Introduction
Osteoarthritis (OA), also known as degenerative
arthritis, is a common chronic and progressive disorder of the
joints in aging populations (1).
It is a leading cause of pain and disability. OA is characterized
by the degeneration and loss of articular cartilage (2). Although the initiation of OA may be
multifactorial, the articular cartilage degradation seems to be a
direct result of infinite proteolytic extracellular matrix
destruction. The cartilage extracellular matrix has a rich
distribution of aggrecan and type 2 collagen. The loss of aggrecan
is considered a reversible event in OA cartilage degradation
(2). A previous study suggested
that a disintegrin and metalloproteinase with thrombospondin
motifs-5 (ADAMTS-5) is the major aggrecanase in cartilage (3), and that the inhibition of the
expression of the ADAMTS-5 gene is likely to represent a novel
therapeutic strategy for the treatment of OA by preventing
cartilage aggrecan breakdown. A previous study on a murine model of
OA revealed a significantly reduced level of cartilage degradation
in ADAMTS-5 knockout mice compared with wild-type mice (4). However, this study proved to be
costly and time consuming. Since RNA interference (RNAi) was
initially discovered by Fire et al (5), this sequence-specific gene-silencing
technology has received a great deal of attention from the
scientific community (6,7). This method provides a specific,
effective and simple way of silencing a target gene (8). Small interfering RNA (siRNA) can be
delivered by stable viral-mediated transfection. Lentiviral vectors
derived from HIV-1 are able to infect a broad variety of cells,
including dividing and non-dividing cells (9). siRNA can stably integrate into the
host genome, resulting in the long-term expression of the transgene
(10).
In the present study, we investigated the protective
effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage
degradation in a rat model of OA.
Materials and methods
Animals
In this study, male Sprague-Dawley rats (acquired
from Wuhan University Animal Experiment Center, Wuhan, China) were
allowed to reach a body weight of 250–300 g before surgery and were
kept under controlled environmental conditions (i.e., 22°C, 40–60%
relative humidity). Food and water were provided ad libitum.
All in vivo experiments were performed in accordance with
the Hubei Province Regulations for the Administration of
Experimental Animals. The animal protocols were approved by the
Animal Care and Use Committee of Tongji Medical College, Wuhan,
China.
Isolation and cultivation of rat
articular chondrocytes
Articular cartilage from the knees of male
Sprague-Dawley rats (15–20 g, 1 week old) was dissected after the
animals were sacrificed by cervical dislocation. Tissues were cut
into 1-mm3 samples, and the cells were isolated using a
sequential proteinase and collagenase digestion technique, as
previously described by Hu et al (11). Cartilage samples were collected
and washed twice for 10 min in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 44 mM NaHCO3 (Sigma Aldrich,
St. Louis, MO, USA), 20 mM
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), pH
7.4, containing the following antibiotics: 100/ml penicillin G
sodium, MgCl2 (0.23 mmol/l, Sigma Aldrich) and 100 μg/ml
streptomycin sulfate (medium A) (12).
The cartilage sections were initially treated with
trypsin (0.25 g/ml, Sigma Aldrich) for 30 min at 37°C. Trypsin was
inhibited by incubation in medium A containing 12% fetal calf serum
(FCS; Gibco, Invitrogen, Grand Island, NY, USA). Digested tissue
was transferred into medium A plus 12% FCS containing 0.2%
collagenase 2 and 0.5% hyaluronidase, and incubated for 4 h at 37°C
with constant stirring. A 100-μm nylon-mesh strainer was used for
filtering the digestion mixture. Cells were collected by
centrifugation (1,500 rpm, 5 min) and washed twice with medium A.
The chondrocytes were suspended in medium A and expended in
monolayer culture in medium A plus 12% FCS at 37°C under a
humidified atmosphere containing 5% CO2. The cells were
seeded in a 6-well plate at a density of 4×105/well
prior to transfection.
Establishment of siRNAs for ADAMTS-5
oligonucleotide and transient transfection
Three pairs of RNA oligonucleotides specific for the
ADAMTS-5 coding region were designed using the online tool
(http://www.invitrogen.com/site/us/en/home/References/Ambion-Tech-Support/rnai-sirna/general-articles/-sirna-design-guidelines.html)
and were constructed from a completely homologous region of
sequences in the human, mouse and rabbit ADAMTS-5 gene from the
National Center for Biotechnology Information (NCBI) website
(http://www.ncbi.nlm.nih.gov/index.html). The sequences
for the ADAMTS-5 siRNA oligonucleotides used in all further
experiments were as follows: sense, 5′-UCGAUCCCUAGCUGUCUUUTT-3′ and
antisense, 5′-TTAGCUAGGGAUCGACAGAAA-3′ for siRNA1; sense,
5′-CACGCAUCCUGCAUGUCUATT-3′ and antisense,
5′-TTGUGCGUAGGACGUACAGAU-3′ for siRNA2; and sense,
5′-CAGGAUGGAAACAGGAAAUTT-3′ and antisense,
5′-TTGUCCUACCUUUGUCCUUUA-3′ for siRNA3. These siRNAs were
chemically synthesized by GenePharma Co. Ltd. (Shanghai, China).
After 6 h of incubation, we detected the transfection efficiency by
fluorescence analysis. The cells were harvested after 48 h of
incubation, and the protein and mRNA expression level of ADAMTS-5
was assessed by western blot analysis and quantitative real-time
PCR (qRT-PCR), respectively as previously described (13).
RNA isolation and qRT-PCR
As previously described by Hummon et al
(14), total cellular RNA was
extracted and isolated from the chondrocytes after 48 h of
transfection using TRIzol reagent (Invitrogen) according to the
manufacturer’s instructions. The RNA concentration was measured
using the BioPhotometer Plus (Eppendorf, Hamburg, Germany), and all
samples were stored at -80°C until further treatment.
Complementary DNA (cDNA) was obtained by reverse
transcription of 1 μg total RNA using specific target primers.
These primers were 18–20 mers, designed using Primer 5 software
(Premier Biosoft, Palo Alto, CA, USA) to amplify the rat ADAMTS-5
gene and the housekeeping gene, glyceraldehyde-3-phosphate
dehydrogenase (GADPH). The sequences for the primers used in this
study were as follows: forward, 5′-TGTGGTGCGCCAAGGCCAAA-3′ and
reverse, 5′-CCCT GTGCAGTAGCGGCCAC-3′ for ADAMTS-5; and forward,
5′-CTCATGACCACAGTCCATGC-3′ and reverse, 5′-TTCAGC TCTGGGATGACCTT-3′
for GADPH. cDNA was synthesized in a total volume of 20 μl at 42°C
for 1 h using the Toyobo high-efficiency reverse transcriptase kit
(Toyobo Co. Ltd., Osaka, Japan). The mRNA expression levels were
determined by qRT-PCR and FastStart DNA SYBR-Green reagent (Roche,
Basel, Switzerland). qRT-PCR was performed as follows: 5 min at
95°C (1 cycle), 20 sec at 94°C, 20 sec at 59°C, 20 sec at 72°C, and
reading plate (40 cycles). Raw data of cycle threshold (Ct) values
for ADAMTS-5 in each group were normalized to those of GADPH.
ADAMTS-5 mRNA levels were determined by column diagram
analysis.
Western blot analysis
The protein expression of ADAMTS-5 was evaluated by
western blot analysis. Total proteins from chondrocytes in each
group were extracted using a protein extraction kit (ProMab
Biotechnologies, Albany, CA, USA) 48 h after transfection. The
cellular proteins were then denatured and resolved by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
After electrophoretic separation, the proteins were transferred
onto nitrocellulose membranes (Invitrogen). The membranes were
blocked with 5% non-fat skim milk in Tris-buffered saline (TBS)
containing Tween-20 buffer at normal temperature for 1 h. The
membranes were then incubated overnight with anti-ADAMTS-5
(ab41037; Abcam, Cambridge, UK) or anti-GADPH (sc-365062; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) monocolonal antibody
at 4°C. After being washed, the protein of interest was visualized
using the BeyoECL Plus enhanced chemiluminescence western blotting
detection system (Beyotime, Shangshai, China). The expression level
of ADAMTS-5 was calculated using Gel-Pro Analyzer 4.0 Image
Analysis Software (Media Cybernetics, Inc., Bethesda, MD, USA) and
was normalized to the GADPH level.
Construction of lentiviral vector
expressing siRNA targeting ADAMTS-5
siRNA1 was converted into short hairpin RNA (shRNA)
according to the stem-loop-stem structure followed by the addition
of AgeI [R0552v, New England Biolabs (NEB), Ipswich, MA] and
EcoRI (NEB, R0101v) restriction sites at the 5′ and 3′ end,
respectively. DNA oligonucleotides (GeneChem Co. Ltd., Shanghai,
China) were annealed and inserted into pGCSIL-GFP (GeneChem)
vectors by double digestion with AgeI and EcoRI with
T4 DNA ligase (NEB, M0202v). The vectors were transformed into
DH5α-competent Escherichia coli cells; we then confirmed the
insertions by restriction enzyme analysis and DNA sequencing.
Lentiviruses were co-transfected into 293T cells
using Lipofectamine 2000 (Invitrogen) and were used to infect the
chondrocytes at a multiplicity of infection (MOI) of 40 (15).
Surgically induced OA in a rat model
Briefly, the animals were anesthetized using chloral
hydrate (350 mg/kg). OA pathogenesis was surgically induced in 80
of the rats, using open surgery involving anterior cruciate
ligament transection (ACL-T) and partial medial meniscectomy (PM),
implemented as previously described by Appleton et al
(16). Surgery was performed only
on the right knee. All animals were administered antibiotics and
analgesics following surgery. The animals were randomly divided
into 4 groups: a negative control group, an experimental group, a
blank control group and a sham operated group. The experimental
group was administered an intra-articular injection of
lentivirus-mediated ADAMTS-5 siRNA (20 μl, 1×108 TU/ml).
However, the negative control group was injected with the same dose
of empty vector control plasmid DNA. The sham operated group
underwent a similar incision in the right knee joint, although
ACL-T and PM were not performed; the blank control group did not
undergo a surgical procedure.
Four animals were used per time point in each
treatment group. Forced mobilization (FM) was used to accelerate OA
onset and severity, beginning 1 week prior to surgery. A rotating
animal cage apparatus (17,18) with divided lanes was manufactured
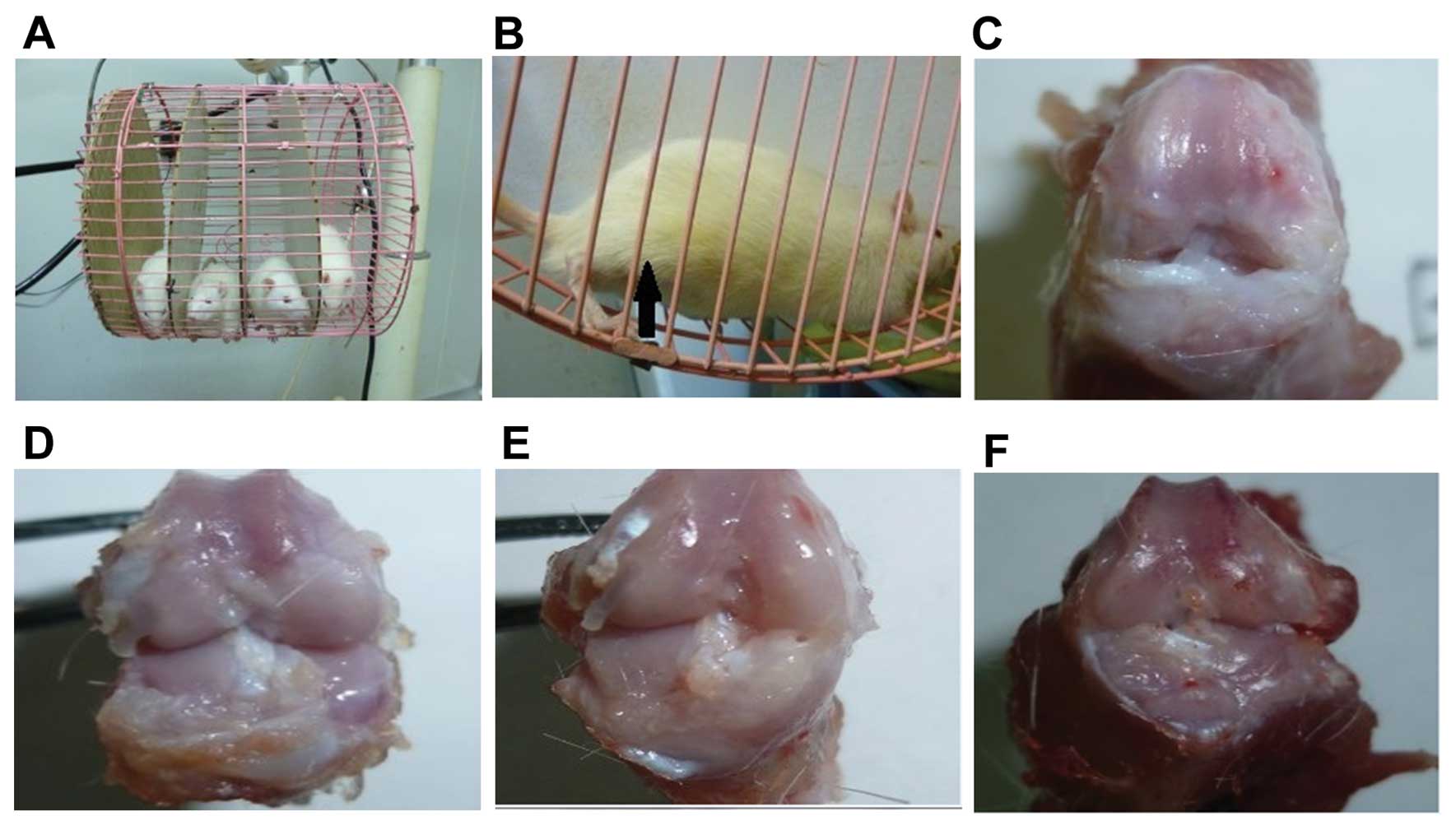
for FM (Fig. 1A). This device
rotated toward the rats at a rate of 5 rpm and forced the animals
to flex and extend the knee joint as they walked in the cage
(Fig. 1B). Each rat completed a
30-min session of FM each week on Tuesdays, Thursdays and
Saturdays. The animals were sacrificed at 2, 4, 8, 12 and 16 weeks
after surgery. The tibiofemoral joints were dissected from each rat
and were processed for histological evaluation.
Western blot analysis in vivo
The protein expression of ADAMTS-5 in vivo
was evaluated by western blot analysis. Total ADAMTS-5 protein in
each experimental group was extracted using a protein extraction
kit (ProMab) at 2, 4, 8, 12, and 16 weeks after transfection. The
cellular proteins were then denatured and resolved by 12% SDS-PAGE.
Following electrophoretic separation, proteins were transferred
onto nitrocellulose membranes (Invitrogen). The membranes were
blocked with 5% non-fat skim milk in TBS containing Tween-20 buffer
at normal temperature for 1 h. The membranes were then incubated
overnight with anti-ADAMTS-5 (ab41037; Abcam) or anti-GADPH
(sc-365062; Santa Cruz Biotechnology Inc.) monocolonal antibody at
4°C. After being washed, the protein of interest was visualized
using the BeyoECL Plus enhanced chemiluminescence western blotting
detection system. The expression level of ADAMTS-5 was calculated
using Gel-Pro Analyzer 4.0 Image Analysis Software and normalized
to the GADPH level.
Processing and scoring of histological
samples
The knee cartilage samples were fixed in 4%
paraformaldehyde in phosphate-buffered saline for 24 h at each time
point; the cartilage samples were then decalcified in 10% nitric
acid in neutral-buffered formalin (containing 4% formaldehyde) for
24–72 h. Finally, the decalcified samples were embedded in wax
blocks for sectioning. Serial sections (6 μm), through the
mid-joint edge, were stained with toluidine blue. A total of 5
stained sections per sample were used for histological scoring.
The progression of OA in all samples was assessed
and compared according to the new Osteoarthritis Research Society
International (OARSI) Cartilage Histopathology Assessment System
(OOCHAS) (19). The system uses a
24-point scale based on a combination of OA grade (0–6 points) and
OA stage (0–4 points). Both the tibia and femur tissues were
evaluated independently in 5 stained sections from each sample by
an observer blinded to the histological data.
Statistical analysis
Statistical analysis software [Statistical Package
for the Social Sciences (SPSS) version 19.0, IBM, Armonk, NY, USA]
was used for all statistical tests. OOCHAS histological grading and
staging scores were performed with analysis of variance (ANOVA) to
determine whether the time point was significant. In addition, the
Student-Newman-Keuls test was used to compare differences between
groups. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
Morphological observation and
identification of rat articular chondrocytes
Original generation chondrocytes are small and have
a uniform distribution. These cells are almost triangular or
polygonal and have strong refractive indices. The cytoplasm shows a
rich distribution of secretory vesicles, endoplasmic reticulum and
Golgi apparatus (Fig. 2A).
Immunohistochemistry revealed higher levels of type 2 collagen in
the nuclear membrane edge (Fig.
2B).
Effect of siRNA on rat ADAMTS-5 gene
expression in rat chondrocytes
Three different siRNA constructs, siRNA1, siRNA2 and
siRNA3, were used to silence the mRNA expression of ADAMTS-5 in the
rat chondrocytes. A non-targeting, scrambled siRNA was used as the
control. The transfection efficiency was observed under a
fluorescence microscope 6 h following transfection. In the siRNA1
group, the green fluorescence cells were considered to be
transfected successfully (Fig. 3A and
B). At 48 h after transfection, all animals in the 3
experimental groups that had received ADAMTS-5 siRNA showed a
downregulation in the constitutive mRNA expression of ADAMTS-5
(Fig. 3C). The ADAMTS-5 mRNA
expression in the siRNA1 group (1.10±0.16) was significantly lower
than that in the negative control group (5.54±0.68) and the blank
control group (5.54±0.62) (p<0.05).
Western blot analysis showed that the protein
expression level of ADAMTS-5 in the siRNA1 group [integrated
optical density (IOD) value, 133.73] was significantly lower than
that in the negative control group (IOD value, 323.95) and the
blank control group (IOD value, 320.79) (Fig. 3D). We selected the most effective
siRNA (siRNA1) and constructed the lentivirus-mediated siRNA
targeting ADAMTS-5 for stable transfection.
Successful construction of lentiviral
vector expressing siRNA targeting ADAMTS-5
We confirmed the succesful insertion of lentiviral
vector expressing siRNA targeting ADAMTS-5 by DNA sequencing
(Fig. 4A) and infected the
chondrocytes at a MOI of 40 (Fig.
4B).
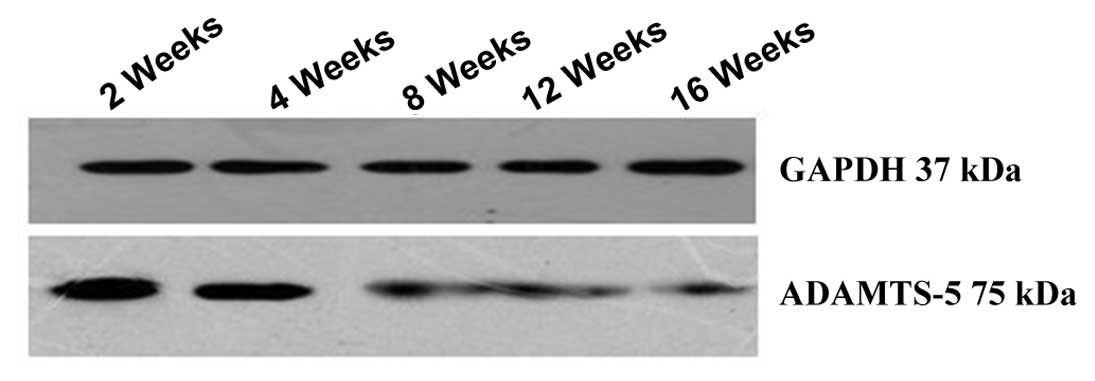
Western blot analysis in vivo
The protein expression of ADAMTS-5 in vivo
was evaluated by western blot analysis (Fig. 5).
Histological changes in rat articular
joints
All animals remained healthy during this study and
no significant differences in body weight were observed among the
groups (data not shown). We confirmed that ACL-T and PM surgery in
all the rats induced OA-like changes in the articular cartilage.
Fig. 1C-F shows the macroscopic
findings in the negative control group and the blank control group
at 2 and 4 weeks following surgery. In the first group (Fig. 1C and D), healthy articular
cartilage was present, while in the negative control group
(Fig. 1E and F) changes
representing early OA were observed.
Normal cartilage has a smooth, uninterrupted surface
and a uniform distribution of chondrocytes, often arranged in
columns (20). A healthy
appearance was observed in all the animals in the blank groups
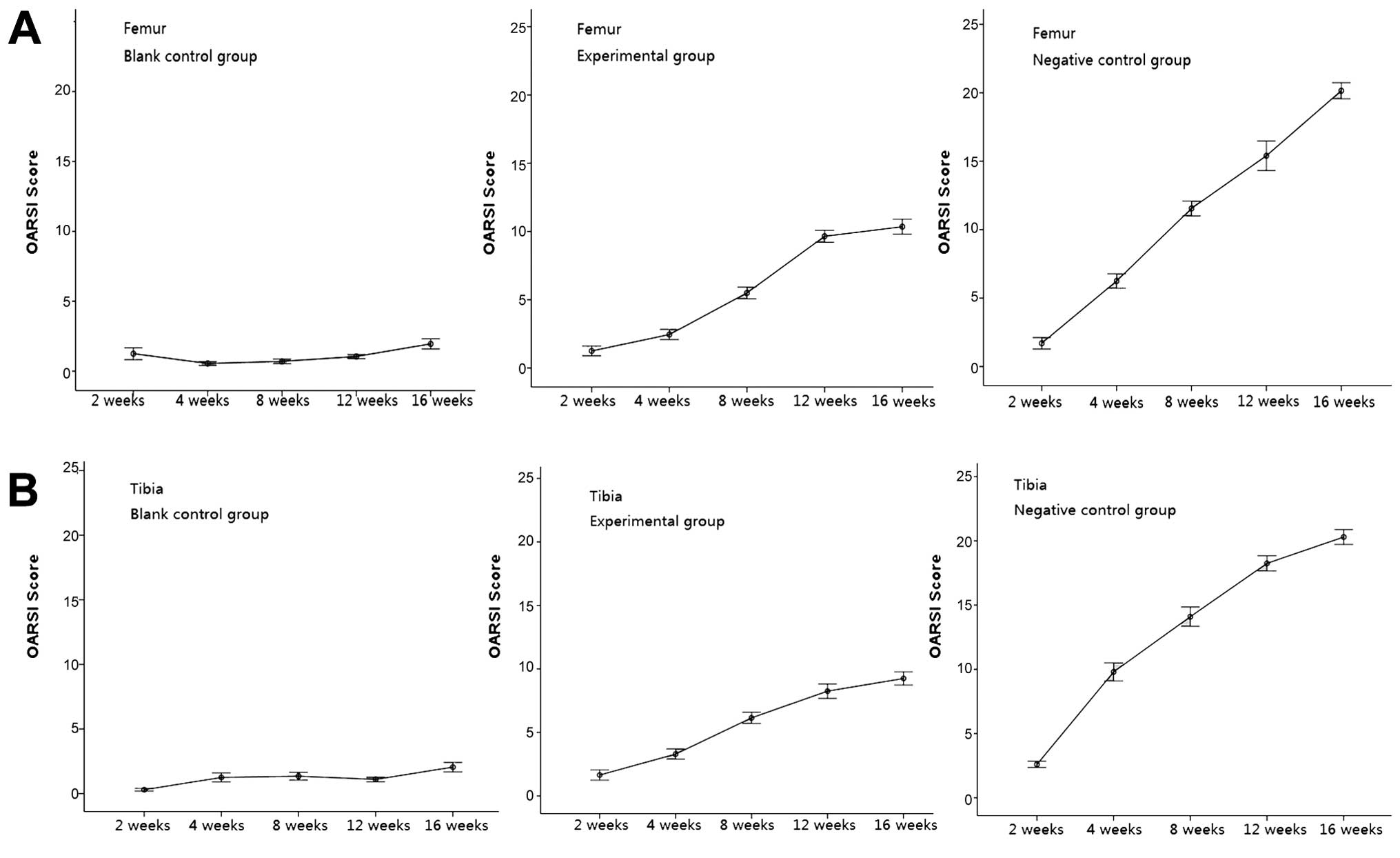
throughout the experimental period (Figs. 1 and 6). The OARSI scores demonstrated these
observations and indicated that changes during early OA did not
appear at any point up to 16 weeks in either joint surface (tibial
or femoral) (Fig. 7).
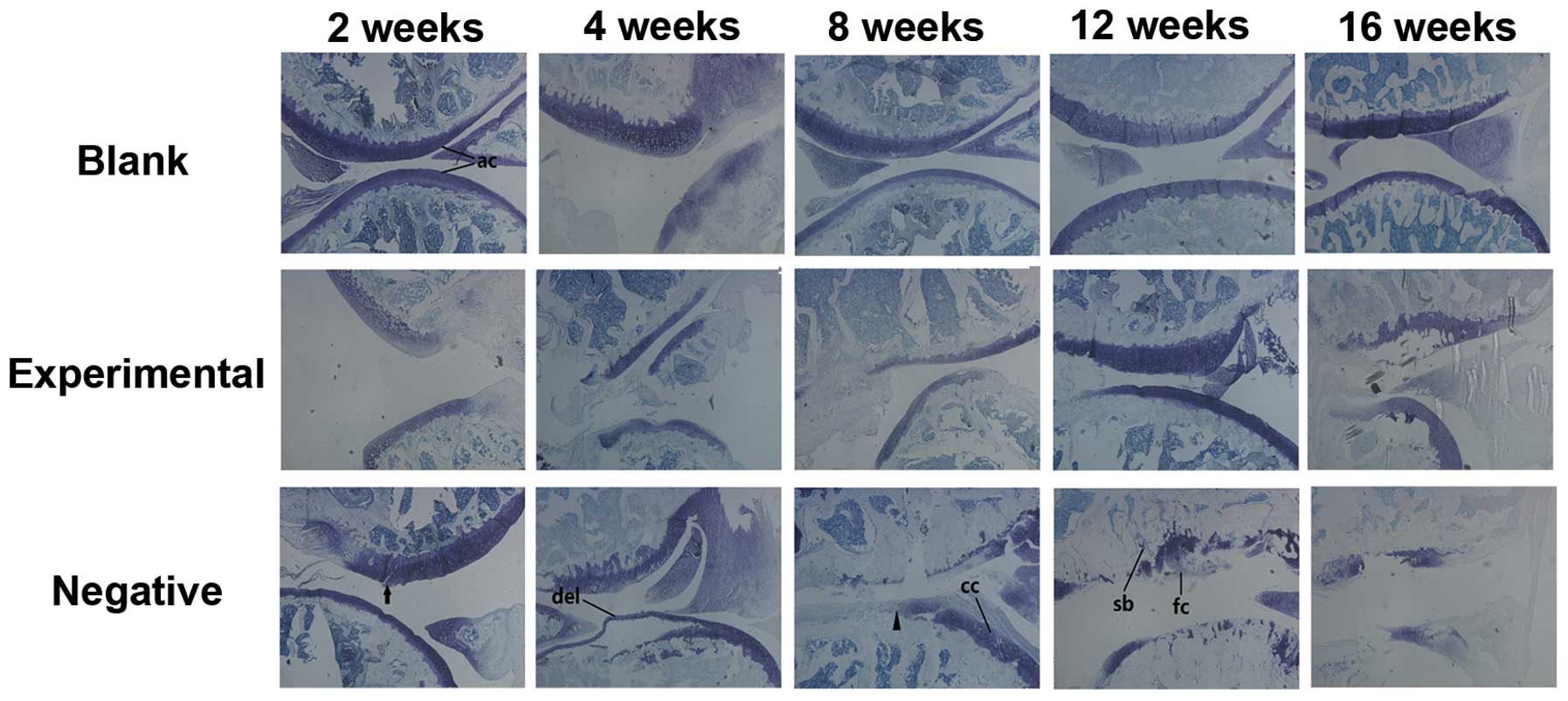 | Figure 6Histological analysis revealed
patterns of articular degradation over time. Sagittal sections from
the blank group, the experimental group, and the negative group of
animals were analyzed over a 16-week time period. Sections were
stained with toluidine blue. Knee joint tissue from each group is
shown at 2, 4, 8, 12 and 16 weeks following surgery in the upper
row of each panel. Examples of morphologically normal articular
cartilage (ac), vertical fissures (black arrow head), surface
discontinuity (black arrow), delamination (del), chondrocyte
clusters (cc), sclerotic bone (sb) and fibrocartilage-like tissue
(fc) are indicated. |
Only slight damage to the articular cartilage (such
as vertical fissures and delamination) was observed in the
experimental joint surfaces at 8 and 16 weeks (Fig. 6). However, these changes did not
worsen over time. The OARSI histopathological scoring demonstrated
no significant progression of OA in either joint surface (Fig. 7). Our results demonstrated that
the experimental group developed minor but non-advancing OA
characteristics at up to 16 weeks. A direct injection of
lentivirus-mediated ADAMTS-5 siRNA in the rats with OA was
effective in suppressing the degeneration of articular
cartilage.
Degradation in the negative control group was
severe. Surface discontinuity and delamination were observed at 4
weeks (Fig. 6). A greater
progression of degradation was observed in the negative control
group compared to the experimental control group after 4 weeks
(Fig. 6). For instance, there was
a sharp increase in the breadth and depth of vertical fissures and
cartilage denudation (Fig.
6).
Discussion
Despite the prevalence of OA in the elderly
population, there are no effective treatments. The first-line
therapy indicated for OA treatment is the analgesic, acetaminophen
(21). However, it is used for
pain management and has no disease-modifying benefits. The
treatment of the vast majority of OA patients, particularly at the
early stage of OA pathogenesis, focuses on relieving symptoms, such
as pain and swelling. ADAMTS-5, a member of the ADAMTS family, has
received much attention in OA research (22). As previously reported, a single
injection of ADAMTS-5 siRNA suppressed the disc degeneration of
nucleus pulposus tissue in a rabbit model of anular puncture disc
degeneration (13). The
double-knockout of ADAMTS-4 and ADAMTS-5 in mice has been shown to
prevent the progression of OA (23). These data suggest that ADAMTS-5 is
the most likely candidate for a role in the pathological mechanisms
of OA.
In the present study, we hypothesized that the
silencing of ADAMTS-5 expression in articular cartilage by
lentivirus-mediated siRNA would inhibit the progression of OA in
rats. We successfully constructed a siRNA oligonucletide for the
rat ADAMTS-5 gene. According to the results of qRT-PCR and western
blot analysis, ADAMTS-5 siRNA-transfected rat chondrocytes showed
>75% silencing efficiency of ADAMTS-5 mRNA compared with the
control group. Furthermore, we successfully constructed
lentivirus-vector-mediated siRNA targeting ADAMTS-5. Using a rat
model of OA, this study investigated the effects of a direct
intra-articular injection of lentivirus-mediated ADAMTS-5 siRNA
into the knee on the delay and attenuation of articular cartilage
degeneration.
Histological findings from toluidine blue staining
demonstrated the maintenance of articular cartilage in the group
treated with lentivirus-mediated ADAMTS-5 siRNA. However, the OARSI
scores in the experimental group were still higher than those of
the blank control group. It has been suggested that ADAMTS-5 and
ADAMTS-4 are important mediators of aggrecan loss in normal
cartilage (22). The silencing of
ADAMTS-4 or ADAMTS-5, or both, may be worthy of further
investigation. This method may lead to the development of novel
therapeutic strategies for the treatment of OA in humans.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Hubei Province (no. ZRY0558).
References
|
1
|
Sarzi-Puttini P, Cimmino MA, Scarpa R, et
al: Osteoarthritis: an overview of the disease and its treatment
strategies. Semin Arthritis Rheum. 35(1 Suppl 1): 1–10. 2005.
View Article : Google Scholar
|
|
2
|
Hayami T, Pickarski M, Wesolowski GA, et
al: The role of subchondral bone remodeling in osteoarthritis:
reduction of cartilage degeneration and prevention of osteophyte
formation by alendronate in the rat anterior cruciate ligament
transection model. Arthritis Rheum. 50:1193–1206. 2004. View Article : Google Scholar
|
|
3
|
Stanton H, Rogerson FM, East CJ, et al:
ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in
vitro. Nature. 434:648–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glasson SS, Askew R, Sheppard B, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin SE and Caplen NJ: Applications of
RNA interference in mammalian systems. Annu Rev Genomics Hum Genet.
8:81–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campbell TN and Choy FY: RNA interference:
past, present and future. Curr Issues Mol Biol. 7:1–6.
2005.PubMed/NCBI
|
|
8
|
Sen GL and Blau HM: A brief history of
RNAi: the silence of the genes. FASEB J. 20:1293–1299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naldini L, Blomer U, Gallay P, et al: In
vivo gene delivery and stable transduction of nondividing cells by
a lentiviral vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zufferey R, Dull T, Mandel RJ, et al:
Self-inactivating lentivirus vector for safe and efficient in vivo
gene delivery. J Virol. 72:9873–9880. 1998.PubMed/NCBI
|
|
11
|
Hu DN, Yang PY, Ku MC, Chu CH, Lim AY and
Hwang MH: Isolation and cultivation of human articular
chondrocytes. Kaohsiung J Med Sci. 18:113–120. 2002.PubMed/NCBI
|
|
12
|
Durigova M, Troeberg L, Nagase H, Roughley
PJ and Mort JS: Involvement of ADAMTS5 and hyaluronidase in
aggrecan degradation and release from OSM-stimulated cartilage. Eur
Cell Mater. 21:31–45. 2011.PubMed/NCBI
|
|
13
|
Seki S, Asanuma-Abe Y, Masuda K, et al:
Effect of small interference RNA (siRNA) for ADAMTS5 on
intervertebral disc degeneration in the rabbit anular
needle-puncture model. Arthritis Res Ther. 11:R1662009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hummon AB, Lim SR, Difilippantonio MJ and
Ried T: Isolation and solubilization of proteins after TRIzol
extraction of RNA and DNA from patient material following prolonged
storage. Biotechniques. 42:467–470. 4722007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gouze E, Pawliuk R, Pilapil C, et al: In
vivo gene delivery to synovium by lentiviral vectors. Mol Ther.
5:397–404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Appleton CT, McErlain DD, Pitelka V, et
al: Forced mobilization accelerates pathogenesis: characterization
of a preclinical surgical model of osteoarthritis. Arthritis Res
Ther. 9:R132007. View
Article : Google Scholar
|
|
17
|
Rozas G, Guerra MJ and Labandeira-Garcia
JL: An automated rotarod method for quantitative drug-free
evaluation of overall motor deficits in rat models of parkinsonism.
Brain Res Brain Res Protoc. 2:75–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martins MA, de Castro Bastos L and Tonussi
CR: Formalin injection into knee joints of rats: pharmacologic
characterization of a deep somatic nociceptive model. J Pain.
7:100–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Custers RJ, Creemers LB, Verbout AJ, van
Rijen MH, Dhert WJ and Saris DB: Reliability, reproducibility and
variability of the traditional Histologic/Histochemical Grading
System vs the new OARSI Osteoarthritis Cartilage Histopathology
Assessment System. Osteoarthritis Cartilage. 15:1241–1248. 2007.
View Article : Google Scholar
|
|
20
|
Broom ND: Further insights into the
structural principles governing the function of articular
cartilage. J Anat. 139:275–294. 1984.PubMed/NCBI
|
|
21
|
Jones MD, Tran CW, Li G, Maksymowych WP,
Zernicke RF and Doschak MR: In vivo microfocal computed tomography
and micro-magnetic resonance imaging evaluation of antiresorptive
and antiinflammatory drugs as preventive treatments of
osteoarthritis in the rat. Arthritis Rheum. 62:2726–2735. 2010.
View Article : Google Scholar
|
|
22
|
Song RH, Tortorella MD, Malfait AM, et al:
Aggrecan degradation in human articular cartilage explants is
mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum.
56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majumdar MK, Askew R, Schelling S, et al:
Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in
physiologically normal animals and prevents the progression of
osteoarthritis. Arthritis Rheum. 56:3670–3674. 2007. View Article : Google Scholar : PubMed/NCBI
|