Introduction
Endometriosis is a complex and chronic gynecological
disorder characterised by the presence of endometrial tissue
outside the uterus (1). Genetic,
hormonal and environmental factors contribute to the susceptibility
to endometriosis; however, the pathogenesis of this disease has not
yet been fully elucidated.
Although endometriotic cells are not characterised
by uncontrolled proliferation, they show some properties of
malignant tissues, such as invasion, induction of metastasis, and
the ability to evade apoptosis (2,3).
In particular, it is known that the ability of endometriotic cells
to invade surrounding tissue is induced by a group of proteins
termed metastasis-inducing proteins (MIPs), such as osteopontin
(OPN) (4).
OPN, a 70-kDa secreted glycoprotein, is mainly
involved in cell adhesion and migration (5), and it has been found to be expressed
in endometrial epithelium in normally cycling fertile women
(6). However, various studies on
the endometrial expression of OPN in patients with endometriosis
have provided controversial results. A previous study demonstrated
that the OPN protein is densely expressed in eutopic normal
endometrium, as well as in epithelial cells of endometriotic cysts
(7). Moreover, OPN mRNA
expression, as well as its plasma levels, have been shown to be
higher in patients with endometriosis compared to normal subjects
(8). It has been reported that
OPN mRNA levels are reduced during the early secretory phase of
women with moderate-to-severe endometriosis (9,10).
Another feature of endometriosis is represented by
its stem cell origin (11). It
has been hypothesised that endometriosis may be caused by a
dysregulation of stem cell function (12).
Prominin-1 (CD133), a stem cell-associated antigen,
is a 120-kDa glycoprotein, and a member of the prominin family of
pentaspan membrane proteins (13). CD133 has been shown to be
localised in glandular and luminal epithelial cells of the normal
endometrium (14).
The spreading of endometrial epithelial progenitor
cells may represent one of the mechanisms involved in the
pathogenesis of endometriosis, a disease characterised by a dense
vascularisation of its lesions (15). It is known that OPN may influence
the angiogenesis, proliferation and migration of endothelial
progenitor cells, acting as a regulator of CD133+
progenitor cells (16,17).
The present study aimed to determine whether OPN and
CD133 expression is altered in the human ectopic endometrium, and
whether the expression of these two molecules correlates with the
clinical features of endometriosis. The expression profiles of OPN
and CD133 were analysed in ectopic lesions, as well as in normal
endometrium by real-time RT-PCR and immunohistochemistry.
Furthermore, we also evaluated the plasma levels of OPN in patients
with endometriosis.
Materials and methods
Patient selection
Sixty-one women were enrolled in this study after
providing written informed consent. Thirty-one patients underwent
laparoscopic surgery at the Department of Obstetrics and
Gynecology, Cannizzaro Hospital, Catania, Italy. As control
subjects, 30 women with benign non-endometriotic ovarian cysts were
enrolled in this study. Clinical data including, age, history of
pregnancy, parity, body mass index (BMI) and serum CA125 levels
were collected at surgery. Endometriosis was confirmed by a
histopathological examination of samples and the extent of the
disease was evaluated according to the revised classification of
endometriosis provided by the American Society of Reproductive
Medicine (18). Twenty-two cases
were classified as minimal-to-mild disease (stage I and II) and 9
cases were classified as moderate-to-severe disease (stage III and
IV). All the patients were in the proliferative phase of the
menstrual cycle. The study protocol was approved by the local
ethics committee.
RNA extraction and real-time RT-PCR
Fresh endometrial specimens were immediately
transferred in RNAlater™ (Sigma-Aldrich, St. Louis, MO, USA) and
stored at −80°C until RNA extraction. Tissue specimens were
pulverised and then dissolved in TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), according the manufacturer’s instructions. The
concentration of the purified RNA was determined by
spectrophotometry. For further analysis, equal RNA loading and
integrity was confirmed by showing consistent intensities of 28S
and 18S rRNA bands on RNase-free agarose gel electrophoresis. A
total of 2 μg of RNA from each sample was reverse transcribed into
cDNA using the SuperScript III First-Strand Synthesis System
(Invitrogen) according to the manufacturer’s instructions. mRNA
expression was measured by SYBR-Green quantitative real-time RT-PCR
using the Rotor-Gene Q thermal cycler (Qiagen, Valencia, CA, USA).
The primers used for PCR amplification were: CD133 forward,
TTTCAAGGACTTGCG AACTCTCTT and reverse, GAACAGGGATGATGTTGGG TCTCA
(167 bp); OPN forward, AGACCTGACATCC AGTACCCTG and reverse,
GTGGGTTTCAGCACTCTGGT (188 bp). The PCR reaction was carried out in
25 μl buffer, containing 50 ng cDNA, 1 μM of each primer and 12.5
μl 2X Rotor-Gene SYBR-Green PCR Master Mix (Qiagen). The thermal
cycling conditions were as follows: denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation for 10 sec at 95°C and
annealing and extension for 15 sec at 60°C. As the housekeeping
gene, glyceraldehyde-3-phosphate dehydrogenase (GADPH; QuantiTect
Primer assay, Qiagen) was amplified in order to normalise the
amount of total RNA present in each reaction. The quantification of
the transcripts was carried out utilizing the dComparative
QuantitationT software supplied with Rotor-Gene Q. Endometrial
tissue from a normal subject was used as calibrator, and the mean
efficiency of the take-off point of the cycling curves was used to
calculate the fold change according to the formula: fold change=
efficiencyCt1–Ct2, where Ct1 and Ct2 are the take-off
values of the cycling curves being compared. Each real-time RT-PCR
reaction was conducted in duplicate, in order to evaluate data
reproducibility. The results are expressed as means ± SEM and the
Student’s t-test was used to compare the means of two samples.
Significance was accepted at the 5% level.
Plasma OPN measurement
Peripheral blood samples were collected from
patients with endometriosis and control subjects by venous
puncture, and immediately centrifuged at 1,500 × g at +4°C for 10
min. Plasma was stored at −80°C until analysis. Plasma OPN levels
were measured using the commercially available Quantikine™ Human
Osteopontin ELISA kit (R&D Systems, Minneapolis, MN, USA),
according to the manufacturer’s instructions. Samples were run in
duplicate, and the results are expressed in ng/ml. Data are
presented as the means ± SEM. The Student’s t-test was used to
compare the means of two samples. Significance was accepted at the
5% level.
Immunohistochemical analysis
Five-micrometer-thick paraffin-embedded sections
were mounted on silanized slides. Following section
deparaffinization and rehydration through a graded ethanol series
at room temperature, antigen retrieval was performed in Tris-EDTA
buffer (pH 9.0, 30 min) and in citrate buffer (pH 6.0, 20 min, 20
min) for OPN and CD133 immunostaining, respectively. As primary
antibodies, rabbit polyclonal anti-OPN, diluted 1:1,000 (AB1870;
Chemicon, Temecula, CA, USA) and anti-prominin-1, diluted 1:200
(PAB12663; Abnova, Taipei, Taiwan) were used. All the
immunohistochemical steps were carried out by the fully automated
Menarini Bond™ autostainer (Menarini Diagnostics, Florence, Italy).
For the controls, the primary antibody was substituted with
non-immune serum and the primary antibody was omitted, thus
incubating the slides only with buffer. For the evaluation of
immunoreactivity, staining intensities were scored on the basis of
the percentage of positive cells for OPN and CD133 as follows: −,
<5%; +, 5–50%; and ++, >50%. Immunohistochemical
semiquantitative analysis was performed by comparing the results
using the χ2 test. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Clinical and demographic features of
patients with endometriosis and control subjects
Table I displays
the demographic and clinical characteristics of the patients and
the control subjects. The mean age of the patients affected by
endometriosis and the controls was 36.45 years (SD ±9.18) and 33.77
years (SD ±8.09), respectively. The mean number of pregnancies, as
well as the parity was not statistically significant between the
patients and the healthy control subjects. The difference in BMI
between the patients and control groups was not significant.
Finally, serum CA125 levels were higher in the patients in
comparison to the controls: 59.44±45.56 vs. 19.37±21.97 IU/ml,
respectively (p=0.0001).
 | Table IDemographic and clinical
characteristics of the patients with endometriosis and the control
subjects. |
Table I
Demographic and clinical
characteristics of the patients with endometriosis and the control
subjects.
| Characteristics | Control subjects
(n=30) | Patients with
endometriosis (n=31) | P-value |
|---|
| Age, years | 33.77±8.09 | 36.45±9.18 | 0.25 |
| Number of
pregnancies | 0.74±1.02 | 0.93±1.39 | 0.56 |
| Parity | 0.59±0.89 | 0.86±1.27 | 0.36 |
| Serum CA125 levels
(IU/ml) | 19.37±21.97 | 59.44±45.56 | 0.0001 |
| BMI
m2/kg | 24.51±2.53 | 23.32±7.56 | 0.44 |
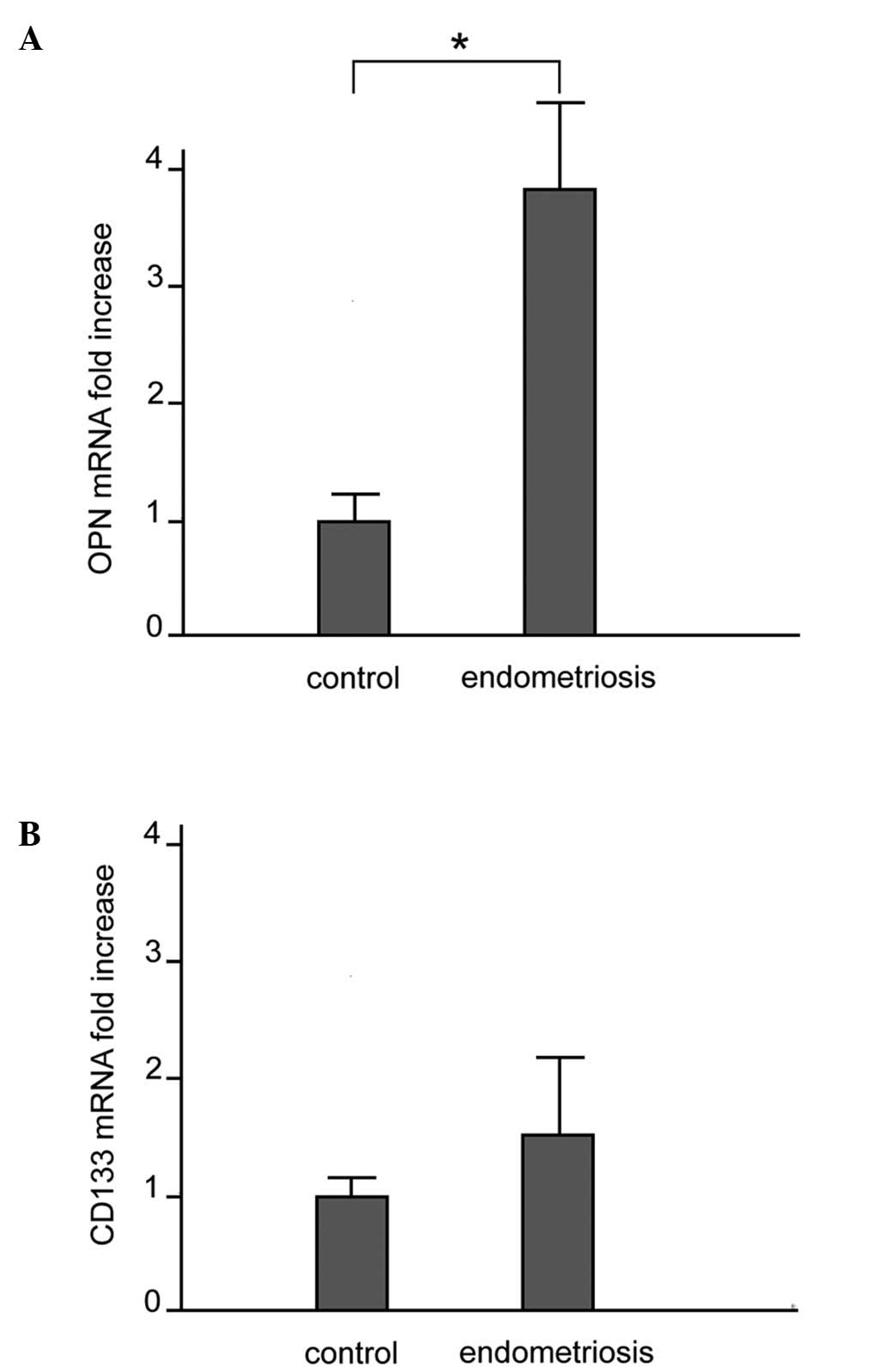
mRNA expression of OPN and CD133
The mRNA expression of OPN and CD133 in the control
and ectopic endometrial tissues was examined by quantitative
real-time RT-PCR. As shown in Fig.
1A, OPN mRNA expression was significantly higher in the
patients with endometriosis compared to the controls:
(3.79±1.30-fold increase vs. control subjects, p<0.010). A
comparison of CD133 mRNA expression between the patients with
endometriosis and the controls did not reveal any significant
difference (Fig. 1B). A
comparison of the OPN mRNA levels between the patients with stage
I-II disease and those with stage III-IV disease did not reveal any
significant difference (p=0.24) (data not shown).
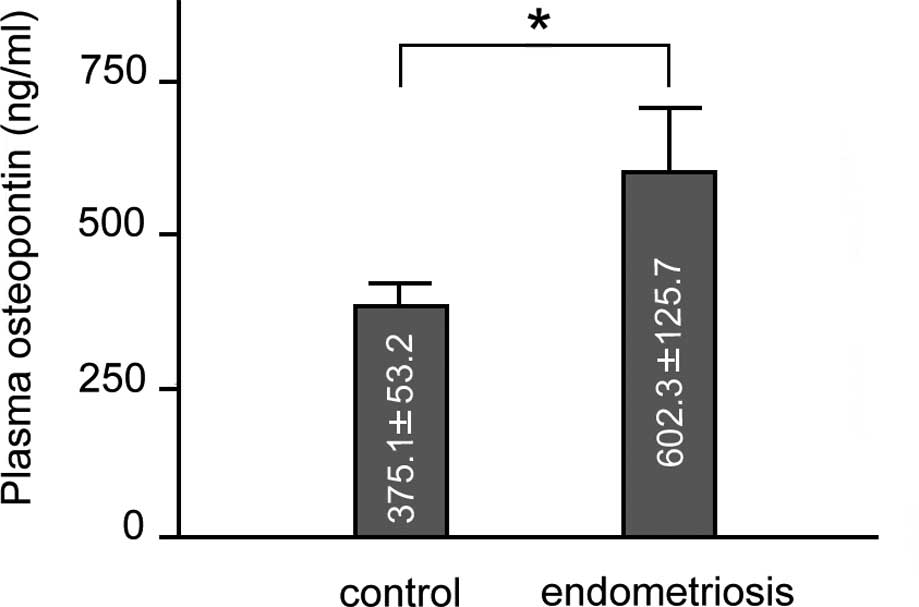
Plasma OPN levels
As shown in Fig.
2, plasma OPN levels (means ± SEM) were higher in patients with
endometriosis in comparison to the controls (602.3±125.7 vs.
375.1±53.2 ng/ml; p<0.01). A comparison of the OPN plasma levels
between the patients with stage I-II disease and those with stage
III-IV disease and the control group revealed similar results to
those obtained between the total number of patients and the control
group. Finally, a comparison of the plasma OPN levels between the
patients with stage I-II disease and those with stage III-IV
disease revealed no significant difference (data not shown). We
analysed the correlation between plasma OPN and serum CA125 levels.
A positive correlation between plasma OPN and serum CA125 levels
was observed in the total number of endometriosis patients
(Pearson’s test, r=0.41, p<0.05). However, a comparison between
the 2 groups of patients (those with stage I-II and stage III-IV
disease) did not reveal any statistically significant
difference.
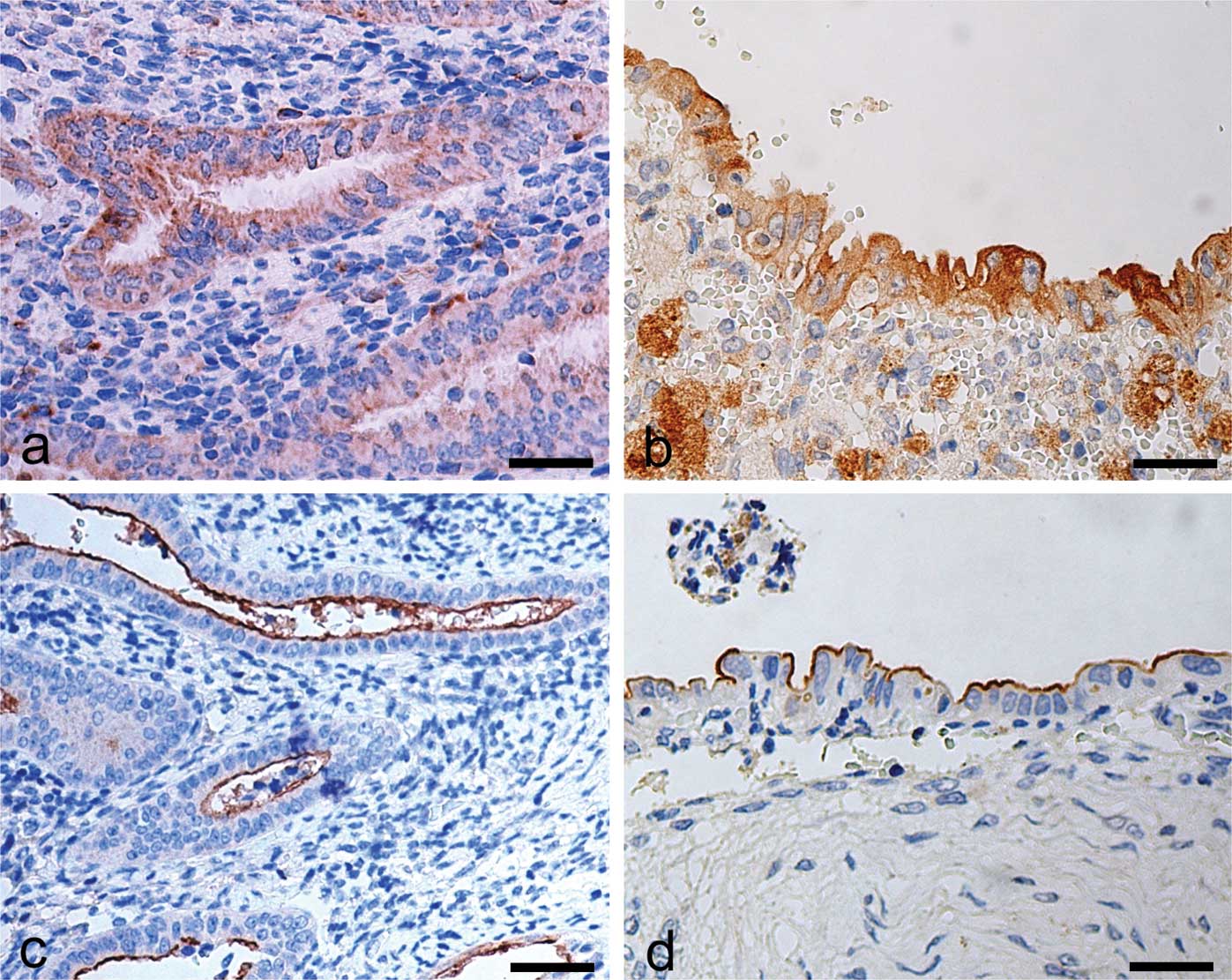
OPN and CD133 immunohistochemical
analysis
In control sections, OPN immunostaining was
localised in the cytoplasm of epithelial cells of the functional
layer. Stromal cells were devoid of immunostaining for OPN
(Fig. 3a). In the endometriotic
tissue, OPN expression was higher in comparison to the control
samples, and was localised in the cytoplasm of epithelial gland
cells, as well as in several stromal macrophages (Fig. 3b). CD133 immunostaining in the
control samples was exclusively localised on the surface of the
epithelial cells lining the lumen of the functional layer (Fig. 3c). No immunostaining for CD133 was
observed in the stroma. In the endometriotic lesion tissue, the
immunohistochemical pattern for CD133 was similar to that observed
in the control tissue (Fig. 3d).
The results of the analysis of the intensity of the
immunohistochemical reactions for OPN and CD133 are summarised in
Table II.
 | Table IIEvaluation of immunostaining intensity
of OPN and CD133 in normal tissue and endometriotic lesions. |
Table II
Evaluation of immunostaining intensity
of OPN and CD133 in normal tissue and endometriotic lesions.
| | Staining
intensity |
|---|
| |
|
|---|
| | OPN | CD133 |
|---|
| |
|
|
|---|
| n | − | + | ++ | | − | + | ++ | |
|---|
| Control
endometrium | 30 | 3 | 27 | 0 | - | 5 | 24 | 1 | - |
| Stage I-II
endometriosis | 22 | 13 | 9 | 0 |
p<0.01a | 4 | 18 | 0 | NS |
| Stage III-IV
endometriosis | 9 | 0 | 6 | 3 |
p<0.01a | 2 | 7 | 0 | NS |
Comparison of clinical findings of
patients with endometriosis according to the severity of
disease
No significant differences were observed between the
age, number of pregnancies, parity, serum CA125 levels and BMI of
the patients with endometriosis with stage I-II and stage III-IV
disease. In particular, the mean age was 36±2.48 years in the
patients with stage I-II disease vs. 35.75±5.94 years in the
patients with stage III-IV disease (p=0.96); the number of
pregnancies was 1.40±0.40 in the patients with stage I-II disease
vs. 1.67±0.89 in the patients with stage III-IV disease (p=0.75);
parity was 1.20±0.28 in the patients with stage I-II disease vs.
1.34±0.67 in the patients with stage III-IV disease (p=0.82); serum
CA125 levels were 47.71±7.72 IU/ml in the patients with stage I-II
disease vs. 72.96±38.74 IU/ml in the patients with stage III-IV
disease (p=0.36); BMI was 25.28±3.08 m2/kg in the
patients with stage I-II disease vs. 20.07±3.30 m2/kg in
the patients with stage III-IV disease (p=0.33).
Discussion
The pathological processes involved in endometriosis
have not yet been fully elucidated. However, endometriosis has been
found to be associated with changes in the expression of several
genes, including cytokines (19),
such as the multifunctional cytokine OPN, which has been
intensively investigated in endometriosis (7,8,10,20).
The results from the present study revealed that OPN
mRNA expression, as well as its release in the blood, was
significantly increased in endometriotic lesions in comparison to
normal tissue. Our findings on OPN mRNA expression are in agreement
with those of other studies, which have demonstrated an increased
OPN mRNA expression, as well as increased OPN plasma levels in
patients with endometriosis in comparison to control subjects,
regardless of the phase of the menstrual cycle and diseases stage
(8). Similarly, by means of
oligonucleotide microarray analysis, complimentary DNA microarrays
and quantitative real-time RT-PCR, OPN gene expression has been
shown to be increased in endometriotic lesions in a rat model of
endometriosis in comparison to normal rats (21,22). On the contrary, with the use of
microarray analysis, a downregulation of OPN expression has been
observed during the secretory phase in endometriotic lesions
(9).
As regards immunohistochemical analysis, we found a
higher semiquantitative expression of OPN in both groups of
endometriosis patients (the first including patients with stage
I-II disease and the second group including patients with stage
III-IV disease) in comparison to the eutopic endometrium of the
control subjects. Moreover, the OPN staining pattern obtained in
the present study, was similar to that observed in the study by
Odagiri et al(7), who
demonstrated that this molecule was mainly immunolocalised in
normal and ectopic endometrial epithelial cells. However, these
authors did not find a significant difference in the staining
intensity between endometriotic lesions and control samples.
Moreover, another immunohistochemical study demonstrated different
OPN expression intensities in endometriotic lesions in comparison
to normal specimens, according to the histological grade (10). Recently, Casals et
al(20) demonstrated no
statistically significant difference in OPN expression between
patients and the control subjects.
Such discrepancies may arise from the heterogeneity
of the endometriotic samples included in the above studies. For
this reason, we restricted the sampling of specimens from women who
were in the proliferative phase, and subdivided the patients into 2
groups, one group including those patients with minimal-to-mild
disease and the other group including patients with
moderate-to-severe disease.
Since it is well known that the functional role of
OPN includes cell adhesion, migration, differentiation and
regulation of the metastatic spread of tumour cells, it can be
hypothesised that the expression of OPN is increased in patients
with endometriosis, which would enhance endometrial invasiveness,
proliferation and survival in ectopic lesions.
A recent study revealed that a subset of
endometriotic cells displayed certain features characteristic of
somatic stem cells, such as the presence of CD133 (23). This cell subset seems to originate
from bone marrow, and to display endothelial progenitor cell-like
features (24). The proliferation
and migration of endothelial progenitor cells are influenced by OPN
(16,17), which may be required for the
homing of these cells (25). OPN
shows opposite effects depending on tissue and the physiological
state of the cell. In general, OPN is a potent stimulator of cell
proliferation, although it represents a negative regulator of
hematopoietic stem cell proliferation (26). Possibly, such a mechanism may be
mediated by β1-integrins (27),
and OPN may induce a lateral organization of lipids and membrane
proteins in lipid rafts (28), in
order to activate associated signal transduction pathways (29). Although the role of CD133 in stem
cells remains unclear, there is much evidence suggesting that this
protein plays a potential role as an organiser of specific membrane
domains (13), which seems
essential for the maintenance of stem cell properties (30). Although we observed the presence
of CD133+ cells in the normal endometrium, as well as in
endometriosis specimens, we did not observe a significant
quantitative variation of this protein in patients with
endometriosis. However, the presence of the somatic stem cell
marker, CD133, suggests the occurrence of a stem cell origin in the
pathogenesis of the disease. There is emerging evidence for the
occurrence of endometrial cancer stem cells within
CD133+ and side population cells (31), which could explain the fact that
endometriosis is associated with 10–15% of ovarian cancer cases
(32).
Thus, further studies are required to determine
whether CD133 is expressed and localised in endometrial stem cells,
and whether this molecule may be used as a marker of stemness in
the eutopic and ectopic endometrium.
In conclusion, the results from our study confirm
that OPN is involved in the development of endometriosis by
enhancing the invasiveness, proliferation and survival of
endometrial cells in ectopic lesions. Furthermore, we suggest that
the protein, CD133, cannot be used as a disease marker for
endometriosis. Future studies are required in order to further
clarify the possible role and specific mechanisms of action of
these molecules in the pathogenesis of endometriosis, in order to
improve the quality of life of patients with this debilitating and
complex disease.
References
|
1
|
de Ziegler D, Borghese B and Chapron C:
Endometriosis and infertility: pathophysiology and management.
Lancet. 376:730–738. 2012.PubMed/NCBI
|
|
2
|
Swiersz LM: Role of endometriosis in
cancer and tumor development. Ann NY Acad Sci. 955:281–292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghese B, Mondon F, Noël JC, Fayt I,
Mignot TM, Vaiman D and Chapron C: Gene expression profile for
ectopic versus eutopic endometrium provides new insights into
endometriosis oncogenic potential. Mol Endocrinol. 22:2557–2562.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anborgh PH, Mutrie JC, Tuck AB and
Chambers AF: Role of the metastasis-promoting protein osteopontin
in the tumour microenvironment. J Cell Mol Med. 14:2037–2044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liaw L, Skinner MP, Raines EW, Ross R,
Cheresh DA, Schwartz SM and Giachelli CM: The adhesive and
migratory effects of osteopontin are mediated via distinct cell
surface integrins. Role of alpha v beta 3 in smooth muscle cell
migration to osteopontin in vitro. J Clin Invest. 95:713–724. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apparao KB, Murray MJ, Fritz MA, Meyer WR,
Chambers AF, Truong PR and Lessey BA: Osteopontin and its receptor
alphav beta(3) integrin are coexpressed in the human endometrium
during the menstrual cycle but regulated differentially. J Clin
Endocrinol Metab. 86:4991–5000. 2001.PubMed/NCBI
|
|
7
|
Odagiri K, Konno R, Fujiwara H, Netsu S,
Ohwada M, Shibahara H and Suzuki M: Immunohistochemical study of
osteopontin and L-selectin in a rat endometriosis model and in
human endometriosis. Fertil Steril. 88:1207–1211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho S, Ahn YS, Choi YS, Seo SK, Nam A, Kim
HY, Kim JH, Park KH, Cho DJ and Lee BS: Endometrial osteopontin
mRNA expression and plasma osteopontin levels are increased in
patients with endometriosis. Am J Reprod Immunol. 61:286–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burney RO, Talbi S, Hamilton AE, Vo KC,
Nyegaard M, Nezhat CR, Lessey BA and Giudice LC: Gene expression
analysis of endometrium reveals progesterone resistance and
candidate susceptibility genes in women with endometriosis.
Endocrinology. 148:3814–3826. 2007. View Article : Google Scholar
|
|
10
|
Wei Q, St Clair JB, Fu T, Stratton P and
Nieman LK: Reduced expression of biomarkers associated with the
implantation window in women with endometriosis. Fertil Steril.
91:1686–1691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008.PubMed/NCBI
|
|
12
|
Maruyama T and Yoshimura Y: Stem cell
theory for the pathogenesis of endometriosis. Front Biosci (Elite
Ed). 4:2854–2863. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corbeil D, Karbanová J, Fargeas CA and
Jászai J: Prominin-1 (CD133): molecular and cellular features
across species. Adv Exp Med Biol. 777:3–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwab KE, Hutchinson P and Gargett CE:
Identification of surface markers for prospective isolation of
human endometrial stromal colony-forming cells. Hum Reprod.
23:934–943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasson IE and Taylor HS: Stem cells and
the pathogenesis of endometriosis. Ann NY Acad Sci. 1127:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Yan W, Lu X, Qian C, Zhang J, Li
P, Shi L, Zhao P, Fu Z, Pu P, Kang C, Jiang T, Liu N and You Y:
Overexpression of osteopontin induces angiogenesis of endothelial
progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in
glioma cells. Eur J Cell Biol. 90:642–648. 2011.PubMed/NCBI
|
|
17
|
Yu M, Liu Q, Yi K, Wu L and Tan X: Effects
of osteopontin on functional activity of late endothelial
progenitor cells. J Cell Biochem. 112:1730–1736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Revised American Society for Reproductive
Medicine classification of endometriosis: 1996. Fertil Steril.
67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eyster KM, Klinkova O, Kennedy V and
Hansen KA: Whole genome deoxyribonucleic acid microarray analysis
of gene expression in ectopic versus eutopic endometrium. Fertil
Steril. 88:1505–1533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casals G, Ordi J, Creus M, Fábregues F,
Carmona F, Casamitjana R and Balasch J: Expression pattern of
osteopontin and αvβ3 integrin during the implantation window in
infertile patients with early stages of endometriosis. Hum Reprod.
27:805–813. 2012.
|
|
21
|
Flores I, Rivera E, Ruiz LA, Santiago OI,
Vernon MW and Appleyard CB: Molecular profiling of experimental
endometriosis identified gene expression patterns in common with
human disease. Fertil Steril. 87:1180–1199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konno R, Fujiwara H, Netsu S, Odagiri K,
Shimane M, Nomura H and Suzuki M: Gene expression profiling of the
rat endometriosis model. Am J Reprod Immunol. 58:330–343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan RW, Ng EH and Yeung WS:
Identification of cells with colony-forming activity, self-renewal
capacity, and multipotency in ovarian endometriosis. Am J Pathol.
178:2832–2844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masuda H, Matsuzaki Y, Hiratsu E, Ono M,
Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, Ito M,
Yoshimura Y, Maruyama T and Okano H: Stem cell-like properties of
the endometrial side population: implication in endometrial
regeneration. PLoS One. 5:e103872010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leen LL, Filipe C, Billon A, Garmy-Susini
B, Jalvy S, Robbesyn F, Daret D, Allières C, Rittling SR, Werner N,
Nickenig G, Deutsch U, Duplàa C, Dufourcq P, Lenfant F, Desgranges
C, Arnal JF and Gadeau AP: Estrogen-stimulated endothelial repair
requires osteopontin. Arterioscler Thromb Vasc Biol. 28:2131–2136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus
T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR and
Scadden DT: Osteopontin is a hematopoietic stem cell niche
component that negatively regulates stem cell pool size. J Exp Med.
201:1781–1791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nilsson SK, Johnston HM, Whitty GA,
Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ,
Simmons PJ and Haylock DN: Osteopontin, a key component of the
hematopoietic stem cell niche and regulator of primitive
hematopoietic progenitor cells. Blood. 106:1232–1239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JL, Wang MJ, Sudhir PR and Chen JY:
CD44 engagement promotes matrix derived survival through the
CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol. 28:5710–5723.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jacobson K and Dietrich C: Looking at
lipid rafts? Trends Cell Biol. 9:87–91. 1999. View Article : Google Scholar
|
|
30
|
Bauer N, Fonseca AV, Florek M, Freund D,
Jászai J, Bornhäuser M, Fargeas CA and Corbeil D: New insights into
the cell biology of hematopoietic progenitors by studying
Prominin-1 (CD133). Cells Tissues Organs. 188:127–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kyo S, Maida Y and Inoue M: Stem cells in
endometrium and endometrial cancer: accumulating evidence and
unresolved questions. Cancer Lett. 308:123–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kokcu A: Relationship between
endometriosis and cancer from current perspective. Arch Gynecol
Obstet. 284:1473–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|