Introduction
The important role of microvasculature in tumor
growth has been well established (1). Tumors are known to be
angiogenesis-dependent, and thus, the occurrence of endothelial
cell (EC) apoptosis in the tumor microvasculature is a critical
homeostatic factor regulating the rate of tumor growth. As a
result, the effectiveness of tumor therapy may be determined by the
responsiveness of the tumor microvascular endothelium, as well as
tumor cells themselves to treatment. The activated vascular
endothelium is an attractive therapeutic target as it is easily
accessible for drugs, is genetically stable (less likely to develop
resistance), homogeneous, and cells are in a proliferative state
primarily within tumor tissue (2). Garcia-Barros et al reported
that microvascular damages regulated tumor cell response to
radiation (1).
Telomeres consist of short tandemly repeated DNA
sequences. In humans, this sequence is TTAGGG, and the average
telomere length is 5–15 kb (3).
When the telomere length is short, it signals the arrest of cell
proliferation, senescence and apoptosis (4). Telomere sequences are mainly
synthesized by a cellular reverse transcriptase, telomerase, which
is an RNA-dependent DNA polymerase that adds telomeric DNA onto
telomeres (3). In humans,
telomerase has a minimum of two essential components: a functional
RNA component (hTER) that serves as a template for telomeric
DNA synthesis and a catalytic protein component with reverse
transcriptase activity (hTERT) that adds the telomeric repeats onto
the end of the chromosome (5).
Telomerase is highly expressed in the advanced
stages of the majority of cancers. Previous studies have shown that
targeting telomerase with antisense oligonucleotides against
hTERT, as well as pharmacological and genetic approaches,
may be a promising cancer therapeutic strategy (6). Vascular ECs have been observed to
express hTERT mRNA by in situ hybridization in human
astrocytoma (7). There was a
significant correlation observed between the level of hTERT
mRNA expression and the proliferation rate of the ECs within the
tumor vasculature. In addition, there was a significant correlation
observed between the hTERT mRNA expression level and the
histological grade of the tumor. Falchetti et al reported
that the inhibition of telomerase in human umbilical vein ECs
(HUVECs) completely suppressed the angiogenic behavior of these
cells in tumor xenografts (8).
The hTERT inhibitor, BIBR1532, has been shown to induce vascular
smooth muscle cell senescence (9). These findings suggest a contribution
of telomerase activity to angiogenesis in ECs in vitro and
in vivo.
Tumor angiogenesis involves a number of angiogenic
factors, including vascular endothelial growth factor (VEGF),
angiopoietin, basic fibroblast growth factor (FGF), and
platelet-derived growth factor. VEGF exerts a variety of effects on
vascular ECs that together promote the formation of new blood
vessels, stimulate ECs to migrate and divide, and profoundly alter
their pattern of gene expression (10,11). VEGF has been reported to delay the
onset of senescence in microvascular ECs and to reverse the
senescence process (12). VEGF
has also been shown to exert a radio-protective effect and to
promote post-radiation survival in the tumor endothelium (2). Li et al showed that VEGF
receptor (VEGFR)2 blockade using monoclonal antibody in ECs
attenuated the proliferation, reduced migration and disrupted the
differentiation of cells (2).
Tie-1 and Tie-2 are tyrosine kinases with immunoglobulin-like and
EGF-like domains (13). FGF
upregulates the telomerase activity of HUVECs (14). Tie-2 is required for EC
maintenance and proliferation. The angiopoietins and the Tie-2
receptor are considered key regulators of tumor-induced
angiogenesis, cancer growth and metastasis (15).
It has been suggested that the tumor endothelium is
quantitatively different from the endothelium derived from normal
tissue. However, the normal and tumor endothelium have many
similarities, sharing many EC-specific markers. St Croix et
al reported 15 pan-endothelial markers that are expressed at
substantially higher levels in both normal and tumor-associated
endothelium compared with other tissues (16). Pan-endothelial markers are
involved in the regulation of tumor angiogenesis (17,18). Among them, collagen type IV, α 2
(COL4α2) inhibits EC migration and proliferation and induces EC
apoptosis (19). Collagen type
XVIII, α 1 (COL18α1), a highly selective inhibitor of angiogenesis,
increases EC apoptosis and decreases the tumor cell expression of
several pro-angiogenic and invasive molecules (20). Collagen type VI, α 1 (COL6α1) is
known to govern cell anchorage to the extracellular matrix and is
downregulated in multiple myeloma ECs (21). Insulin-like growth factor-binding
protein (IGFBP)4, IGFBP7, connective tissue growth factor (CTGF),
interferon-induced transmembrane protein 1 (9–27),
von Willebrand factor (vWF), and melanoma cell adhesion molecule
(MCAM) have also been identified as pan-endothelial markers
(16).
Ionizing radiation (IR) exerts powerful antitumor
effects as it induces cytotoxicity via DNA damage. IR targets both
tumors and ECs. The radiosensitivity of the tumor microvasculature
and microvascular damage significantly contributes to tumor
response to radiation (11).
Thus, targeting an intrinsic treatment threshold in tumor
vasculature ECs sensitizes the tumor cells to IR. Considering that
IR directly induces DNA double-strand breaks, it is possible that
cellular senescence is activated under these conditions (22). EC senescence may be an important
factor for determining angiogenic activity following IR. Igarashi
et al reported that the majority of growing ECs (80–90%)
exhibited the senescence phenotype 3–5 days following exposure to 8
Gy of IR (23). It has been
reported that the DNA damage response elicited by IR-induced DNA
double-strand breaks is associated with telomere-initiated cellular
senescence (24).
However, the mechanisms of action of IR in tumor
angiogenesis are largely unknown (23). We hypothesized that the effect of
IR-induced EC senescence may be associated with changes in
telomerase- and angiogenesis-related gene expression. To examine
this hypothesis, we investigated the effects of IR on telomerase-
and angiogenesis-related gene expression in HUVECs in
vitro.
Materials and methods
Cell lines
HUVECs were obtained from ATCC (Manassas, VA, USA).
HUVECs were grown in Ham’s F12K medium (Gibco, Invitrogen, Grand
Island, NY, USA) with 2 mM L-glutamine adjusted to contain 1.5 g/l
sodium bicarbonate and supplemented with 10% heat-inactivated fetal
bovine serum (Omega Scientific, Inc., Tarzana, CA, USA), 100 U/ml
penicillin, 100 μg/ml streptomycin and 50 μg/ml endothelial growth
supplement (BD Biosciences, Bedford, MA, USA). Cells were incubated
in a humidified atmosphere of 5% CO2 and 95% air in a
37°C incubator.
At confluence (70–80%), cells were harvested by
treatment with 0.05% Trypsin-0.02% ethylenediaminetetraacetic acid
(EDTA). Trypsin was inactivated by the addition of 1.25 mg of
soybean trypsin inhibitor, and the cells were routinely subcultured
at a constant 1:5 split ratio. The passage number (PN) was defined
as the number of times cells have been subcultured into a new
vessel. HUVECs were used between the first and third PNs.
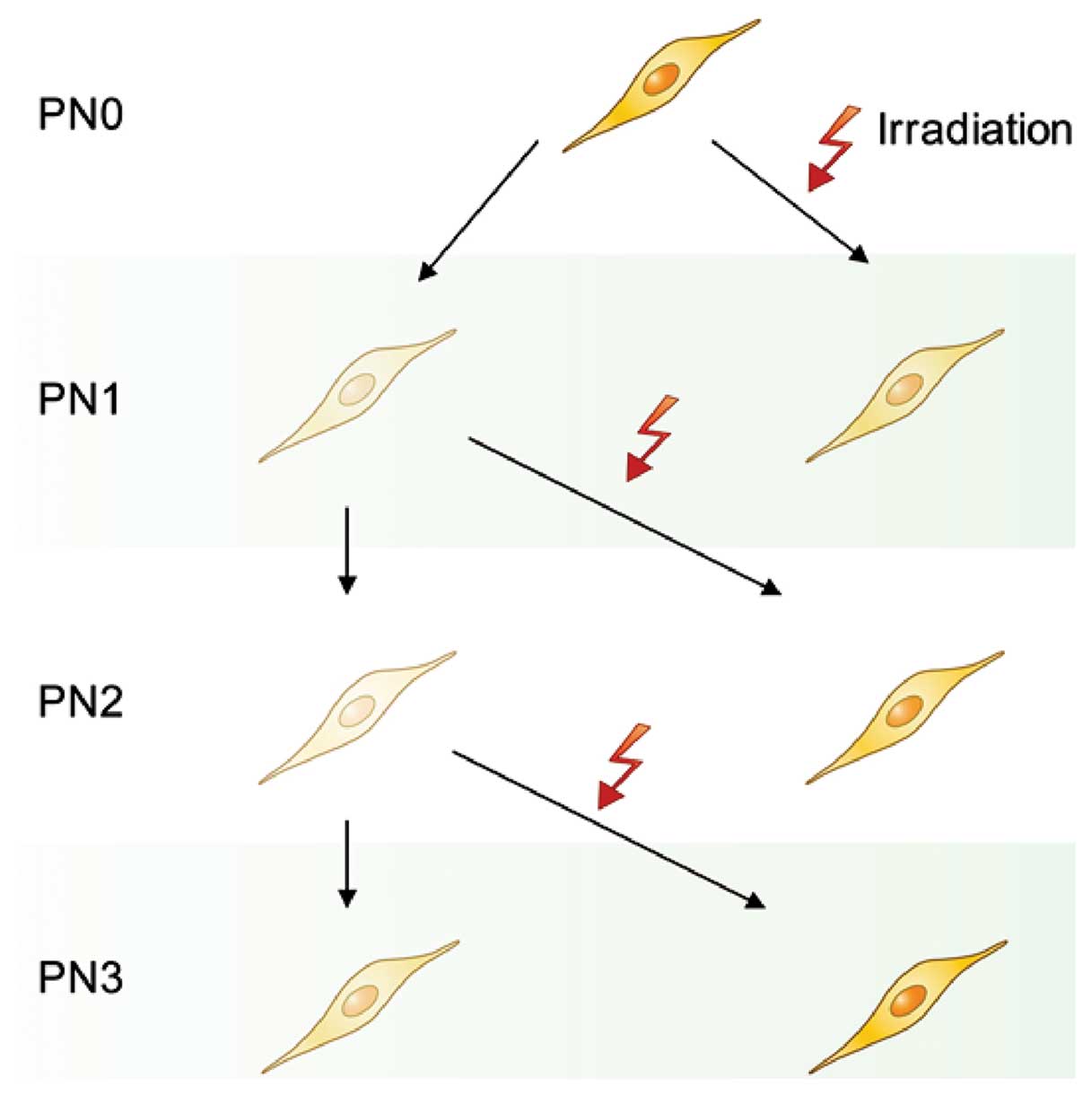
HUVECs at PN1, PN2 and PN3 were irradiated at room
temperature at 2 Gy/min with a PRIMART linear accelerator (Siemens
AG, Erlangen, Germany). Irradiated HUVECs were cultured for 8 days
and were then harvested for the evaluation of gene expression
(Fig. 1).
Measurement of growth rate
The growth rate of the HUVECs was examined by
proliferation assay. Cells were seeded at 20% confluence
(5×103 cells) in a 24-well plate, and cell numbers were
counted using methylene blue staining every 24 h for 8 days. The
growth curve was drawn using the mean cell number of duplicated
determinations, and the growth rate was calculated as follows:
growth rate = ln (N2/N1)/(t2 - t1); where N1 and N2 were the cell
numbers at time 1 (t1) and time 2 (t2), respectively.
Senescence-associated β-galactosidase
assay
Senescence- associated (SA) β-galactosidase-positive
cells were detected using the method described below. Briefly, cell
monolayers were washed twice with phosphate-buffered saline (PBS)
and fixed with 2% formaldehyde/0.2% glutaraldehyde for 5 min. The
cells were then washed twice with PBS, and staining solution [1
mg/ml 5-bromo-4-chloro-3-indolyl β-D-galacto-pyranoside (X-Gal) in
dimethylformamide (20 mg/ml, stock), 40 mM citric acid/sodium
phosphate buffer (pH 6.0), 5 mM potassium ferrocyanide, 5 mM
potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2] was
added. The cells were then incubated at 37°C for 16 h and washed
with PBS, and the number of stained cells was counted.
Telomerase assay
Briefly, the telomeric repeat amplification protocol
(TRAP) was performed according to the method described by Kim et
al (25), with some
modifications. Cells were washed in ice-cold wash buffer [10 mM
HEPES-KOH (pH 7.5), 1.5 mM MgCl2, 10 mM KCl, 1 mM
dithiothreitol], and then with 100 μl of ice-cold lysis buffer [10
mM Tris-HCl (pH 7.5), 1 mM MgCl2, 1 mM EGTA, 5 mM
2-mercaptoethanol, 0.1 mM PMSF, 0.5% CHAPS and 10% glycerol] in
Kontes tubes. The homogenate was placed on ice for 30 min and
centrifuged at 14,000 × g for 30 min at 4°C. Supernatants were
transferred into frozen vials and stored at −80°C. Protein
concentration was measured using the Bio-Rad protein assay kit
(Bio-Rad Laboratories, Munich, Germany). Telomerase activity was
assayed by the TRAP method. A total of 6 μg of sample was mixed in
50 μl of reaction mixture [forward telomerase substrate (TS) primer
(5′-AATCCGTCGAGCAGAGTT-3′) (0.1 μg), 20 mM Tris-HCl (pH 8.3), 1.5
mM MgCl2, 63 mM KCl, 1 mM EGTA, 0.005% Tween-20, 0.1 mg
bovine serum albumin and 50 μM dNTP including
32P-labeled dCTP], and incubated at 20°C for 30 min for
the telomerase-mediated extension of TS primers. After heating the
mixture at 94°C for 3 min to inactivate telomerase, 1 μg of CX
primer [5′-(CCCTTA)3CCCTAA-3′] and 2 U of Taq DNA polymerase were
added, and the mixture was then subjected to 30 cycles at 94°C for
30 sec, 50°C for 30 sec and 72°C for 90 sec. Polymerase chain
reaction (PCR) products were analyzed by electrophoresis on 12%
polyacrylamide non-denaturing gels. The gels were dried and exposed
overnight on X-ray films. The criterion for a positive TRAP assay
was a hexanucelotide ladder of 3 or more bands. A total of 293
human embryonic kidney cell extracts were used as the positive
control. The standard 6 μg of protein extract was serially diluted
10- and 100-fold to remove possible false-negative results from the
Taq polymerase inhibitor and to create a relative comparison
system.
Real-time PCR
Total RNA was collected from the cells using TRIzol
reagent. RNA integrity was initially checked on a 1% agarose gel by
confirming the presence of the 18S and 28S ribosomal RNA bands.
Approximately 5 μg of total RNA was used to create cDNA using a
First Strand cDNA Synthesis kit (MBI Fermentas, Vilnius, Lithuania)
according to the manufacturer’s instructions. For quantitative
real-time PCR, primers and the QuantiTect SYBR-Green PCR kit
(Qiagen, Santa Clarita, CA, USA) were used with the Rotor-Gene
2072D real-time PCR machine (Corbett Research, Sydney, Australia).
Briefly, in a total reaction mixture volume of 20 μl composed of 1X
QuantiTect SYBR-Green PCR Master Mix containing HotStarTaq DNA
polymerase, QuantiTect SYBR-Green PCR buffer, dNTP mix including
dUTP, SYBR Green, ROX (passive reference dye), 5 mM
MgCl2, 0.5 μM primers and 0.5 μg of cDNA, PCR was
performed as follows: 15 min at 95°C and then 45 cycles of 15 sec
at 94°C, 15 sec at 60°C, and 20 sec at 72°C. The primers used are
listed in Table I. The relative
expression level of each gene was calculated by dividing the gene
expression of the irradiated HUVECs by that of the control HUVECs
at the same PN.
 | Table IPrimers used for real-time PCR. |
Table I
Primers used for real-time PCR.
| Gene | Sense sequence
(5′-3′) | Antisense sequence
(5′-3′) |
|---|
| hTER |
CTAACCCTAACTGAGAAGGGCGTAG |
GAAGGCGGCAGGCCGAGGCTTTTCC |
| hTERT |
CGGAAGAGTGCTCTGGAGCAA |
GGATGAAGCGGACTCTGGA |
| hTEP |
TCAAGCCAAACCTGAATCTGAG |
CCCGAGTGAATCTTTCTACGC |
| c-Myc |
AAGTCCTGCGCCTCGCAA |
GCCTGTGGCCTCCAGCAGA |
| Mad1 |
TTCAGACTTGGACTGTGTCA |
GAAGGAAGTCCAGAAGGTTT |
| VEGF |
GTGGACATCTTCCAGGAGTA |
TCTGCATTCACATTTGTTGT |
| VEGFR-1 |
GGCTCTGTGGAAAGTTCAGC |
AATCACTTGGAAGAGGGGCT |
| VEGFR-2 |
CCCACCCCCAGAAATAAAAT |
ACATTTGCCGCTTGGATAAC |
| VEGFR-3 |
GCTGAAGCAGAGAGAGAGAA |
GTCACACTCCTTGTCCACTT |
| Tie-1 |
GTCCTTTGGAGTCCTTCTTT |
AAGTTCTCAAACAGCGACAT |
| Tie-2 |
CAAAGATGATCACAGGGACT |
GAAGGAAGTCCAGAAGGTTT |
| COL18α1 |
CTCCCTGCTCTACACAGAAC |
CTCTGGAACTCCTCACAGTC |
| COL4α2 |
GACATCGGGGACACTATAAA |
ACCTTCTGTTCCCTTCTCTC |
| COL6α1 |
ATGCCATGGACTTTATCAAC |
GAGTTGCCATCTGAGAAGAG |
| CTGF |
CCTCAATTTCTGAACACCAT |
AACAATCTGTTTTGACGGAC |
| IGFBP4 |
CACGAGGACCTCTACATCAT |
GTCCACACACCAGCACTT |
| IGFBP7 |
GGGTCACTATGGAGTTCAAA |
TGTAATTTTTGCTGATGCTG |
| 9–27 |
TTACTGGTATTCGGCTCTGT |
CACTGTAGACAGGTGTGTGG |
| MCAM |
CTGTAAATACCTGGCTCCTG |
CACAGGAGACTTTGAAGAGG |
| vWF |
GAACGGGTATGAGTGTGAGT |
CAAGGTGACTTTCTTTCCTG |
| β-actin |
GGGAATTCAAAACTGGAACGGTGAAGG |
GGAAGCTTATCAAAGTCCTCGGCCACA |
Statistical analysis
An unpaired two-tailed Student’s t-test was used for
the evaluation of the senescence rate; a P-value <0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS software for Windows (version
12.0, SPSS, Chicago, IL, USA).
Results
Changes in biological phenotypes
following IR
Following IR, the growth rate was delayed. The mean
growth rate of the control cells was 0.156/day at PN1, 0.132/day at
PN2 and 0.098/day at PN3. The mean growth rate of the irradiated
cells was 0.086/day at PN1, 0.042/day at PN2 and 0.042/day at PN3.
The growth inhibition rates following IR treatment were 44.8, 62.2
and 57.1 % at PN1, PN2 and PN3, respectively.
Appearance of senescence following
IR
The number of IR-induced senescent cells was
significantly increased in the irradiated HUVECs at all PNs (mean ±
SD; PN1, 7.12±1.1 vs. 32.4±4.4%, P<0.001; PN2, 11.7±4.3 vs.
30.9±6.2%, P<0.05; PN3, 18.6±3.1 vs. 43.1±5.5%, P<0.01) (n=3
for each passage) (Fig. 2).
Telomerase activity following IR
In the control cells, there was no difference
observed in telomerase activity as the PN increased. However,
following IR treatment, there was a 20% decrease in telomerase
activity in the irradiated cells compared to the control cells at
PN1, a 20% reduction at PN2 and a 25% reduction at PN3.
Changes in telomerase-related gene
expression following IR
Compared to the control cells at the corresponding
PNs, the downregulation of hTERT and hTER was
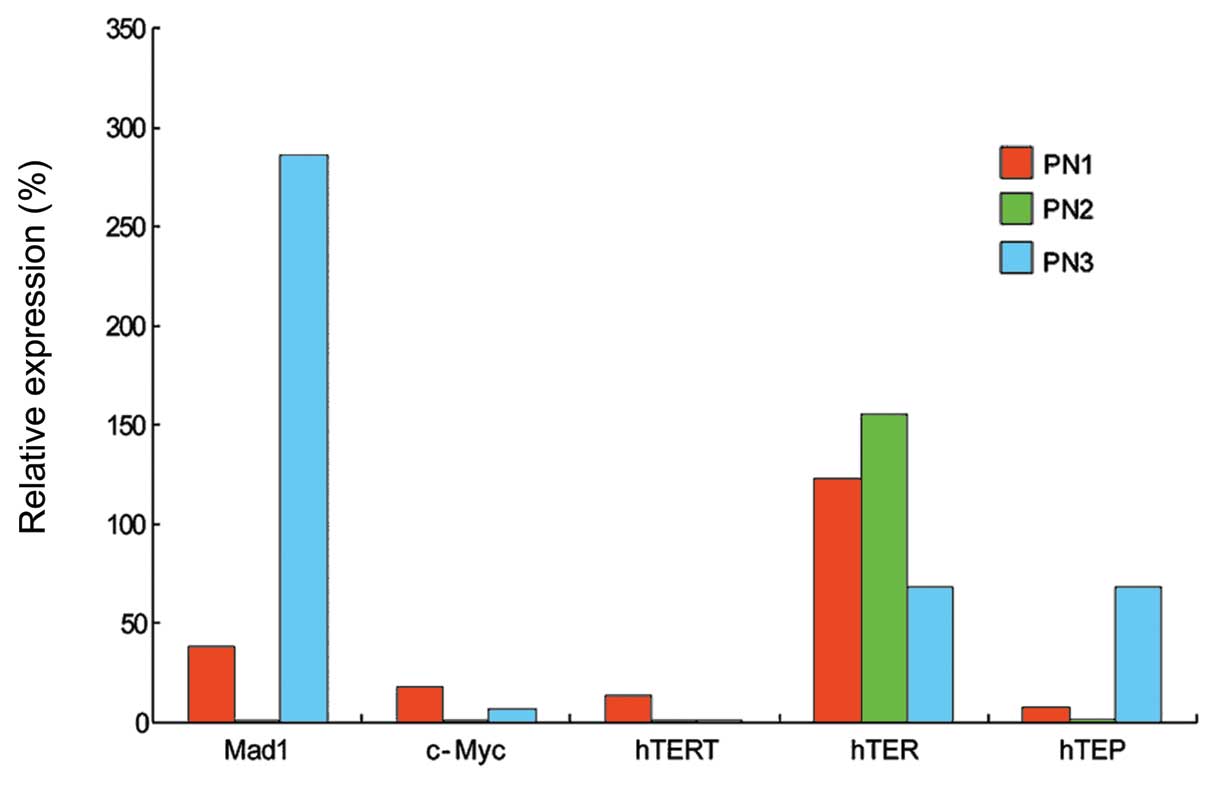
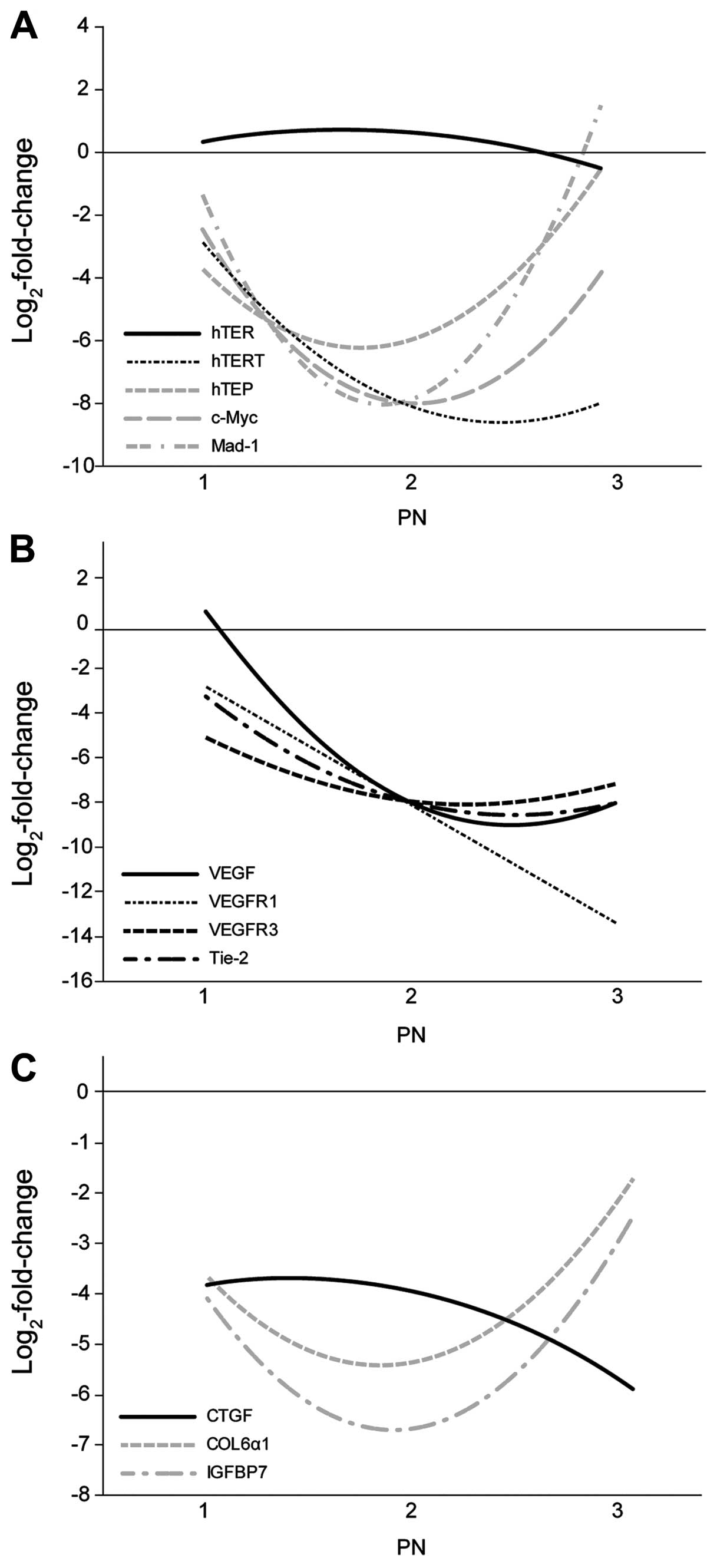
observed in the irradiated HUVECs (Figs. 3 and 6A). The hTERT expression level
continuously decreased at all PNs. The expression of Mad1
decreased at PN1 and 2, but increased by approximately 3-fold in
the irradiated cells compared to the control cells at PN3 (Figs. 3 and 6A). c-Myc was continuously
downregulated at all PNs.
Changes in angiogenesis-related gene
expression following IR
Compared to the control cells at the corresponding
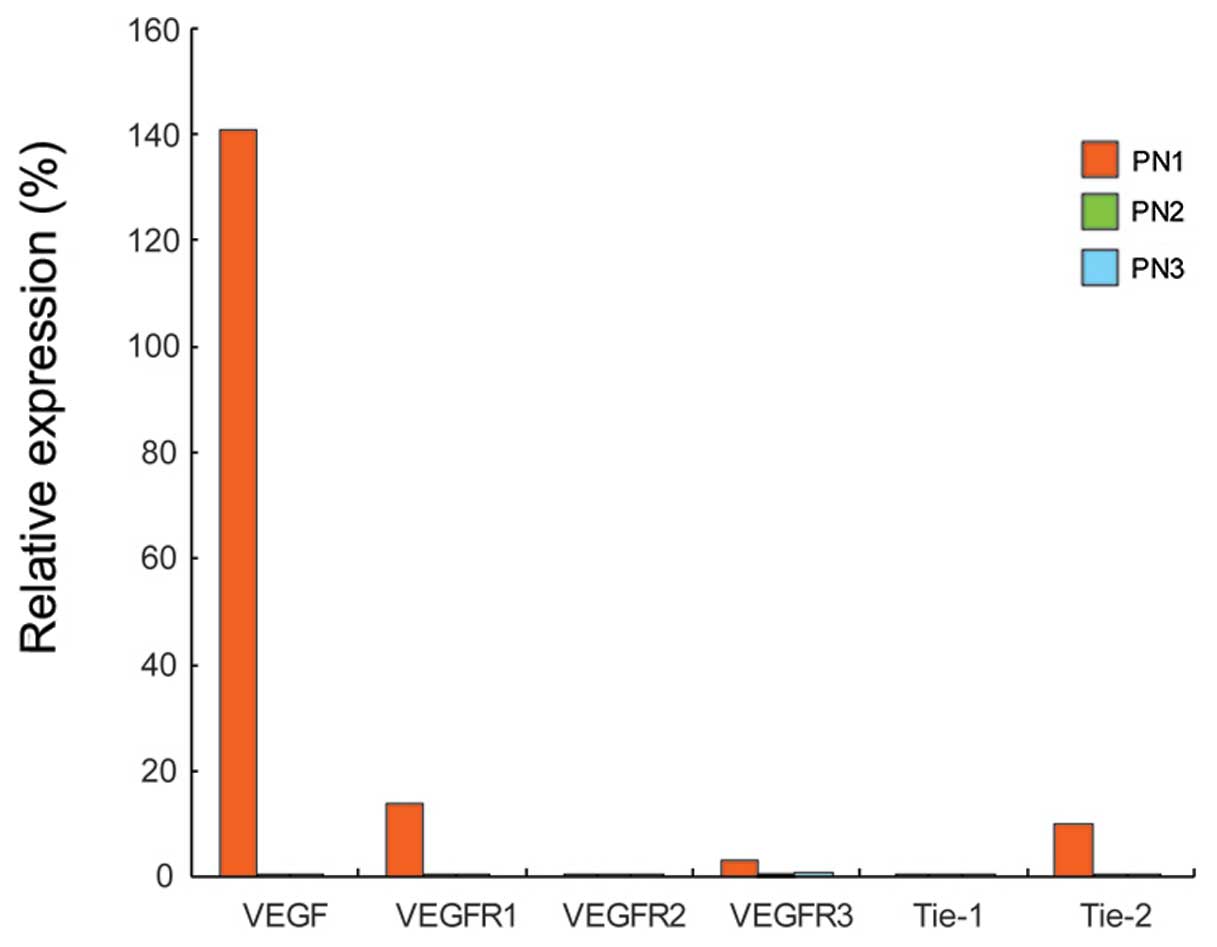
PNs, the downregulation of VEGFR1, VEGFR2,
VEGFR3, Tie-1 and Tie-2 was observed in the
irradiated HUVECs (Figs. 4 and
6B). VEGF expression
decreased as the PN increased compared to the control cells. Among
the pan-endothelial markers, COL4α2, COL18α1 and
COL6α1 were downregulated in the irradiated HUVECs at all
PNs (Figs. 5 and 6C). The levels of IGFBP4,
IGFBP7, CTGF, MCAM, 9–27 and vWF
were also downregulated in the irradiated HUVECs (Figs. 5 and 6C).
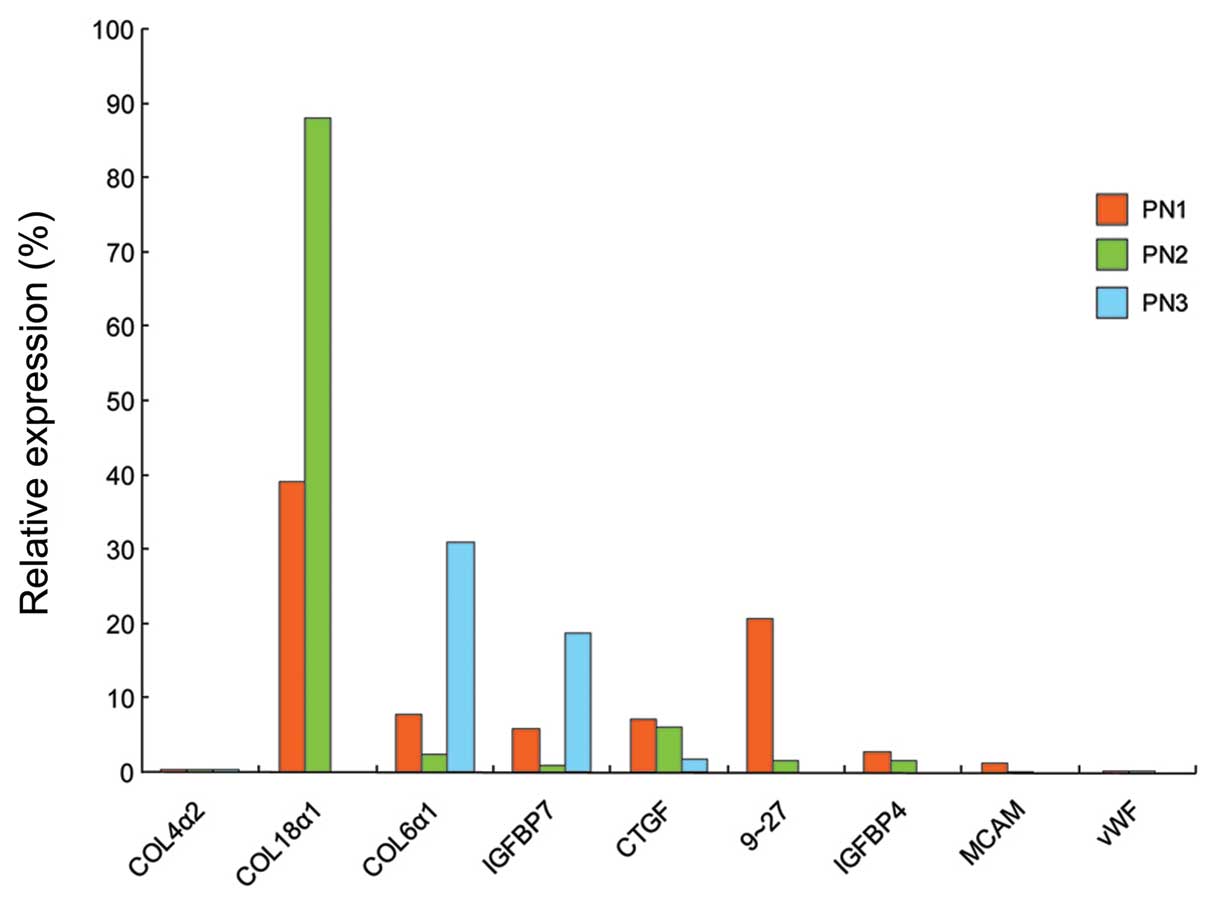 | Figure 5Changes in relative gene expression
in COL4α2, COL18α1, COL6α1, IGFBP7,
CTGF, 9–27, IGFBP4, MCAM and
vWF. HUVECs were irradiated at room temperature at 2 Gy/min.
Irradiated HUVECs were cultured for 8 days and then harvested for
the evaluation of gene expression. Real-time PCR was carried out
once for each angiogenesis-related gene. Relative gene expression
was the ratio of expression of irradiated HUVECs and that of the
control HUVECs at the same PNs. COL18α1, 9–27,
IGFBP4, MCAM and vWF gene expression in
irradiated HUVECs were not evaluated at PN3. PN, passage number;
COL4α2, collagen type IV, α 2; COL18α1,
collagen type XVIII, α 1; COL6α1, collagen type VI, α
1; IGFBP, insulin-like growth factor-binding protein;
CTGF, connective tissue growth factor; MCAM, melanoma
cell adhesion molecule; vWF, von Willebrand factor. |
Discussion
This study demonstrates that IR is a potent inducer
of EC senescence, as well as the downregulation of
telomerase-related genes. Telomere and hTERT are associated with
cellular senescence. It has been reported that telomeres in
senescent cells directly play a role in DNA damage response, and
that uncapped telomeres are associated with DNA damage response
proteins (24). Takano et
al reported that hTERT induced a delay in senescence, and that
hTERT-overexpressed ECs appeared more resistant to stress (26). Certain studies have demonstrated
that telomerase inhibitors increase the sensitivity of cancer cells
to IR. Nakamura et al showed that HeLa cells lacking
hTERT treated with small interfering RNAs had a decreased
telomerase activity and a significantly increased sensitivity to
radiation compared with control cells (6). Wu et al reported that
treatment with imetelstat, a telomerase antagonist, reduced the
telomerase activity of esophageal cells by more than 70% compared
to the controls. In addition, imetelstat increased the number and
size of 53BP1 foci following IR (27).
In our study, the number of senescent cells
increased in the irradiated HUVECs, while the telomerase activity
and hTERT expression were decreased. c-Myc expression
was also downregulated following IR. By contrast, the expression of
Mad1 in the irradiated cells increased by approximately
3-fold compared to the control cells at PN3. The regulation of
hTERT expression is a major control mechanism of telomerase
activity (3). The hTERT
promoter contains several binding sites for transcription factors,
including c-Myc and Mad1 (28).
Mad1 is a transcriptional repressor that represses the
c-Myc-mediated transactivation by competing for the ubiquitous
binding partner, Max, preventing it from binding to c-Myc. The
hTERT promoter contains 2 E-box consensus sites. One is
located close to the translational initiation codon at position −29
to −34 (proximal E-box), and the other is located at position −238
to −243 with regard to the ATG (distal E-box). c-Myc and Mad1 exert
their transcriptional effects by binding to the same site in the
hTERT promoter (3). Though
we have no direct evidence that IR directly regulates c-Myc
and Mad1 expression, these data suggest that the
upregulation of Mad1 and the downregulation of c-Myc
in irradiated HUVECs deactivate hTERT.
In the present study, VEGF, as well as
c-Myc were downregulated in the irradiated HUVECs during
serial passage. c-Myc has been shown to increase VEGF production in
several cell types (29).
Myc-overexpressing B cells have been shown to increase VEGF
production during the early stages of lymphomagenesis in
Eμ-c-Myc mice (29). The
VEGF promoter contains a consensus Myc-binding site. In
addition, VEGF induces hTERT expression and telomerase
activity in human ECs (30). We
suggested that the downregulation of c-Myc in irradiated
HUVECs was associated with the downregulation of VEGF, which
contributed to the IR-induced hTERT downregulation. However,
there is little evidence that c-Myc directly induces VEGF
mRNA transcription (29). Further
studies are required to evaluate the correlation among c-Myc, hTERT
and VEGF expression in irradiated HUVECs.
The expression of endothelial markers, such as
VEGFR2, vWF and MCAM, is known to increase during endothelial
progenitor cell differentiation toward ECs (31). Considering that IR decreased the
expression of these markers in our study, IR may negatively
modulate the differentiation of endothelial progenitor cells toward
ECs. IR may hinder the ability of endothelial progenitor cells to
adhere, migrate and form a capillary-like structure (31). This may be one of the mechanisms
of action of IR in tumor angiogenesis.
In conclusion, IR can induce human microvascular EC
senescence at doses relevant to clinical radiotherapy. During the
serial passage of irradiated HUVECs, the expression of
hTERT, c-Myc and VEGF was downregulated. The
data presented in this study may aid in the understanding of the
mechanisms behind IR-induced EC senescence and telomerase- and
angiogenesis-related gene response.
Acknowledgements
This study was supported by a grant from the Korea
Health 21 R&D Project, Ministry of Health and Welfare, Republic
of Korea (0405-BC01-0604-0002). We are grateful to Mr. Dong-Su Jang
for the illustrations.
Abbreviations:
|
FGF
|
fibroblast growth factor
|
|
CTGF
|
connective tissue growth factor
|
|
EC
|
endothelial cell
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
IGFBP4
|
insulin-like growth factor-binding
protein4
|
|
IR
|
ionizing radiation
|
|
MCAM
|
melanoma cell adhesion molecule
|
|
PBS
|
phosphate-buffered saline
|
|
PN
|
passage number
|
|
VEGF
|
vascular endothelial growth factor
|
|
vWF
|
von Willebrand factor
|
References
|
1
|
Garcia-Barros M, Paris F, Cordon-Cardo C,
Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z and Kolesnick R:
Tumor response to radiotherapy regulated by endothelial cell
apoptosis. Science. 300:1155–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Huang S, Armstrong EA, Fowler JF and
Harari PM: Angiogenesis and radiation response modulation after
vascular endothelial growth factor receptor-2 (VEGFR2) blockade.
Int J Radiat Oncol Biol Phys. 62:1477–1485. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunes C, Lichtsteiner S, Vasserot AP and
Englert C: Expression of the hTERT gene is regulated at the level
of transcriptional initiation and repressed by Mad1. Cancer Res.
60:2116–2121. 2000.PubMed/NCBI
|
|
4
|
Calado RT and Young NS: Telomere diseases.
N Engl J Med. 361:2353–2365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grimes A and Chandra SB: Significance of
cellular senescence in aging and cancer. Cancer Res Treat.
41:187–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura M, Masutomi K, Kyo S, Hashimoto
M, Maida Y, Kanaya T, Tanaka M, Hahn WC and Inoue M: Efficient
inhibition of human telomerase reverse transcriptase expression by
RNA interference sensitizes cancer cells to ionizing radiation and
chemotherapy. Hum Gene Ther. 16:859–868. 2005. View Article : Google Scholar
|
|
7
|
Pallini R, Pierconti F, Falchetti ML,
D’Arcangelo D, Fernandez E, Maira G, D’Ambrosio E and Larocca LM:
Evidence for telomerase involvement in the angiogenesis of
astrocytic tumors: expression of human telomerase reverse
transcriptase messenger RNA by vascular endothelial cells. J
Neurosurg. 94:961–971. 2001. View Article : Google Scholar
|
|
8
|
Falchetti ML, Mongiardi MP, Fiorenzo P,
Petrucci G, Pierconti F, D’Agnano I, D’Alessandris G, Alessandri G,
Gelati M, Ricci-Vitiani L, Maira G, Larocca LM, Levi A and Pallini
R: Inhibition of telomerase in the endothelial cells disrupts tumor
angiogenesis in glioblastoma xenografts. Int J Cancer.
122:1236–1242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bu DX, Johansson ME, Ren J, Xu DW, Johnson
FB, Edfeldt K and Yan ZQ: Nuclear factor {kappa}B-mediated
transactivation of telomerase prevents intimal smooth muscle cell
from replicative senescence during vascular repair. Arterioscler
Thromb Vasc Biol. 30:2604–2610. 2010.
|
|
10
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: a critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar
|
|
11
|
Damianovich D and Tebbutt NC: Role of
novel targeted agents in the treatment of metastatic colorectal
cancer. Asia Pac J Clin Oncol. 3:2–11. 2007. View Article : Google Scholar
|
|
12
|
Watanabe Y, Lee SW, Detmar M, Ajioka I and
Dvorak HF: Vascular permeability factor/vascular endothelial growth
factor (VPF/VEGF) delays and induces escape from senescence in
human dermal microvascular endothelial cells. Oncogene.
14:2025–2032. 1997. View Article : Google Scholar
|
|
13
|
Seegar TC, Eller B, Tzvetkova-Robev D,
Kolev MV, Henderson SC, Nikolov DB and Barton WA: Tie1-Tie2
interactions mediate functional differences between angiopoietin
ligands. Mol Cell. 37:643–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurz DJ, Hong Y, Trivier E, Huang HL,
Decary S, Zang GH, Luscher TF and Erusalimsky JD: Fibroblast growth
factor-2, but not vascular endothelial growth factor, upregulates
telomerase activity in human endothelial cells. Arterioscler Thromb
Vasc Biol. 23:748–754. 2003. View Article : Google Scholar
|
|
15
|
Oliner J, Min H, Leal J, Yu D, Rao S, You
E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E,
Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T,
Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu
J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S,
Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A,
Hayward I, Radinsky R, Boone T and Kendall R: Suppression of
angiogenesis and tumor growth by selective inhibition of
angiopoietin-2. Cancer Cell. 6:507–516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B and Kinzler KW: Genes expressed in human tumor
endothelium. Science. 289:1197–1202. 2000.PubMed/NCBI
|
|
17
|
Kalluri R: Basement membranes: structure,
assembly and role in tumour angiogenesis. Nat Rev Cancer.
3:422–433. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durai R, Yang SY, Sales KM, Seifalian AM,
Goldspink G and Winslet MC: Insulin-like growth factor binding
protein-4 gene therapy increases apoptosis by altering Bcl-2 and
Bax proteins and decreases angiogenesis in colorectal cancer. Int J
Oncol. 30:883–888. 2007.
|
|
19
|
Roth JM, Akalu A, Zelmanovich A,
Policarpio D, Ng B, MacDonald S, Formenti S, Liebes L and Brooks
PC: Recombinant alpha2(IV)NC1 domain inhibits tumor
cell-extracellular matrix interactions, induces cellular
senescence, and inhibits tumor growth in vivo. Am J Pathol.
166:901–911. 2005. View Article : Google Scholar
|
|
20
|
Itasaka S, Komaki R, Herbst RS, Shibuya K,
Shintani T, Hunter NR, Onn A, Bucana CD, Milas L, Ang KK and
O’Reilly MS: Endostatin improves radioresponse and blocks tumor
revascularization after radiation therapy for A431 xenografts in
mice. Int J Radiat Oncol Biol Phys. 67:870–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ria R, Todoerti K, Berardi S, Coluccia AM,
De Luisi A, Mattioli M, Ronchetti D, Morabito F, Guarini A,
Petrucci MT, Dammacco F, Ribatti D, Neri A and Vacca A: Gene
expression profiling of bone marrow endothelial cells in patients
with multiple myeloma. Clin Cancer Res. 15:5369–5378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong EH, Lee SJ, Kim JS, Lee KH, Um HD,
Kim JH, Kim SJ, Kim JI and Hwang SG: Ionizing radiation induces
cellular senescence of articular chondrocytes via negative
regulation of SIRT1 by p38 kinase. J Biol Chem. 285:1283–1295.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Igarashi K, Sakimoto I, Kataoka K, Ohta K
and Miura M: Radiation-induced senescence-like phenotype in
proliferating and plateau-phase vascular endothelial cells. Exp
Cell Res. 313:3326–3336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
d’Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.PubMed/NCBI
|
|
25
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takano H, Murasawa S and Asahara T:
Functional and gene expression analysis of hTERT overexpressed
endothelial cells. Biologics. 2:547–554. 2008.PubMed/NCBI
|
|
27
|
Wu X, Smavadati S, Nordfjall K, Karlsson
K, Qvarnstrom F, Simonsson M, Bergqvist M, Gryaznov S, Ekman S and
Paulsson-Karlsson Y: Telomerase antagonist imetelstat inhibits
esophageal cancer cell growth and increases radiation-induced DNA
breaks. Biochim Biophys Acta. 1823:2130–2135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin SY and Elledge SJ: Multiple tumor
suppressor pathways negatively regulate telomerase. Cell.
113:881–889. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mezquita P, Parghi SS, Brandvold KA and
Ruddell A: Myc regulates VEGF production in B cells by stimulating
initiation of VEGF mRNA translation. Oncogene. 24:889–901. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaccagnini G, Gaetano C, Della Pietra L,
Nanni S, Grasselli A, Mangoni A, Benvenuto R, Fabrizi M, Truffa S,
Germani A, Moretti F, Pontecorvi A, Sacchi A, Bacchetti S,
Capogrossi MC and Farsetti A: Telomerase mediates vascular
endothelial growth factor-dependent responsiveness in a rat model
of hind limb ischemia. J Biol Chem. 280:14790–14798. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian F, Liang PH and Li LY: Inhibition of
endothelial progenitor cell differentiation by VEGI. Blood.
113:5352–5360. 2009. View Article : Google Scholar : PubMed/NCBI
|