Introduction
Osteoclasts are bone-resorbing, multinucleated giant
cells differentiated from monocyte-lineage hematopoietic cells
(1,2). Disruption of bone resorption leads
to sclerotic bone, as seen in osteopetrosis, whereas excessive
resorption is central to the pathogenesis of osteoporosis,
arthritis and periodontal disease. Thus, the elucidation of
regulatory mechanisms involved in osteoclastogenesis is important
to gain a deeper understanding of the health and disease of the
skeletal system.
Osteoclast differentiation occurs in a series of
events (3). First, precursors
alter gene and protein expression to establish a fusion-competent
status, enabling cell-cell recognition and attachment. Second,
mononuclear preosteoclasts fuse together to become nonfunctional
multinucleated osteoclasts that are polykaryons lacking ruffled
borders. Finally, nonfunctional multinucleated osteoclasts are
activated into functional bone resorbing osteoclasts by various
factors.
Receptor activator of nuclear factor-κB
(RANK)-ligand (RANKL) is an essential factor for osteoclast
differentiation (4). RANKL
signaling induces the key transcription factor, nuclear factor of
activated T cells c1 (NFATc1), which regulates a large number of
the osteoclast-associated genes required for osteoclast
differentiation and function (5,6).
Mechanical stress is an important regulatory factor
in bone homeostasis (7). Lack of
stress causes bone loss and even osteoporosis in some cases,
whereas stress overload leads to pathological bone modeling and
remodeling (8). Previous studies
investigated the responses of bone and bone cells to mechanical
stresses, such as sheer stress (fluid flow) (9–11),
compressive force (12–15), tensile force (16–18), hydrostatic pressure (19), microgravity (20), and others (21–23). Several reports have focused on the
effect of mechanical stimulation on osteoblast-like cells, but the
direct effect of mechanical stimulation on the behavior of
osteoclast-like cells has only been investigated in a few studies.
Therefore, we examined the effect of mechanical stress on
osteoclast differentiation using a Flexercell tension system. We
previously showed that mechanical stress (48–96 h) directly
suppresses osteoclast differentiation and fusion (24,25). However, the early phase effect of
osteoclasts in response to direct mechanical stress has not been
elucidated. Thus, this study investigated the effects of short-term
mechanical stress (up to 24 h) on osteoclast differentiation and
fusion.
Materials and methods
Cell culture and osteoclast
differentiation
Mouse monocyte/macrophage RAW264.7 (RAW) cells (no.
TIB-71™; ATCC, Manassas, VA, USA) were used as osteoclast
precursors. RAW cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) (Wako Pure Chemical, Osaka, Japan)
supplemented with 10% fetal bovine serum (FBS) (Invitrogen,
Carlsbad, CA, USA) and 66.7 μg/ml kanamycin sulfate (Meiji
Seika, Japan) in a humidified atmosphere with 5% CO2 at
37°C. For osteoclast differentiation, RAW cells were plated at
9.0×104 cells/well in type I collagen-coated BioFlex
Culture Plates (Flexcell International, Hillsborough, NC, USA) and
were cultured with α-minimum essential medium (α-MEM) (Wako Pure
Chemical) supplemented with 10% FBS, 2 mM L-alanyl-L-glutamine
(Sigma-Aldrich, St. Louis, MO, USA), 284 μM L-ascorbic acid
2-phosphate (Sigma-Aldrich) and 66.7 μg/ml kanamycin sulfate
in the presence of 50 ng/ml RANKL (Oriental Yeast Co., Ltd., Tokyo,
Japan).
Flexercell tension system
Mechanical stress was applied to RAW cells with the
FX-3000™ Flexercell Strain Unit (Flexcell International). RAW cells
stimulated by RANKL were pre-cultured for three days and subjected
to cyclical tensile force [10% elongation at 30 cycles/min (0.5
Hz)] for up to 24 h. Control cells were plated on similar plates
and kept in the same incubator, but were not subjected to
strain.
Tartrate-resistant acid phosphatase
(TRAP) staining
RAW cells were subjected to mechanical stress for 24
h and fixed with 10% neutral formalin. They were then washed with
distilled water and stained with the TRAP staining solution (pH
5.0) supplemented with Fast Red Violet LB Salt (Sigma-Aldrich).
TRAP-positive cells with more than two nuclei were counted under
the microscope. The counted range was 1 cm2 dividing the
circle in a fan-shape (24,25).
RNA isolation and quantitative
RT-PCR
RAW cells were subjected to mechanical stress for 0,
6, 12 and 24 h. Total RNA was extracted from osteoclastogenic
cultures at different time points using the TRIzol reagent
isolation kit (Invitrogen). First-strand cDNA was synthesized using
ReverTra Ace-α FSK-101 (Toyobo, Osaka, Japan). We used specific
primers for osteoclast-associated genes [TRAP, matrix
metalloproteinase-9 (MMP-9), Cathepsin-K (Cath-K), calcitonin
receptor (CTR), ATPase H+ transporting vacuolar proton
pump member I (ATP6i), chloride channel 7 (ClC7), dendritic
cell-specific transmembrane protein (DC-STAMP), osteoclast
stimulatory transmembrane protein (OC-STAMP), E-cadherin, Integrin
αV, Integrin β3, NFATc1, NFATc2 and NFATc3] as shown in Table I. Real-time quantitative PCR
reactions were performed using the ABI PRISM 7300 sequence
detection system (Applied Biosystems, Foster City, CA, USA). Data
were normalized to the expression of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) for each sample and are shown as relative
quantitation of target expression (2−ΔΔCt method)
relative to control after normalization against GAPDH
expression.
 | Table I.Assey IDs of the primers used. |
Table I.
Assey IDs of the primers used.
| Gene | Assay ID |
|---|
| TRAP | Mm00475698_m1 |
| MMP-9 | Mm00432271_m1 |
| Cath-K | Mm00484036_m1 |
| CTR | Mm00432271_m1 |
| ATP6i | Mm00469395_g1 |
| ClC7 | Mm00442400_m1 |
| DC-STAMP | Mm01168058_m1 |
| OC-STAMP | Mm00512445_m1 |
| E-cadherin | Mm01247357_m1 |
| Integrin αV | Mm00434506_m1 |
| Integrin β3 | Mm00443980_m1 |
| NFATc1 | Mm00479445_m1 |
| NFATc2 | Mm00477776_m1 |
| NFATc3 | Mm01249194_m1 |
| GAPDH | Mm99999915_g1 |
Western blot analysis
RAW cells were subjected to mechanical stress for 24
h and washed with ice-cold PBS and lysed in extraction buffer (50
mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1.0% Nonidet P-40 and
protease inhibitors). Cell lysates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western
blotting. The following antibodies were used as primary antibodies:
anti-DC-STAMP antibody (clone 1A; Millipore, Billerica, MA, USA),
anti-E-cadherin antibody (24E10; Cell Signaling, Danvers, MA, USA),
anti-Integrin αV antibody (no. 3919-1; Cell Signaling),
anti-Integrin β3 antibody (no. 4702; Cell Signaling) and
anti-β-actin antibody (AC-15; Sigma-Aldrich). Signals were detected
and analyzed with a LAS1000 luminescent image analyzer (Fuji,
Tokyo, Japan). Densitometric quantitation of western blot analyses
was determined with the ImageJ software (http://rsbweb.nih.gov/ij/).
Luciferase reporter gene assay
RAW cells seeded on Flexercell 6-well plates were
transiently cotransfected with luciferase reporter plasmids [100 ng
each of pNF-AT-TA-Luc (Clontech Laboratories, Inc., Mountain View,
CA, USA) and 10 ng of pRL-SV40 (Toyo Ink, Tokyo, Japan) as an
internal control] using FuGENE® HD (Promega, Madison,
WI, USA) according to manufacturer’s protocol. Following
transfection, RAW cells were subjected to mechanical stress for 24
h, and the luciferase activity was performed with the
Dual-Luciferase® Reporter Assay system (Promega).
Statistical analysis
All results are given as the means ± SD. Comparisons
between two groups were analyzed using the two-tailed unpaired
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
The number of osteoclasts decreases in
response to mechanical stress during osteoclast
differentiation
To investigate the effect of short-term mechanical
stress on osteoclast differentiation, we assessed the number of
TRAP-positive multinucleated osteoclasts (Fig. 1). Mechanical stress was applied
for 24 h, while the control cells were grown in the same
conditions, but without mechanical stress. Mechanical stress
clearly inhibited osteoclastogenesis in RAW cells by >40%
compared with the control cells. Furthermore, the number of large
osteoclasts with >8 nuclei was also significantly decreased by
mechanical stress.
Expression of osteoclast-associated
genes
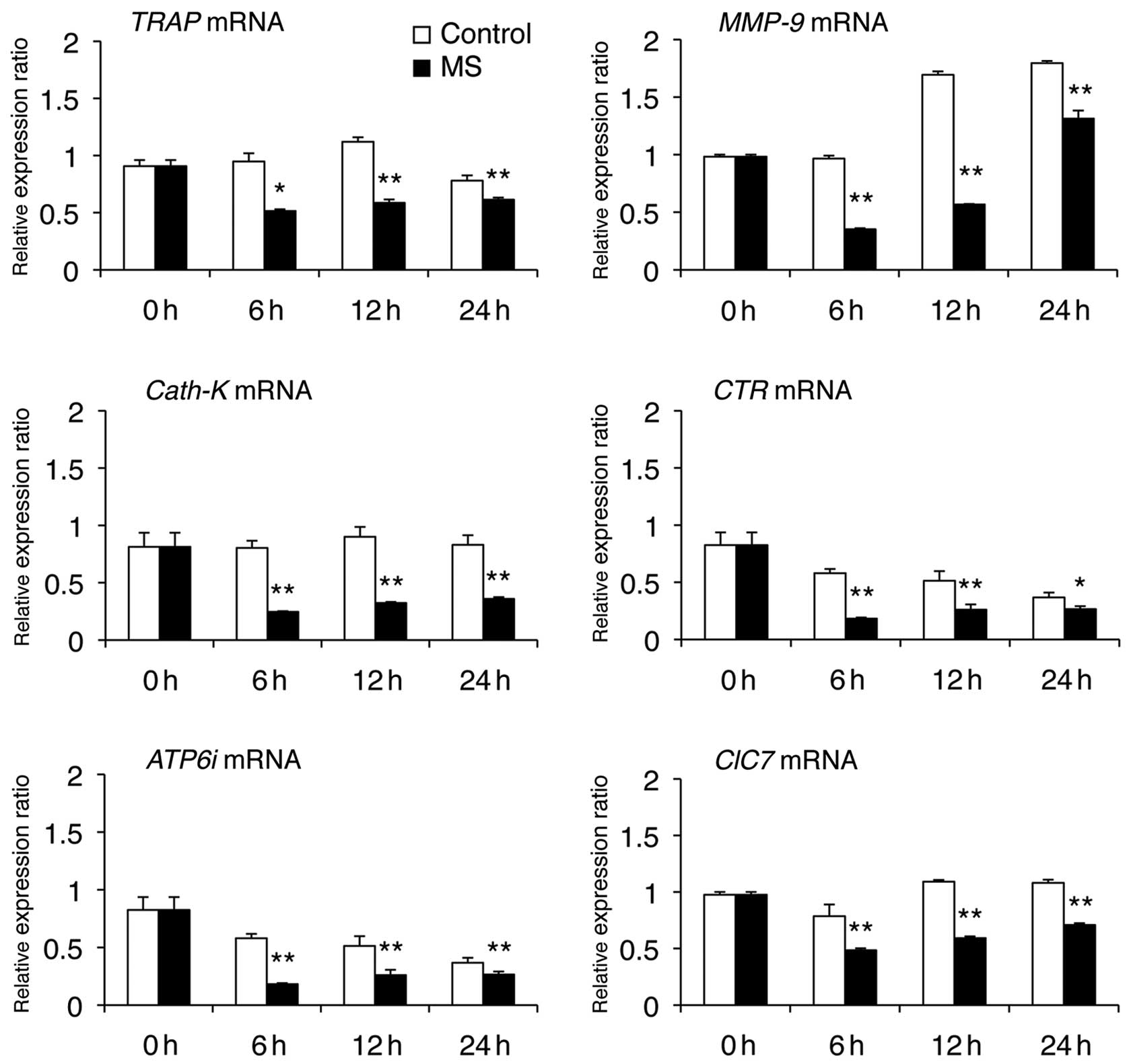
We next examined the effect of mechanical stress (6,
12 or 24 h) on the mRNA levels of osteoclast-specific genes (TRAP,
CTR, MMP-9, Cath-K, ClC7 and ATP6i) and fusion-related molecules
(DC-STAMP, OC-STAMP, E-cadherin, Integrin αV and Integrin β3) that
are required for osteoclast differentiation and bone resorption.
Real-time PCR analysis revealed that mechanical stress caused
downregulation of mRNA levels of osteoclast-specific genes
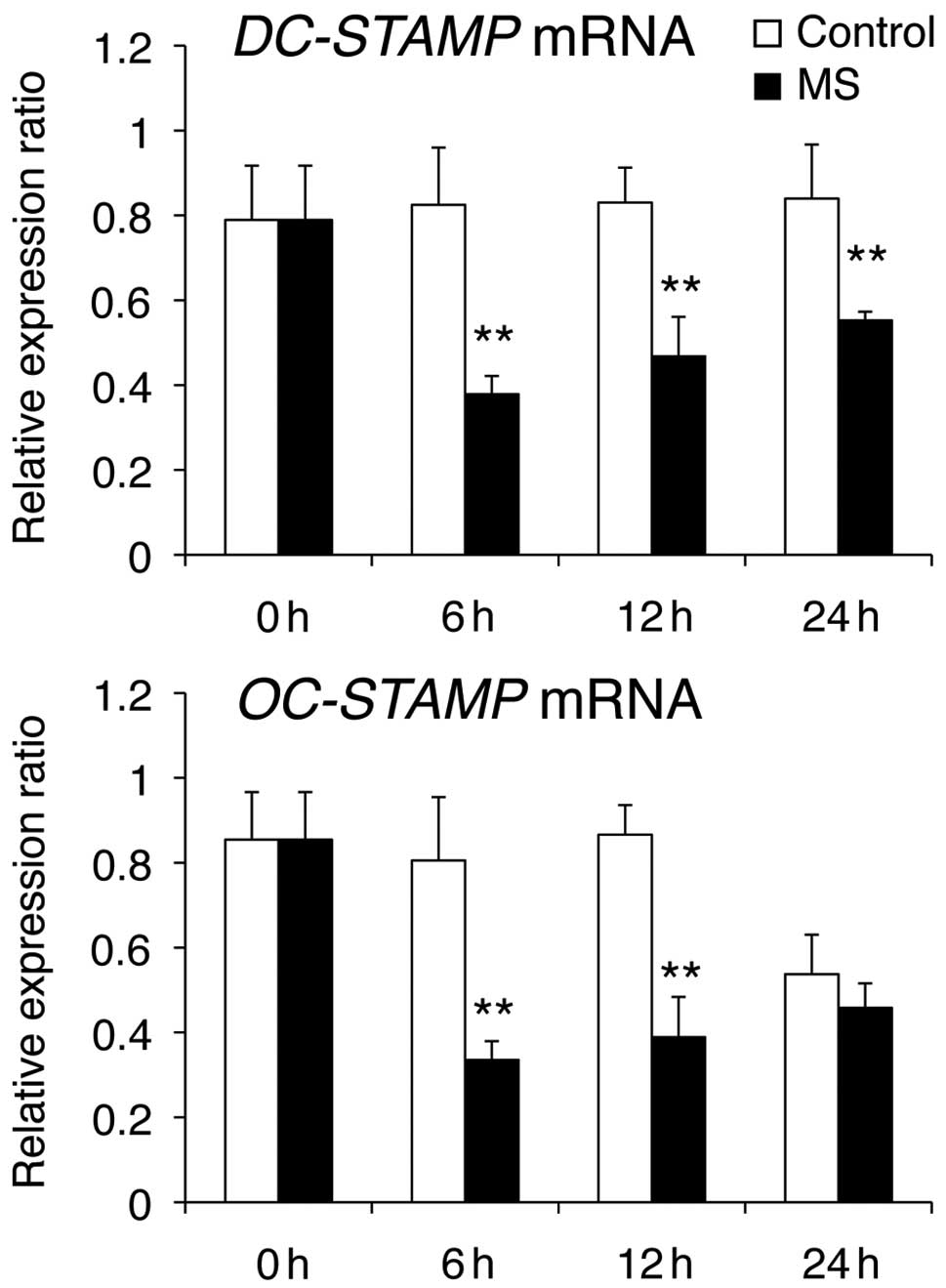
(Fig. 2), DC-STAMP (Fig. 3) and Integrin β3 (Fig. 4). OC-STAMP mRNA levels were
markedly reduced at 6 and 12 h compared with those in control cells
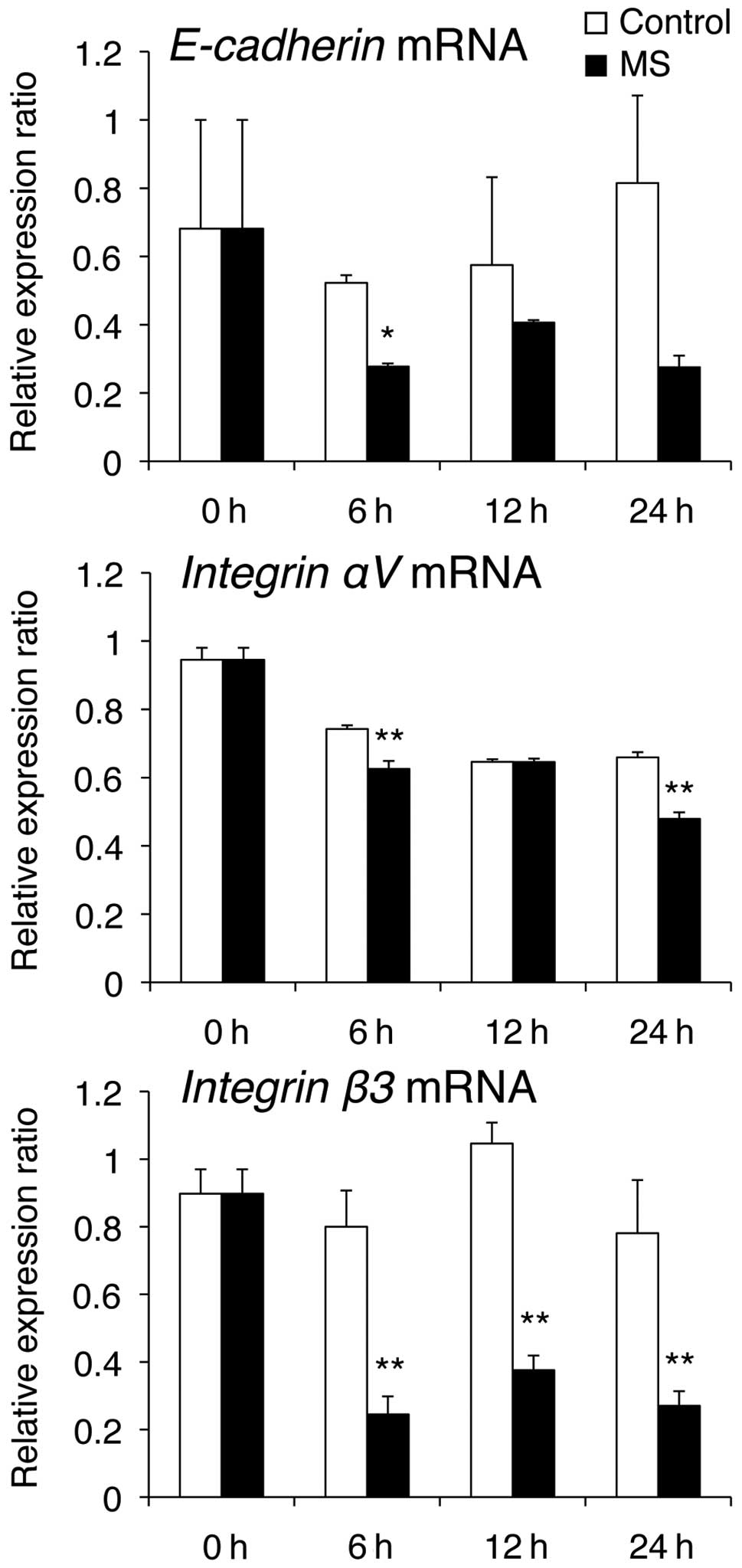
(Fig. 3). E-cadherin mRNA levels
were markedly reduced at 6 h compared with those in the control
cells, while Integrin αV mRNA levels were decreased at 6 and 24 h,
compared with those in control cells (Fig. 4).
Protein levels of DC-STAMP, E-cadherin,
Integrin αV and Integrin β3
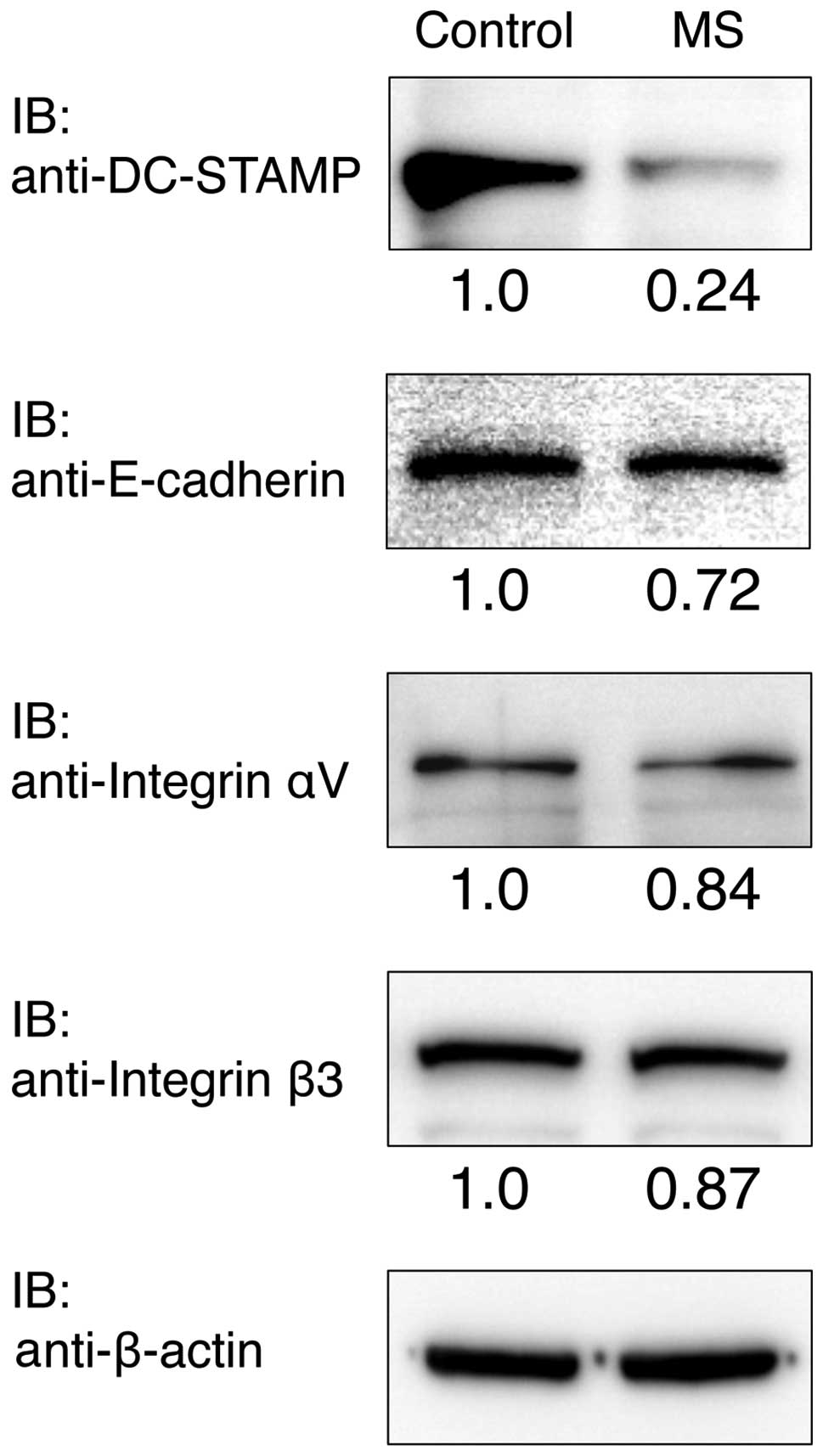
RAW cells were cultured with or without mechanical
stress for 24 h, and the protein expression levels of Integrin αV,
Integrin β3, E-cadherin and DC-STAMP were determined by western
blot analysis (Fig. 5). The
protein expression level of DC-STAMP was clearly reduced compared
with the control after culturing for 24 h. Integrin αV, Integrin β3
and E-cadherin protein expressions were slightly reduced compared
with the control.
Gene expression and transcriptional
activity of NFAT
The transcription factor NFATc1 plays an essential
role in osteoclast differentiation and regulates a number of
osteoclast-associated genes, such as TRAP, CTR, MMP-9, Cath-K,
ClC7, ATP6i, DC-STAMP, OC-STAMP and Integrin β3. To explore the
relationship between mechanical stress and NFATc1, we investigated
the effect of mechanical stress on mRNA levels and transcriptional
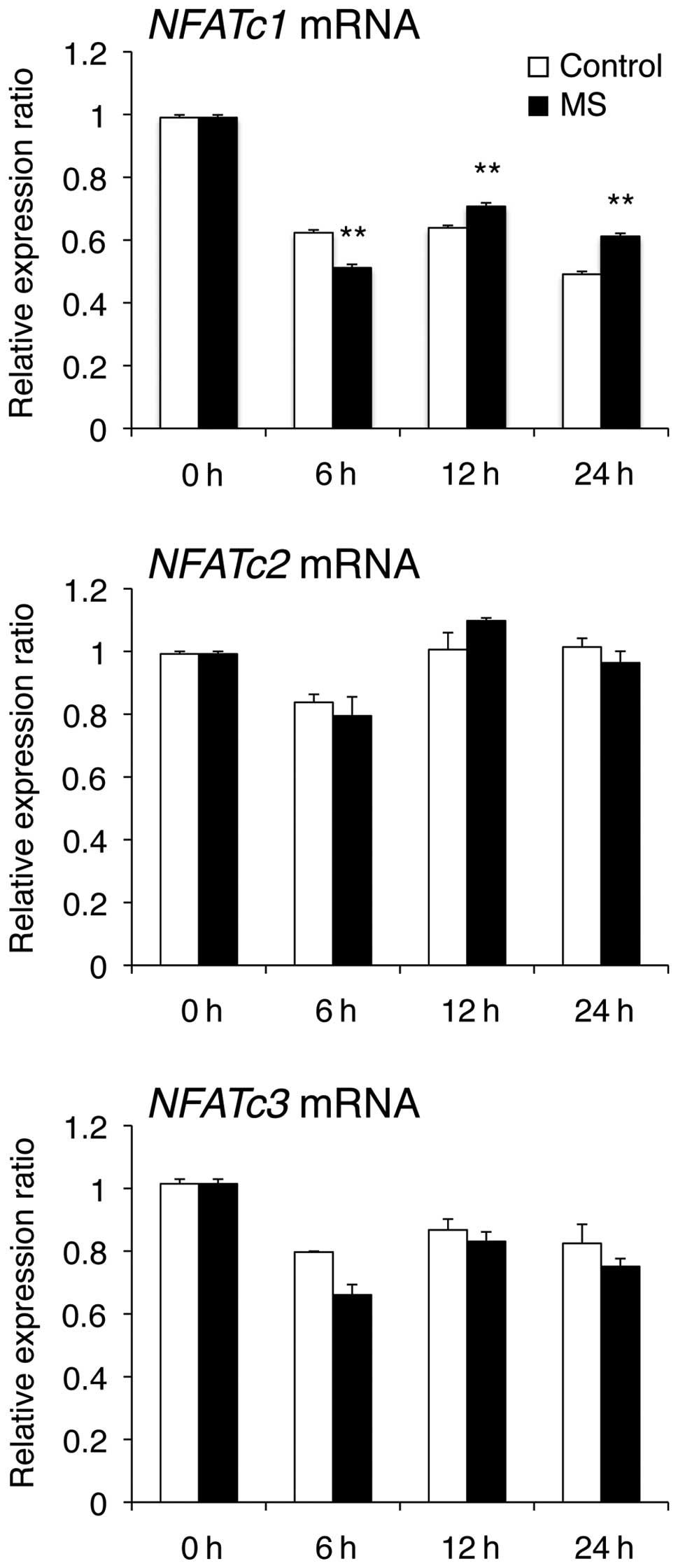
activity of NFAT. NFATc1 mRNA levels were decreased when compared
at 6 h and increased at 12 and 24 h compared with those in control
cells (Fig. 6). However, NFATc2
and NFATc3 mRNA levels were unchanged when compared with those in
control cells. Furthermore, mechanical stress markedly enhanced
NFAT transcriptional activity at 24 h compared with the control
(Fig. 7).
Discussion
This study demonstrated that tensile force inhibits
osteoclast differentiation and fusion by suppressing several
osteoclast-associated genes in the early phase, which is consistent
with results from previous reports (24,25). Furthermore, mRNA and protein
levels of DC-STAMP, which is essential for cell-cell fusion of
osteoclasts, decreased in response to short-term mechanical
stress.
Cell-cell fusion is a critical event for the
development of multinucleated osteoclasts, which occurs in a
multi-step process that involves cell-cell and cell-matrix
interactions, cell spreading and membrane-membrane fusion (3,26).
To date, several molecules have been identified as fusion-related
elements in osteoclasts. DC-STAMP was originally identified in
dendritic cells as an interleukin (IL)-4 inducible protein
consisting of seven transmembrane domains (27). DC-STAMP is also found in
macrophages and osteoclasts and is essential for the
multinucleation of osteoclasts in the presence of RANKL and
macrophage colony-stimulating factor (M-CSF) (28). Studies with mice that are
genetically deficient for DC-STAMP suggest a critical role for
osteoclast precursor (OCP) fusion, since these mice have few
multinucleated TRAP-positive osteoclasts and have increased bone
mineral density (28–31). Conversely, experiments in DC-STAMP
transgenic mice revealed accelerated bone resorption and a
concomitant decrease in bone mass (32). NFATc1 binds to the promoter
regions of DC-STAMP in osteoclasts and directly induces the
expression of DC-STAMP (33).
Thus, the NFATc1-DC-STAMP signaling axis plays a key role in the
osteoclast multinucleation process that is essential for efficient
bone resorption. A previous study showed that RANKL induces
OC-STAMP, which is a multipass transmembrane protein that promotes
the formation of multinucleated osteoclasts (34). Cell-cell fusion in osteoclasts is
modulated cooperatively by OC-STAMP and DC-STAMP, both of which are
induced by the RANKL-NFATc1 axis (35). In this study, the mRNA levels of
DC-STAMP and OC-STAMP decreased in response to mechanical stress
along with a decrease in the number of osteoclasts. Furthermore,
the protein levels of DC-STAMP were also clearly reduced in
response to mechanical stress compared with those in control cells.
These results suggest that mechanical stress suppresses
osteoclastogenesis, possibly through the inhibition of cell-cell
fusion via the downregulation of DC-STAMP and OC-STAMP.
E-cadherin and Integrin αVβ3 are also related to
osteoclast fusion. Cadherins are calcium-dependent adhesion
molecules that mediate cell-cell adhesion. Mbalaviele et al
(36) reported that blocking
E-cadherin suppresses macrophage fusion in vitro, supporting
a key role for E-cadherin in the attachment of precursors prior to
fusion. When precursors arrive at bone, integrin-mediated
attachment is required for differentiation. Integrin αVβ3 is the
most important integrin for proliferation of osteoclast precursor
cells to form multinuclear cells and for subsequent bone resorption
(37). In this study, the mRNA
and protein levels of E-cadherin, Integrin αV and Integrin β3
decreased in response to mechanical stress compared with the
control. These results suggest that mechanical stress may suppress
expressions of E-cadherin, Integrin αV and Integrin β3.
We found that there is a discrepancy between mRNA
expression of NFATc1-mediated genes and NFAT transcriptional
activity; mechanical stress suppressed the mRNA expression of
DC-STAMP and other osteoclast-associated genes that are
transcriptionally regulated by NFATc1, whereas NFAT-reporter assay
revealed that NFAT transcriptional activity was enhanced by
mechanical stress. The following may be possible causes of this
discrepancy; the first cause is that some negative transcriptional
regulator of NFATs may be induced by mechanical stress and this
factor suppresses osteoclast-associated genes. This possibility
seems to be low as several osteoclast-associated genes that have
different promoters are downregulated in response to mechanical
stress. The other is that mechanical stress may induce epigenetic
changes in osteoclast-associated genes. Indeed, it has been
reported that epigenetic mechanisms including histone acetylation
and methylation are important for osteoclast differentiation
(38). Furthermore, demethylation
of H3K27me3 in the Nfatc1 gene locus by Jmjd3 plays a critical role
in RANKL-induced osteoclast differentiation (39), and JMJD5 negatively regulates
osteoclastogenesis as a post-translational co-repressor for NFATc1
(40). It is possible that there
is an association between mechanical stress and the epigenetic
regulation. Although further investigations are required to clarify
this discrepancy, our results suggest that mechanical stress
induces a negative regulatory mechanism that cancels the
enhancement of NFAT transcriptional activity.
During osteoclastogenesis, NFATc1 is a crucial
transcriptional factor and autoregulates its own promoter (38). In this study, NFATc1 mRNA was
decreased at 6 h, compared with control, whereas NFATc1 mRNA was
increased at 12 and 24 h. Although these mRNA changes have
statistical significance, these slight changes may not explain the
NFAT transcriptional activation in response to mechanical stress.
These data suggest that NFAT transcriptional activation in response
to mechanical stress was caused by NFAT functional change, not by
induction of NFAT mRNA.
Tension stress promotes bone formation. Distraction
osteogenesis (DO) is the process of generating new bone in a gap
between two bone segments in response to the application of
graduated tensile stress across the bone gap (41–43). The application of DO offers new
possibilities for the treatment of orofacial anomalies, such as
mandibular widening in transverse direction or lengthening of the
vertical and horizontal mandibular ramus. Widening of the maxilla
by means of DO is the most common application. Similarly,
orthodontic tooth movement necessitates bone resorption at the
pressure side with concomitant bone formation at the tension side
of periodontal ligament. The present study helps to elucidate the
mechanism of bone formation with tension stress and may have
therapeutic implications.
In conclusion, this study demonstrated that
short-term mechanical stress strongly inhibits osteoclast
differentiation and fusion through the downregulation of DC-STAMP
and other fusion-related molecules. Moreover, it induces a negative
regulatory mechanism that cancels the enhancement of NFAT
transcriptional activity.
Acknowledgements
We are grateful to Dr K. Shibata for
the technical advice and support. This study was supported in part
by the Japan Society for the Promotion of Science Grants-in-Aid for
Scientific Research (C) nos. 22592274 to Y.Y., 24659906 to T.K. and
24593071 to M.M.
References
|
1.
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Helming L and Gordon S: Molecular
mediators of macrophage fusion. Trends Cell Biol. 19:514–522. 2009.
View Article : Google Scholar
|
|
4.
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009.
|
|
6.
|
Nakashima T and Takayanagi H: New
regulation mechanisms of osteoclast differentiation. Ann NY Acad
Sci. 1240:E13–E18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Al Nazer R, Lanovaz J, Kawalilak C,
Johnston JD and Kontulainen S: Direct in vivo strain measurements
in human bone-a systematic literature review. J Biomech. 45:27–40.
2012.PubMed/NCBI
|
|
9.
|
Mehrotra M, Saegusa M, Wadhwa S,
Voznesensky O, Peterson D and Pilbeam C: Fluid flow induces Rankl
expression in primary murine calvarial osteoblasts. J Cell Biochem.
98:1271–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tan SD, de Vries TJ, Kuijpers-Jagtman AM,
Semeins CM, Everts V and Klein-Nulend J: Osteocytes subjected to
fluid flow inhibit osteoclast formation and bone resorption. Bone.
41:745–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kulkarni RN, Bakker AD, Everts V and
Klein-Nulend J: Mechanical loading prevents the stimulating effect
of IL-1β on osteocyte-modulated osteoclastogenesis. Biochem Biophys
Res Commun. 420:11–16. 2012.PubMed/NCBI
|
|
12.
|
Ichimiya H, Takahashi T, Ariyoshi W,
Takano H, Matayoshi T and Nishihara T: Compressive mechanical
stress promotes osteoclast formation through RANKL expression on
synovial cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
103:334–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang F, Wang CL, Koyama Y, Mitsui N,
Shionome C, Sanuki R, Suzuki N, Mayahara K, Shimizu N and Maeno M:
Compressive force stimulates the gene expression of IL-17s and
their receptors in MC3T3-E1 cells. Connect Tissue Res. 51:359–369.
2010. View Article : Google Scholar
|
|
14.
|
Cho ES, Lee KS, Son YO, Jang YS, Lee SY,
Kwak SY, Yang YM, Park SM and Lee JC: Compressive mechanical force
augments osteoclastogenesis by bone marrow macrophages through
activation of c-Fms-mediated signaling. J Cell Biochem.
111:1260–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kaneuji T, Ariyoshi W, Okinaga T,
Toshinaga A, Takahashi T and Nishihara T: Mechanisms involved in
regulation of osteoclastic differentiation by mechanical
stress-loaded osteoblasts. Biochem Biophys Res Commun. 408:103–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rubin J, Murphy TC, Fan X, Goldschmidt M
and Taylor WR: Activation of extracellular signal-regulated kinase
is involved in mechanical strain inhibition of RANKL expression in
bone stromal cells. J Bone Miner Res. 17:1452–1460. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Koike M, Shimokawa H, Kanno Z, Ohya K and
Soma K: Effects of mechanical strain on proliferation and
differentiation of bone marrow stromal cell line ST2. J Bone Miner
Metab. 23:219–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kanzaki H, Chiba M, Sato A, Miyagawa A,
Arai K, Nukatsuka S and Mitani H: Cyclical tensile force on
periodontal ligament cells inhibits osteoclastogenesis through OPG
induction. J Dent Res. 85:457–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rubin J, Biskobing D, Fan X, Rubin C,
McLeod K and Taylor WR: Pressure regulates osteoclast formation and
MCSF expression in marrow culture. J Cell Physiol. 170:81–87. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Makihira S, Kawahara Y, Yuge L, Mine Y and
Nikawa H: Impact of the microgravity environment in a 3-dimensional
clinostat on osteoblast- and osteoclast-like cells. Cell Biol Int.
32:1176–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kadow-Romacker A, Hoffmann JE, Duda G,
Wildemann B and Schmidmaier G: Effect of mechanical stimulation on
osteoblastand osteoclast-like cells in vitro. Cells Tissues Organs.
190:61–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kurata K, Uemura T, Nemoto A, Tateishi T,
Murakami T, Higaki H, Miura H and Iwamoto Y: Mechanical strain
effect on bone-resorbing activity and messenger RNA expressions of
marker enzymes in isolated osteoclast culture. J Bone Miner Res.
16:722–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yan YX, Gong YW, Guo Y, Lv Q, Guo C,
Zhuang Y, Zhang Y, Li R and Zhang XZ: Mechanical strain regulates
osteoblast proliferation through Integrin-mediated ERK activation.
PLoS One. 7:e357092012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Suzuki N, Yoshimura Y, Deyama Y, Suzuki K
and Kitagawa Y: Mechanical stress directly suppresses osteoclast
differentiation in RAW264.7 cells. Int J Mol Med. 21:291–296.
2008.PubMed/NCBI
|
|
25.
|
Shibata K, Yoshimura Y, Kikuiri T,
Hasegawa T, Taniguchi Y, Deyama Y, Suzuki K and Iida J: Effect of
the release from mechanical stress on osteoclastogenesis in
RAW264.7 cells. Int J Mol Med. 28:73–79. 2011.PubMed/NCBI
|
|
26.
|
Oursler MJ: Recent advances in
understanding the mechanisms of osteoclast precursor fusion. J Cell
Biochem. 110:1058–1062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hartgers FC, Vissers JL, Looman MW, van
Zoelen C, Huffine C, Figdor CG and Adema GJ: DC-STAMP, a novel
multimembrane-spanning molecule preferentially expressed by
dendritic cells. Eur J Immunol. 30:3585–3590. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K,
Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K,
Oike Y, Takeya M, Toyama Y and Suda T: DC-STAMP is essential for
cell-cell fusion in osteoclasts and foreign body giant cells. J Exp
Med. 202:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kukita T, Wada N, Kukita A, Kakimoto T,
Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H,
Hieshima K, Yoshie O and Nomiyama H: RANKL-induced DC-STAMP is
essential for osteoclastogenesis. J Exp Med. 200:941–946. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yagi M, Miyamoto T, Toyama Y and Suda T:
Role of DC-STAMP in cellular fusion of osteoclasts and macrophage
giant cells. J Bone Miner Metab. 24:355–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yagi M, Ninomiya K, Fujita N, Suzuki T,
Iwasaki R, Morita K, Hosogane N, Matsuo K, Toyama Y, Suda T and
Miyamoto T: Induction of DC-STAMP by alternative activation and
downstream signaling mechanisms. J Bone Miner Res. 22:992–1001.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Iwasaki R, Ninomiya K, Miyamoto K, Suzuki
T, Sato Y, Kawana H, Nakagawa T, Suda T and Miyamoto T: Cell fusion
in osteoclasts plays a critical role in controlling bone mass and
osteoblastic activity. Biochem Biophys Res Commun. 377:899–904.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell-specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:176–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yang M, Birnbaum MJ, MacKay CA,
Mason-Savas A, Thompson B and Odgren PR: Osteoclast stimulatory
trans-membrane protein (OC-STAMP), a novel protein induced by RANKL
that promotes osteoclast differentiation. J Cell Physiol.
215:497–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Miyamoto H, Suzuki T, Miyauchi Y, Iwasaki
R, Kobayashi T, Sato Y, Miyamoto K, Hoshi H, Hashimoto K, Yoshida
S, Hao W, Mori T, Kanagawa H, Katsuyama E, Fujie A, Morioka H,
Matsumoto M, Chiba K, Takeya M, Toyama Y and Miyamoto T: Osteoclast
stimulatory transmembrane protein and dendritic cell-specific
transmembrane protein cooperatively modulate cell-cell fusion to
form osteoclasts and foreign body giant cells. J Bone Miner Res.
27:1289–1297. 2012. View Article : Google Scholar
|
|
36.
|
Mbalaviele G, Chen H, Boyce BF, Mundy GR
and Yoneda T: The role of cadherin in the generation of
multinucleated osteoclasts from mononuclear precursors in murine
marrow. J Clin Invest. 95:2757–2765. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Miyamoto T, Arai F, Ohneda O, Takagi K,
Anderson DM and Suda T: An adherent condition is required for
formation of multinuclear osteoclasts in the presence of macrophage
colony-stimulating factor and receptor activator of nuclear factor
kappa B ligand. Blood. 96:4335–4343. 2000.PubMed/NCBI
|
|
38.
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Yasui T, Hirose J, Tsutsumi S, Nakamura K,
Aburatani H and Tanaka S: Epigenetic regulation of osteoclast
differentiation: possible involvement of Jmjd3 in the histone
demethylation of Nfatc1. J Bone Miner Res. 26:2665–2671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Youn MY, Yokoyama A, Fujiyama-Nakamura S,
Ohtake F, Minehata K, Yasuda H, Suzuki T, Kato S and Imai Y: JMJD5,
a Jumonji C (JmjC) domain-containing protein, negatively regulates
osteoclastogenesis by facilitating NFATc1 protein degradation. J
Biol Chem. 287:12994–13004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ilizarov GA: The tension-stress effect on
the genesis and growth of tissues. Part I. The influence of
stability of fixation and soft-tissue preservation. Clin Orthop
Relat Res. 238:249–281. 1989.PubMed/NCBI
|
|
42.
|
Ilizarov GA: The tension-stress effect on
the genesis and growth of tissues: Part II. The influence of the
rate and frequency of distraction. Clin Orthop Relat Res.
239:263–285. 1989.PubMed/NCBI
|
|
43.
|
Ilizarov GA: Clinical application of the
tension-stress effect for limb lengthening. Clin Orthop Relat Res.
250:8–26. 1990.PubMed/NCBI
|