Introduction
Pancreatic cancer is one of the most common
malignant tumors in the world. In addition, it is the fourth most
common cause of cancer-related deaths with an overall 5-year
survival rate of <2% (1).
Surgical resection represents the main treatment, but only a
minority (10–20%) of patients are eligible for surgical treatment
due to difficulties in early diagnosis (2–4).
The results of chemotherapy and radiotherapy are not satisfactory
because some tumor cells are capable of evading drug or
radiation-induced cell death (5,6).
Alpinia katsumadai
Hayata, as a traditional medicine with low toxicity,
has notable of antitumor and anti-oxidative effects (7,8).
Alpinetin, 7-hydroxy-5-methoxyflavanone (molecular formula
C16H14O4, molecular weight
270.28), found in Alpinia katsumadai Hayata, is a novel
plant-derived flavonoid and is believed to be the major active
ingredient of Alpinia katsumadai Hayata (9,10).
Previous studies demonstrated blockade of the proliferation of the
human tumor cells by alpinetin, indicating the potential anticancer
properties of this compound. The anticancer capability of alpinetin
has also been confirmed in the treatment of breast cancer,
hepatoma, leukemia, carcinoma of the colon and pulmonary cancer
(11–13). However, the antitumor effect of
alpinetin on pancreatic cancer cells and the detailed mechanisms
involved in it remain largely unknown.
It has been suggested that pancreatic cancer cells
have protective mechanisms against the mitochondrial pathway of
apoptosis through overexpression of Bcl-family proteins or XIAP to
block activation of caspases (14). Previous studies also proved that
Bcl-2 and XIAP protein are two important targets for antitumor
medicines (15,16).
The aim of this study was to investigate the
anticancer effect and the possible mechanisms of alpinetin on
pancreatic cancer cells. BxPC-3 is an extremely metastatic human
pancreatic cancer cell line, chosen for detailed study. We found
that alpinetin can induce human pancreatic cancer cells apoptosis,
possibly through regulation of the Bcl-2 family and XIAP expression
and of the release of cytochrome c.
Materials and methods
Cell culture, antibodies and
reagents
The BxPC-3, PANC-1 and AsPC-1 human pancreatic
cancer cell lines were purchased from the American Type Culture
Collection (ATCC). Cells were cultured in RPMI-1640 medium with 10%
fetal bovine serum (FBS) and maintained at 37°C in 5%
CO2. Alpinetin (≥98% purity) was obtained from the
National Institute for Food and Drug Control (Beijing, China).
Bcl-2, Bcl-xL, Bax, XIAP and GAPDH antibodies were from Cell
Signaling Technology, Inc. (USA). Propidium iodide (PI) and Annexin
V- fluorescein isothiocyanate (FITC) were from Sigma (USA). Hoechst
33342 was from Beyotime (China). Fluorogenic caspase substrates
Ac-DEVD-AMC (acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin),
Ac-IETD-AMC (acetyl-Ile-Glu-Thr-Asp-aminomethylcoumarin) and
Ac-LEHD-AMC (acetyl-Leu-Glu-His-Asp-aminomethylcoumarin) were from
Alexis Biochemicals (San Diego, CA).
Cell proliferation assay
The effect of alpinetin on cell proliferation was
detected using methyl-thiazolyl-terazolium (MTT) (Sigma) assay.
Cells growing in logarithmic phase were seeded in the 96-well plate
and then treated with alpinetin. Twenty microliters of MTT (0.5
mg/ml) was added to each well followed by incubation at 37°C for 4
h to allow the yellow dye to be transformed into blue crystals. The
medium was removed and 200 μl of dimethyl sulfoxide (DMSO) (Sigma)
was added to each well to dissolve the dark blue crystals. Finally,
the optical density was measured with a microtiter plate reader at
570 nm. Six replicates were prepared for each condition.
Hoechst 33342 nuclear staining
Pancreatic cancer cells were plated in 6-well plates
with poly-lysine-coated coverslips and cultured for 24 h. Then the
cells were treated with or without alpinetin for 24 h. The
untreated and treated cells were washed twice with PBS and
incubated with 8 μg/ml Hoechst 33342 (Sigma) at 37°C for 20 min,
and fluorescent images were obtained using a fluorescence
microscope (Leica Microsystems, Germany).
Annexin V-FITC/PI double-labeled
detection of apoptosis
The protocol was based on the use of Annexin V-FITC
and PI staining according to the manufacturer's instructions. The
results were analyzed by flow cytometry to differentiate the types
of cell death. Cells that were Annexin V-FITC-positive and
PI-negative were classified as apoptotic or early-stage apoptotic
cells. Briefly, cells were digested with 0.25% trypsin and washed
three times with PBS. Unfixed cells were stained by adding the
Annexin V-FITC reaction mixture (10 μl Annexin V-FITC, 5 μl
propidium iodide) and incubated at room temperature for 15 min in
the dark. The stained cells were subjected to flow cytometric
analysis with a FACSCalibur (Becton-Dickinson, USA).
Caspase activity assay
The activities of caspases-3, −8 and-9 were checked
according to the procedures of Köhler et al with minor
modifications (17). Briefly, the
cells were collected and suspended in extraction buffer (50 mmol/l
Tris-HCl, pH 7.4; 10 mmol/l EGTA; 1 mmol/l EDTA; 10 mmol/l DTT; 1%
(v/v) Triton X-100). Subsequently, the supernatants were stained by
20 mmol/l fluorogenic peptide substrates, Ac-DEVD-AMC (caspase-3),
Ac-IETD-AMC (caspase-8) and Ac-LEHD-AMC (caspase-9) for 30 min at
37°C. Fluorescence was checked on a Perkin-Elmer LS-50B
spectrofluorimeter, setting excitation at 380 nm and emission at
460 nm. Changes in caspase activities were determined by comparing
the levels of the alpinetin-treated cells with the controls.
Mitochondria separation, protein
extraction and western blot analysis
Mitochondria in BxPC-3 cells were separated and
extraction was performed according to the procedures described in
our previous study (18).
Briefly, cells were washed once with ice-cold PBS containing 100 mM
sodium orthovanadate and solubilized in lysis buffer (50 mM
Tris-HCl, 137 mM NaCl, 10% glycerol, 100 mM sodium orthovanadate, 1
mM phenylmethylsulphonyl fluoride (PMSF), 10 mg/ml aprotinin, 10
mg/ml leupeptin, 1% Nonidet P-40; pH 7.4). After centrifugation at
12,000 × g for 20 min, the supernatant was collected. Cells were
dissolved in sample buffer containing 65 mM Tris-HCl (pH 6.8), 3%
SDS, 10% glycerol and 6 M urea. After determination of the protein
concentration (BCA kit; Pierce, Rockford, IL, USA),
β-mercaptoethanol and bromophenol blue were added to the sample
buffer for electrophoresis. The protein was separated by 12%
SDS-polyacrylamide gel electrophoresis (PAGE) and transblotted to
polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA).
The blots were incubated at 4°C overnight with antibodies, and the
resulting bands were detected using enhanced chemiluminescence.
Intensities of the bands were quantified using an image-analysis
system.
Analysis of the cell cycle by flow
cytometry
Cells in the logarithmic phase were seeded in 6-well
plates and then treated with alpinetin. After collection by
centrifugation and washing twice with PBS, the cell pellets were
suspended in 1 ml ice-cold 70% ethanol at 4°C. After 1 h, the fixed
cells were spun by centrifugation and the pellets were washed with
PBS. After resuspension with 1 ml PI integration staining solution,
the cells were incubated with RNase A (10 mg/l), PI (50 mg/l), 1%
Triton X-100 and sodium citrate (1 g/l) shaken for 30 min at 37°C
in the dark. The stained cells were analyzed using a FACSCalibur
flow cytometer (Becton-Dickinson).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). One-way ANOVA and the unpaired Student's t-test was
used to test for differences between the two groups. A P-value
<0.05 was considered statistically significant.
Results
Alpinetin inhibits the growth of
pancreatic cancer cells
To investigate the effect of alpinetin on
proliferation and viability of pancreatic cancer cells, BxPC-3,
PANC-1 and AsPC-1 pancreatic cancer cells were treated with
alpinetin at different doses (0, 20, 40, 60, 80 μg/ml) for 24, 48
or 72 h, and then an MTT assay was performed to determine cell
viability. The results suggested that the viability of three
alpinetin-treated pancreatic cancer cell lines was greatly
decreased with increased alpinetin dose and treatment time
(Fig. 1). At concentrations
ranging from 0 to 40 μg/ml, slight growth inhibition effects on the
pancreatic cancer cells was observed. The concentration of
alpinetin needed to significantly inhibit cell growth of BxPC-3 and
PANC-1, AsPC-1 cells was 60 μg/ml. The effect of inhibition in
pancreatic cancer cells increased proportionately when treated with
at a range from 20 to 80 μg/ml alpinetin for 48 h. BxPC-3 cells are
an extremely metastatic human pancreatic cancer cell line compared
to the other two cell lines examined herein. We thus chose to treat
BxPC-3 cells with 20 to 80 μg/ml alpinetin for 48 h to further
investigate its detailed mechanism of action.
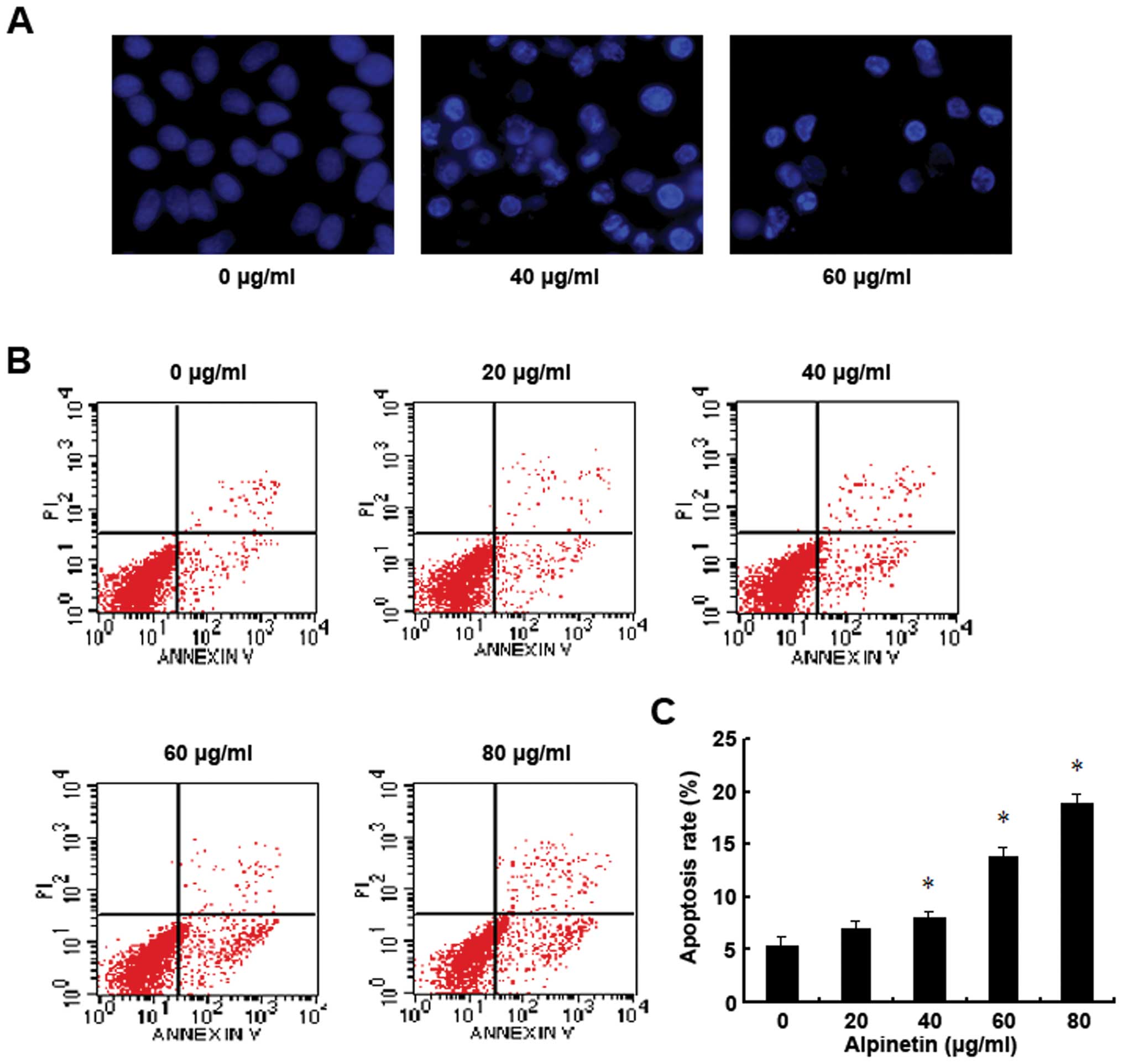
Alpinetin induces BxPC-3 pancreatic
cancer cells apoptosis
To explore whether the inhibitory effects of
alpinetin on pancreatic cancer cells was attributed to the
induction of apoptosis, BxPC-3 cells were treated with alpinetin at
different doses for 48 h. Then the alpinetin-treated cells were
identified using Hoechst 33342 nuclear staining and analyzed by
Annexin V-FITC/PI double staining flow cytometry (Fig. 2). As shown in our results, the
condensed chromatin of apoptotic cells in the 40 and 60 μg/ml
alpinetin-treated groups was significantly brighter than the
chromatin of normal cells in the control group (Fig. 2A). Furthermore, alpinetin-treated
BxPC-3 cells had notably higher apoptosis rates which were
dose-dependent compared to the control group (Fig. 2B and C). These results indicate
that alpinetin dose-dependently induced cell apoptosis to inhibit
proliferation of pancreatic cancer cells.
Effect of alpinetin on caspase activation
in BxPC-3 cells
To detect the apoptotic pathway induced by
alpinetin, BxPC-3 cells were treated with different concentrations
of alpinetin for 48 h, and then the activities of caspases-3, −8
and −9 were determined by the fluorogenic peptide substrates
Ac-IETD, Ac-IETD-AMC and Ac-LEHD-AMC, respectively (Fig. 3). Our data show that the caspase-8
and −9 activities were significantly increased in BxPC-3 cells
treated with 60 μg/ml alpinetin. As a downstream effector of
caspase-8 and −9, the activity of caspase-3 was found to be 3- to
4-fold higher after treatment with 40 μg/ml alpinetin and reached
its peak at 60 μg/ml in alpinetin-treated BxPC-3 cells. Our data
suggest that alpinetin could induce caspase activation and
caspase-dependent apoptosis in BxPC-3 cells.
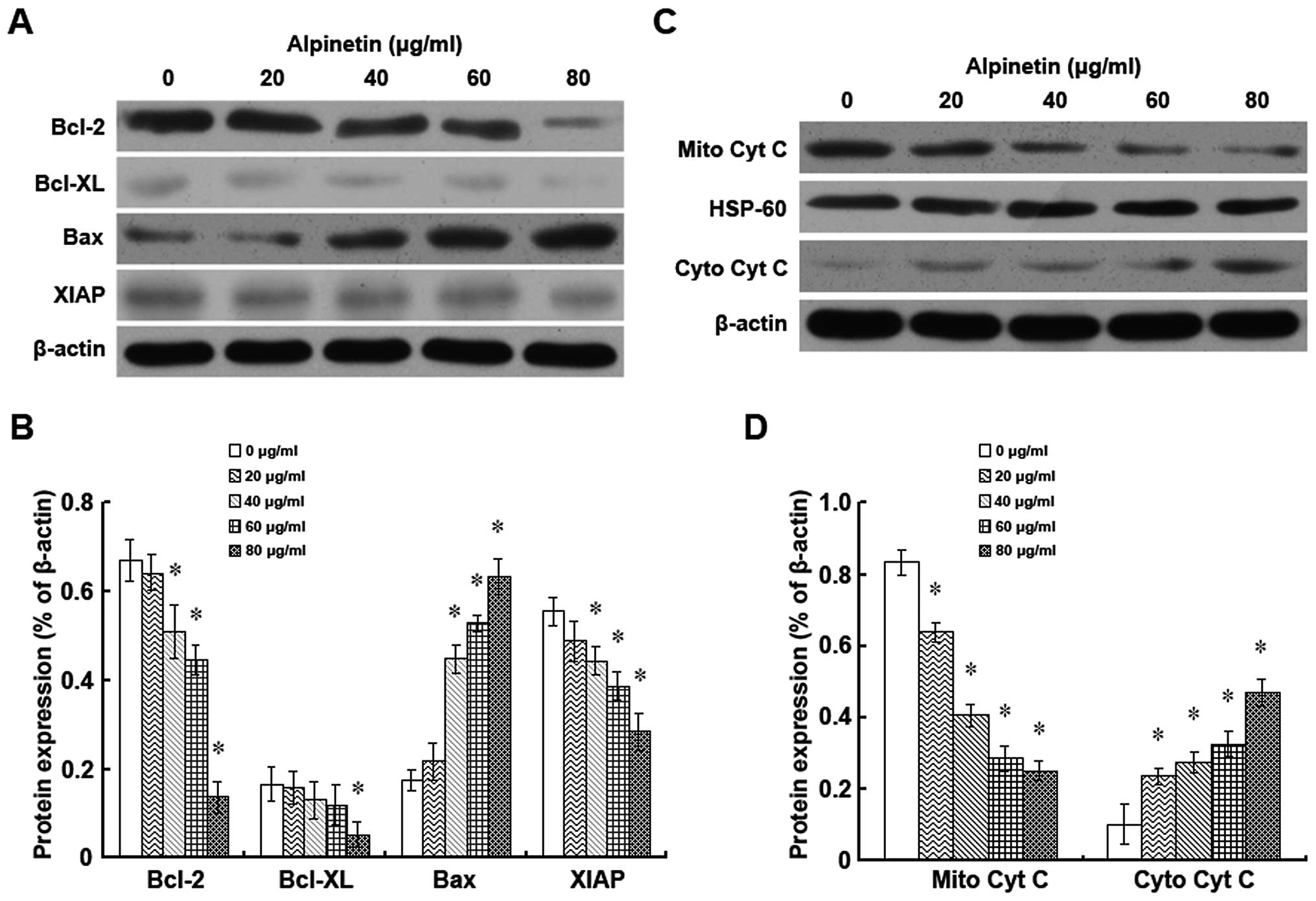
Alpinetin regulates the expression of the
Bcl-2 family members and of XIAP and the release of cytochrome c in
BxPC-3 cells
To further study the mechanism involved in
alpinetin-induced cell growth inhibition and apoptosis, the changes
in the expression of the Bcl-2 family members and XIAP and in the
release of cytochrome c were assessed by western blot analysis
following treatment with alpinetin for 48 h (Fig. 4). Our data showed that the
expression of the anti-apoptotic proteins Bcl-2, Bcl-xL and XIAP in
BxPC-3 cells were downregulated by alpinetin in a dose-dependent
manner. Conversely, the expression of the pro-apoptotic protein Bax
was upregulated in alpinetin-treated BxPC-3 cells (Fig. 4A and B). Also, the expression of
cytochrome c in mitochondria was remarkably decreased while a
significant increase of cytochrome c protein levels in the
cytoplasm was observed after alpinetin treatment (Fig. 4C and D). All these results
indicate that alpinetin could activate the mitochondrial apoptotic
pathway which could be responsible for its ability to suppress
proliferation of BxPC-3 cells.
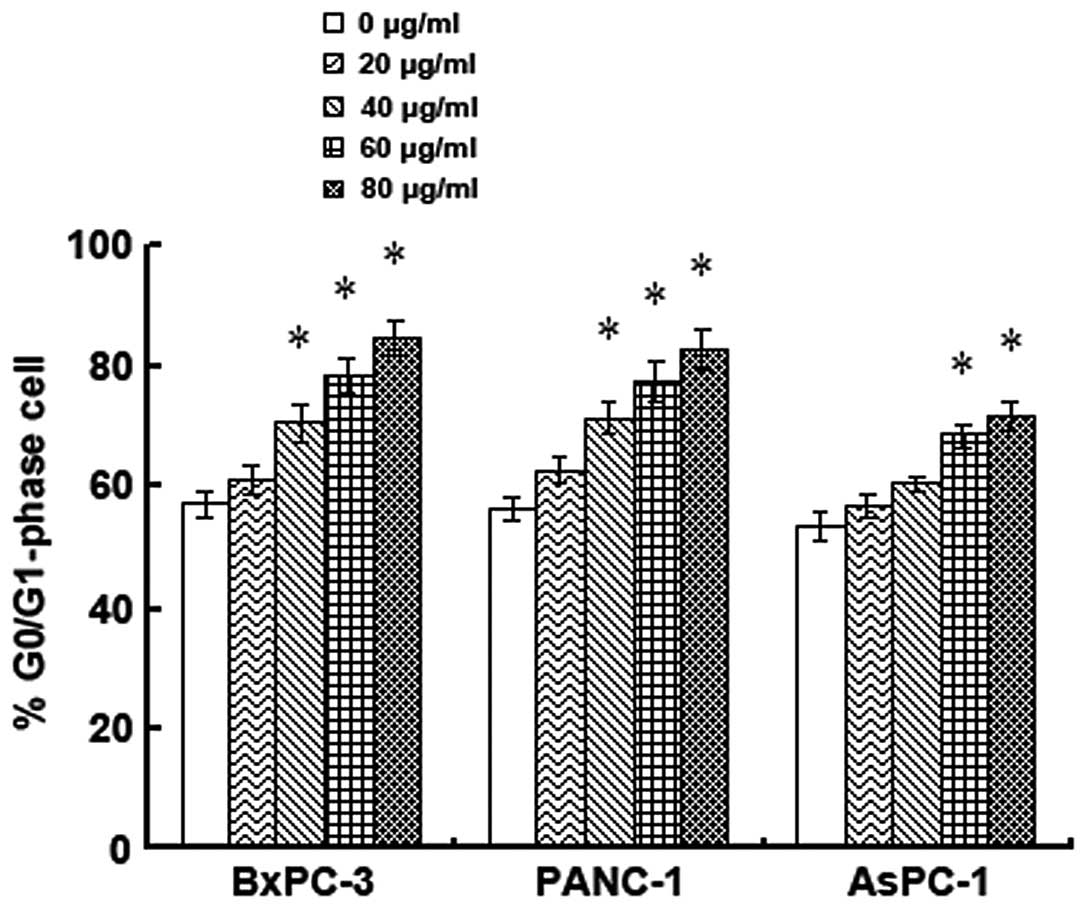
Effects of alpinetin on cell cycle phase
distribution
To investigate whether alpinetin could regulate cell
cycle phase distribution in human pancreatic cancer cells, cell
cycle progression in 3 alpinetin-treated pancreatic cancer cell
lines was assessed using flow cytometry. The percentage of
different treatment groups in the G0/G1 phase are shown by
histograms (Fig. 5). As shown in
our results, an arrest in the G0/G1 phase was observed after
treatment with 40 μg/ml alpinetin for 48 h in BxPC-3 cells.
Meanwhile, the percentage of PANC-1 cells in the G0/G1 phase was
also higher in the 40 μg/ml alpinetin-treated group than in the
untreated cells. In AsPC-1 cells, alpinetin concentrations of 60
μg/ml and higher caused an arrest in the G0/G1 phase. Our data
implies that the anti-proliferation effect induced by alpinetin
also possibly occurs through inducing G0/G1 phase arrest.
Discussion
Our present study shows that alpinetin, the extract
of a traditional Chinese herb, is able to remarkably inhibit the
growth and viability of three human pancreatic cancer cells.
Furthermore, it has been indicated that alpinetin induces BxPC-3
cells apoptosis through upregulating pro-apoptotic Bax expression
and downregulating anti-apoptotic Bcl-2, Bcl-xL and XIAP expression
and results in the release of cytochrome c to the cytoplasm and the
activation of caspases-3, −8, −9. Moreover, alpinetin can also
induce G0/G1 phase arrest in human pancreatic cancer cells. These
results suggest that the effect of alpinetin on suppressing the
proliferation and viability of pancreatic cancer cells may be
mediated by regulating Bcl-2 family and XIAP expression, releasing
of cytochrome c and activation of caspases.
It has been revealed that alpinetin exerts
anti-proliferative activity against various types of tumors such as
hepatoma, breast carcinoma and leukemia (11). Keeping in line with these
observations, we confirmed that alpinetin showed strong antitumor
activity in three human pancreatic cancer cell lines. However, less
is known regarding defined signaling pathways involved in these
processes. In this study, Hoechst 33342 nuclear staining and FACS
analysis revealed that treatment of BxPC-3 pancreatic cancer cells
with alpinetin for 48 h increased condensed chromatin and Annexin
V-positive and PI-negative populations in a dose-dependent manner,
which suggested that part of the alpinetin induced suppression was
mediated through the induction of apoptosis rather than the
necrosis. The observation that alpinetin treatment induced cell
cycle arrest at the G0/G1 phase further supports that
alpinetin-induced BxPC-3 pancreatic cancer cell death occurs
through regulation of apoptosis.
Apoptosis is essential for the fundamental antitumor
mechanism which prevents tumorigenesis of normal cells (6). There are two alternative
intracellular pathways, the intrinsic or mitochondrial pathway and
the extrinsic or death-receptor pathway to initiate apoptosis
(19). The Bcl-2 family, which is
associated with the intrinsic apoptosis pathway, plays a crucial
role in the control of apoptosis by the activation of caspases or
by regulating the release of cytochrome c (20–22). This family includes numerous
proteins with homologous amino acid sequences and some of them have
the ability to promote apoptosis, such as Bax, while others have
anti-apoptotic effects, like Bcl-2, Bcl-xL and XIAP. The relative
effect of these pro- and anti-apoptotic Bcl-2 family members
regulates the death signaling pathways in cells (23,24). Many studies have shown that
pancreatic cancer cells, like other tumor cells, could alter the
balance of pro- and anti-apoptotic proteins, therefore attenuating
apoptosis processes induced by anticancer drugs (25,26). Previous studies also indicated
that pancreatic cancer cells suppress the mitochondrial apoptotic
pathway by overexpression of Bcl-2, Bcl-xL or XIAP to block the
activation of caspases (14). In
this study, to confirm the possible mechanism involved in
alpinetin-induced apoptosis, the expression of the anti-apoptotic
proteins Bcl-2, Bcl-xL and XIAP as well as of the pro-apoptotic
protein Bax were examined. We found that the protein expression of
Bcl-2, Bcl-xL and XIAP were significantly suppressed in
alpinetin-treated BxPC-3 cells. Meanwhile, the expression of Bax
was remarkably upregulated after treatment with alpinetin. The data
were consistent with the previous reports that overexpression of
anti-apoptotic proteins such as Bcl-2 and Bcl-xL repress the
function of Bax. Conversely, upregulation of Bax expression
promotes cell death (27,28).
Caspases, a family of cysteine proteases which
cleave protein substrates after their Asp residues and play
important roles in the extrinsic and intrinsic apoptotic response.
In the mitochondrial-dependent apoptotic pathway, Bcl-2 reduces the
mitochondrial permeability and prevents cytochrome c release to the
cytoplasm while Bax has the converse effect. Cytochrome c released
from the mitochondria can activate the initiator caspase-9 and the
effector caspase-3, which is an important step in the apoptotic
pathway (29,30). Our present study showed that the
activations of caspases-3, −8 and −9 in alpinetin-treated BxPC-3
cells were enhanced in a dose-dependent manner. Moreover, the
release of cytochrome c from the mitochondria to the cytoplasm was
also relatively increased. Meanwhile, activated caspase-8, as an
upstream signaling molecule in caspase cascades, could induce
caspase-9 activation. These findings are similar to a study of
apoptosis in breast cancer cells (31).
In conclusion, we have demonstrated that alpinetin
inhibit the proliferation and viability of pancreatic cancer cells
in a dose- and time-dependent manner through regulating Bcl-2
family and XIAP expression, releasing of cytochrome c and
activation of caspases. Alpinetin may be a potential traditional
Chinese medicine for the future development of pancreatic cancer
therapy.
Acknowledgements
This study was supported by the National High
Technology Research and Development Program (863 Program) funding
(2006AA02A309) and the Natural Science Foundation of China (no.
30870719).
References
|
1
|
A JemalR SiegelE WardT MurrayJ XuMJ
ThunCancer statistics, 2007CA Cancer J
Clin574366200710.3322/canjclin.57.1.43
|
|
2
|
AC LockhartML RothenbergJD BerlinTreatment
for pancreatic cancer: current therapy and continued
progressGastroenterology12816421654200510.1053/j.gastro.2005.03.03915887156
|
|
3
|
CJ WraySA AhmadJB MatthewsAM LowySurgery
for pancreatic cancer: recent controversies and current
practiceGastroenterology12816261641200510.1053/j.gastro.2005.03.03515887155
|
|
4
|
B GudjonssonPancreatic cancer: survival
errors and evidenceEur J Gastroenterol
Hepatol2113791382200910.1097/MEG.0b013e328323aab719907226
|
|
5
|
S FuldaTumor resistance to apoptosisInt J
Cancer124511515200910.1002/ijc.2406419003982
|
|
6
|
D HanahanRA WeinbergThe hallmarks of
cancerCell1005770200010.1016/S0092-8674(00)81683-9
|
|
7
|
S VogelS OhmayerG BrunnerJ HeilmannNatural
and non-natural prenylated chalcones: synthesis, cytotoxicity and
anti-oxidative activityBioorg Med
Chem1642864293200810.1016/j.bmc.2008.02.07918343123
|
|
8
|
J TangN LiH DaiK WangChemical constituents
from seeds of Alpinia katsumadai, inhibition on NF-kappaB
activation and antitumor effectZhongguo Zhong Yao Za
Zhi35171017142010(In Chinese)
|
|
9
|
S WangL ZhouW HeZ HuSeparation and
determination of alpinetin and cardamonin by reverse micelle
electrokinetic capillary chromatographyJ Pharm Biomed
Anal4315571561200710.1016/j.jpba.2006.11.02117240102
|
|
10
|
W HeY LiJ TangF LuanJ JinZ HuComparison of
the characterization on binding of alpinetin and cardamonin to
lysozyme by spectroscopic methodsInt J Biol
Macromol39165173200610.1016/j.ijbiomac.2005.11.00316828496
|
|
11
|
SN MalekCW PhangH IbrahimAW NorhanomKS
SimPhytochemical and cytotoxic investigations of Alpinia
mutica
rhizomesMolecules16583589201110.3390/molecules1601058321240148
|
|
12
|
LL InMN AzmiH IbrahimK AwangNH
Nagoor1′S-1′-acetoxyeugenol acetate: a novel phenylpropanoid from
Alpinia conchigera enhances the apoptotic effects of
paclitaxel in MCF-7 cells through NF-κB inactivationAnticancer
Drugs224244342011
|
|
13
|
ZH HeW GeGG YueCB LauMF HePP
ButAnti-angiogenic effects of the fruit of Alpinia
oxyphyllaJ
Ethnopharmacol132443449201010.1016/j.jep.2010.08.02420723592
|
|
14
|
R HamacherRM SchmidD SaurG
SchneiderApoptotic pathways in pancreatic ductal adenocarcinomaMol
Cancer764200810.1186/1476-4598-7-6418652674
|
|
15
|
J CuiR SunY YuS GouG ZhaoC
WangAntiproliferative effect of resveratrol in pancreatic cancer
cellsPhytother Res2416371644201010.1002/ptr.315721031621
|
|
16
|
K ShojiM TsubakiY YamazoeT SatouT ItohY
KideraY TanimoriM YanaeH MatsudaA TagaMangiferin induces apoptosis
by suppressing Bcl-xL and XIAP expressions and nuclear entry of
NF-κB in HL-60 cellsArch Pharm Res34469475201121547680
|
|
17
|
C KöhlerS OrreniusB ZhivotovskyEvaluation
of caspase activity in apoptotic cellsJ Immunol
Methods265971102002
|
|
18
|
B TangY ZhangR LiangP YuanJ DuH WangL
WangActivation of the δ-opioid receptor inhibits serum
deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathwayInt J Mol
Med28107710852011
|
|
19
|
A ArltSS MüerkösterH SchäferTargeting
apoptosis pathways in pancreatic cancerCancer LettNov 132010(Epub
ahead of print)
|
|
20
|
A BurlacuRegulation of apoptosis by bcl-2
family proteinsJ Cell Mol
Med7249257200310.1111/j.1582-4934.2003.tb00225.x14594549
|
|
21
|
S CoryJM AdamsThe Bcl2 family: regulators
of the cellular life-or-death switchNat Rev
Cancer2647656200210.1038/nrc88312209154
|
|
22
|
T KuwanaMR MackeyG PerkinsMH EllismanM
LatterichR SchneiterDR GreenDD NewmeyerBid, Bax, and lipids
cooperate to form supramolecular openings in the outer
mitochondrial
membraneCell111331342200210.1016/S0092-8674(02)01036-X12419244
|
|
23
|
EH ChengB LevineLH BoiseCB ThompsonJM
HardwickBax-independent inhibition of apoptosis by
Bcl-xLNature379554556199610.1038/379554a08596636
|
|
24
|
XM YinZN OltvaiSJ KorsmeyerBH1 and BH2
domains of Bcl-2 are required for inhibition of apoptosis and
heterodimerization with
BaxNature369321323199410.1038/369321a08183370
|
|
25
|
HH WongNR LemoinePancreatic cancer:
molecular pathogenesis and new therapeutic targetsNat Rev
Gastroenterol
Hepatol6412422200910.1038/nrgastro.2009.8919506583
|
|
26
|
S FuldaApoptosis pathways and their
therapeutic exploitation in pancreatic cancerJ Cell Mol
Med1312211227200910.1111/j.1582-4934.2009.00748.x19382915
|
|
27
|
JM AdamsS CoryThe Bcl-2 protein family:
arbiters of cell
survivalScience28113221326199810.1126/science.281.5381.13229735050
|
|
28
|
JC ReedDouble identity for proteins of the
Bcl-2 familyNature387773776199710.1038/428679194558
|
|
29
|
SJ RiedlY ShiMolecular mechanisms of
caspase regulation during apoptosisNat Rev Mol Cell
Biol5897907200410.1038/nrm149615520809
|
|
30
|
M ChenJ WangInitiator caspases in
apoptosis signaling
pathwaysApoptosis7313319200210.1023/A:1016167228059
|
|
31
|
SY ChienYC WuJG ChungJS YangHF LuMF TsouWG
WoodSJ KuoDR ChenQuercetin-induced apoptosis acts through
mitochondrial- and caspase-3-dependent pathways in human breast
cancer MDA-MB-231 cellsHum Exp
Toxicol28493503200910.1177/096032710910700219755441
|