Introduction
Acute liver failure (ALF) is a complex multisystemic
disease with degeneration and necrosis of the liver which induces
serious hepatosis, disturbances of blood coagulation, jaundice,
hepatic encephalopathy and high mortality rates (1). Worldwide, the most frequent cause of
ALF is viral hepatitis, such as hepatitis B and hepatitis C
(2). Recent research suggests
that apoptosis, infiltration of inflammatory cells and
microcirculatory disturbance play important roles in the process of
ALF (3,4). Apoptosis of hepatocytes was mediated
by death receptors such as Fas, tumor necrosis factor (TNF)-α, and
TNF related apoptosis-inducing ligand (TRAIL) (5,6),
which have been implicated in hepatitis including hepatitis B virus
(HBV) and hepatitis C virus (HCV) infection (7,8).
Caspase-8, a key protease in the death receptor
signaling pathways, is activated when receiving apoplectic signals
and is turned into cleaved caspase-8. Cleaved caspase-8 may
activate caspase-3, caspase-9, eliminate Bcl-2 and may initiate
apoptosis (9). Thus, caspase-8 is
an ideal target factor for inhibiting apoptosis. The discovery of
small non-coding RNA called microRNA (miRNA) has greatly expanded
our understanding of the cellular mechanisms that regulate gene
expression and immunology (10,11). Many studies have found that the
miRNA expression profile is altered in liver diseases (12,13). Lanford et al reported that
SPC3649 blocked miR-122 and effectively inhibited HCV replication
and improved the pathological state of the liver in HCV model
animals (14). Yoo et al
unraveled a novel mechanism by which increased RNA-induced
silencing complex (RISC) activity might contribute to
hepatocarcinogenesis (15).
Our previous studies found that the expression
profile of hepatic miRNAs in ALF mice is significantly altered. We
found that hepatic miR-122, a liver specific miRNA, was decreased
and correlated reversely with hepatic damage (16). We also found miRNAs including
miR-155, miR-146a, miR-125a, miR-15b and miR-16 were up-regulated
and miR-1187 was down-regulated significantly during ALF in mice
(17). These studies suggest that
miRNAs play regulatory roles in ALF. However, it is still unclear
whether down-regulation of miR-1187 plays a role in hepatocyte
apoptosis. In the current study, we report a possible role of
miR-1187 in hepatocyte apoptosis in ALF mice.
Materials and methods
Animal model of ALF
Male BALB/c mice (10-weeks-old) weighing 20–22 g,
obtained from Shanghai SLAC Laboratory Animal Co., Ltd., (Shanghai,
China), were housed under conventional laboratory conditions with
food and water ad libitum. Experiments adhered to the
guidelines of the Shanghai Jiaotong University Animal Ethics
Committee.
A murine ALF model was induced by intraperitoneal
injection of D-GalN (Sigma-Aldrich, St. Louis, MO, USA) (900 μg/kg
of body weight) and LPS (Sigma-Aldrich) (10 μg/kg body weight) as
described (18), whereas the
control group was given saline only. The challenged mice were
sacrificed at different time points (n=8 per group). Part of the
liver was stored in liquid nitrogen for qRT-PCR and Western
blotting, whereas another part was fixed in 10% formalin for
histopathology.
Histopathology
Formalin fixed livers were embedded in paraffin, for
routine histological analysis, 5 μm sections were cut and stained
with hematoxylin and eosin (H&E).
RNA extraction and quantitative real-time
reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from liver tissue or cells,
using Trizol (Invitrogen, Paisley, UK) according to the
manufacturer’s instructions. qPCR was used to confirm the
expression of miR-1187 and caspase-8 mRNA. cDNA was synthesized
using reverse transcriptase (37°C for 30 min, 85°C for 5 min;
Takara Bio, Inc., Ohtsu, Shiga, Japan). qRT-PCR was performed with
the SYBR-Premix Ex TaqII (Takara Bio, Inc.) with the ABI 7500 qPCR
system (95°C for 30 sec, 95°C for 5 sec, and 60°C for 34 sec)
(Applied Biosystems, Foster City, CA, USA) (19). qPCR was performed, using primers
described in Table I to detect
mouse miR-1187 with U6 as an internal control, while for caspase-8
β-actin was used as internal control. The quantity of miRNA was
calculated with the formula 2−ΔΔCT (the CT
value represents fluorescence reached the threshold number of
cycles required for PCR) (20).
 | Table IPrimers for qRT-PCR and sequences of
miR-1187 mimic and non-specific mimic. |
Table I
Primers for qRT-PCR and sequences of
miR-1187 mimic and non-specific mimic.
| Name | Sequence
(5′-3′) |
|---|
| miR-1187 | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTTACAC |
| F:
CCCGGCTATGTGTGTGTGTATGT |
| R:
GTGCAGGGTCCGAGGT |
| miR-1187 mimic | F:
UAUGUGUGUGUGUAUGUGUGUAA |
| R:
AUACACACACACAUACACACAUU |
| Non-specific
mimic | F:
UCACAACCUCCUAGAAAGAGUAGA |
| R:
AGUGUUGGAGGAUCUUUCUCAUCU |
| U6 | F:
CTCGCTTCGGCAGCACA |
| R:
AACGCTTCACGAATTTGCGT |
| Caspase-8 | F:
TGCCCTCAAGTTCCTGTGCTTGGAC |
| R:
GGATGCTAAGAATGTCATCTCC |
| β-actin | F:
CTAGGCACCAGGGTGTGAT |
| R:
TGCCAGATCTTCTCCATGTC |
Cell culture and apoptosis induction
The normal mouse embryonic liver cell line (BNLCL2)
(21) obtained from the Cell
Bank, Chinese Academy of Science, was maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA)
supplemented with 10% FBS (Gibco-Invitrogen, Carlsbad, CA, USA) and
1% streptomycin (Gibco-Invitrogen) at 37°C with 5% CO2.
TNF-α (10 μg/ml; Sigma-Aldrich) and D-GalN (1.0 mg/ml;
Sigma-Aldrich) were added into cell culture medium for induction of
apoptosis (22), whereas the
mock-treated cells were given culture medium only. Part of the
cells was collected for total RNA extraction, while the other was
collected for Annexin-V-FITC/PI-labeled (Mai Bio, Shanghai, China)
flow cytometric analysis. FITC-positive and PI-negative cells were
considered as apoptotic cells and PI-positive cells were considered
as necrotic, while unstained cells were normal viable cells.
FCSExpressV3Full software (De Novo Software, Thornhill, Canada) was
requested for apoptotic profiles analysis.
Transfection of the miR-1187 mimic and
non-specific mimic
miRNA mimics are small double-stranded RNA
oligonucleotides designed to mimic (overexpression) endogenous
mature miRNA molecules when transfected into cells. The synthesized
miR-1187 mimic and non-specific mimic (NSM) were purchased from
RiboBio (Guangzhou, China). BNLCL2 cells (50–70% confluent) were
transiently transfected with miR-1187 mimic (50 nM) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s instruction; NSM (50 nM) was transfected as a
negative control. The efficiency of transfection was analysed using
qRT-PCR.
Western blot analysis
Total protein from BNLCL2 cells was extracted with
extraction buffer RIPA plus the protease inhibitor PMSF (Bocai Bio
Co., Shanghai, China) and quantified by the bicinchoninic acid
(BCA) method (Pierce, Rockford, IL, USA). Protein samples were
size-fractionated on 8% SDS-polyacrylamide gels and transferred to
PVDF membranes (Amersham, Buckinghamshire, UK). The membranes were
blocked with 5% dry milk for 1 h in Tris-saline buffer and 0.1%
Tween-20 (TBS/Tween-20) (Dako, Carpinteria, CA, USA). After washing
in TBS/Tween-20, the membranes were incubated with rabbit
anti-mouse caspase-8 polyclonal antibody or rabbit anti-mouse
β-actin polyclonal antibody (1:2,000, Abcam, Cambridge, UK)
overnight at 4°C. After washing with TBS/Tween-20, the membranes
were incubated for 1 h at room temperature with HRP-conjugated goat
anti-rabbit IgG (1:2,000, Abcam, Cambridge, UK), washed with
TBS/Tween-20 and the proteins of interest on the membrane were
detected with ECL Plus™ Western blotting detection reagents
(Amersham).
Statistical analysis
All data are presented as means ± SD (standard
deviation). Two-way ANOVA or Student’s t-test was performed for
statistical analysis, using Graphpad Prism 5 (GraphPad Software,
Inc.; La Jolla, CA, USA). Differences between group means with
P<0.05 were regarded as being statistically significant.
Results
miR-1187 was significantly decreased
during ALF and was predicted to target the caspase-8 mRNA
3′-untranslated region (3′UTR)
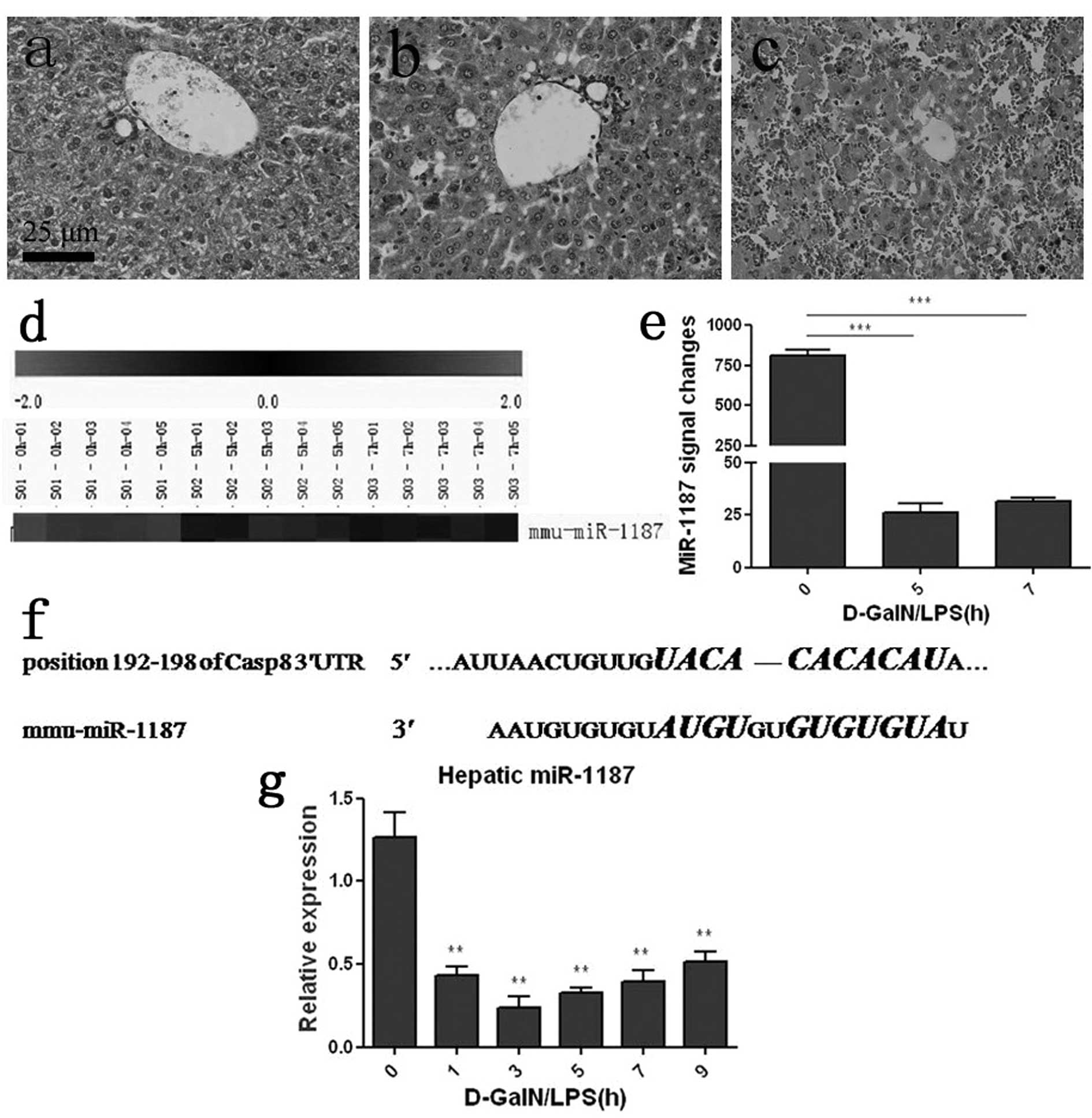
After D-GalN/LPS induction, the mice mortality rate
was 50% at 7 h and over 80% at 24 h (data was shown). A
histopathology study detected obvious damage in the liver 5 h
post-challenge, which showed a large quantity of inflammatory cells
and disordered hepatic lobules (Fig.
1b). At 7 h, histopathological change was pronounced and severe
congestion and destructed hepatic lobules were detected (Fig. 1c). LNA-based microarray analysis
showed that hepatic miR-1187 was dramatically decreased with
ongoing ALF (Fig. 1d). The
miR-1187 expression signal was quantified and about a 97 or 96%
decrease at 5 or 7 h post challenge respectively was noted compared
with 0 h (saline control) (P<0.001, Fig. 1e). qRT-PCR was applied to verify
the expression of miR-1187, and the result was consistent with the
microarray data (P<0.01, Fig.
1g). TargetScan (http://www.targetscan.org/) revealed that 7
nucleotides in the seed region of miR-1187 were complementary to
the position 192–198 of caspase-8 mRNA 3′UTR in mice (Fig. 1f).
Overexpression of miR-1187 suppressed the
level of caspase-8 mRNA as well as protein in BNLCL2 cells induced
by D-GalN/TNF
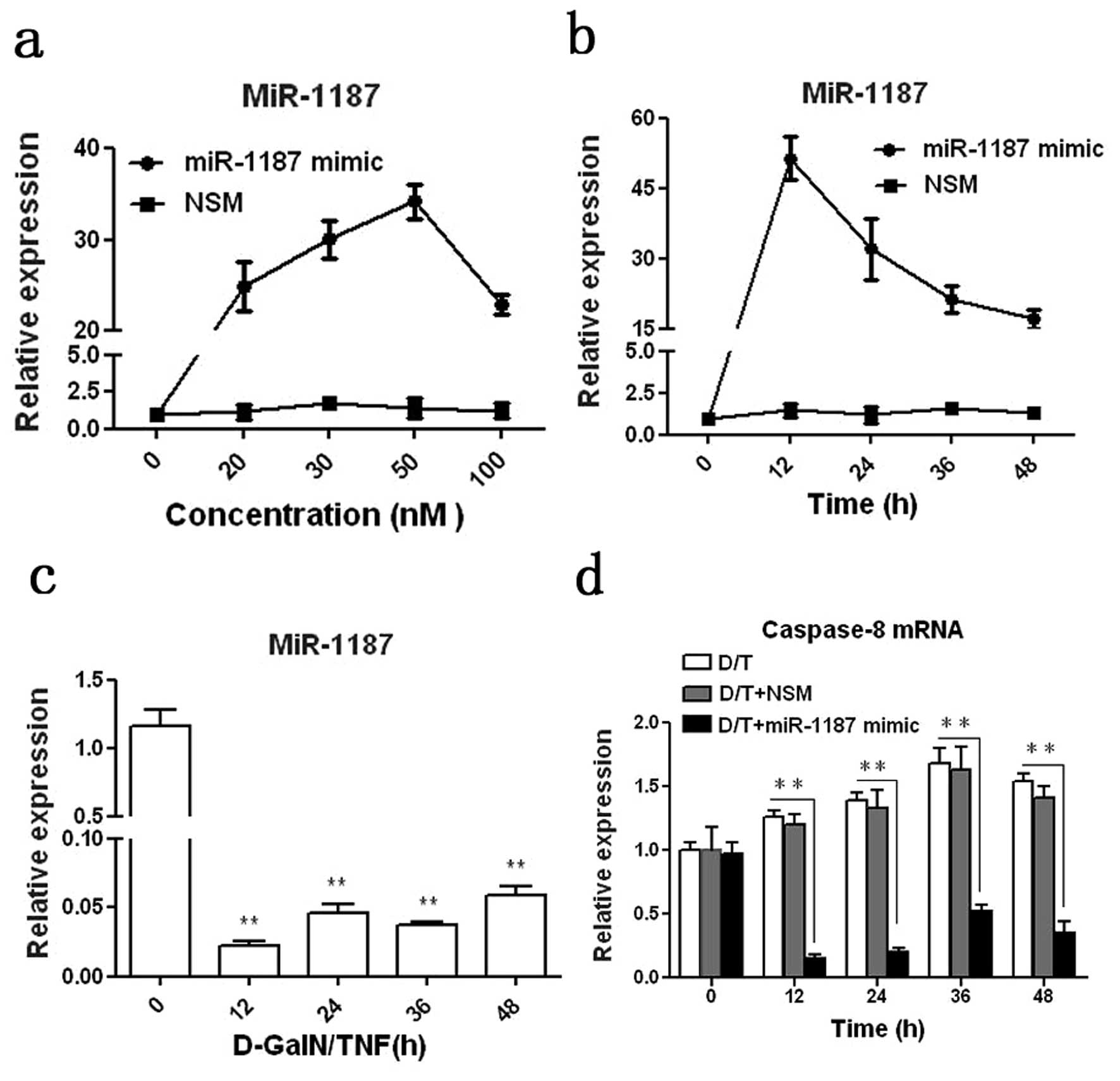
In order to study the regulatory role of miR-1187 in
caspase-8, we applied a miR-1187 mimic for study. It was shown that
miR-1187 was overexpressed in BNLCL2 cells transfected with
miR-1187 mimic compared with the cells transfected with NSM. At 50
nM the miRA-1187 mimic with 12 h transfection resulted in the
highest expression of miR-1187 in BNLCL2 cells (P<0.001,
Fig. 2a and b). Thus we used this
condition for the whole experiment. miR-1187 was dramatically
decreased in the cells induced with D-GalN/TNF (P<0.01, Fig. 2c), correlating with our in
vivo data above (Fig 1d, e and
g). Inversely, caspase-8 mRNA was up-regulated in BNLCL2 cells
induced with D-GalN/TNF (P<0.05), but overexpression of miR-1187
reduced caspase-8 mRNA significantly (P<0.01, Fig. 2d).
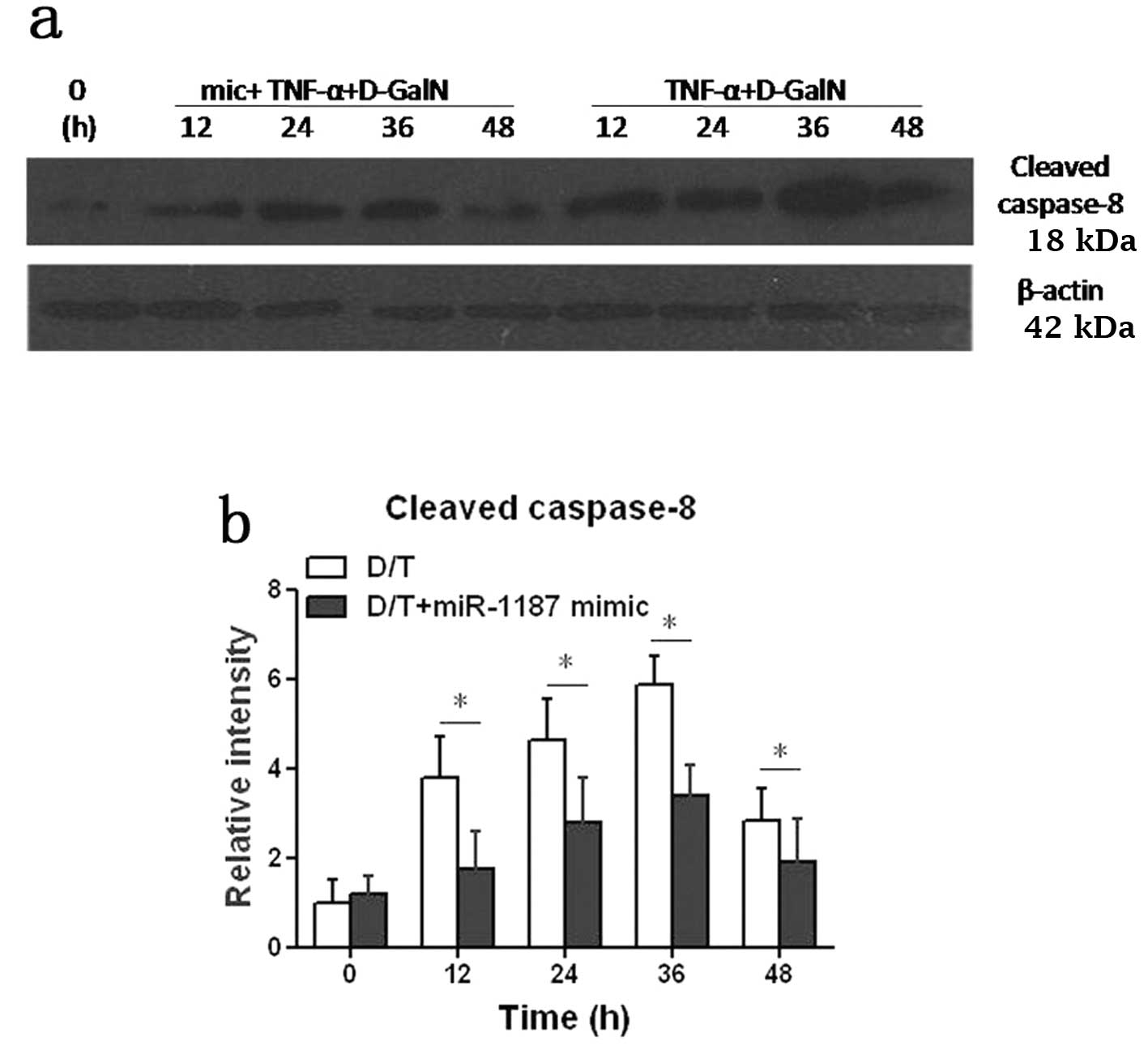
Cleaved caspase-8 protein was increased in BNLCL2
cells induced by D-GalN/TNF, but it was significantly suppressed
(P<0.05) when the cells were transfected with the miR-1187 mimic
(D/T+1187 mimic) (Fig. 3). Taken
together, it was presumed that miR-1187 regulated caspase-8 by mRNA
degradation and the level of protein was suppressed
accordingly.
Up-regulated miR-1187 attenuated
apoptosis of BNLCL2 cells
Following the data above, it is of interest to
investigate if miR-1187 regulates BNLCL2 cells apoptosis. The flow
cytometry data showed that the apoptotic rate of BNLCL2 cells was
increased in a time-dependent manner; it was 26, 32, 37 or 42%
following the D-GalN/TNF treatment for 12, 24, 36 or 48 h
respectively. However, overexpression of miR-1187 attenuated the
apoptotic rate significantly, i.e. by about 21, 24, 29 and 36%,
respectively (P<0.05, Fig.
4).
 | Figure 4Apoptosis of BNLCL2 cells with
different treatments were analyzed. (a) Apoptosis of BNCL2 cells
was quantified by Annexin-V/PI labelled flow cytometric analysis in
which Annexin-V-FITC was used to label apoptotic cells and PI was
used to label necrotic cells in the treatment of the apoptosis of
the cells treated with D-GalN/TNF for 12, 36 and 48 h (D/T 12, 24,
36, 48 h) or the cells transfected with miR-1187 mimic for 12 h
then treated with D-GalN/TNF for another 12, 24, 36 and 48 h (mimic
+ D/T 12, 24, 36, 48 h) was detected. The mock treatment was used
as a negative control. (b) Based on the flow cytometry data, the
apoptosis rates were calculated. Data represent the mean ± SD of 8
independent experiments. *P<0.05. |
Discussion
Death receptor-mediated apoptosis of hepatocytes
contributes to ALF (23). Recent
studies found miRNAs are responsible for a number of liver diseases
(24). Increasing evidence
demonstrates that miRNAs regulate death receptor-mediated
hepatocytes apoptosis in ALF (25,26). However, the regulatory role of
miRNAs in TNF-α-dependent hepatocytes apoptosis in ALF remains
unclear.
In the current study, the D-GalN/LPS induced ALF
mice model was used for investigation. It was detected that
miR-1187 was reduced in the liver of ALF mice by qRT-PCR. This data
was consistent with our microarray data. Furthermore, using the
TargetScan database, it was found that caspase-8 was a putative
target of miR-1187. Fas, TNF-α and TRAIL are important genes in the
death receptor-mediated apoptosis signaling pathway in human acute
viral hepatitis (27,28), and caspase-8 is a key gene
involved in this pathway. Caspase-8 has been implicated in
hepatocyte apoptosis in liver injury (29), anti-caspase-8 could effectively
inhibit apoptosis of Hepa 1-6 cells induced by TNF-α (30) and caspase-8 small interfering RNA
prevents mice from acute liver failure (31). Thus, it is reasonable to conclude
that miR-1187 contributes to hepatocyte apoptosis via targeting
caspase-8 during ALF.
In order to study the role of miR-1187 in
hepatocytes apoptosis, D-GalN/TNF was applied to induce BNLCL2 to
apoptosis. After induction, a down-regulation of miR-1187 but an
up-regulation of caspase-8 (both mRNA and protein level) was
detected. Moreover, overexpression of miR-1187 reduced the levels
of caspase-8 mRNA and protein and attenuated the apoptotic rate as
well. Taken together, we speculate that overexpression of miR-1187
suppresses caspase-8, then down-regulates downstream genes
including caspase-3, caspase-7 and caspase-9, consequently
attenuating the apoptosis of hepatocytes. However, the relationship
between miR-1187 and other factors related with immunization and
apoptosis and how miR-1187 plays a role in an in vivo
environment, remain to be further investigated.
In summary, our study demonstrated that miR-1187
regulated hepatocytes apoptosis via targeting caspase-8. miR-1187
acted as an inhibitor of hepatocyte apoptosis which shed light on
the treatment of ALF.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NSFC81071358), the National Science
and Technology Major Project grants (2008ZX10002-005 and
2008ZX10002-007) and a Shanghai Science and Technology Committee
grant (10411966800).
References
|
1
|
JG O’GradyAcute liver failurePostgrad Med
J811481542005
|
|
2
|
HC LeeAcute liver failure related to
hepatitis B virusHepatol
Res38S9S13200810.1111/j.1872-034X.2008.00420.x19125959
|
|
3
|
Z ZhangZS ZouJL FuSevere dendritic cell
perturbation is actively involved in the pathogenesis of
acute-on-chronic hepatitis B liver failureJ
Hepatol49396406200810.1016/j.jhep.2008.05.01718644645
|
|
4
|
K RyoY KamogawaI IkedaSignificance of Fas
antigen-mediated apoptosis in human fulminant hepatic failureAm J
Gastroenterol9520472055200010.1111/j.1572-0241.2000.02268.x10950056
|
|
5
|
B AlbertsA JohnsonJ LewisMolecular Biology
of the Cell4th editionGarland ScienceNew York2002
|
|
6
|
Y AkazawaGJ GoresDeath receptor-mediated
liver injurySemin Liver
Dis27327338200710.1055/s-2007-99151017979070
|
|
7
|
ME GuicciardiGJ GoresApoptosis: a
mechanism of acute and chronic liver
injuryGut5410241033200510.1136/gut.2004.05385015951554
|
|
8
|
H MalhiGJ GoresCellular and molecular
mechanisms of liver
injuryGastroenterology13416411654200810.1053/j.gastro.2008.03.00218471544
|
|
9
|
SM ElbashirJ HarborthW LendeckelDuplexes
of 21-nucleotide RNAs mediate RNA interference in cultured
mammalian cellsNature411494498200110.1038/3507810711373684
|
|
10
|
M YangY LiRW PadgettMicroRNAs: small
regulators with a big impactCytokine Growth Factor
Rev16387393200510.1016/j.cytogfr.2005.02.00815869899
|
|
11
|
KD TaganovMP BoldinD BaltimoreMicroRNAs
and immunity: tiny player in a big
fieldImmunity26133137200710.1016/j.immuni.2007.02.00517307699
|
|
12
|
S UraM HondaT YamashitaDifferential
microRNA expression between hepatitis B and hepatitis C leading
disease progression to hepatocellular
carcinomaHepatology4910981112200910.1002/hep.2274919173277
|
|
13
|
O CheungP PuriC EickenNonalcoholic
steatohepatitis is associated with altered hepatic microRNA
expressionHepatology4818101820200810.1002/hep.2256919030170
|
|
14
|
RE LanfordES Hildebrandt-EriksenA
PetriTherapeutic silencing of microRNA-122 in primates with chronic
hepatitis C virus
infectionScience327198201201010.1126/science.117817819965718
|
|
15
|
BK YooPK SanthekadurR GredlerIncreased
RNA-induced silencing complex (RISC) activity contributes to
hepatocellular
carcinomaHepatology5315381548201110.1002/hep.2421621520169
|
|
16
|
FM AnDS YuQ XieThe role of miRNA-122
expression during the acute liver failure in mice induced by
D-GalN/LPSZhonghua Gan Zang Bing Za Zhi185275322010(in Chinese)
|
|
17
|
FM AnDS YuBD GongThe expression profile
and roles of microRNA in tumor necrosis factor-α mediated acute
liver failure in mouse modelChin J Infect Dis285495542010(In
Chinese)
|
|
18
|
H MatsumotoS TamuraY KamadaAdiponectin
deficiency exacerbates lipopolysaccharide/D-galactosamine-induced
liver injury in miceWorld J Gastroenterol1233523358200616733851
|
|
19
|
BD GongQ XieL WangReal-time quantification
of microRNAs in Huh7 cells by stem-loop reverse transcriptase
polymerase chain reactionZhonghua Gan Zang Bing Za
Zhi176036062009(In Chinese)
|
|
20
|
KJ LivakTD SchmittgenAnalysis of relative
gene expression data using real-time quantitative PCR and the
2(-Delta Delta C(T))
methodMethods25402408200110.1006/meth.2001.126211846609
|
|
21
|
MA ReddySD ShuklaPotentiation of
mitogen-activated protein kinase by ethanol in embryonic liver
cellsBiochem
Pharmacol51661668199610.1016/S0006-2952(95)02239-28615903
|
|
22
|
GQ ZhangH YuXQ ZhouTNF-α induced apoptosis
and necrosis of mice hepatocytesWorld Chinese J
Digestol83033062000(In Chinese)
|
|
23
|
E HatanoTumor necrosis factor signaling in
hepatocyte apoptosisJ Gastroenterol
Hepatol22S43S44200710.1111/j.1440-1746.2006.04645.x17567463
|
|
24
|
S BalaM MarcosG SzaboEmerging role of
microRNAs in liver diseasesWorld J
Gastroenterol1556335640200910.3748/wjg.15.563319960558
|
|
25
|
S YoonTH KimA NatarajanAcute liver injury
upregulates microRNA-491-5p in mice, and its overexpression
sensitizes Hep G2 cells for tumour necrosis factor-alpha-induced
apoptosisLiver
Int30376387201010.1111/j.1478-3231.2009.02181.x20015148
|
|
26
|
AD SharmaN NarainEM HändelMicroRNA-221
Regulates FAS-Induced Fulminant Liver
FailureHepatology5316511661201110.1002/hep.2424321400558
|
|
27
|
B MundtF KühnelL ZenderInvolvement of
TRAIL and its receptors in viral hepatitisFASEB
J179496200312475902
|
|
28
|
K StreetzL LeifeldD GrundmannTumor
necrosis factor alpha in the pathogenesis of human and murine
fulminant hepatic
failureGastroenterology119446460200010.1053/gast.2000.936410930380
|
|
29
|
T Ben MosheH BarashTB KangRole of
caspase-8 in hepatocyte response to infection and injury in
miceHepatology4510141024200717385212
|
|
30
|
S LinX LiuR YinInhibitory effects of short
hairpin RNA against caspase-8 on apoptosis of murine hepatoma
Hepa1–6 cellsBiosci Trends35357200920103947
|
|
31
|
L ZenderS HutkerC LiedtkeCaspase 8 small
interfering RNA prevents acute liver failure in miceProc Natl Acad
Sci USA10077977802200310.1073/pnas.133092010012810955
|