Introduction
Stroke is a leading cause of serious and long-term
disability in adults. One of the most physically debilitating
disabilities is spasticity, defined as movement disorder (1). As one component of the upper motor
neuron syndrome (UMNS) (2),
spasticity is characterized by a velocity-dependent increase in
tonic stretch reflexes (muscle tone) with exaggerated tendon jerks,
resulting from hyperexcitablility of the stretch reflex. Spasticity
one year after the event of stroke has been reported to occur in up
to 38% of patients (3–5). Spasticity has a disabling effect on
patients through reduced mobility and pain, which may slow down the
potential success of rehabilitation (6), and eventually significantly affects
the quality of life in stroke survivors.
Although the pathogenic mechanisms of spasticity are
not yet well understood, glutamate-induced excitotoxicity is
considered to be involved in this condition; thus, the suppression
of excitation (glutamate) is considered as a means of treating
spasticity (7,8). Glutamate functions as a
neurotransmitter in the majority of excitatory synaptic signals in
the mammalian brain. It is the major excitatory neurotransmitter in
the central nervous system (CNS), and is the agonist of two types
of glutamate receptors, metabotropic and ionotropic glutamate
receptors. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
(AMPA) receptors are one of the ionotropic glutamate receptors for
the fast excitatory synaptic transmission in the CNS. The binding
of glutamate to post-synaptic AMPA-type glutamate receptors induces
depolarizations leading to neuronal firing. AMPA receptor subunits
(GluR1, GluR2, GluR3 and GluR4) play a crucial role in motor
function following cerebral ischemia. It has been shown that AMPA
subunits are upregulated in immunoreactive hypertrophic astrocytes
in the CA1 hippocampal region after transient forebrain ischemia
(9). Moreover, AMPA subunit
expression has been shown to be significantly increased in reactive
astrocytes during the chronic stages of ischemic spastic paraplegia
(10), indicating that AMPA
receptors are strongly associated with spasticity following
cerebral ischemia.
A variety of therapeutic approaches are available
for post-stroke spasticity, including physical and occupational
therapy, neurosurgery and orthopedic surgery, as well as oral
medications. The mainstay of pharmacotherapy includes botulinum
toxin, which functions by diminishing peripheral cholinergic
activity at the neuromuscular junction, dantrolene sodium which
inhibits the release of calcium from the sarcoplasmic reticulum,
and a group of medications such as baclofen, diazepam and clonidine
which act centrally (11–14). However, these medication
procedures generally require repetition and may have troubling
side-effects, such as permanent weakness, dysesthesias and
causalgia (15).
Natural products, including traditional Chinese
medicine (TCM), have relatively fewer side-effects as compared to
modern chemotherapeutics and have been used for thousands of years
as important alternative remedies for a variety of diseases. Gua
Lou Gui Zhi decoction (GLGZD) is a well-known traditional Chinese
formula that was first recorded in ‘Essentials from the Golden
Cabinet’ written during the Eastern Han Dynasty, around 210 AD.
GLGZD consists of a combination of six herbs, including
Trichosanthis Radix, Ramulus Cinnamomi, Paeonia lactiflora,
Glycyrrhiza, Zingiber officinale Roscoe and Fructus
Jujubae. GLGZD has long been used clinically in China to treat
muscular spasticity following stroke, epilepsy or spinal cord
injury (16–18). Our previous study demonstrated
that GLGZD is effective in the treatment of post-stroke spasticity,
improving the Fugl-Meyer score and Barthel Index score (unpublished
data). In order to further elucidate the mode of action of GLGZD,
in the present study, we used a focal cerebral ischemia/reperfusion
(I/R) inujry rat model to evaluate the therapeutic efficacy of
GLGZD against cerebral ischemia and spasticity, and investigated
the underlying molecular mechanisms.
Materials and methods
Materials and reagents
GluR1, GluR2, GluR3 and GluR4 antibodies were
provided by Abcam (Cambridge, MA, UK). β-actin antibodies and
horseradish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling Technology (Beverly, MA, USA). All
other chemicals used, unless otherwise stated, were obtained from
Sigma Chemical Co. (St. Louis, MO, USA).
Animals
Male Sprague-Dawley rats (with an initial body
weight of ~250 g) were obtained from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and housed in standard cages. A
12-h light/dark cycle was used throughout. Food and water were
provided ad libitum during the experiment. All animal
treatments were strictly in accordance with International Ethics
Guidelines and the National Institutes of Health Guidelines
Concerning the Care and Use of Laboratory Animals, and the
experiments were approved by the Institutional Animal Care and Use
Committee of Fujian University of Traditional Chinese Medicine,
Fuzhou, China.
Establishment of cerebral ischemic
spasticity and animal grouping
A cerebral ischemia model was established by middle
cerebral artery occlusion (MCAO) as described previously (19). Briefly, after the rats were
anesthetized with 10% chloral hydrate by intraperitoneal injection,
the left common carotid artery (CCA), the left external carotid
artery (ECA) and internal carotid artery (ICA) were carefully
exposed by a midline neck incision. The left middle cerebral artery
(MCA) was occluded by introducing an embolus through the ICA. Focal
cerebral ischemia commenced when the tip of the catheter reached
the origin of the MCA (~18–22 mm). Mild resistance indicated that
the embolus was properly lodged in the anterior cerebral artery,
thus blocking blood flow to the MCA. Reperfusion was achieved by
pulling out the thread after 120 min of occlusion to restore blood
supply to the MCA area, and the left CCA and ECA were ligated. The
rectal temperature of the rats was maintained at 37°C during the
surgical procedures. After surgery the rats were allowed to recover
in pre-warmed cages. Following the induction of ischemia,
spasticity were examined by a screen test and Hoffman’s reflex
(H-reflex).
The rats were randomly divided into three groups
(n=8) as follows: i) sham-operated control (SC) group: rats
underwent a neck dissection and coagulation of the ECA, but no
occlusion of the MCA; ii) ischemia control (IC) group: the blood
flow of the left MCA was blocked for 120 min, followed by
reperfusion; iii) GLGZD: the surgical procedure in the GLGZD group
was the same as that in the IC group. Immediately after recovery
from surgery, the rats received GLGZD at a concentration of 1.16
g/ml daily for a period of seven days.
Preparation of herbal extracts
According to the original prescription from
‘Essentials from the Golden Cabinet’, the decoction comprised:
Trichosanthis Radix, Ramulus Cinnamomi, Paeonia lactiflora,
Glycyrrhiza, Zingiber officinale Roscoe and Fructus
Jujubae at a ratio of 3:3:3:2:3:3. Dried crude drugs were
purchased from Tongrentang Chinese Medicine Pharm (Fuzhou, China),
a well-known and time-honored brand name in the TCM industry in
China. They were identified and confirmed by the College of
Pharmacology, Fujian University of Traditional Chinese Medicine.
Ramulus Cinnamomi was pulverized to 100 mesh. The residual
five dried materials were extracted twice with boiling water for
1.5 h. The obtained solution was combined, filtrated (cotton gauze)
and concentrated by using a rotary evaporator to a final
concentration of 1.16 g/ml. The powder of Ramulus Cinnamomi
was accurately weighed and added to the drug solution by stirring
vigorously to produce a more uniform solution. The decoction was
obtained for further use.
High-performance liquid chromatography
(HPLC) fingerprint
An HPLC fingerprint was used to control the quality
of the GLGZD extract in our study. A Sampark LC-20A series HPLC
system (Sampark Technology, Japan) with a PDA detector and a
Diamonsil C18 column (4.6×250 mm, 5 μm particle size) was used for
HPLC analysis. The UV spectra were recorded in the range 230–400
nm, and the chromatographic peaks were measured at a wavelength of
230 nm. The mobile phase consisted of solvent A (acetonitrile;
Merck, USA) and solvent B (0.1% phosphoric acid/water, v/v). All
agents were HPLC grade. The gradient procedure was used as follows:
5% A for 0 min, 32% A for 0–45 min, 48% A for 45–60 min and 48% A
for 60–65 min. The flow rate was 1.0 ml/min and the column
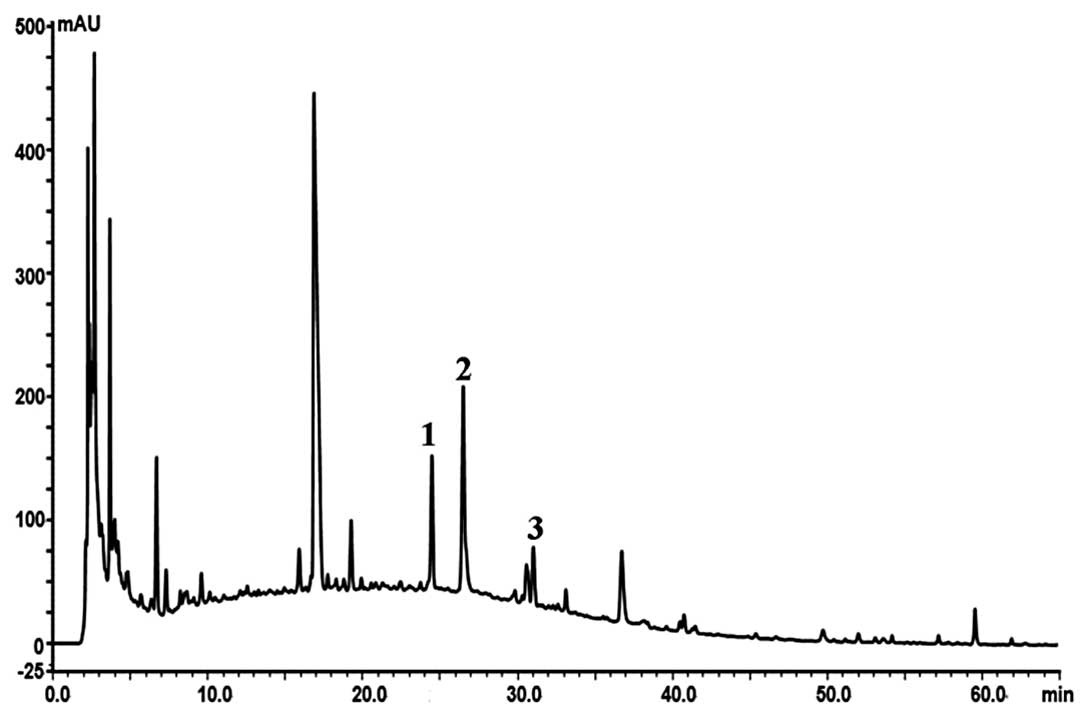
temperature was set at 30°C. Based on the fingerprint we
established an optimum and easily controlled procedure for
preparing the GLGZD extract as mentioned above (Fig. 1). If the peak areas of R1, R2 and
R3 are in an optimum ratio of 15:21:7, all results from the
experiment would readily be reproducible.
Scoring of neurological deficits
At 2 h after cerebral ischemia and for the following
seven days, the neurological deficit score was examined in a
blinded fashion as described previously (19): score 0, no neurological deficit;
score 1 (failure to extend the right forepaw fully), mild deficits;
score 2 (circling to the right) and score 3 (falling to the right),
moderate deficits; and score 4 (loss of walking), severe deficits.
In brief, the rats with a score of 0 or 4 were excluded from the
experiment.
Scoring of screen test
At 2 h after cerebral ischemia and for the following
seven days, the screen test scores were examined in a blinded
fashion as described previously (20). To measure the muscle tone,
strength, stamina and balance, a net screen was used. The net
screen was made of 50×40 cm barbed wire with 1×1 cm areole. The
trial started after the rats were placed on the horizontal screen
on the ground. The screen was turned over 90° within 2 sec by
raising one side of it gradually and maintaining this position for
5 sec. The time by which the rats took to hold onto the net screen
was recorded in seconds. The scoring criterion was: 5, holding on
the screen and climbing upward; 4, holding on the screen with
forelimbs and not falling down within 5 sec; 3, holding on the
screen temporally and slipping off a certain distance; 2, falling
down to the ground within 5 sec; 1, falling down to the ground
immediately as soon as the screen was set at a vertical
position.
Measurement of infarct volumes
Following cerebral ischemia injury for seven days,
the rats were anaesthetized with 10% chloral hydrate by
intraperitoneal injection. The rats were perfused transcardiacally
with 0.9% NaCl and the brains were quickly removed for
2,3,5-triphenyl tetrazolium chloride (TTC) staining. Thereafter,
six serial coronal sections of 2-mm thickness were prepared. Brain
slices were incubated in a 0.2% TTC solution (Sigma, St. Louis, MO,
USA) in phosphate-buffered saline (PBS) at 37°C for 20 min and
fixed by immersion in 4% buffered formaldehyde solution. The normal
area of brain was stained dark red based on intact mitochondrial
function, whereas the infarct area remained unstained. Each brain
slice was scanned by a high-resolution digital camera (Cannon
S×20), and the infarct volume was quantified using the Motic Med
6.0 System, which was represented as a percentage of the total
brain volume.
H-reflex recording
The H-reflex was recorded as previously described
(10). Briefly, the rats were
anaesthetized with 10% chloral hydrate by intraperitoneal
injection. The right hindlimbs of the rats were secured, and a pair
of stimulating needle electrodes was transcutaneously inserted into
the surroundings of the tibial nerve. Moreover, a pair of silver
needle electrodes was inserted into the interosseous muscles
between the fourth and the fifth or the first and the second
metatarsal right foot muscles for recording. The tibial nerve was
stimulated using square pulses with increasing stimulus intensity
(0.1–10 in 0.5 mA increments, 0.1 Hz, 0.2 msec; RM6240; Chengdu
Instrument Factory, Chengdu, China), and responses were recorded
automatically. The threshold for both the M and H waves was
determined, and the Hmax/Mmax ratio was calculated.
Determination of glutamate level in
cerebrospinal fluid (CSF)
The CSF was collected by cisternal puncture under
light general anesthesia using 10% chloral hydrate by
intraperitoneal injection. The derivatization process was performed
by mixing 1 μl of sample or glutamate standard solution, 1 μl of
freshly prepared methanolic OPA and 5 μl borate buffer (pH 9.5).
This final solution was vortexed and analyzed after 1 min. The
mobile phase that was used on the FLD system was composed of a
mixture of 0.1 M sodium acetate, 1.5 ml tetrahydrofuran, 90 μl
triethylamine (pH 7.20±0.05, 1–2% acetic acid) and HPLC grade as
phase A and methanol-acetonitrile-0.10.1 M sodium acetate (pH
7.20±0.05, 1–2% acetic acid; 2:2:1 V/V) as phase B. Mobile phase
and solvents were filtered through Millipore 0.45 μm Durapore
membrane filters and vacuum degassed prior to use. The gradient
system was: 0 min, 0% mobile phase B, increased to 60% B at 14 min
(0.45 ml/min), and held at 100% B until 15 min (0.8 ml/min). The
column was maintained at a temperature of 40°C and the fluorescence
detector was set at 340 nm (excitation wavelength) and 450 nm
(emission wavelength).
Western blot analysis
Ischemic cerebral tissues were homogenized in
non-denaturing lysis buffer and centrifuged at 12,000 × g for 15
min. Supernatants were collected and frozen at −80°C until
immunoblot analysis. Protein concentration for each homogenate was
determined. Equal amounts of protein (50 μg) were loaded onto 8–10%
SDS-PAGE gels for electrophoresis and then transferred onto PVDF
membranes. After blocking in 5% non-fat dry milk in 0.1 M
Tris-buffered saline (TBS)-0.1% Tween-20 (TBST), the membranes were
incubated with primary antibodies against GluR1, GluR2, GluR3,
GluR4 and β-actin overnight on a shaker at 4°C. Membranes were
washed three times for 10 min with TBST and incubated for 1 h on a
shaker at room temperature with goat anti-rabbit HRP-conjugated
secondary antibody. Blots were developed using enhanced
chemiluminescence, and images were taken using a Bio-Image Analysis
System (Bio-Rad, Hercules, CA, USA).
Perfusion fixation and fluorescent
immunohistochemistry
The rats were anesthetized and perfused
transcardiacally with 0.9% NaCl and 4% paraformaldehyde through the
left ventricle and the brains were removed. Samples were fixed in
cold 4% paraformaldehyde and then processed into 5-μm-thick
sections. For staining, the slides were placed in PBS containing
10% normal goat serum (NGS), and incugated for 1 h at 37°C to block
non-specific protein activity. This was followed by an incubation
at 4°C overnight with the primary antibodies, rabbit anti-GluR1
(1:40), rabbit anti-GluR2 (1:50), rabbit anti-GluR3 (1: 300) and
rabbit anti-GluR4 (1:300) (all from Abcam). After incubation with
primary antibodies, the sections were washed three times in PBS and
incubated with secondary goat anti-rabbit antibodies conjugated to
a fluorescent marker (Alexa 488; Abcam). The nuclei of all cells
were visualized by DAPI staining. After staining, the sections were
dried at room temperature and covered with ProLong antifade medium
(Invitrogen). Slides were analyzed using a Leica fluorescence
microscope. Some slides were selected for confocal imaging using a
confocal fluorescence microscope (Leiss LSM710).
Statistical analysis
Statistical data are expressed as the means ± SD.
Statistical analysis was performed using the Student’s t-test and
ANOVA using the SPSS package for Windows (version 16.0).
Differences with P<0.05 were considered to be statistically
significant.
Results
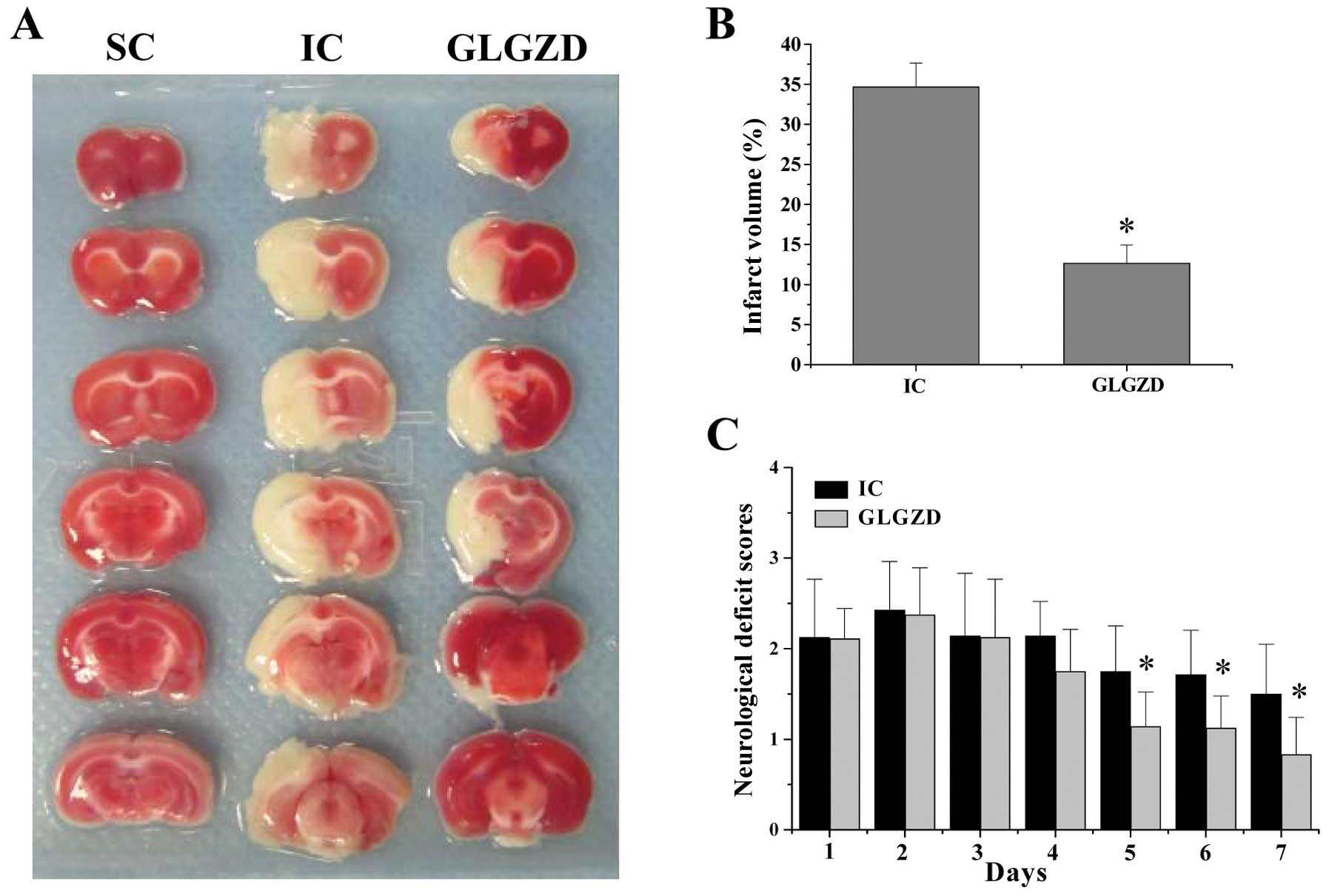
GLGZD ameliorates neurological deficits
and cerebral infarction
Following I/R injury by MCAO, the rats received
GLGZD and its neuroprotective effects were evaluated by examining
the neurological deficit scores. As expected, the rats in the SC
group did not show any manifestation of neurological deficits
(Fig. 2A), whereas all the rats
in the IC and GLGZD-treated groups displayed obvious signs of
cerebral injury (P<0.05, vs. SC group). However, after treatment
with GLGZD for five days, the neurological deficit scores of the
rats were significantly ameliorated (Fig. 2A) (P<0.05, vs. IC group). To
further verify these results, we evaluated the effect of GLGZD on
cerebral infarction. GLGZD treatment profoundly reduced cerebral
infarct volumes in the I/R-injured rats (P<0.05, vs. IC group)
(Fig. 2B and C). Taken together,
these results deomonstrate that GLGZD exerts therapeutic effects
against cerebral I/R injury.
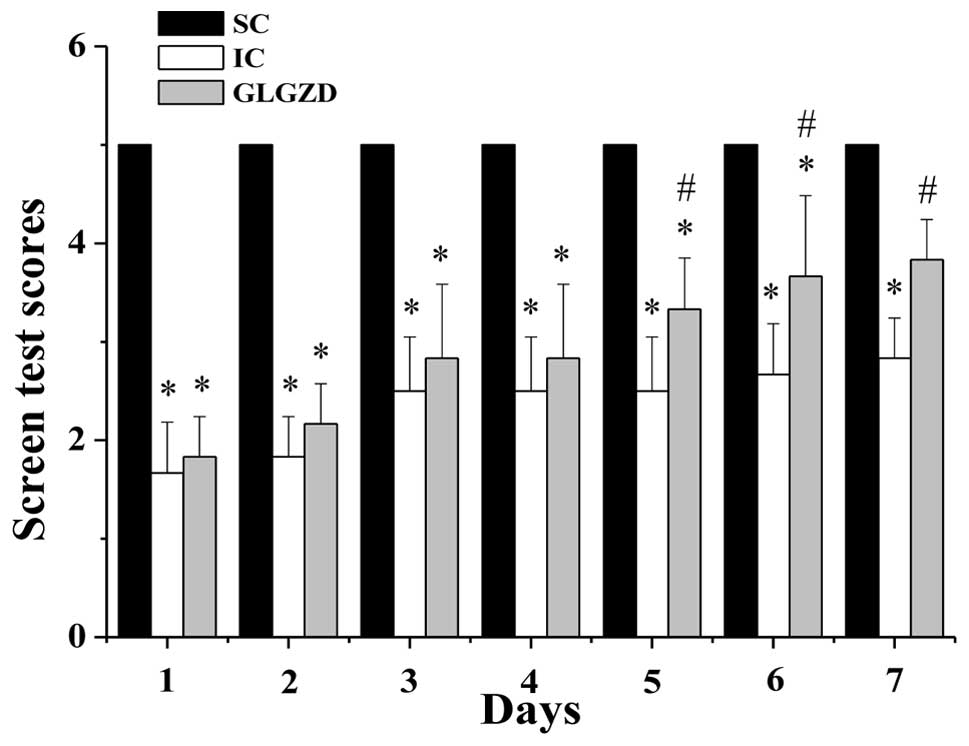
GLGZD reduces cerebral ischemic
spasticity
Cerebral I/R injury usually leads to spasticity, a
motor disorder that is characterized by a velocity-dependent
increase in muscle tone (hypertonia) exhibiting resistance to
stretching. To evaluate the effect of GLGZD on cerebral ischemic
spasticity, we performed a screen test to measure the muscle tone,
strength, stamina and balance in the rats from all the experimental
groups. MCAO model construction significantly decreased the screen
test score in the IC and GLGZD group rats (P<0.05, vs. SC
group), indicating that cerebral I/R injury induced spasticity
(Fig. 3). However, following
treatment with GLGZD for five days, the screen test scores in the
GLGZD-treated rats were significantly enhanced, as compared with
those in the rats from the IC group (P<0.05), suggesting that
GLGZD treatment alleviates the severity of cerebral ischemic
spasticity.
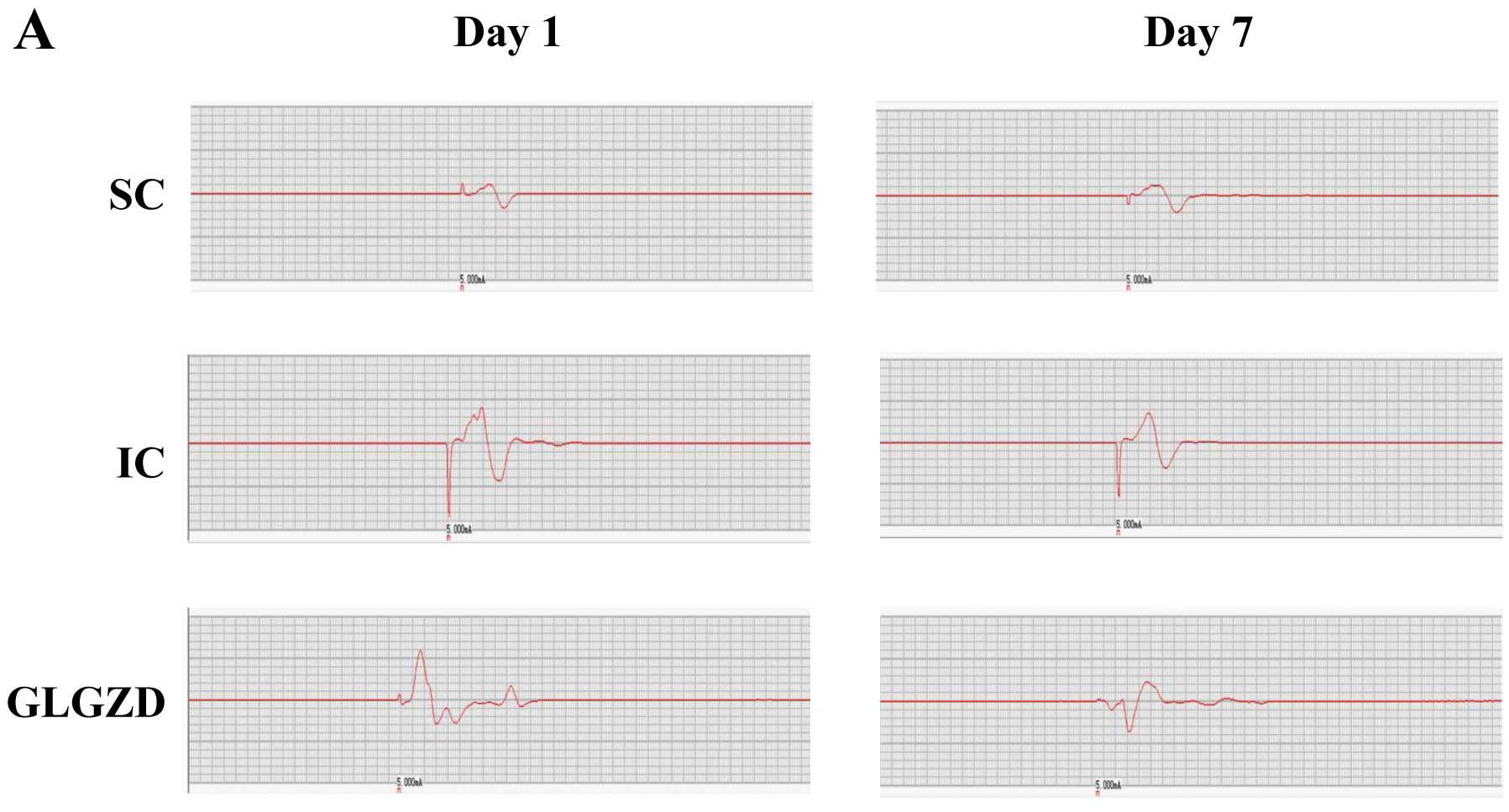
To confirm the above observations, we determined the
effect of GLGZD on the H-reflex that represents monosynaptic reflex
and thus indicates the degree of excitability of motor neurons.
Cerebral I/R injury resulted in an obvious increase in the
amplitude and decrease in the latency of the H-reflex wave, as well
as a significant increase in the Hmax/Mmax ratio, demonstrating the
occurrence of spasticity (Fig.
4). However, GLGZD treatment ameliorated the spasticity in
cerebral I/R-injured rats.
GLGZD inhibits I/R-induced elevation of
glutamate levels in CSF
Glutamate is the principal fast excitatory
neurotransmitter in the mammalian brain. Cerebral ischemia or brain
injury has been shown to cause a marked elevation in glutamate
concentrations (21), which is
responsible for neuronal injury or death due to excitotoxicity
(22). The suppression of
excitation (suppression of glutamate levels) is considered as a
means of treating spasticity (7,8).
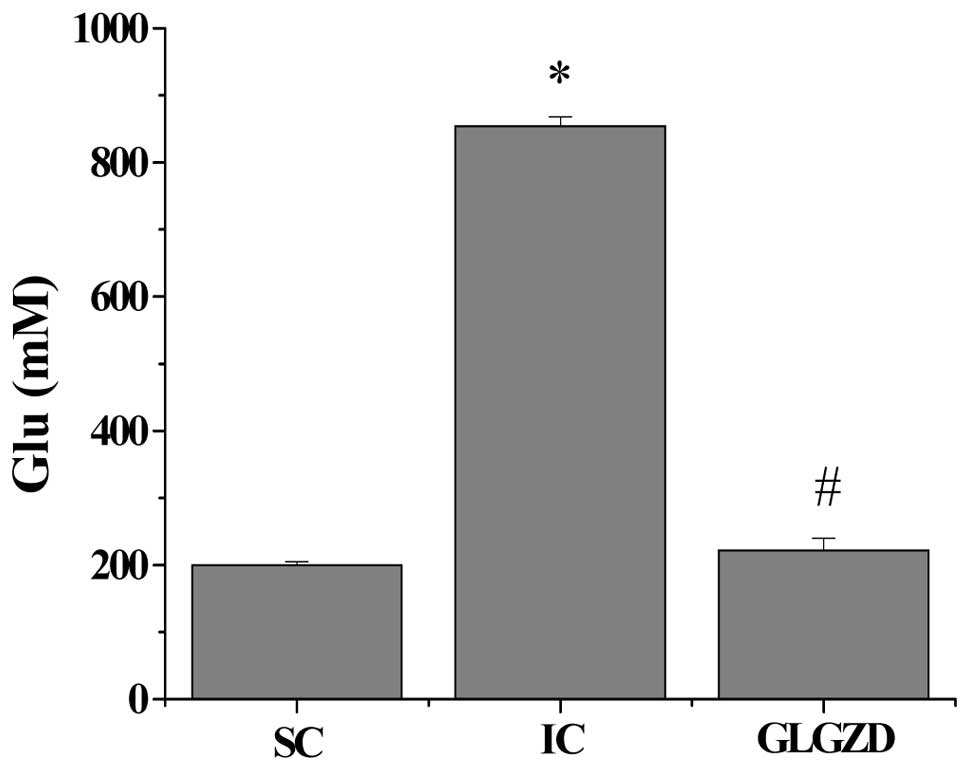
By HPLC analysis, we found that cerebral I/R injury significantly
enhanced the glutamate concentration in the CSF (Fig. 5) (SC group, 201.16±3.69 mM; IC
group, 855.05±13.20 mM; P<0.05). However, the administration of
GLGZD markedly reduced the levels of glutamate to 222.97±16.93 mM
(P<0.05, vs. IC group).
GLGZD alters the expression of AMPA
receptor subunits following cerebral ischemia
AMPA receptors are one of the ionotropic glutamate
receptors for the fast excitatory synaptic transmission in the CNS.
AMPA subunit expression is altered in cerebral ischemia tissues
(9), playing an important role in
ischemic spasticity (10). To
further explore the mechanisms mediating the neuroprotective and
anti-spasticity effects of GLGZD, we examined its effect on the
expression of AMPA receptor subunits in ischemic cerebral tissues.
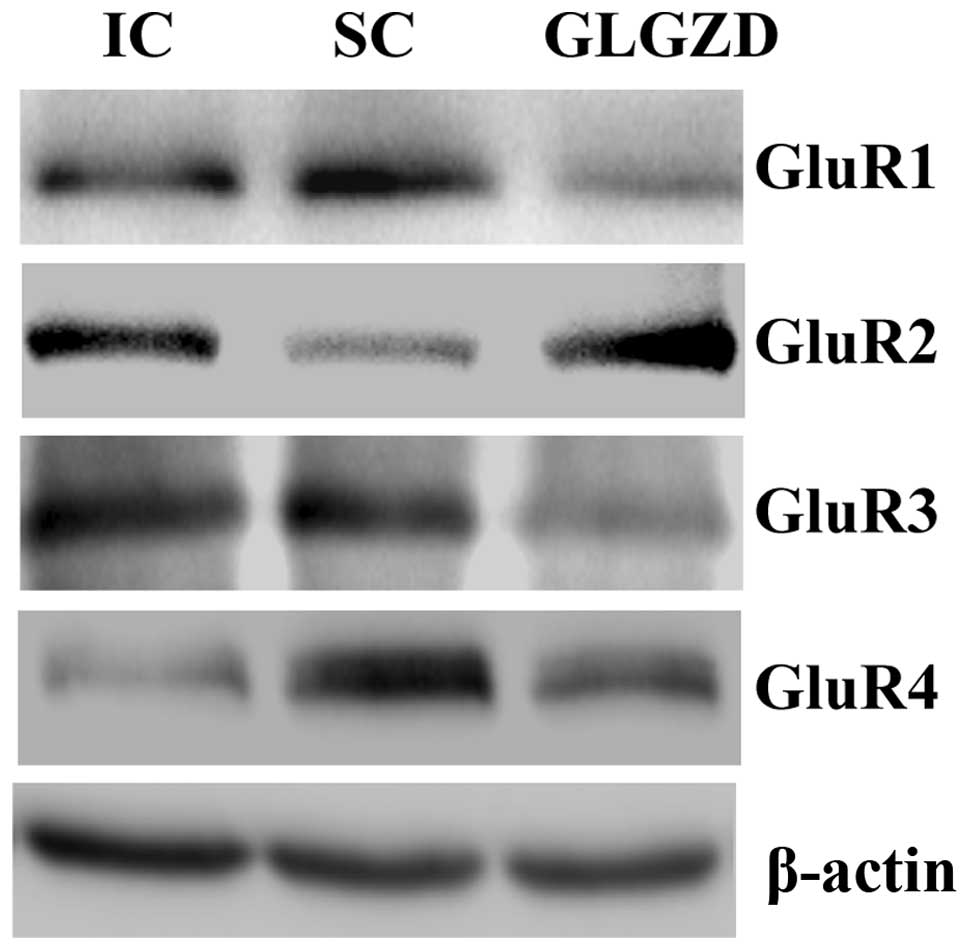
Data from both western blot analysis and immunofluorescence showed
that, as compared with the SC group, the protein expression of
GluR1, GluR3 and GluR4 was increased, whereas that of GluR2 was
reduced in the rats from the IC group. However, the I/R-induced
alteration in the expression of AMPA receptor subunits was
neutralized by GLGZD treatment (Figs.
6 and 7).
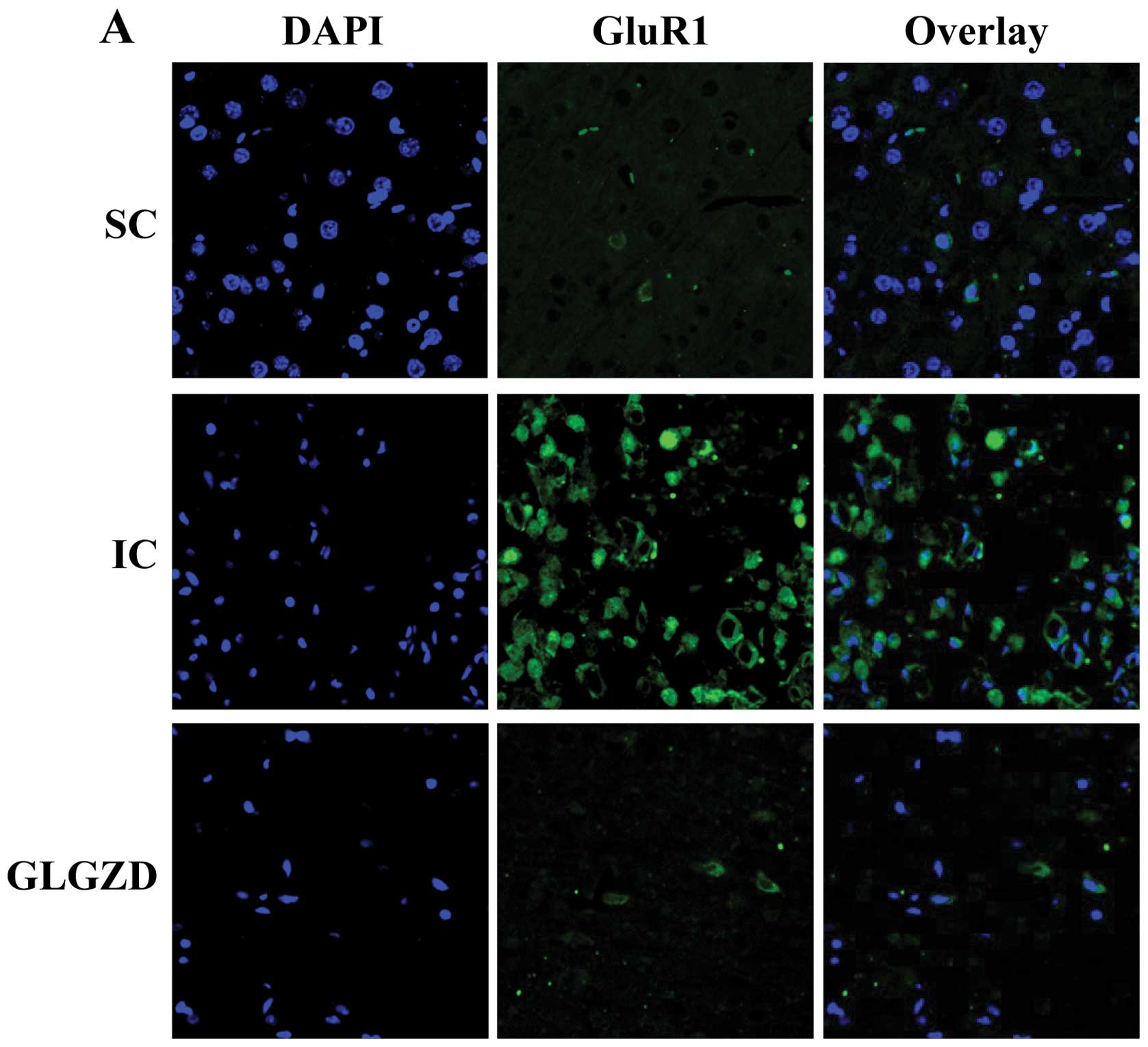 | Figure 7Effect of Gua Lou Gui Zhi decoction
(GLGZD) on AMPA expression using fluorescent immunohistochemistry.
At the end of the experiment, cerebral tissues from each group
(n=5) were processed for fluorescent immunohistochemistry. Nuclei
of all cells were visualized by DAPI staining and the green
fluorescence of AMPA receptors was detected by a confocal
fluorescence microscope. AMPA-positive cells were counted at four
arbitrarily selected microscopic fields at a magnification of ×400.
SC, sham operation control; IC, ischemic control; GLGZD, Gua Lou
Gui Zhi Decoction. (A) Fluorescent microscope images showing
GluR1-immunoreactive cells (green) and nucleus of neurons (blue)
between different groups. After ischemia, strong expression of
GluR1 can be seen while after treatment of GLGZD, expression of
GluR1 was decreased. (B) GluR2-immunoreactive cells (green) and
nucleus of neurons (blue) of different groups. After ischemia,
decreased expression of GluR2 was seen. After GLGZD, expression of
GluR2 was increased. (C) Strong GluR3-immunoreactive cells was
evident after ischemia. After GLGZD, expression of GluR3 was
decreased. (D) GluR4-immunoreactive cells was also noted to be
strongly expressed after ischemia. After GLGZD, expression of GluR4
was decreased. (E) Positive rate was expressed as the ratio of
green-stained cells to the blue DAPI-stained total cells. Data are
the means ± SE (error bars). *P<0.05, vs. SC group;
#P<0.05, vs. IC group. |
Discussion
Spasticity is a serious post-stroke physical
disability and may slow down the potential success of
rehabilitation. Glutamate and AMPA receptors have been shown to
play a crucial role in spasticity following cerebral I/R injury.
Glutamate is one of the most abundant excitatory neurotransmitters
in the mammalian CNS and is responsible for sending signals between
nerve cells. At normal concentrations it plays a critical role in
learning and memory. However, under pathological conditions such as
ischemic stroke, glutamate in the extracellular fluid usually
accumulates to reach aberrantly high concentrations, which can lead
to overexcitation and eventually the death of nerve cells. This
pathological process is termed excitotoxicity that is associated
with spasticity (7,23). As well as pathologically high
glutamate levels, excitotoxicity can be induced by the
overactivation of glutamate receptors, such as the AMPA receptors.
The binding of glutamate to its receptors can cause high levels of
Ca2+ to influx into cells, initiating the process of
cell apoptosis. AMPA receptors are composed of four types of
subunits (known as GluR1–4) that mostly exist as heterotetramers,
consisting of symmetric ‘dimer of dimers’ of GluR2 and either
GluR1, GluR3 or GluR4. Although the four subunits of AMPA receptor
family are of similar size and are approximately 70% homologous,
their functions differ. Unlike the GluR1, GluR3 and GluR4 subunits
that facilitate Ca2+ influx, the GluR2 subunit almost
always prevents calcium from entering the cell. Therefore, the
permeability of AMPA receptors to Ca2+ is determined by
the GluR2 subunit. If an AMPA receptor lacks a GluR2 subunit, it
will be permeable to Ca2+; whereas GluR2-containing AMPA
receptors are unfavorable for calcium influx. AMPA receptor
subunits have been found on spinal α-motoneurons as well as on
presynaptic Ia afferents, consistent with their demonstrated role
in motor function. It has been shown that the expression of AMPA
receptors can affect the clinical signs of spasticity and rigidity
following cerebral ischemia; the intrathecal or systemic delivery
of the selective AMPA receptor antagonist, NGX424, represents an
effective therapy for modulating chronic spasticity in
baclofen-tolerant animals (24,25).
GLGZD is a classical TCM that was first prescribed
in the Eastern Han Dynasty, around 210 AD. As shown in our previous
study (unpublished data), GLGZD exerts significant therapeutic
effects on spasticity in stroke patients. However, the mode of
action of its neuroprotective and anti-spasticity effects remains
poorly understood. In the present study, using a focal cerebral
ischemia rat model, we demonstrate that GLGZD exerts
neuroprotective effects by improving neurological deficits and
reducing the cerebral infarct volume. In addition, GLGZD displays
anti-spasticity effects by improving the screen test and H-reflex
scores. Moreover, our results demonstrate that GLGZD significantly
decreases the cerebral I/R-induced overexpression of glutamate in
CSF. Furthermore, GLGZD downregulates the expression of the AMPA
receptor subunits, GluR1, GluR3 and GluR4, but increases GluR2
expression in cerebral I/R-injured rats.
In conclusion, to our knowledge, in the present
study, we report for the first time that GLGZD exerts
neuroprotective and therapeutic effects against spasticity in an
ischemic stroke model via the inhibition of glutamate/AMPA
receptor-mediated excitotoxicity. These data suggest that GLGZD may
be a potential therapeutic agent for cerebral ischemia and
spasticity.
Acknowledgements
This study was sponsored by the Guidance Project of
the Fujian Provincial Department of Science and Technology (no.
2012D011), and the Key Project of the Department of Health of
Fujian Province (no. zlckf01).
Abbreviations:
|
GLGZD
|
Gua Lou Gui Zhi decoction
|
|
MCAO
|
middle cerebral artery occlusion
|
|
CSF
|
cerebrospinal fluid
|
|
UMNS
|
upper motor neuron syndrome
|
|
AMPA
|
α-amino-3-hydroxy-5-methyl-
4-isoxazolepropionic acid
|
|
TTC
|
2,3,5-triphenyl tetrazolium
chloride
|
References
|
1
|
Mayer NH, Esquenazi A and Childers MK:
Common patterns of clinical motor dysfunction. Muscle Nerve Suppl.
20:S21–S35. 1997. View Article : Google Scholar
|
|
2
|
Lance JW: What is spasticity? Lancet.
335:6061990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lundstrom E, Terent A and Borg J:
Prevalence of disabling spasticity 1 year after first-ever stroke.
Eur J Neurol. 15:533–539. 2008.PubMed/NCBI
|
|
4
|
Watkins CL, Leathley MJ, Gregson JM, Moore
AP, Smith TL and Sharma AK: Prevalence of spasticity post stroke.
Clin Rehabil. 16:515–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sommerfeld DK, Eek EU, Svensson AK,
Holmqvist LW and von Arbin MH: Spasticity after stroke: its
occurrence and association with motor impairments and activity
limitations. Stroke. 35:134–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duncan PW, Zorowitz R, Bates B, et al:
Management of adult stroke rehabilitation care a clinical practice
guideline. Stroke. 36:e100–e143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gracies JM, Nance P, Elovic E, McGuire J
and Simpson DM: Traditional pharmacological treatments for
spasticity. Part II: General and regional treatments. Muscle Nerve
Suppl. 20:S92–S120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davidoff RA: Antispasticity drugs:
mechanisms of action. Ann Neurol. 17:107–116. 2004. View Article : Google Scholar
|
|
9
|
Gottlieb M and Matute C: Expression of
ionotropic glutamate receptor subunits in glial cells of the
hippocampal CA1 area following transient forebrain ischemia. J
Cereb Blood Flow Metab. 17:290–300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hefferan MP, Kucharova K, Kinjo K, et al:
Spinal astrocyte glutamate receptor 1 overexpression after ischemic
insult facilitates behavioral signs of spasticity and rigidity. J
Neurosci. 27:11179–11191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saulino M and Jacobs BW: The
pharmacological management of spasticity. J Neurosci Nurs.
38:456–459. 2006.
|
|
12
|
Hesse S and Werner C: Poststroke motor
dysfunction and spasticity: novel pharmacological and physical
treatment strategies. CNS Drugs. 17:1093–1107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meythaler JM, Guin-Renfroe S, Johnson A
and Brunner RM: Prospective assessment of tizanidine for spasticity
due to acquired brain injury. Arch Phys Med Rehabil. 82:1155–1163.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meythaler JM, Guin-Renfroe S, Law C, Grabb
P and Hadley MN: Continuously infused intrathecal baclofen over 12
months for spastic hypertonia in adolescents and adults with
cerebral palsy. Arch Phys Med Rehabil. 82:155–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ford B, Greene P, Louis ED, et al: Use of
intrathecal baclofen in the treatment of patients with dystonia.
Arch Neurol. 53:1241–1246. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X: Research on formula treating
paralysis and spasticity from ‘treatise on febrile and
miscellaneous diseases’. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.(In Chinese).
|
|
17
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi decoction on c-fos and c-jun in epileptic rats. Sichuan Hua xi
Zhong Yi Yao Yan Jiu Suo. 23:21–22. 2005.(In Chinese).
|
|
18
|
Yang C, Chen L and Tao J: New usage of a
classical formula - Gua Lou Gui Zhi decoction. Liaoning Zhong Yi Za
Zhi. 8:166–167. 2012.(In Chinese).
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Liu L, Ma C, Xu B, Duan X and Wang
B: Effect of restraint stress on depression-like behaviors in rats
after transient focal cerebral ischemic injury. Neural Regen Res.
2:390–394. 2007. View Article : Google Scholar
|
|
21
|
Parelkar NK and Wang JQ: Upregulation of
metabotropic glutamate receptor 8 mRNA expression in the rat
forebrain after repeated amphetamine administration. Neurosci Lett.
433:250–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonde C, Noraberg J, Noer H and Zimmer J:
Ionotropic glutamate receptors and glutamate transporters are
involved in necrotic neuronal cell death induced by oxygen-glucose
deprivation of hippocampal slice cultures. Neuroscience.
136:779–794. 2005. View Article : Google Scholar
|
|
23
|
Abbruzzese G: The medical management of
spasticity. Eur J Neurol. 9(Suppl 1): S30–S34. 2002. View Article : Google Scholar
|
|
24
|
Oshiro M, Hefferan MP, Kakinohana O, et
al: Suppression of stretch reflex activity after spinal or systemic
treatment with AMPA receptor antagonist NGX424 in rats with
developed baclofen tolerance. Br J Pharmacol. 161:976–985. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gómez-Soriano J, Goiriena E and Taylor J:
Spasticity therapy reacts to astrocyte GluA1 receptor upregulation
following spinal cord injury. Br J Pharmacol. 161:972–975.
2010.PubMed/NCBI
|