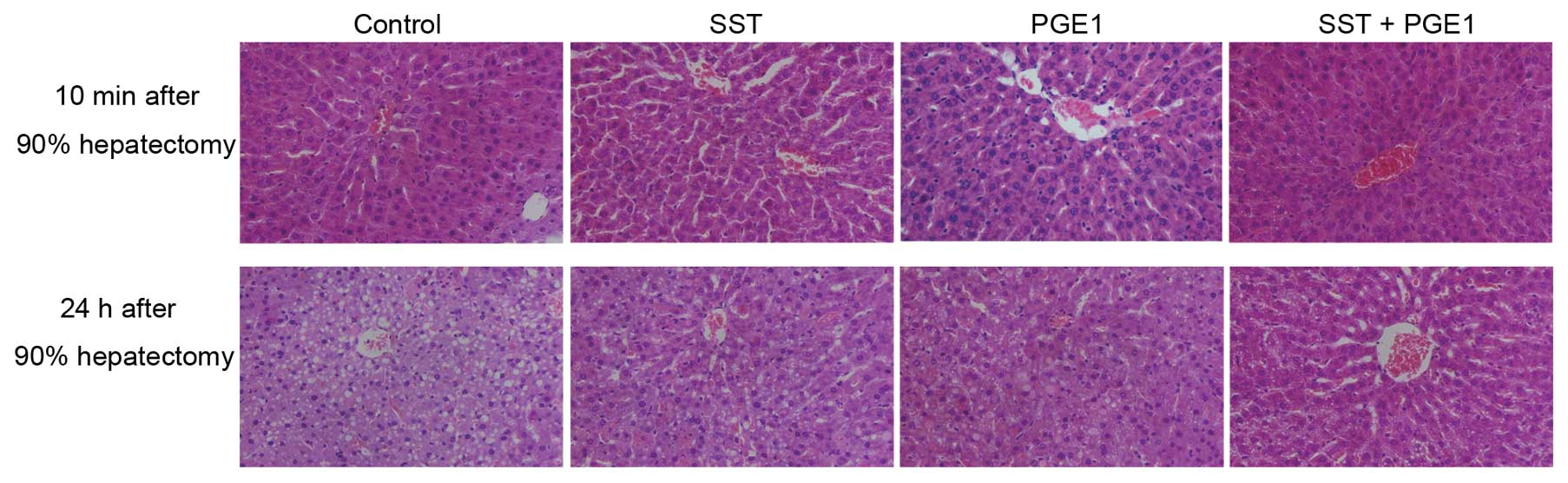
and C). H&E staining results showed that no obvious
histological alterations were observed 10 min after 90% hepatectomy
(Fig. 3). However, pathological
changes, such as disorder structure of hepatic lobules, enlarged
hepatic sinus, liver cell swelling and partial necrosis, appeared
24 h after massive hepatectomy in the control group (Fig. 3). Co-administration of PGE1 with
SST abated these changes more efficiently than either PGE1 or SST
alone (Fig. 3). Collectively, our
data demonstrate that co-administration of PGE1 with SST attenuates
liver injury in rats after massive hepatectomy.
Production of inflammatory cytokines is
suppressed in rats after massive hepatectomy by co-administration
of PGE1 with SST
Inflammatory cytokines, such as TNF-α and IL-6, have
been shown to be involved in liver failure after massive resection
(31,32). Therefore, contents of TNF-α and
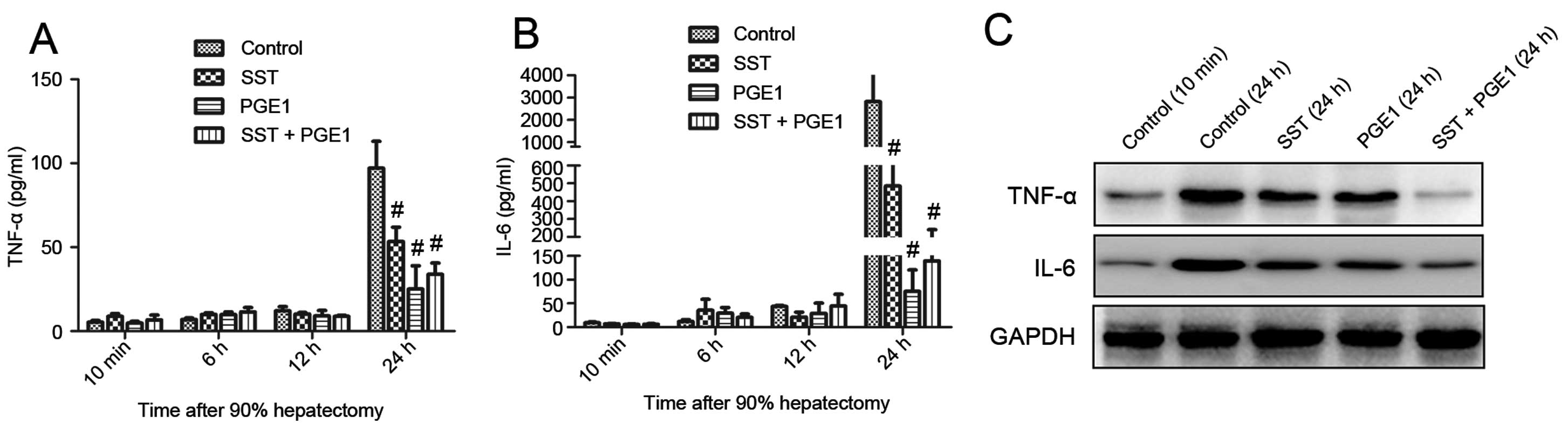
IL-6 were determined in serum and the remnant liver. As shown in
Fig. 4A and B, TNF-α and IL-6
were minimally expressed 10 min after 90% hepatectomy in serum from
all groups. Twenty-four hours postoperatively, the levels of TNF-α
and IL-6 were greatly increased in the control group. By contrast,
these increases were significantly attenuated by administration of
PGE1 and SST. Pretreatment with PGE1 alone was more effective than
combined with SST in terms of inhibiting inflammatory cytokine
accumulation in serum. We found similar results in remnant liver,
except that co-administration of PGE1 with SST exhibited the most
effective inhibition effect than either drug alone (Fig. 4C).
Co-administration of PGE1 with SST
inhibits apoptosis in rats after massive hepatectomy
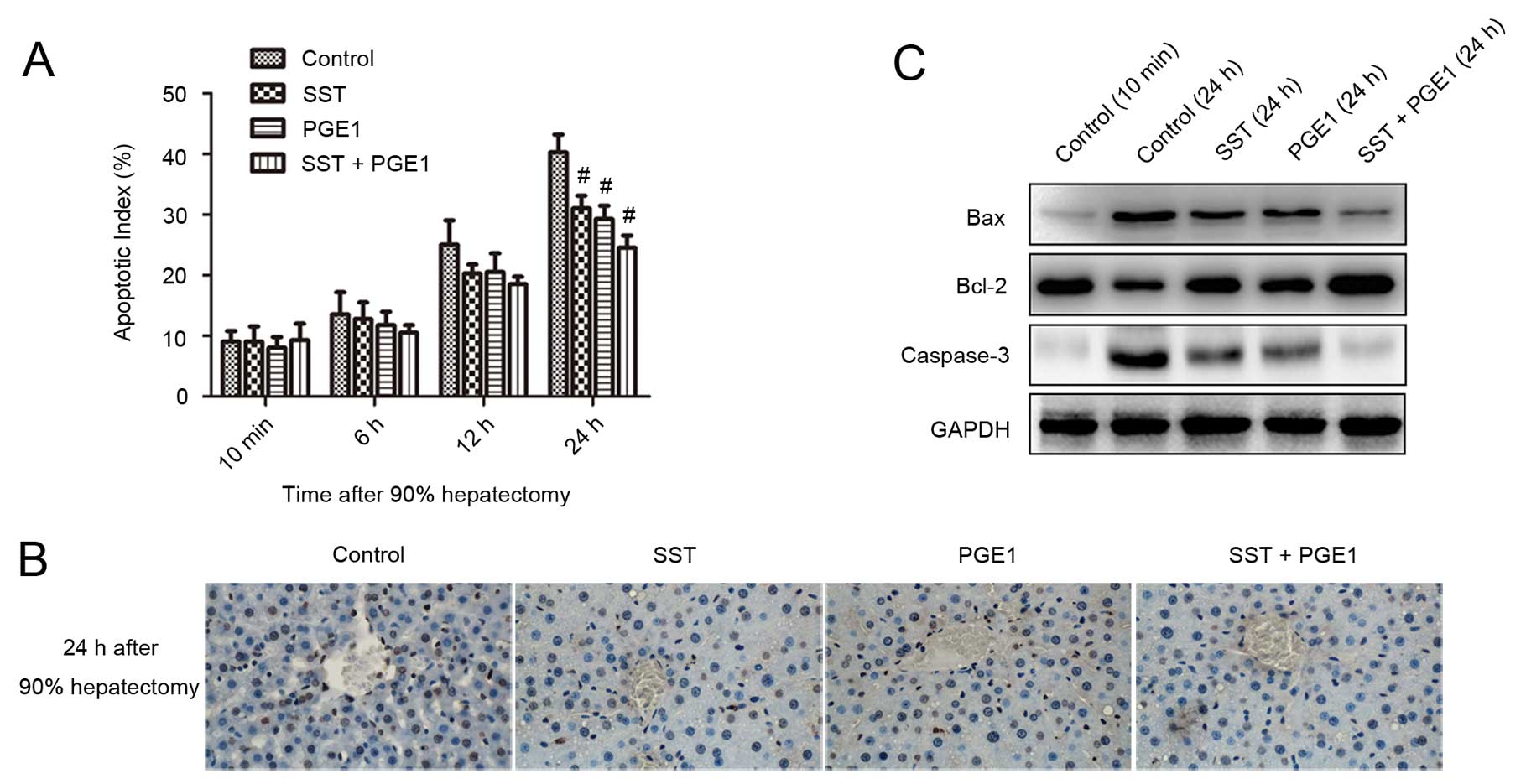
To investigate the effects of PGE1 and SST on
apoptosis, TUNEL assays were performed. The results indicated that
the apoptotic index (AI) increased over time for all groups.
Twenty-four hours postoperatively, the AI value decreased from
40.25±2.99% for the control group to 29.25±2.21, 31±2.16,
24.5±2.08% for the SST, PGE1 and combined treatment groups,
respectively (Fig. 5A).
Co-administration of PGE1 with SST suppressed apoptosis more
efficiently than either alone as evidenced by less TUNEL-positive
staining (Fig. 5B). Furthermore,
we observed that pretreatment with PGE1 and SST downregulated
expression levels of Bax and caspase-3 and upregulated Bcl-2,
particularly in the combined treatment group (Fig. 5C). These results indicate that
co-administration of PGE1 with SST suppresses apoptosis in rats
after liver resection.
Co-administration of PGE1 with SST
alleviates ER stress in rats after massive hepatectomy
In order to eliminate ER stress, eukaryotic cells
initiate unfolded protein response (UPR) which involves suspension
of protein synthesis and upregulation of molecular chaperones. To
explore whether PGE1 and SST alleviate ER stress, we analyzed the
expression of UPR related proteins. Western blotting results showed
that the expression levels of two molecular chaperones, HSP70 and
GRP78, were significantly increased by co-administration of PGE1
with SST compared to the control group (Fig. 6). Transcription factor C/EBP
homologous protein (CHOP), whose expression is upregulated by ER
stress, is involved in ER stress-induced apoptosis (33). We found that pretreatment with
PGE1 and SST significantly inhibited the expression of CHOP,
particularly in the combined group (Fig. 6). These data suggest that
co-administration of PGE1 with SST alleviates ER stress in the
remnant liver.
Discussion
In the present study, we examined the protective
effects of PGE1 and SST in rats after 90% hepatectomy. Our data
demonstrated that co-administration of PGE1 with SST attenuates
liver injury and improves the survival rates in rats after massive
hepatectomy more efficiently than either treatment alone. The
underlying mechanisms include inhibition of inflammatory responses
and apoptosis, and alleviation of ER stress.
Liver failure after massive hepatic resection is
considered a major contributor to postoperative mortality (3). The development of liver failure
involves several causes, such as excess inflammatory response,
impaired liver regeneration and induction of apoptosis, abnormal
hemodynamics, I/R injury and sepsis (34). Therefore, drug combinations which
can exert multiple therapeutical actions are preferable. According
to earlier findings, both PGE1 and SST have been shown to protect
against liver injury produced by I/R injury and partial hepatectomy
(13,16,22,25). The results obtained in the present study showed
that application of PGE1 or SST significantly improved survival,
reduced PVP, ALT, AST and alleviated the histological changes of
rats after 90% hepatectomy. In particular, the combination of these
two drugs exhibited a much stronger protective effect than either
drug alone. It should also be noted that co-administration of PGE1
with SST did not show a significant difference in reducing PVP
compared to PGE1 or SST alone. This may be due to the major effect
of PGE1 on liver hemodynamics in increasing portal venous flow
rather than reducing PVP (35).
Typically pro-inflammatory cytokines, such as TNF-α
and IL-6, participate in the initiation of liver regeneration
(36). A previous report showed
that impaired liver regeneration after partial hepatectomy in
ICAM-1 deficient mice is associated with a marked decrease in
tissue TNF-α and IL-6 levels. In addition, injection of recombinant
IL-6 could restore the proliferation of hepatocytes (37). Receptor for advanced glycation end
products (RAGE) inhibits regeneration after massive liver injury by
suppression of TNF-α (38). It
has been shown that cold ischemia suppresses liver regeneration
after partial liver transplantation in rats by decreasing the
production of TNF-α and IL-6 in rats (39). However, excessive releases of
these cytokines are tightly related to liver failure after massive
hepatectomy. Ogata et al (32) reported that suppression of
excessive TNF-α production using ONO-SM362, a TNF-α suppressant,
ameliorates liver failure after 85% hepatectomy. Another study also
showed that enhanced expressions of TNF-α are detected in rats
after 85% hepatectomy which exhibit extensive patchy necrosis as
compared with 70% hepatectomy (31). The results obtained in our present
study are consistent with previous reports. We found that
expressions of TNF-α and IL-6 were significantly increased 24 h
after 90% hepatectomy both in serum and the remnant liver.
Furthermore, application of PGE1 and SST was able to downregulate
the excessive expressions of TNF-α and IL-6 and thus alleviate
liver injury. In addition, these cytokine levels were relatively
higher even after administration of PGE1 and SST. This may be a
benefit for maintaining and promoting regeneration of the remnant
liver. However, the mechanisms animals use to maintain the balance
of TNF-α and IL-6 between liver injury and regeneration remain to
be elucidated. Co-administration of PGE1 with SST inhibited the
production of TNF-α and IL-6 in the remnant liver more efficiently
than in serum.
Apoptosis and necrosis of the remnant hepatocytes
further aggravate liver injury and eventually result in liver
failure following massive hepatectomy (8). Upregulation of caspases and Fas and
downregulation of Bcl-2 have been found in rats which died of
hepatic failure within 96 h after 95% hepatectomy (40). A previous study demonstrated that
pretreatment with PGE1 significantly increases the expression
levels of Bcl-xL in rats after 95% hepatectomy and thus improves
the survival of these animals (16). Our results further confirmed the
above findings. We observed that apoptosis increased significantly
in rats 24 h after 90% hepatectomy. However, co-administration of
PGE1 with SST reduced the occurrence of apoptosis markedly compared
to the control group. Further analyses demonstrated that
administration of PGE1 or SST in rats after 90% hepatectomy
downregulated the expression levels of pro-apoptotic Bax and
caspase-3, whereas it induced the accumulation of anti-apoptotic
Bcl-2 and eventually inhibited apoptosis. Therefore, PGE1 and SST
exert protective effects on rats after massive hepatectomy at least
partially by inhibiting apoptosis.
The accumulation of misfolded proteins in the lumen
of the ER results in ER stress. UPR, including suspension of
protein synthesis, upregulation of molecular chaperones and
accumulation of folding enzymes, is activated to eliminate such
stress (41). ER stress has been
observed among various liver diseases, such as chronic viral
hepatitis B and C, I/R injury and alcohol-induced liver injury
(42). However, the precise role
of ER stress in rats after massive hepatectomy has yet to be
demonstrated. HSP70 and GRP78 are two molecular chaperones that
facilitate protein folding and thus eliminate ER stress. Enhanced
expression of HSP70 has been found in rats after 95% hepatectomy
(18). However, sustained ER
stress can lead to apoptosis (41). It has been shown that deletion of
the transcription factor CHOP protects against ER stress-induced
apoptosis (33). In the present
study, we found that co-administration of PGE1 with SST greatly
upregulated the protein expression levels of HSP70 and GRP78 and
thus may contribute to alleviating the ER stress induced by
hepatectomy. Moreover, CHOP was significantly inhibited when
pretreated with the combination of PGE1 and SST. Therefore,
co-administration of PGE1 with SST may exert a protective effect
against rats after massive hepatectomy partially by inhibiting
apoptosis induced by excessive ER stress.
In conclusion, our data demonstrated that
co-administration of PGE1 with SST attenuated the acute liver
damage of rats after 90% hepatectomy. The potential mechanisms
included inhibition of inflammatory responses and apoptosis, and
alleviation of ER stress induced by hepatectomy. Our findings have
broad implications in promoting the application of the combination
of these two drugs in clinical settings against liver failure after
massive hepatectomy.
References
|
1.
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hammond JS, Guha IN, Beckingham IJ and
Lobo DN: Prediction, prevention and management of postresection
liver failure. Br J Surg. 98:1188–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jarnagin WR, Gonen M, Fong Y, et al:
Improvement in perioperative outcome after hepatic resection:
analysis of 1,803 consecutive cases over the past decade. Ann Surg.
236:397–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Belghiti J, Hiramatsu K, Benoist S,
Massault P, Sauvanet A and Farges O: Seven hundred forty-seven
hepatectomies in the 1990s: an update to evaluate the actual risk
of liver resection. J Am Coll Surg. 191:38–46. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Virani S, Michaelson JS, Hutter MM, et al:
Morbidity and mortality after liver resection: results of the
patient safety in surgery study. J Am Coll Surg. 204:1284–1292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
House MG, Ito H, Gonen M, et al: Survival
after hepatic resection for metastatic colorectal cancer: trends in
outcomes for 1,600 patients during two decades at a single
institution. J Am Coll Surg. 210:744–755. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hasegawa S, Kubota T, Fukuyama N, et al:
Apoptosis of hepatocytes is a main cause of inducing lethal hepatic
failure after excessive hepatectomy in rats. Transplant Proc.
31:558–559. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mochida S, Ogata I, Hirata K, Ohta Y,
Yamada S and Fujiwara K: Provocation of massive hepatic necrosis by
endotoxin after partial hepatectomy in rats. Gastroenterology.
99:771–777. 1990.PubMed/NCBI
|
|
10.
|
Leist M, Gantner F, Bohlinger I, Tiegs G,
Germann PG and Wendel A: Tumor necrosis factor-induced hepatocyte
apoptosis precedes liver failure in experimental murine shock
models. Am J Pathol. 146:1220–1234. 1995.PubMed/NCBI
|
|
11.
|
Andiran F, Ayhan A, Tanyel FC, Abbasoglu O
and Sayek I: Regenerative capacities of normal and cirrhotic livers
following 70% hepatectomy in rats and the effect of
alpha-tocopherol on cirrhotic regeneration. J Surg Res. 89:184–188.
2000.
|
|
12.
|
Kobayashi S, Baba H, Takeno K, et al:
Blood flow analysis of compressed nerve root after intravenous
injection of lipo-prostaglandin E1. J Orthop Res. 27:1252–1257.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Li JP, Wang Y, Huang YH, Li HP, Chen W and
Yang JY: Experimental study of protective mechanism of Lipo-PGE(1)
on hepatic ischemia-reperfusion injury. Zhonghua Yi Xue Za Zhi.
89:3371–3374. 2009.(In Chinese).
|
|
14.
|
Matsuo K, Togo S, Sekido H, et al:
Pharmacologic preconditioning effects: prostaglandin E1 induces
heat-shock proteins immediately after ischemia/reperfusion of the
mouse liver. J Gastrointest Surg. 9:758–768. 2005. View Article : Google Scholar
|
|
15.
|
Suehiro T, Shimada M, Kishikawa K, et al:
Effect of intraportal infusion to improve small for size graft
injury in living donor adult liver transplantation. Transpl Int.
18:923–928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ishibe A, Togo S, Kumamoto T, et al:
Prostaglandin E1 prevents liver failure after excessive hepatectomy
in the rat by upregulating Cyclin C, Cyclin D1, and Bclxl. Wound
Repair Regen. 17:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ando K, Miyazaki M, Shimizu H, Okuno A and
Nakajima N: Beneficial effects of prostaglandin E(1) incorporated
in lipid microspheres on liver injury and regeneration after 90%
partial hepatectomy in rats. Eur Surg Res. 32:155–161.
2000.PubMed/NCBI
|
|
18.
|
Togo S, Chen H, Takahashi T, et al:
Prostaglandin E1 improves survival rate after 95% hepatectomy in
rats. J Surg Res. 146:66–72. 2008.PubMed/NCBI
|
|
19.
|
Yoshida N, Iwata H, Yamada T, et al:
Improvement of the survival rate after rat massive hepatectomy due
to the reduction of apoptosis by caspase inhibitor. J Gastroenterol
Hepatol. 22:2015–2021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dasgupta P: Somatostatin analogues:
multiple roles in cellular proliferation, neoplasia, and
angiogenesis. Pharmacol Ther. 102:61–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Pinter E, Helyes Z and Szolcsanyi J:
Inhibitory effect of somatostatin on inflammation and nociception.
Pharmacol Ther. 112:440–456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Landa I, Arias J, Gomez M, Quadros M,
Moreno A and Balibrea JL: Cytoprotective effect of somatostatin in
a rat model of hepatic ischemic reperfusion. Hepatology.
16:1474–1476. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Villanueva C and Balanzo J: Variceal
bleeding: pharmacological treatment and prophylactic strategies.
Drugs. 68:2303–2324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pruthi RS, Farouk M, Tsai WH,
Michalopoulos G and Meyers WC: The effect of octreotide on hepatic
regeneration in rats. Surgery. 113:84–89. 1993.PubMed/NCBI
|
|
25.
|
Yamamoto K, Takenaka K, Matsumata T,
Shimada M and Sugimachi K: The effect of octreotide on
morphological hepatic regeneration and hepatic functional recovery
after a two-thirds hepatectomy in rats. Hepatogastroenterology.
46:1880–1884. 1999.PubMed/NCBI
|
|
26.
|
Hashimoto M, Kothary PC and Raper SE: The
effects of transforming growth factor alpha and somatostatin on
regenerating hepatocytes in the rat. Regul Pept. 44:49–59. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Feng ZY, Xu X, Wu LJ, Wu J, Zhu SM and
Zheng SS: Downregulation of endothelin-1 by somatostatin improves
liver function of recipients undergoing adult-to-adult living donor
liver transplantation. Chin Med J (Engl). 123:1961–1966.
2010.PubMed/NCBI
|
|
28.
|
Xu X, Man K, Zheng SS, et al: Attenuation
of acute phase shear stress by somatostatin improves small-for-size
liver graft survival. Liver Transpl. 12:621–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Koyama S, Sato Y and Hatakeyama K: The
subcutaneous splenic transposition prevents liver injury induced by
excessive portal pressure after massive hepatectomy.
Hepatogastroenterology. 50:37–42. 2003.
|
|
30.
|
Yachida S, Wakabayashi H, Kokudo Y, et al:
Measurement of serum hyaluronate as a predictor of human liver
failure after major hepatectomy. World J Surg. 24:359–364. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Panis Y, McMullan DM and Emond JC:
Progressive necrosis after hepatectomy and the pathophysiology of
liver failure after massive resection. Surgery. 121:142–149. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Ogata T, Yamashita K, Horiuchi H, Okuda K
and Todo S: A novel tumor necrosis factor-alpha suppressant,
ONO-SM362, prevents liver failure and promotes liver regeneration
after extensive hepatectomy. Surgery. 143:545–555. 2008. View Article : Google Scholar
|
|
33.
|
Zinszner H, Kuroda M, Wang X, et al: CHOP
is implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Garcea G and Maddern GJ: Liver failure
after major hepatic resection. J Hepatobiliary Pancreat Surg.
16:145–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Nakadaira K, Tsukada K, Sakaguchi T, et
al: A pharmacological analysis of prostaglandin E1 on portal blood
flow after partial hepatectomy in rats. Surg Today. 23:277–279.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Fausto N and Riehle KJ: Mechanisms of
liver regeneration and their clinical implications. J Hepatobiliary
Pancreat Surg. 12:181–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Selzner N, Selzner M, Odermatt B, Tian Y,
Van Rooijen N and Clavien PA: ICAM-1 triggers liver regeneration
through leukocyte recruitment and Kupffer cell-dependent release of
TNF-alpha/IL-6 in mice. Gastroenterology. 124:692–700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cataldegirmen G, Zeng S, Feirt N, et al:
RAGE limits regeneration after massive liver injury by coordinated
suppression of TNF-alpha and NF-kappaB. J Exp Med. 201:473–484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Selzner N, Selzner M, Tian Y, Kadry Z and
Clavien PA: Cold ischemia decreases liver regeneration after
partial liver transplantation in the rat: A
TNF-alpha/IL-6-dependent mechanism. Hepatology. 36:812–818. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Morita T, Togo S, Kubota T, et al:
Mechanism of postoperative liver failure after excessive
hepatectomy investigated using a cDNA microarray. J Hepatobiliary
Pancreat Surg. 9:352–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Malhi H, Guicciardi ME and Gores GJ:
Hepatocyte death: a clear and present danger. Physiol Rev.
90:1165–1194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar
|