Introduction
The interactions between cells and their surrounding
extracellular matrices (ECMs) play a crucial role in normal
physiological processes such as tissue development, morphogenesis
and cellular differentiation (1–3).
Abnormal changes in cell and ECM interaction have been shown to
cause diverse pathological conditions, including tissue fibrosis
and tumor associated stromagenesis (4,5).
ECM consists of various proteins including collagen
and proteoglycan. The interaction of ECM proteins and the cell is
known to be mediated by integrin and syndecan, a well- known ECM
receptor existing on the plasma membrane (6). The extracellular domain of the
receptors binds to diverse ECM proteins, and induces the
recruitment of various signaling molecules and cytoskeleton
proteins. Ultimately, this physical and mechanical interaction of
the cell and the ECM is connected with functional regulation of
various cellular dynamics and tissue homeostasis (7). In particular, integrins can act as a
mechanosensor, which convert mechanical signals created by the ECM
into biochemical signals in the cells. Series of these events
influence tissue-specific biological events reflected in the
cell-ECM mechanical interaction (8,9).
Fibroblasts cultured in 3D collagen matrices
presented distinct morphological features and signaling compared
with those cultured on the more commonly used 2D surface. Cells
interacting with 2D planar surface formed in a flattened, lamellar
shape with massive focal adhesions and actin stress fibers, while
cells in the 3D collagen matrices were dendritic in shape with
long, slender extensions which are similar to the in situ
appearance of mesenchymal cells and connective tissue fibroblasts
(10). Moreover, dendritic
extensions of fibroblasts in the 3D collagen matrices became
entangled with matrix fibrils, resulting in an integrin-independent
mechanical interaction (11).
However, the underlying molecular mechanisms remain poorly
understood.
The discoidin domain receptor (DDR) family, which
includes the receptor tyrosine kinase, has recently been identified
as a non-integrin receptor for collagen (12). The DDR family is composed of two
members, DDR1 and DDR2. DDR1 is primarily expressed in the
epithelial cells, particularly of the lungs, kidneys and mammary
glands, whereas DDR2 is found in cells which are of mesenchymal
origin, such as fibroblasts and smooth muscle cells (13). It has been clearly shown that the
activation of DDRs is linked to intracellular signaling, resulting
in the control of cell proliferation and transcriptional
regulation. Upon collagen-mediated receptor activation, DDRs become
phosphorylated on several tyrosine residues in their cytoplasmic
regions. These tyrosine residues provide binding sites for a number
of different Src homology-2 (SH2) and phosphotyrosine binding
(PTB)-containing proteins such as Nck, ShcA and PI3 kinase
(14). Although it has been
demonstrated that DDRs also have an ability to regulate cell
adhesion and are active in the remodeling of the ECM, DDRs do not
seem to be required for integrin activity as a co-receptor for ECM
(15). Moreover, the role of DDRs
in the mechanical interaction of cells to the ECM components has
not been studied.
In the current study, we found that DDR2 is a major
phospho-tyrosinated protein in the 3D collagen matrices. Incubation
of DDR2-silenced fibroblasts with 3D collagen matrices demonstrated
that fibroblasts attached to collagen fibrils in 3D collagen
matrices is dependent on DDR2. Finally, we also showed that the
DDR2 silencing influenced the ability of fibroblasts to migrate in
3D environments but did not affect matrix contraction and
remodeling, indicating that active DDR2 is required for the
mechanical interaction of fibroblasts to 3D collagen matrices with
control of the dendritic extensions, which in turn appeared to be
critical for fibroblast migration in 3D collagen matrices.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and 0.25%
trypsin/EDTA and oligofectamine solution were purchased from
Invitrogen (Gaithersburg, MD, USA). Fetal bovine serum (FBS) was
purchased from HyClone (Logan, UT, USA). Platelet-derived growth
factor (PDGF) was obtained from Upstate Biotechnology, Inc. (Lake
Placid, NY, USA). Alexa Fluor 488 phalloidin, Alexa Fluor 594
phalloidin and propidium iodide (PI) were obtained from Molecular
Probes (Eugene, OR, USA). RNase (DNase-free) was purchased from
Roche (Indianapolis, IN, USA). Fluoromount-G was obtained from
Southern Biotechnology Associates, Inc. (Birmingham, AL, USA).
Primary antibodies were: goat anti-human DDR2 (polyclonal) antibody
from R&D Systems (Minneapolis, MN, USA) and Type I rat tail
collagen (10.6 mg/ml) purchased from BD Biosciences (Bedford, MA,
USA). All other chemical reagents were purchased from Sigma (St.
Louis, MO, USA) unless otherwise specified.
Cell culture and nested collagen
matrices
Early passage of human foreskin fibroblast BR5 cells
were cultured in DMEM supplemented with 10% FBS. Cell culture and
experimental incubations were carried out at 37°C in a 5%
CO2 incubator.
For experiments with the collagen matrices, cells in
neutralized solutions of 1 mg/ml of collagen were placed in 24-well
culture plates or seeded on top of collagen matrices following
polymerization (2×104 cells/matrix). Growth factors and
inhibitors were added as described in the figure legend.
To measure cell migration using nested collagen
matrices, floating matrices were precontracted for 4 h in DMEM/10%
FBS, after which the cell-containing contracted matrices (dermal
equivalents) were re-embedded in 200 μl cell-free outer collagen
matrices and then incubated for an additional 24 h in DMEM/BSA + 50
ng/ml PDGF. At the end of the incubations, samples were fixed and
stained with Alexa Fluor-conjugated phalloidin to visualize actin
and PI to detect cell nuclei.
Immunoprecipitation
To identify the collagen-induced phosphorylated
proteins in tyrosine residues, collagen matrices containing BR5
fibroblasts were polymerized for 1 h after which the matrices were
further incubated in DMEM containing 50 ng/ml of PDGF for 4 h.
Samples were lysed using Dounce homogenizer with a modified RIPA
buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2,
5 mM EGTA, 10% glycerol, 1% Triton X-100, 10 μg/ml aprotinin, 10 mM
NaF, 1 mM PMSF and 1 mM sodium orthovanadate). After clearing by
centrifugation, cell lysates (~2 mg) were mixed with anti-tyrosine
(PY20) DDR2 antibodies overnight at 4°C and then with 100 μl of 30%
slurry of protein A-sepharose for 2 h. The beads were washed three
times with the modified RIPA buffer. Samples were extracted by
adding 4X of the sample buffer. Aliquots of the resulting samples
were analyzed by SDS-PAGE and silver staining and then compared to
identify proteins that were present in the collagen matrix samples
but not in the trypsinized ones. Bands corresponding to the major
affinity-selected proteins were identified by matrix-assisted laser
desorption ionization-time of flight (MALDI-TOF) mass
spectrometry.
DDR2 silencing by siRNA
To knock down DDR2, primer pairs were designed by
and obtained from Dharmacon (Chicago, IL, USA). siRNA silencing of
gene expression in the cells was performed as previously described,
with minor modifications (16).
Mock-transfected cells were treated with only the sense direction
oligonucleotide at a double concentration.
Immunofluorescence microscopy
Cell preparations for analysis were fixed for 10 min
with 3% paraformaldehyde in phosphate-buffered saline (PBS),
blocked with 2% BSA/1% glycine in PBS for 30 min, and permeabilized
for 15 min with 0.5% Triton X-100 in PBS. For actin staining,
preparation of samples with Alexa Fluor 488-conjugated phalloidin
and PI was carried out as previously described (17). Microscopic images were captured
using a fluorescent microscope (Eclipse 80i; Nikon) using Plan
Fluor 10Χ/0.30, Plan Apo 20Χ/0.75 and Plan Fluor 40Χ/0.75
infinity-corrected objectives. Images were acquired using a digital
camera (digital sight DS-Qi1Mc; Nikon) and NIS element image
analysis (Nikon). Image processing was carried out using Photoshop
11.0 (Adobe).
Results and Discussion
DDR2 is the major tyrosine-phosphorylated
protein in 3D collagen matrices
Based on the significance of phosphorylation on the
tyrosine residues in a variety of signal proteins to transmit the
intracellular signaling in response to specific environmental cues,
we carried out the immunoprecipitation experiment with
phospho-tyrosine antibodies using cell lysates that were prepared
from 3D collagen matrices to identify the 3D environment-specific
tyrosine-phosphorylated proteins and analyzed by MALDI-TOF mass
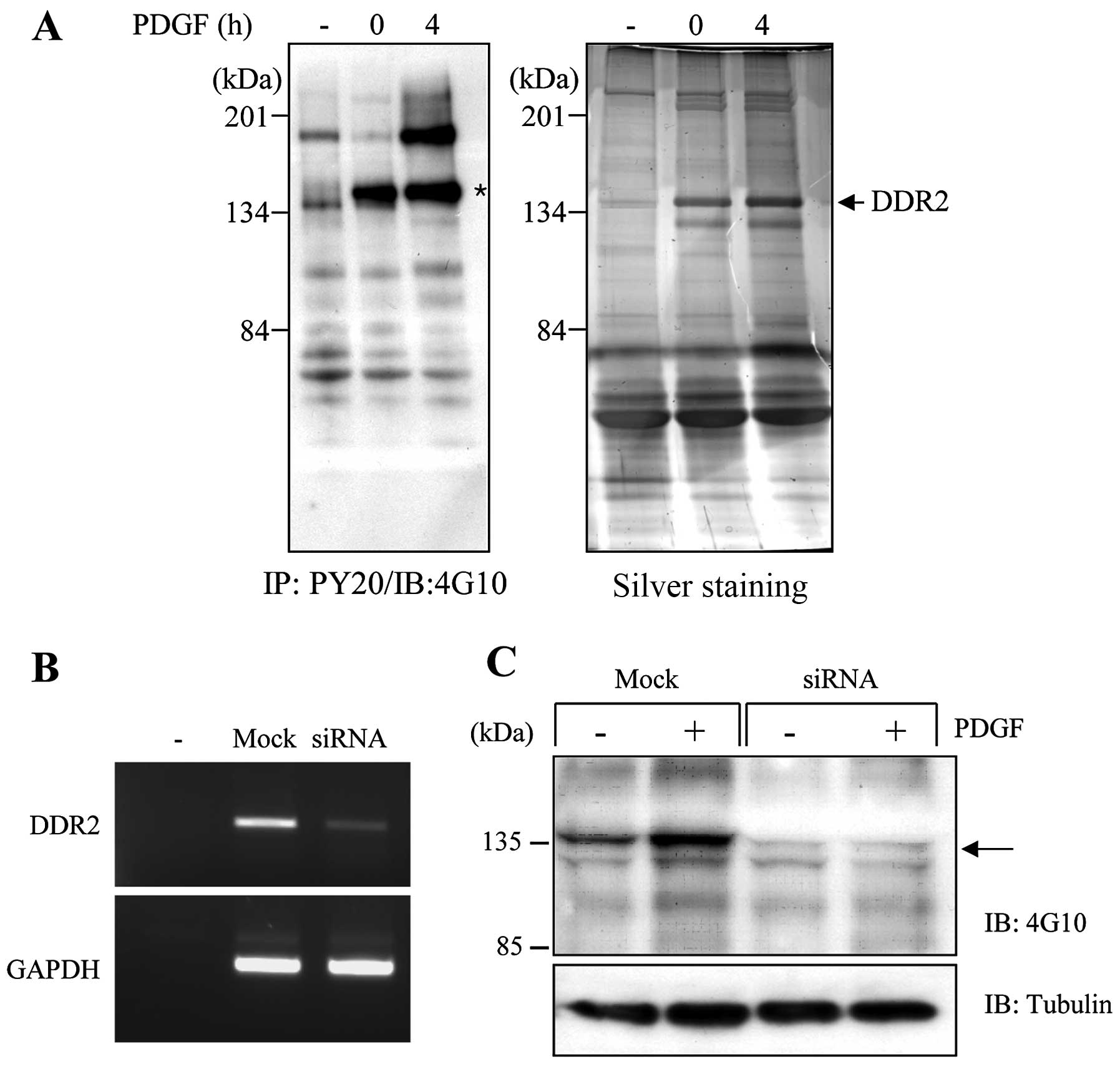
spectrometry. Fig. 1A shows that
several proteins increased the tyrosine phosphorylation in 3D
collagen matrices. DDR2 (~134 kDa) was found to be the most
prominent protein to increase the tyrosine phosphorylation in 3D
collagen matrices. In particular, DDR2 enhanced the phosphorylation
in response to PDGF stimulation. It has been reported that the DDRs
only respond to extracellular components such as fibrillar
collagens, but not to soluble growth factors (12). However, our findings indicate that
DDR2 appeared likely to increase the phosphorylation upon PDGF
stimulation in 3D collagen matrices. Thus, it may be beneficial to
define whether DDR2 can respond to soluble growth factors, such as
PDGF, when cells are in the 3D environment.
To further analyze the role of DDR2 in the 3D
collagen matrices, we used siRNA technology to knock down DDR2
expression in the human fibroblasts. Fig. 1B provides an example of the RT-PCR
analysis performed on the cell lysates prepared from the cells
after 36 h of transfection with DDR2-specific siRNA. The level of
DDR2 mRNA was markedly reduced, by almost 90%, compared with that
of the mock-transfected cells. We also confirmed the knockdown of
DDR2 expression in the human fibroblasts using western blot
analysis (data not shown). When DDR2 silencing cells were cultured
in the 3D collagen matrices, the tyrosine-phosphorylated protein in
a 135 kDa size on the mock-transfected cells completely
disappeared, indicating that DDR2 is the major
tyrosine-phosphorylated protein in 3D collagen matrices.
DDR2-silenced fibroblasts reduce
expansion of dendritic extensions in 3D collagen matrices
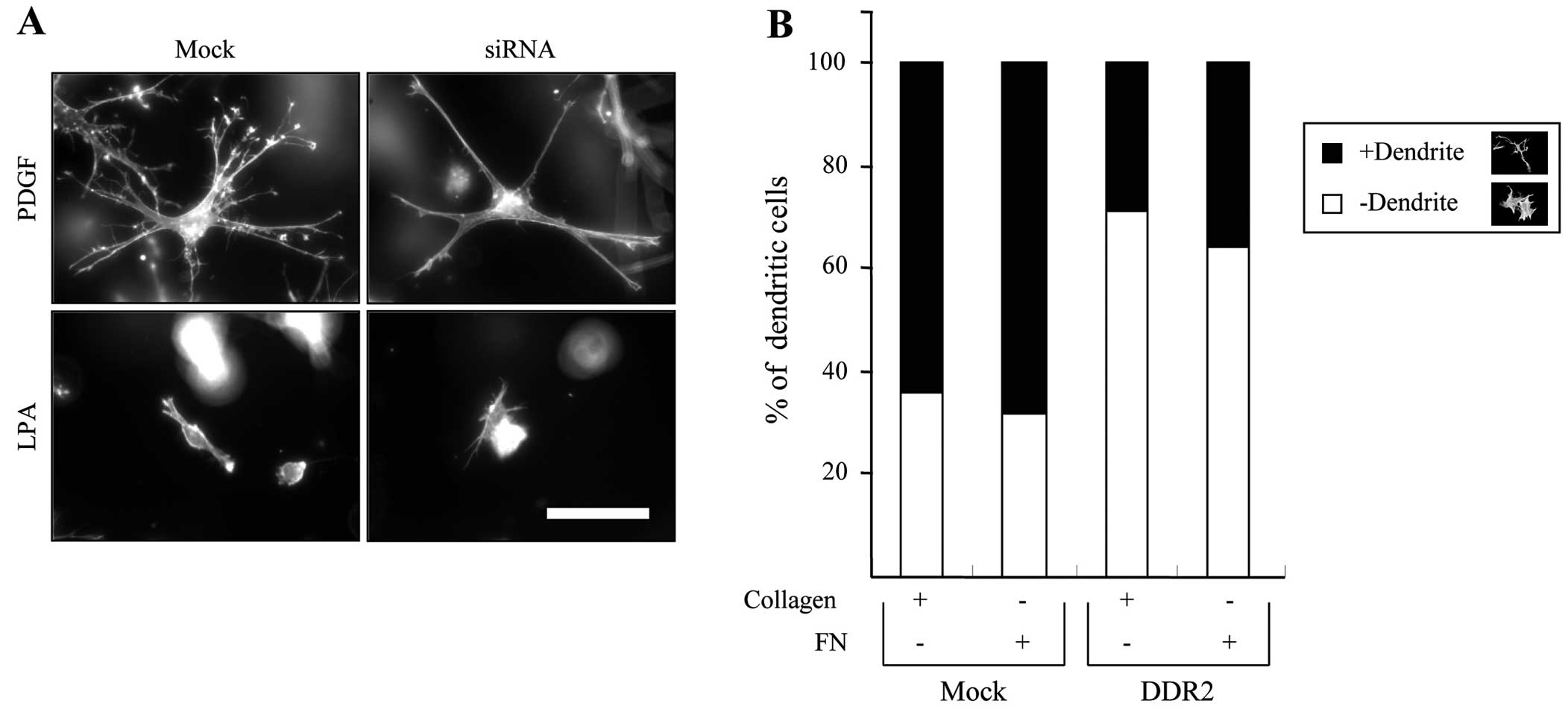
It has been shown that the PDGF causes the expansion
of the dendritic network in fibroblasts, while LPA resulted in the
retraction of dendritic extensions (16). Thus, we first examined the effect
of DDR2 silencing on fibroblast morphology in 3D collagen matrices.
Fig. 2A shows that the
DDR2-silenced fibroblasts appeared to reduce the number of
dendritic extensions, although the projected length of the
dendritic extensions is similar to that of the control cells in
response to PDGF stimulation. However, it had no effect on the
LPA-mediated retraction of dendritic extensions in the fibroblasts.
Fig. 2B shows the morphometric
analysis of a representative experiment, which indicates that
DDR2-silenced cells showed an ~30% decrease in the number of
dendritic extended cells compared with mock-transfected cells
either in collagen or fibronectin matrices. These results suggest
that DDR2 is involved in the regulation of fibroblast dendritic
extensions but not in the formation of dendrites in the 3D collagen
matrices.
We have shown that the microtubule-dependent
mechanism is involved in cell spreading according to the tension
state of the cell matrix interaction in 3D collagen matrices but
actin dynamics are prerequisites for the initial spreading in this
case (18). Our findings indicate
that DDR2 silencing significantly inhibited dendritic extensions,
indicating that it plays a critical role in determining the cell
morphology in the 3D collagen matrices (Fig. 2A). Although there is a lack of
evidence regarding the relationship between DDR2 and cytoskeletal
dynamics during cell spreading, it is possible that DDR may control
the actin dynamics via myosin IIA in a dependent manner (15).
DDR2 silencing decreases the cell-matrix
interaction in 3D collagen matrices
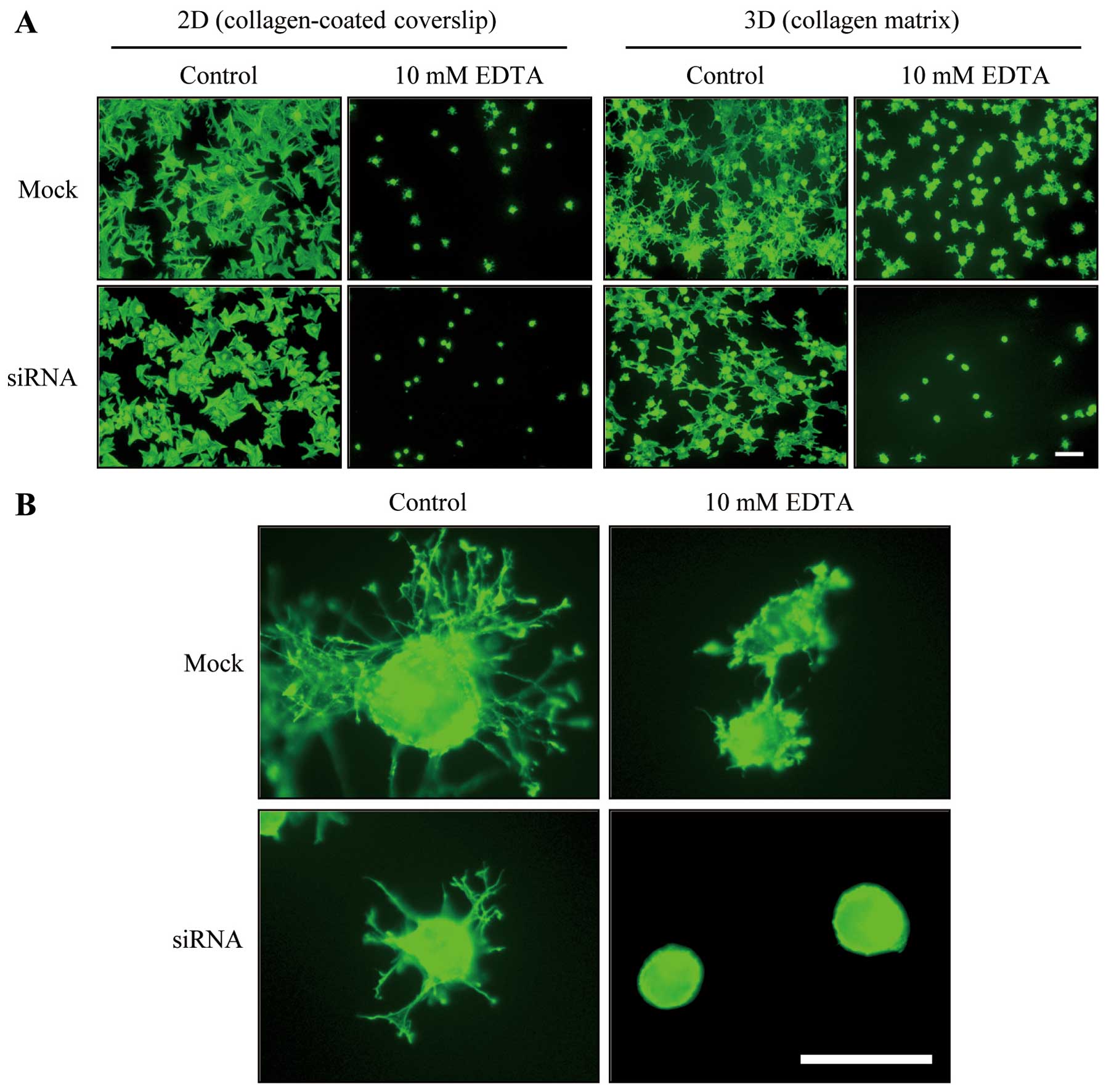
Results indicated that the DDR2 silencing impaired
the regulation of dendritic extensions of fibroblasts. Since it has
been proposed that the attachment of fibroblasts to collagen
fibrils in 3D collagen matrices is required for protruded dendrite
(11), we examined the effect of
DDR2 silencing on fibroblast attachment to 3D collagen matrices.
Fig. 3A shows that the incubation
with 10 mM EDTA caused the removal of either mock or
siRNA-transfected cells that had attached for 30 min to
collagen-coated coverslips (11).
However, fibroblasts that had attached to 3D collagen matrices for
30 min prior to EDTA treatment were unable to be released from the
collagen matrices, whereas DDR2-silenced fibroblasts that were
attached to the collagen matrices for 30 min were easily detached
with the EDTA treatment (Fig.
3A), which suggests that the DDR2-mediated dendritic protrusion
may be involved in the fibroblast and 3D collagen matrix
interaction.
Fig. 3B presents
representative images of mock and DDR2-silenced fibroblasts under
control (PDGF) and EDTA conditions. After 1 h of incubation, the
extensions of the dendrites in the DDR2-silenced cells were
relatively simple and short in length compared with those of the
control cells. However, a notable difference appeared in the
EDTA-treated cells. The addition of EDTA to the control cells
allowed the cells to form a small actin rich membrane protrusion
and ruffles along the peripheral of plasma membrane, while DDR2
silencing resulted in complete inhibition of those morphological
processes suggesting DDR2-mediated signaling is critical for
calcium ion-independent cytoskeletal rearrangement and cell-matrix
interaction.
It has been proposed that the cell adhesion to
collagen matrices occurs in a divalent cation-independent manner
(11). However, our results
clearly showed that DDR2-silenced cell fibroblasts were completely
removed from the 3D collagen matrices under the EDTA conditions. We
also showed that the membrane protrusions in DDR2-silenced
fibroblasts in the EDTA conditions were completely inhibited,
although the mock-transfected cells exhibited tinny extensions of
the membrane protrusions in the same conditions. Therefore,
DDR2-mediated membrane protrusion may be involved in the
integrin-independent cell adhesion in 3D collagen matrices.
DDR2 regulates matrix contraction and
cell migration in 3D collagen matrices
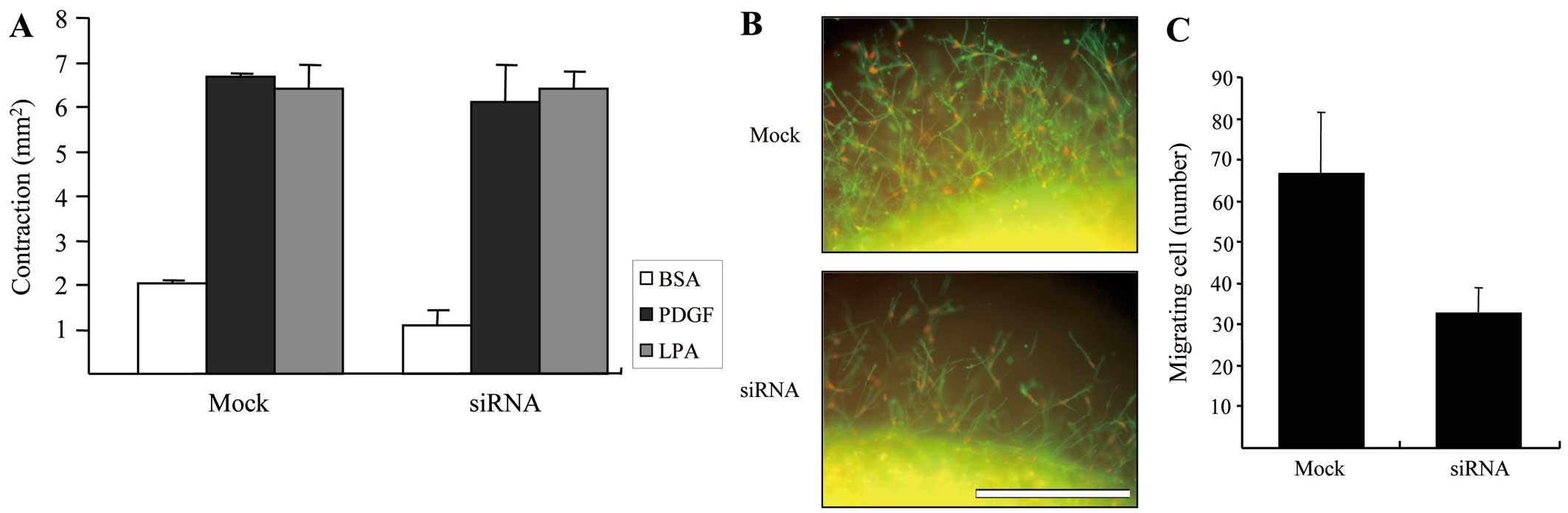
Lastly, we carried out an experiment to determine
the ability of control and DDR2-silenced fibroblasts to contract
the matrix. Fig. 4A shows the
results of floating matrix contraction in response to various
growth factors. Although it has been described in previous studies
that the mechanical interaction of fibroblasts with collagen
fibrils is critical for matrix contraction and cell migration in 3D
collagen matrices (19), our
results showed that the extent of growth factor dependence on
matrix contraction with DDR2- silenced cells is similar to that of
mock-transfected cells, indicating that DDR2-dependent cell-matrix
interaction may not to be required for the matrix contraction
although there may be another mechanism to control.
To analyze the migration of DDR2-silenced and
control fibroblasts in the 3D matrix environments, the
pre-contracted collagen matrices shown in Fig. 4A were embedded in cell-free
matrices to prepare the nested collagen matrices. The migration of
cells in nested collagen matrices can be easily quantified when
double stained for actin and PI by counting the number of nuclei
moving out of the border of inner matrix (20). Fig.
4B shows representative images of the control and DDR2-silenced
fibroblast migration after 24 h of migration in PDGF-containing
medium, and Fig. 4C presents the
quantified results. DDR2-silenced fibroblasts markedly decreased
cell migration in the 3D collagen environment, although they did
not have a significant influence on the floating collagen matrix
remodeling.
In the current study, we showed that DDR2 is
required for the cell-collagen fibril interaction in 3D collagen
matrices. It is unclear if the kinase activity or phosphorylation
of DDR2 is necessary for DDR2 function in initial spreading and
cell-matrix interaction in 3D collagen matrices, since the
autophosphorylation appeared after 2–18 h of collagen stimulation
(12). Previous studies reported
that the tyrosine kinase-independent mechanism is sufficient to
control the collagen fibrillogenesis and cell migration (21,22), supporting the hypothesis that DDR2
for cell spreading and attachment in 3D collagen matrices may be
mediated in a kinase-independent manner. It has been reported that
the numerous signaling proteins, including PI3 kinase, Nck and Shc,
are associated with the cytoplasmic region of DDR1 and form a large
signaling complex either in a kinase-dependent or -independent
manner but relatively little is known about the binding partners of
DDR2 (14). Thus, it is possible
that the signal proteins involved in cytoskeleton rearrangement
could be recruited to the cytoplasmic region of DDR2 through which
the signaling complex may play an important role in cell spreading
and attachment in 3D collagen matrices. The fact that the
DDR2-mediated signaling complex regulates fibroblast attachment to
3D collagen matrices is in agreement with a previous study which
demonstrated that DDR1 regulated cell spreading and motility via
myosin IIA (15). Moreover, DDR2
has an unusually long juxtamembrane domain in the cytoplasmic
region, suggesting that it has an ability to serve as a dock site,
making possible an intermolecular association. Thus, further
studies to define the molecular interaction with the cytoplasmic
domain of DDR2 are warranted.
Acknowledgements
We are particularly grateful to Dr Frederick
Grinnell for the initiative effort toward this study. This study
was supported by a 2010 Chung-Ang University research grant.
References
|
1
|
Engler AJ, Sweeney HL, Discher DE and
Schwarzbauer JE: Extracellular matrix elasticity directs stem cell
differentiation. J Musculoskelet Neuronal Interact.
7:3352007.PubMed/NCBI
|
|
2
|
Friedl P and Bröcker EB: The biology of
cell locomotion within three-dimensional extracellular matrix. Cell
Mol Life Sci. 57:41–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geiger B and Yamada KM: Molecular
architecture and function of matrix adhesions. Cold Spring Harb
Perspect Biol. 3:a0050332011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levental KR, Yu H, Kass L, et al: Matrix
crosslinking forces tumor progression by enhancing integrin
signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castelló-Cros R and Cukierman E:
Stromagenesis during tumorigenesis: characterization of
tumor-associated fibroblasts and stroma-derived 3D matrices.
Methods Mol Biol. 522:275–305. 2009.
|
|
6
|
Hynes RO: The extracellular matrix: not
just pretty fibrils. Science. 326:1216–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larsen M, Artym VV, Green JA and Yamada
KM: The matrix reorganized: extracellular matrix remodeling and
integrin signaling. Curr Opin Cell Biol. 18:463–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bershadsky AD, Ballestrem C, Carramusa L,
et al: Assembly and mechanosensory function of focal adhesions:
experiments and models. Eur J Cell Biol. 85:165–173. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riveline D, Zamir E, Balaban NQ, et al:
Focal contacts as mechanosensors: externally applied local
mechanical force induces growth of focal contacts by an
mDia1-dependent and ROCK-independent mechanism. J Cell Biol.
153:1175–1186. 2001. View Article : Google Scholar
|
|
10
|
Grinnell F, Ho CH, Tamariz E, Lee DJ and
Skuta G: Dendritic fibroblasts in three-dimensional collagen
matrices. Mol Biol Cell. 14:384–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang H and Grinnell F: Cell-matrix
entanglement and mechanical anchorage of fibroblasts in
three-dimensional collagen matrices. Mol Biol Cell. 16:5070–5076.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vogel W, Gish GD, Alves F and Pawson T:
The discoidin domain receptor tyrosine kinases are activated by
collagen. Mol Cell. 1:13–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogel W: Discoidin domain receptors:
structural relations and functional implications. FASEB J.
13(Suppl): S77–S82. 1999.PubMed/NCBI
|
|
14
|
Vogel WF, Abdulhussein R and Ford CE:
Sensing extracellular matrix: an update on discoidin domain
receptor function. Cell Signal. 18:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Arora P, McCulloch CA and Vogel
WF: The collagen receptor DDR1 regulates cell spreading and
motility by associating with myosin IIA. J Cell Sci. 122:1637–1646.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhee S and Grinnell F: P21-activated
kinase 1: convergence point in PDGF- and LPA-stimulated collagen
matrix contraction by human fibroblasts. J Cell Biol. 172:423–432.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, You E and Rhee S: Dynein regulates
cell migration depending on substrate rigidity. Int J Mol Med.
29:440–446. 2012.PubMed/NCBI
|
|
18
|
Rhee S, Jiang H, Ho CH and Grinnell F:
Microtubule function in fibroblast spreading is modulated according
to the tension state of cell-matrix interactions. Proc Natl Acad
Sci USA. 104:5425–5430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Provenzano PP, Inman DR, Eliceiri KW,
Trier SM and Keely PJ: Contact guidance mediated three-dimensional
cell migration is regulated by Rho/ROCK-dependent matrix
reorganization. Biophys J. 95:5374–5384. 2008. View Article : Google Scholar
|
|
20
|
Grinnell F, Rocha LB, Iucu C, Rhee S and
Jiang H: Nested collagen matrices: a new model to study migration
of human fibroblast populations in three dimensions. Exp Cell Res.
312:86–94. 2006.
|
|
21
|
Blissett AR, Garbellini D, Calomeni EP,
Mihai C, Elton TS and Agarwal G: Regulation of collagen
fibrillogenesis by cell-surface expression of kinase dead DDR2. J
Mol Biol. 385:902–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hachehouche LN, Chetoui N and Aoudjit F:
Implication of discoidin domain receptor 1 in T cell migration in
three-dimensional collagen. Mol Immunol. 47:1866–1869. 2010.
View Article : Google Scholar : PubMed/NCBI
|