Introduction
Intracerebral hemorrhage (ICH) represents at least
10% of all cases of stroke, with a 30-day mortality ranging from 35
to 52% (1,2). Nearly 30% of patients with ischemic
stroke have secondary ICH (3),
particularly as a major complication of thrombolytic therapy
(4). Currently, medical therapy
for patients with ICH is generally limited to supportive care or
neurosurgical evacuation of the hematoma. Therefore, development of
alternative therapies for ICH is important.
ICH induces brain injury due to local tissue
deformation and subsequent pathological changes such as
inflammation, excitotoxicity and apoptosis (5). Hematoma-induced injury mainly
involves mechanical disruption of neurons and glia, followed by
mechanical deformation causing oligemia, neurotransmitter release,
mitochondrial dysfunction and membrane depolarization. A secondary
cascade of injury is started by products of coagulation and
hemoglobin breakdown, in particular thrombin, which leads to
microglia activation and neutrophil infiltration 4 h after ICH
(6). Increasing evidence suggest
that ICH-induced inflammation is a key factor in secondary brain
damage. The inflammatory cascade comprises both cellular and
molecular components. Blood components, including erythrocytes,
neutrophils, macrophages, thrombin, and plasmin immediately
infiltrate the brain after ICH. The inflammatory responses involve
enzyme activation, mediator release, inflammatory cell migration
and glial activation (7).
Activated microglia and infiltrating neutrophils release products
that induce breakdown of the blood-brain barrier, vasogenic oedema,
and apoptosis in neurons and glia (6). Therefore, anti-inflammatory
approaches may improve the outcome of ICH, and anti-inflammatory
pharmacologic therapies have been investigated to reduce the brain
injury after ICH (5,8,9).
Mesenchymal stem cells (MSCs), a heterogeneous
population of plastic-adherent cells, have been successfully used
for the treatment of experimental ICH (10–12). In our previous study, we showed
that transplantation of human bone marrow-derived MSCs (hBMSCs)
increased glucose metabolic activity in the cortex and basal
ganglia in the hemorrhagic boundary zone using serial
18F-FDG PET scans, leading to neurological recovery in
Macaca fascicularis monkey models (12). However, the clinical application
of stem cell therapy in stroke has been delayed since the
neuroprotective mechanisms by which MSCs improve functional
recovery remain largely unknown. Recently, MSCs are receiving more
and more attention based on the observation that they can exert
immunoregulatory activity both in vitro and in vivo
(13–15). MSCs have been reported to have
therapeutic effects by downregulating inflammation in a variety of
diseases such as graft-versus-host disease after kidney transplant
(16), lung injury (17), myocardial infarction (18) and experimental autoimmune
encephalomyelitis (19).
Therefore, MSC treatment may inhibit inflammation after ICH and
reduce subsequent brain injury. To examine this hypothesis, we used
a subpopulation of hBMSCs, termed fetal liver kinase
(Flk)-1+ hBMSCs, which share most features of MSCs and
possess marked differentiation potential, even after being expanded
for >50 cell doublings (20,21). Flk-1+ hBMSCs may
represent a very early population in the hierarchy of stem cell
development. This is the first stem cell product approved by the
State Food and Drug Administration (SFDA) of China, and the
feasibility and safety of Flk-1+ hBMSC transplantation
were evaluated in rhesus monkeys and humans (22). Understanding the mechanisms
underlying the beneficial effects of BMSC therapy may greatly
enhance its translation to the clinic.
Materials and methods
Cell culture, flow cytometry and
karyotype analysis
Bone marrow was obtained from the iliac aspirates of
healthy male volunteers after informed consent was obtained. Human
BMSCs were used in accordance with the procedures approved by the
Human Experimentation and Ethics Committee. As described (37), ~20 ml of bone marrow was obtained
from each volunteer and diluted with the same volume of normal
saline. Mononuclear cells were separated using lymphocyte
separation medium and density gradient centrifugation (1.077
g/cm3). The cells were plated at the density of
3×105/cm2 in Dulbecco’s modified Eagle’s
medium/nutrient mixture F-12 (Invitrogen, Carlsbad, CA, USA)
supplemented with 40% MCDB-201 medium (Sigma, St. Louis, MO, USA),
10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 10 ng/ml
epidermal growth factor, 10 ng/ml platelet-derived growth factor,
100 U/ml phytomycin and 100 U/ml penicillin (all were from Sigma),
and cultured at 37°C in a humidified atmosphere containing 5%
CO2. After 24 h, non-adherent cells were washed and
removed. The medium was replaced with the same fresh medium every
third day. The cells were digested with pancreatic enzyme
(Invitrogen) and passaged upon reaching 80% confluence.
At the fifth passage, hBMSCs were collected,
counted, and analyzed by flow cytometry for phycoerythrin
anti-human phycoerythrin-CD29, -CD105, -CD31, -Flk-1, -CD34, -CD44
and human leukocyte antigen-DR (HLA-DR) expression
(Becton-Dickinson Biosciences, San Diego, CA, USA).
For karyotype analysis, metaphases were prepared
from methanol/acetic acid (3:1) fixed cells. Slides were hybridized
by spectral karyotyping. At least 20 metaphase cells were analyzed
from each hBMSC preparation. Breakpoints were assigned based on
G-banded karyotype.
Mixed lymphocyte reaction
Rat peripheral blood mononuclear cells (rPBMCs) from
individual Sprague-Dawley rats were pooled and used as responder
cells. Human peripheral blood mononuclear cells (hPBMCs;
3×105/well) from a healthy volunteer and
Flk-1+ hBMSCs (5×104/well) were used as
stimulator cells in 96-well round-bottom plates. Responder rPBMCs
(3×105/well) were added to irradiated (15 Gy) stimulator
hPBMCs or Flk-1+ hBMSCs in RPMI-1640 medium containing
10% FBS (supplied by Peking Union Medical College). In the mitogen
proliferative assay, responder rPBMCs (3×105/well) were
incubated with 3 μg/ml concanavalin A (ConA; Sigma). Furthermore,
rPBMCs (3×105/well) containing ConA were added to
Flk-1+ hBMSCs in 96-well plates. Following 5 days of
incubation at 37°C in a humidified atmosphere containing 5%
CO2, cells were pulsed with 3H-thymidine (1
μCi/well; supplied by China Atomic Energy Research Institute) for
the last 18 h, harvested, and counted with a Microβ 1450 Trilux
liquid scintillation counter (Wallac Inc., Gaithersburg, MD, USA).
Results were expressed in counts per minute (cpm) and presented as
the means obtained from triplicate cultures.
ICH model and groups
All animal procedures were performed in accordance
with guidelines issued by the Committee on Animal Research of
Peking Union Medical College Hospital and were approved by the
Institutional Ethics Committee (Peking Union Medical College,
Beijing). Adult male Sprague-Dawley rats weighing 190–210 g were
used. ICH was induced via the stereotaxic intrastriatal injection
of collagenase type VII (Sigma), as described previously (9). In brief, rats were anesthetized with
10% chloral hydrate (400 mg/kg, i.p.; Sigma). Rectal temperature
was maintained at 37°C throughout the surgical procedure using a
heating lamp. Animals were placed in a stereotaxic frame and an
incision was made exposing the bregma. One burr hole was drilled at
the injection site: anterior-posterior (AP) -0.2 mm, mediolateral
(ML) 2.9 mm, and dorsoventral (DV) 6.0 mm from the dura.
Collagenase type VII (0.4 IU) in 2 μl saline was injected at a rate
of 0.7 μl/min. The needle was left in place for 2 min prior to
withdrawal. The rats recovered from anesthesia and were returned to
their cages with free access to food and water. On 1 day after ICH,
the modified neurological severity score (mNSS) test was calculated
based on a series of motor, sensory, balance and reflex tests
(33). Rats subjected to ICH with
mNSS 10–14 were selected and were randomly assigned to two groups:
i) the Flk-1+ hBMSC-treated group (ICH + hBMSCs, n=65)
and ii) the saline control group (ICH + saline, n=67).
Cell transplantation
On day 1 after ICH, rats were anesthetized as
described above and received transplantation of Flk-1+
hBMSCs or saline. Animals were placed in a stereotaxic frame and
burr holes were drilled at three injection sites: site 1, AP 1.6
mm, ML 2.5 mm and DV 4.0 mm from dura; site 2, AP 0.2 mm, ML 2.9 mm
and DV 4.0 mm from dura; site 3, AP −1.8 mm, ML 3.8 mm and DV 4.5
mm from dura. Approximately 2×105 Flk-1+
hBMSCs in 15 μl saline or an equal volume of saline was injected at
a rate of 1 μl/min. Following injections, the needle was left in
place for 2 min prior to withdrawal.
Behavioral testing
The mNSS test was performed at 1, 3, 7, 14, 28, 42
and 56 days after ICH by an investigator blinded to the
experimental groups (n=12 for each group). The mNSS test is a
composite of motor, sensory, balance and reflex tests. Neurological
function is graded on a scale of 0–18 (normal score, 0; maximal
deficit score, 18). A single point is awarded for a specific
abnormal behavior or for the lack of a tested reflex; thus, the
higher the score the more severe the injury (33).
Brain water content
The brain water content was measured 3 days after
ICH (n=6 for each group), as previously described (9). In brief, the rats were
over-anesthetized. The brains were removed immediately, and divided
into the ipsilateral and contralateral hemispheres. Each brain
sample was placed on a piece of aluminum foil, and immediately
weighed on an electronic analytical balance to obtain the wet
weight, then dried at 110°C for 24 h to obtain the dry weight. The
brain water content was calculated using the following equation: %
Water = (wet weight − dry weight)/wet weight × 100.
Tissue processing
On days 3, 7, 14, 28 and 56 following ICH, rats (n=6
for each group, respectively) were intracardially perfused with
chilled saline followed by 4% paraformaldehyde in 0.01 M PBS (pH
7.4). The brains were collected and postfixed in 4%
paraformaldehyde at 4°C overnight, then transferred to 30% sucrose
in PBS at 4°C for cryoprotection. Coronal sections (8 μm) were
taken from a +2.0 to −2.0 mm area around the bregma using a
vibratome (Leica, Wetzlar, Germany), thaw-mounted on
gelatine-coated slides and stored at −80°C until processing.
Nissl staining and peroxidase
immunohistochemistry
Nissl staining was used to detect neurons according
to standard methods (38). In
brief, the sections were incubated in 1% cresyl violet for 30 sec,
decolorized in acetic acid, and then dehydrated and covered.
Diaminobenzidine peroxidase immunohistochemistry was used to label
myeloperoxidase (MPO)+ cells (neutrophils). Briefly,
slices were blocked with normal goat serum for 30 min at 37°C, and
then incubated in rabbit anti-MPO IgG (1:200; Abcam, Cambridge, UK)
at 4°C overnight. After three washes in PBS, sections were
incubated in horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (1:200; ZSJQ Corp., Beijing, China) for 30 min at
37°C, and developed using a DAB kit.
Immunofluorescence histochemistry
Slices were blocked with normal goat serum for 30
min at 37°C, and then incubated in mouse anti-ED1 IgG (a marker for
macrophage and activated microglia, 1:150; Millipore, Temecula, CA,
USA) or mouse anti-NeuN IgG (a marker for neurons, 1:200;
Millipore) at 4°C overnight. After three washes in PBS, sections
were incubated in the dark for 30 min at 37°C with
Rhodamine-conjugated or FITC-conjugated goat anti-mouse IgG
(1:150). After washing, sections were covered with mounting medium
containing 4′,6′-diamino-2-phenylindole (DAPI) (both were from ZSJQ
Corp.).
Double-label immunofluorescence was used to analyze
the survival and differentiation of Flk-1+ hBMSCs.
MAB1281 (mouse anti-human nuclear monoclonal antibody, Millipore)
is a marker for human cells (39). In the present study, it was used
to detect Flk-1+ hBMSCs. To visualize the cellular
colocalization of MAB1281 and cell type-specific markers in the
same cells, each section was incubated at 4°C overnight in the
primary MAB1281 (1:100) with microtubule-associated protein 2
(MAP-2) (a neuron marker; rabbit monoclonal IgG, 1:200; Millipore),
glial fibrillary acidic protein (GFAP) (an astrocyte marker; rabbit
monoclonal IgG, 1:400; Abcam), or von Willebrand factor (vWF) (an
endothelial marker; rabbit monoclonal IgG, 1:200; Abcam). After
washing, sections were incubated in the dark for 30 min at 37°C in
FITC-conjugated goat anti-mouse IgG (1:150) with
Rhodamine-conjugated goat anti-rabbit IgG (1:150) (both were from
ZSJQ Corp.).
Terminal deoxynucleotidyltransferase
(TdT)-mediated dUTP-biotin nick-end labeling (TUNEL) staining
TUNEL staining was performed using the In
Situ Cell Death Detection kit (TMR red; Roche Diagnostics,
Mannheim, Germany) according to the manufacturer’s instructions. In
brief, slices were first incubated in permeabilization solution
containing 0.1% sodium citrate and 0.1% Triton X-100 at 4°C for 2
min. Sections were then incubated with TdT enzyme in reaction
buffer containing TMR red labeled dUTP at 37°C for 60 min. The
negative control was incubated in reaction buffer without the TdT
enzyme. After washing with wash buffer, sections were covered with
mounting medium containing DAPI (ZSJQ Corp.).
Hematoxylin and eosin staining
On day 56 following ICH, rats were deeply
anesthetized with chloral hydrate and were fixed by transcardial
perfusion with saline, followed by 4% paraformaldehyde. The brains
were collected and embedded in paraffin. The brain tissue was
dissected into 6 pieces of 2-mm coronal blocks with rodent brain
matrix. A series of adjacent 6-μm sections were cut from each block
(33). The brain sections were
stained with hematoxylin and eosin and photographed with a
microscope (Zeiss, Oberkochen, Germany). Relative hemorrhage volume
was analyzed with ImagePro Plus software (Media Cybernetics, Inc.,
Bethesda, MD, USA). The indirect lesion area, in which the intact
area of the ipsilateral hemisphere was subtracted from the area of
the contralateral hemisphere, was calculated. Relative hemorrhage
volume was presented as the percentage of the volume of the
indirect lesion compared with the contralateral hemisphere
(40).
Quantitative real-time RT-PCR
Total RNA was extracted from ipsilateral brain
tissues (Fig. 5A) by TRIzol
reagent (Invitrogen). RNA concentrations were determined by
absorbance readings at 260 nm with GeneQuant (Amersham Biosciences,
Amersham, UK), and 1 μg total RNA was reverse transcribed to cDNA
using the RevertAid™ First Strand cDNA Synthesis kit with oligo(dT)
primers (Fermentas, Hanover, MD, USA). PCR amplification was
performed in a 50-μl volume containing 2 μl of each primer and 25
μl SYBR-Green qPCR mix (Toyobo, Osaka, Japan). The amplification
procedure consisted of 50°C for 2 min, 95°C for 10 min and 40
cycles of amplification reactions at 95°C for 15 sec, and at 60°C
for 1 min. The primers used were as follows: interleukin (IL)-1β
(100 bp) forward, 5′-CAGCTTTCGACAGTGAGGAGAA-3′ and reverse,
5′-CTCATCTGGACAGCCCAAGTC-3′; IL-2 (100 bp) forward,
5′-GCCACAGAATTGAAACATCTTCAG-3′ and reverse,
5′-GCGTCTTCCAAGTGAAAGCTTT-3′; IL-4 (100 bp) forward,
5′-ACCTCCGTGCTTGAAGAACAA-3′ and reverse,
5′-CATTCACGGTGCAGCTTCTC-3′; IL-6 (100 bp) forward,
5′-CAGCGATGATGCACTGTCAGA-3′ and reverse,
5′-TCAATAGGCAAATTTCCTGGTTATATC-3′; tumor necrosis factor (TNF)-α
(100 bp) forward, 5′-TGTACCTTATCTACTCCCAGGTTCTCT-3′ and reverse,
5′-CTCCTGGTATGAAATGGCAAATC-3′; R-actin β (93 bp) forward,
5′-CAACCGTGAAAAGATGACCCA-3′ and reverse,
5′-AATGCCAGTGGTACGACCAGA-3′. R-actin β cDNA was used as a control.
Relative expression between a given sample and a reference sample
was calculated using the 2 −ΔΔCt method.
Quantification
The sections were examined under a fluorescence
microscope (Eclipse 80i; Nikon, Tokyo, Japan) or under a confocal
laser scanning microscope (Leica TCS SP2; Leica, Wetzlar, Germany).
Images were collected and analyzed with ImagePro Plus software
(Media Cybernetics, Inc., Bethesda, MD, USA) based on evaluation of
an average of 3 slides (8-μm thick, 72-μm interval, every 10th
slide) for each animal. We counted the total number of
MAB1281-positive cells in the consistently selected area, and the
percentage of MAB1281-positive cells colocalized with cell
type-specific markers (MAP-2, GFAP and vWF) by double staining was
investigated in terms of where the transplanted cells were located.
The number of MPO+ and ED1+ cells around the
hemorrhagic lesion, and NeuN+, Nissl+ and
TUNEL+ cells in the ipsilateral cortical area were
counted for six random high-power fields in each section, and these
data were averaged.
Statistical analysis
Data are presented as means ± SD and analyzed by
SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA). For the
behavioral test, analysis of variance (ANOVA) was used to test the
group effect by the repeated time of assessments. The results of
the mixed lymphocyte reaction were analyzed by ANOVA. Brain water
content, relative hemorrhage volume, immunohistochemistry, and
quantitative real-time RT-PCR data were analyzed by the Student’s
2-tailed unpaired t-test. A P-value of <0.05 was considered to
indicate a statistically significant result.
Results
The mNSS test was assessed before rats were selected
and randomly grouped. In this way, we tried to control the quality
and minimize variations of the models. One hundred and twenty of
the 132 ICH rats survived. Among the 12 animals that died
prematurely, 5 were in the Flk-1+ hBMSC-treated group
and 7 were in the control group.
Characteristics and karyotype analysis of
hBMSCs
The phenotypes of hBMSCs used in this study were
positive for Flk-1, CD29, CD44, CD105 and CD106 and were negative
for HLA-DR, CD31 and CD34. They displayed the capacity for
multi-lineage differentiation into adipocytes, osteoblasts and
chondrocytes (23).
Flk-1+ hBMSCs at the fifth passage were used for
karyotype analysis, which was conducted to validate chromosome
stability. No trisomy, tetraploidy or chromosome rearrangement was
observed (24).
Neurological behavior, brain water
content, and hemorrhage volume
The mNSS test was compared between the
Flk-1+ hBMSC-treated and control rats. There was no
difference in neurological outcome before cell transplantation;
however, significant functional improvement appeared in the
Flk-1+ hBMSC-treated group at each time point starting
at 3 days after ICH compared with the control group (P<0.05)
(Fig. 1A).
Brain water content in the ipsilateral (hemorrhagic)
hemisphere at 3 days post-ICH was significantly reduced in the
Flk-1+ hBMSC-treated group (80.6±0.8%) compared with the
controls (81.8±0.6%) (P<0.05) (Fig. 1B). However, no difference was
observed in the contralateral hemisphere (79.5±0.4% in the control
group and 79.2±0.5% in the Flk-1+ hBMSC-treated group)
(P>0.05) (Fig. 1B).
Following hematoxylin and eosin staining,
hemorrhagic lesions mainly appeared in the striatum 56 days after
ICH. Hemorrhagic core tissues were transformed into cysts. The
damaged tissue consisted of a central cavity surrounded by a scar
border. Although lower hemorrhagic volume was detected in the
Flk-1+ hBMSC-treated rats (23.8±5.8%) when compared with
that in the controls (28.2±4.2%), no significant difference was
found between the two groups (P>0.05) (Fig. 1C).
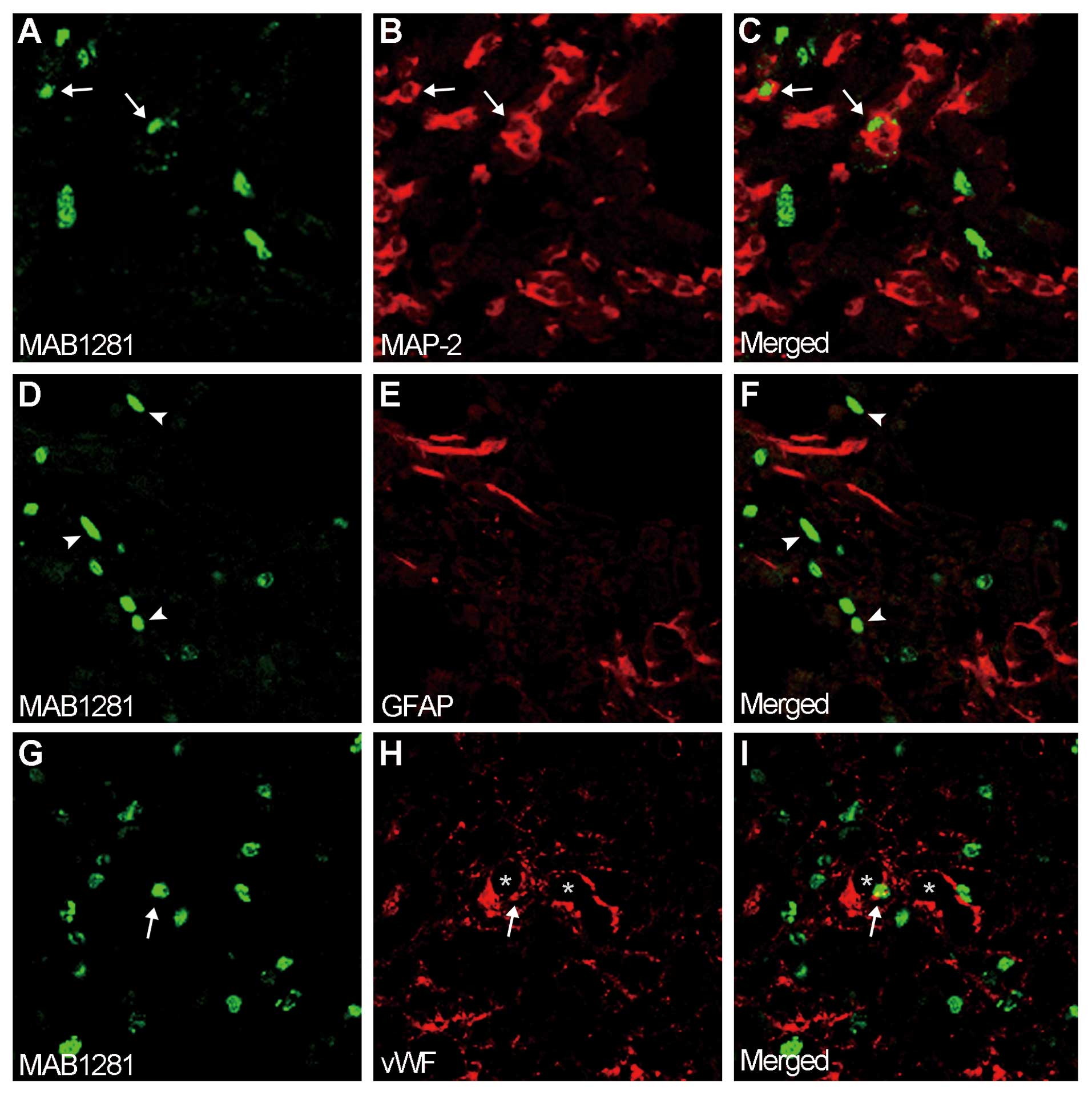
Identification of donor cells in
recipients
Flk-1+ hBMSCs survived and the majority
of the cells were distributed close to the hemorrhagic boundary
zone 55 days after transplantation. Few Flk-1+ hBMSCs
were observed in the contralateral hemisphere. Double-label
immunohistochemistry revealed that Flk-1+ hBMSCs
(Fig. 2A and G) were reactive for
neuron marker, MAP-2 (Fig. 2B and
C), and endothelial marker, vWF (Fig. 2H and I); however, no
Flk-1+ hBMSCs (Fig.
2D) were colocalized with astrocyte marker, GFAP (Fig. 2E and F). The hemorrhagic boundary
zone was divided into three different areas (striatum, cortex and
corpus callosum). In the striatum, the percentages of
Flk-1+ hBMSCs that expressed MAP-2, vWF and GFAP were
~4.3, 1.2 and 0% respectively. In the cortex, the percentages of
Flk-1+ hBMSCs that expressed MAP-2, vWF and GFAP were
~5.8, 2.5 and 0%, respectively. In the corpus callosum, the
percentages of Flk-1+ hBMSCs that expressed MAP-2, vWF
and GFAP were ~1.6, 1.6 and 0%, respectively. The control rats were
evaluated, and no hBMSCs were detected.
Immunomodulatory effect of
Flk-1+ hBMSCs in vitro
To confirm the in vitro immunomodulatory
effect of Flk-1+ hBMSCs, Flk-1+ hBMSCs and
hPBMCs were added to mixed lymphocyte cultures of rPBMCs,
respectively. Human PBMCs induced the proliferation of rPBMCs,
whereas Flk-1+ hBMSCs did not elicit a proliferative
response of rPBMCs. Furthermore, Flk-1+ hBMSCs
significantly inhibited the proliferation of rPBMCs induced by ConA
(P<0.05) (Fig. 3).
Anti-inflammatory effect of
Flk-1+ hBMSCs in vivo
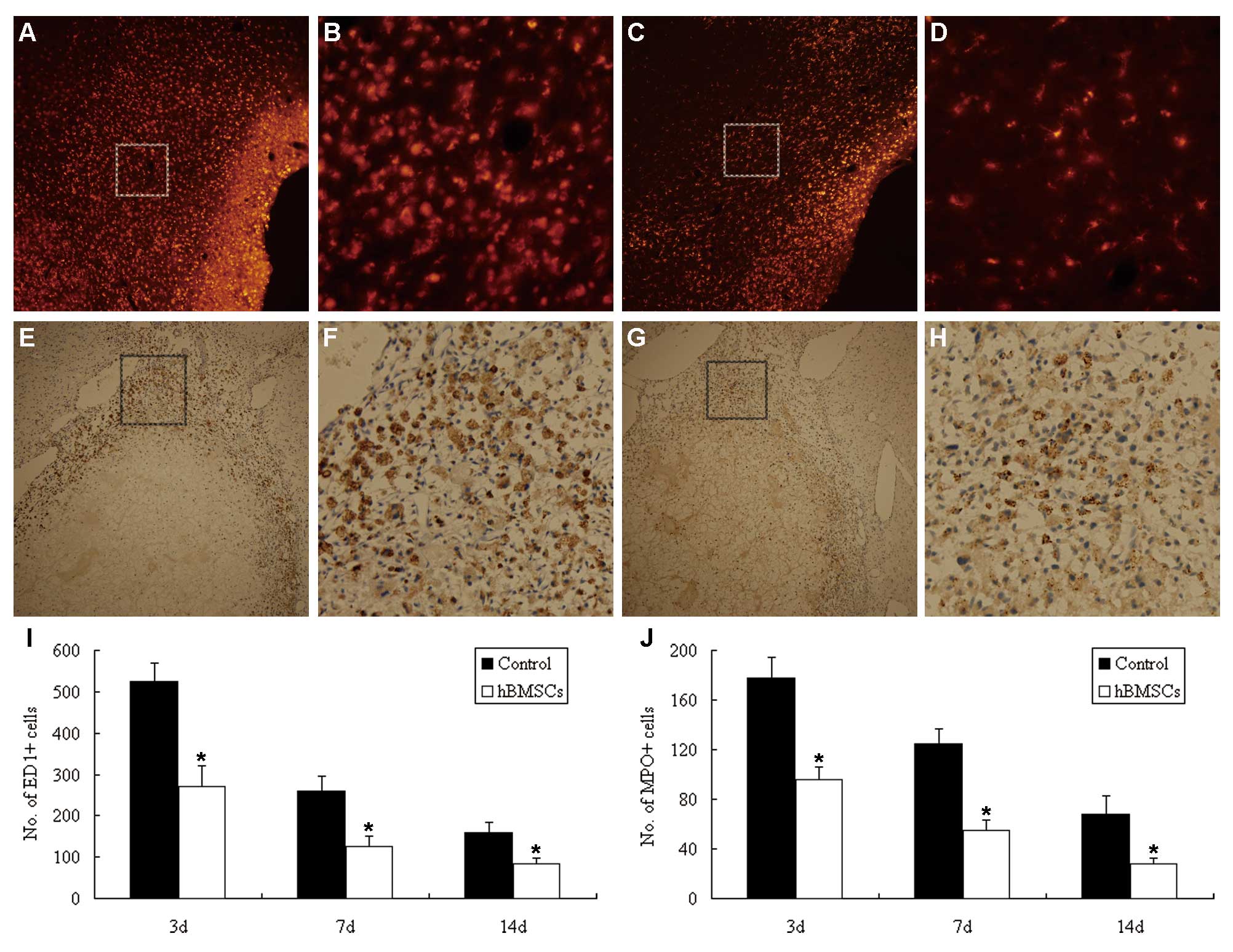
Microglial activation and neutrophil infiltration
are main inflammatory responses after ICH, with a peak at 3 to 7
days (25). In the present study,
microglia and neutrophils were shown by ED1 and MPO staining,
respectively. ED1+ and MPO+ cells in the
hemorrhagic boundary zone were significantly decreased in the
Flk-1+ hBMSC-treated rats when compared with the
controls (P<0.05) (Fig.
4A–J).
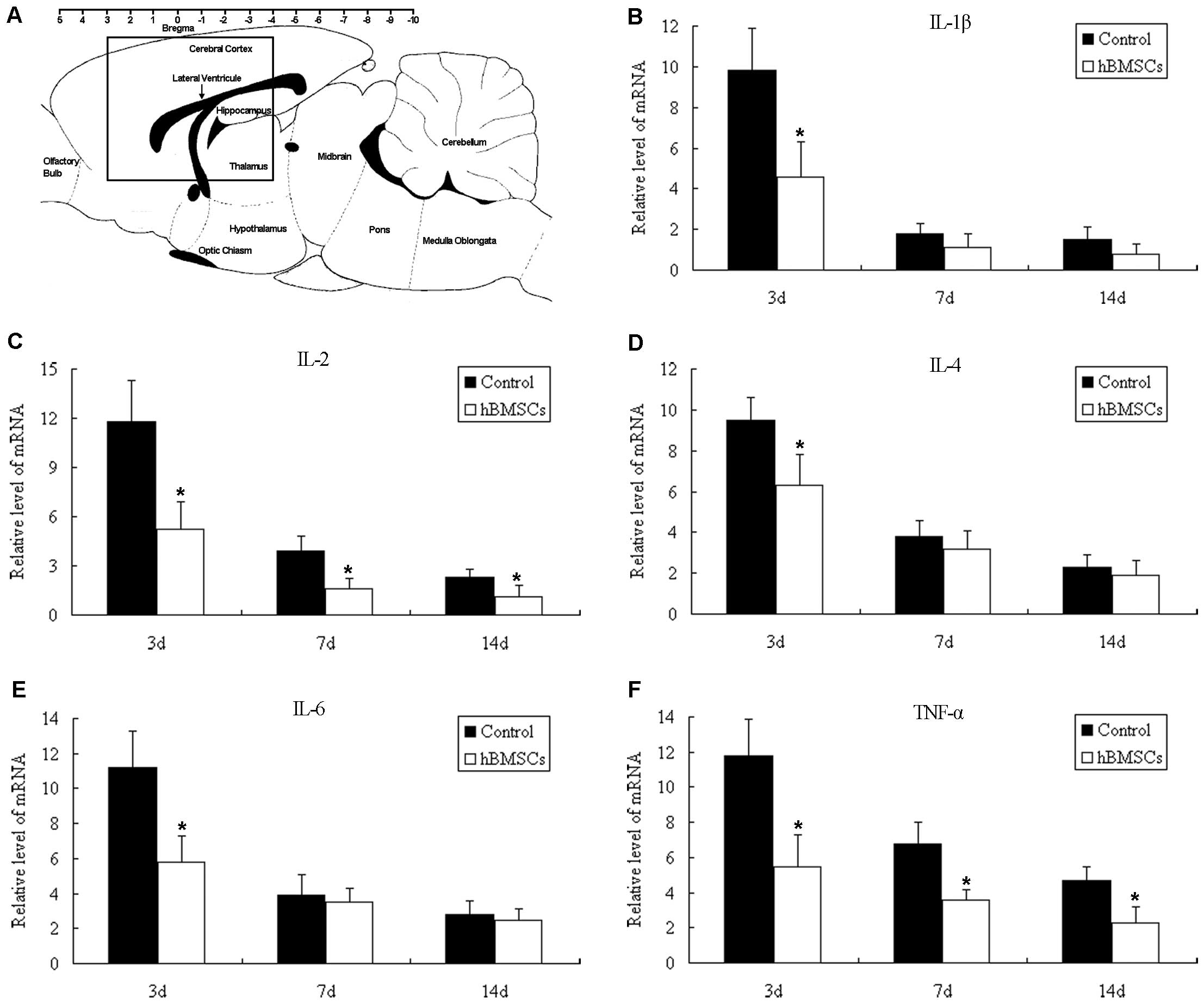
Real-time reverse transcription-polymerase chain
reaction assay (RT-PCR) was used to analyze changes in the levels
of inflammatory mediators, including IL-1β, IL-2, IL-4, IL-6 and
TNF-α. On day 3, the mRNA levels of IL-1β, IL-2, IL-4, IL-6 and
TNF-α were all downregulated in the Flk-1+ hBMSC-treated
rats. On days 7 and 14, significantly lower levels of IL-2 and
TNF-α were detected in the rats transplanted with Flk-1+
hBMSCs (Fig. 5B–F).
Flk-1+ hBMSC treatment
promotes angiogenesis and reduces cell apoptosis
To determine whether Flk-1+ hBMSC
treatment induces angiogenesis in the hemorrhagic boundary zone, we
performed immunofluorescence staining and blood vessel density
assays. Quantitative analysis of blood vessel density examined by
vWF immunofluorescence showed that ICH rats treated with
Flk-1+ hBMSCs had significantly increased neovasculature
in the hemorrhagic boundary region when compared with the controls
(P<0.05) (Fig. 6A–C).
TUNEL staining was used to investigate neuron
apoptosis. The number of TUNEL+ cells in the cortical
hemorrhagic boundary was significantly decreased in the
Flk-1+ hBMSCs-treated rats compared with this value in
the controls (P<0.05) (Fig.
7A–C). The number of survival neurons in the cortical
hemorrhagic boundary, stained by NeuN (Fig. 7D and E) or Nissl (Fig. 7G and H), was higher in rats
receiving Flk-1+ hBMSCs when compared with this value in
controls at 56 days after ICH (P<0.05) (Fig. 7F and I).
Discussion
In the present study, we demonstrated that
transplantation of Flk-1+ hBMSCs into ipsilateral brain
parenchyma reduced inflammatory infiltration and promoted
angiogenesis in a rat ICH model, which was accompanied by the
reduction in brain edema, a decrease in cell apoptosis and improved
neurological function.
Similar to MSCs, Flk-1+ hBMSCs have
unique immunologic characteristics, such as low immunogenicity and
immunoregulatory property. They express negligible levels of major
histocompatibility complex (MHC) class I and no MHC class II or Fas
ligand, nor do they express CD80, CD86, CD40 or CD40L (13–15). It has been demonstrated that MSCs
produce hepatocyte growth factor and transforming growth factor-β
(TGF-β) to mediate T-cell suppression, while other studies have
emphasized that cell contact is also important in the
immunoregulatory property of MSCs (26). In our previous study, we found
that Flk-1+ BMSCs, partly via cell-cell contact, drive
mature dendritic cells to differentiate into a novel
Jagged-2-dependent regulatory dendritic cell population and escape
their apoptotic fate, and thus revealed a new mechanism of their
immunoregulation (15). In the
present study, Flk-1+ hBMSCs significantly inhibited the
proliferation of rPBMCs induced in a mixed lymphocyte reaction.
Consistently, we found a significant anti-inflammatory effect of
Flk-1+ hBMSCs in the ICH brain, including decreased
neutrophil infiltration and microglial activation in the
perihematomal areas, and downregulation of inflammatory mediators,
IL-1β, IL-2, IL-4, IL-6 and TNF-α. These immunological
characteristics may allow for their use in allogeneic or xenogeneic
transplantation without any immunosuppressant and they may have an
anti-inflammatory effect on brain damage after ICH or other
immunological diseases (16,19,23). These findings suggest that
Flk-1+ hBMSCs are likely to interact with inflammatory
cascades after ICH. However, further research is required to
identify the intrinsic anti-inflammatory mechanisms of
Flk-1+ hBMSCs in vivo.
Our previous studies revealed that Flk-1+
hBMSCs differentiate into brain parenchymal cells both in
vitro (20,21) and in vivo (27,28), and replacement of neural cells was
often considered to be the main goal of cell therapy for stroke
(29). However, the number of
BMSCs that survived after transplantation was relatively small and
was miniscule compared to the ~25–30% hemispheric brain tissue
damage observed after ICH. Furthermore, only a small percentage of
BMSCs express neural protein, too few to replace the injured tissue
(10,11), while expression of phenotypic
brain cell markers does not indicate true differentiation and may
be a misinterpretation of spontaneous cell fusion (30,31). In the present study, the number of
Flk-1+ hBMSCs that survived 55 days after
transplantation was relatively small and only a small percentage of
Flk-1+ hBMSCs expressed proteins phenotypic of
neural-like cells. The death of Flk-1+ hBMSCs was
primarily attributed to the deleterious microenvironment after ICH.
Functional recovery was observed within days after
Flk-1+ hBMSC treatment. It is highly unlikely that these
cells integrated into the cerebral tissue and made appropriate
connections within days after transplantation.
Another mechanism that mediated the benefit of
Flk-1+ hBMSCs following ICH may be attributed to
angiogenesis. We previously reported that Flk-1+ hBMSCs
transdifferentiate into vascular endothelial cells both in
vitro and in vivo (20,21). Additionally, transplantation of
Flk-1+ hBMSCs to the rat ischemic brain significantly
promoted vascular endothelial cell proliferation and induced
angiogenesis in the ischemic boundary zone (24,28). In the present study, we observed
that Flk-1+ hBMSCs were located in the vessel wall and
differentiated into vascular endothelial cells in the hemorrhagic
boundary region, indicating that increased angiogenesis following
Flk-1+ hBMSC treatment may be associated with direct
incorporation into cerebral vasculature. However, the percentage of
Flk-1+ hBMSCs that expressed an endothelial phenotype
was extremely small, consistent with previous studies (32,33). These findings indicate that
Flk-1+ hBMSC-induced angiogenesis may largely depend on
the proliferation of endogenous endothelial cells or recruitment of
endothelial progenitor cells toward the hemorrhagic brain, rather
than the transdifferentiation of grafted Flk-1+ hBMSCs.
BMSC-induced proliferation of endothelial cells is, at least
partially, attributed to trophic factors (32). Flk-1+ BMSCs secrete
multiple trophic factors in rat brain, including vascular
endothelial growth factor (VEGF), brain-derived neurotrophic factor
(BDNF), neurotrophin-3 (NT-3), insulin-like growth factor-1
(IGF-1), basic fibroblast growth factor (bFGF), glia-derived
neurotrophic factor (GDNF) and TGF (24,28). VEGF is the most important mitogen
in the process of angiogenesis (34), and VEGF expression was
significantly upregulated in the brain of rats receiving
Flk-1+ hBMSCs in our previous studies (24,28). Furthermore, BMSC treatment of
cerebral ischemia was found to promote vascular stabilization and
to decrease VEGF-induced blood-brain barrier leakage, by increasing
angiopoietin-1/Tie2 and VEGF/Flk-1 expression (35).
In conclusion, BMSCs show promise as a potential
therapy for restoration of function after cerebral hemorrhage.
However, most stem cell-based therapies remain in the early stages
of development. Recommendations and guidelines for translation of
laboratory studies with stem cells to patients have been published
following the ‘Stem Cell Therapies as an Emerging Paradigm in
Stroke (STEPS)’ conference (36).
Understanding the mechanisms underlying the beneficial effects of
these therapies will greatly enhance their translation to the
clinic.
Acknowledgements
This study was supported by a grant from the
National High Technology Research Project (2011AA020112 and
2006AA02A115) and the National Natural Science Foundation of China
(81200916 and 81100869).
References
|
1
|
Heiskanen O: Treatment of spontaneous
intracerebral and intracerebellar hemorrhages. Stroke. 24:I94–I95.
1993.PubMed/NCBI
|
|
2
|
Qureshi AI, Tuhrim S, Broderick JP, Batjer
HH, Hondo H and Hanley DF: Spontaneous intracerebral hemorrhage. N
Engl J Med. 344:1450–1460. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyden PD and Zivin JA: Hemorrhagic
transformation after cerebral ischemia: mechanisms and incidence.
Cerebrovasc Brain Metab Rev. 5:1–16. 1993.PubMed/NCBI
|
|
4
|
Hacke W, Kaste M, Bluhmki E, et al:
Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic
stroke. N Engl J Med. 359:1317–1329. 2008. View Article : Google Scholar
|
|
5
|
Aronowski J and Hall CE: New horizons for
primary intracerebral hemorrhage treatment: experience from
preclinical studies. Neurol Res. 27:268–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J and Doré S: Inflammation after
intracerebral hemorrhage. J Cereb Blood Flow Metab. 27:894–908.
2007.
|
|
8
|
Kim J, Lee S, Kon C, et al: Systemic
transplantation of human adipose stem cells attenuated cerebral
inflammation and degeneration in a hemorrhagic stroke model. Brain
Res. 1183:43–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee S, Chu K, Jung K, et al:
Anti-inflammatory mechanism of intravascular neural stem cell
transplantation in haemorrhagic stroke. Brain. 131:616–629. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seyfried D, Ding J, Han Y, Li Y, Chen J
and Chopp M: Effects of intravenous administration of human bone
marrow stromal cells after intracerebral hemorrhage in rats. J
Neurosurg. 104:313–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Huang Z, Xu Y and Zhang S:
Differentiation and neurological benefit of the mesenchymal stem
cells transplanted into the rat brain following intracerebral
hemorrhage. Neurol Res. 28:104–112. 2006. View Article : Google Scholar
|
|
12
|
Feng M, Zhu H, Zhu Z, et al: Serial
18F-FDG PET demonstrates benefit of human mesenchymal
stem cells in treatment of intracerebral hematoma: a translational
study in a primate model. J Nucl Med. 52:90–97. 2011.
|
|
13
|
Deng W, Han Q, Liao L, et al: Allogeneic
bone marrow-derived Flk-1+Sca-1− mesenchymal
stem cells leads to stable mixed chimerism and donor-specific
tolerance. Exp Hematol. 32:861–867. 2004.PubMed/NCBI
|
|
14
|
Xu G, Zhang L, Ren G, Yuan Z, Zhang Y,
Zhao RC and Shi Y: Immunosuppressive properties of cloned bone
marrow mesenchymal stem cells. Cell Res. 17:240–248.
2007.PubMed/NCBI
|
|
15
|
Zhang B, Liu R, Shi D, et al: Mesenchymal
stem cells induce mature dendritic cells into a novel
Jagged-2-dependent regulatory dendritic cell population. Blood.
113:46–57. 2009. View Article : Google Scholar
|
|
16
|
Tan J, Wu W, Xu X, et al: Induction
therapy with autologous mesenchymal stem cells in living-related
kidney transplants: a randomized controlled trial. JAMA.
307:1169–1177. 2012. View Article : Google Scholar
|
|
17
|
Saito S, Nakayama T, Hashimoto N, et al:
Mesenchymal stem cells stably transduced with a dominant-negative
inhibitor of CCL2 greatly attenuate bleomycin-induced
lung damage. Am J Pathol. 179:1088–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar
|
|
19
|
Morando S, Vigo T, Esposito M, et al: The
therapeutic effect of mesenchymal stem cell transplantation in
experimental autoimmune encephalomyelitis is mediated by peripheral
and central mechanisms. Stem Cell Res Ther. 3:32012. View Article : Google Scholar
|
|
20
|
Fang B, Liao L, Shi M, Yang S and Zhao RC:
Multipotency of Flk1+CD34− progenitors
derived from human fetal bone marrow. J Lab Clin Med. 143:230–240.
2004.PubMed/NCBI
|
|
21
|
Fang B, Shi M, Liao L, Yang S, Liu Y and
Zhao RC: Multiorgan engraftment and multilineage differentiation by
human fetal bone marrow
Flk1+/CD31−/CD34− progenitors. J
Hematother Stem Cell Res. 12:603–613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Sun Z, Chen B, et al: Ex vivo
expansion and in vivo infusion of bone marrow-derived
Flk-1+CD31−CD34− mesenchymal stem
cells: feasibility and safety from monkey to human. Stem Cells Dev.
15:349–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou H, Guo M, Bian CJ, et al: Efficacy of
bone marrow-derived mesenchymal stem cells in the treatment of
sclerodermatous chronic graft-versus-host disease: clinical report.
Biol Blood Marrow Transplant. 16:403–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao X, Feng M, Wei J, et al:
Transplantation of Flk-1+ human bone marrow-derived
mesenchymal stem cells promotes angiogenesis and neurogenesis after
cerebral ischemia in rats. Eur J Neurosci. 34:87–98. 2011.
|
|
25
|
Gong C, Hoff JT and Keep RF: Acute
inflammatory reaction following experimental intracerebral
hemorrhage in rat. Brain Res. 871:57–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nauta AJ and Fibbe WE: Immunomodulatory
properties of mesenchymal stromal cells. Blood. 110:3499–3506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Zhu H, Liu Y, et al: Human
mesenchymal stem cell transplantation protects against cerebral
ischemic injury and upregulates interleukin-10 expression in
Macaca fascicularis. Brain Res. 1334:65–72. 2010. View Article : Google Scholar
|
|
28
|
Bao X, Wei J, Feng M, et al:
Transplantation of human bone marrow-derived mesenchymal stem cells
promotes behavioral recovery and endogenous neurogenesis after
cerebral ischemia in rats. Brain Res. 1367:103–113. 2011.
View Article : Google Scholar
|
|
29
|
Li Y, Chen J, Chen XG, et al: Human marrow
stromal cell therapy for stroke in rat: neurotrophins and
functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terada N, Hamazaki T, Oka M, et al: Bone
marrow cells adopt the phenotype of other cells by spontaneous cell
fusion. Nature. 416:542–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castro RF, Jackson KA, Goodell MA,
Robertson CS, Liu H and Shine HD: Failure of bone marrow cells to
transdifferentiate into neural cells in vivo. Science.
297:12992002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Zhang ZG, Li Y, et al: Intravenous
administration of human bone marrow stromal cells induces
angiogenesis in the ischemic boundary zone after stroke in rats.
Circ Res. 92:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen LH, Li Y, Chen J, et al: One-year
follow-up after bone marrow stromal cell treatment in middle-aged
female rats with stroke. Stroke. 38:2150–2156. 2007.PubMed/NCBI
|
|
34
|
Schott RJ and Morrow LA: Growth factors
and angiogenesis. Cardiovasc Res. 27:1155–1161. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zacharek A, Chen J, Cui X, et al:
Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies
angiogenesis and vascular stabilization after stroke. J Cereb Blood
Flow Metab. 27:1684–1691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stem Cell Therapies as an Emerging
Paradigm in Stroke Participants. The STEPS Participants: Stem cell
therapies as an emerging paradigm in stroke (STEPS): bridging basic
and clinical science for cellular and neurogenic factor therapy in
treating stroke. Stroke. 40:510–515. 2009. View Article : Google Scholar
|
|
37
|
Han Q, Sun Z, Liu L, Chen B, Cao Y, Li K
and Zhao RC: Impairment in immuno-modulatory function of
Flk1+ CD31−CD34− MSCs from MDA-RA
patients. Leuk Res. 31:1469–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parent JM, Valentin VV and Lowenstein DH:
Prolonged seizures increase proliferating neuroblasts in the adult
rat subventricular zone-olfactory bulb pathway. J Neurosci.
22:3174–3188. 2002.PubMed/NCBI
|
|
39
|
Henriksson HB, Svanvik T, Jonsson M,
Hagman M, Horn M, Lindahl A and Brisby H: Transplantation of human
mesenchymal stems cells into intervertebral discs in a xenogeneic
porcine model. Spine. 34:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Swanson RA, Morton MT, Tsao-Wu G, Savalos
RA, Davidson C and Sharp FR: A semiautomated method for measuring
brain infarct volume. J Cereb Blood Flow Metab. 10:290–293.
1990.PubMed/NCBI
|