Introduction
The skin is the largest organ of the body and its
function in the immune system has attracted the attention of both
immunologists and dermatopathologists (1,2).
Ultraviolet (UV) radiation is the major environmental cause of skin
damage (3,4).
UVA (320.400 nm) and UVB (280.320 nm) radiations
reach the earth’s surface in amounts sufficient to have important
biological consequences for the skin (5). UVB, in particular, has a wide
spectrum of biological effects on the skin, and acute exposure
causes a variety of adverse skin reactions, including erythema,
edema, sunburn, hyperplasia, inflammation and immunosuppression
(6). Furthermore, chronic UVB
exposure leads to skin carcinogenesis and premature skin aging
(6,7).
In addition, exposure of cells to UVB radiation
results in the loss of keratinocyte viability, an increase in
membrane blebbing (8),
cytoskeletal molecular changes (9–12)
and apoptosis (13,14). The whole plant of Ixeris
dentata (I. dentata), a typical Oriental herb, has been
used for the treatment of indigestion, pneumonia, hepatitis,
contusions and tumors (15,16); it is also used in folk medicine in
Korea for the treatment of inflammatory diseases.
Recent pharmacological studies of I. dentata
showed that water or organic solvent extracts of whole herbal
medicine lower lipid concentrations and act as an antioxidant
(17), antiallergic (18), monamine oxidase (19), anti-inflammatory (20,21) antimutagenic and anticancer
(22) activity. Although crude
extracts of a single herbal medicine or herbal formula can exhibit
striking biological effects, their mechanisms cannot be fully
elucidated as they can contain innumerable compounds (23).
Guaiane-type sesquiterpene lactones were isolated
from the whole extract of I. dentata. Subsequently, the
effects of tectroside (TES) isolated from I. dentata on
UVB-induced pro-inflammatory mediators were evaluated by inhibiting
mitogen-activated protein kinases (MAPKs) in a human keratinocyte
cell line, HaCaT.
The effect on skin inflammation has yet to be
reported; therefore, as part of our ongoing screening program to
evaluate anti-inflammatory potential of natural compounds, we
investigated the in vitro anti-inflammatory activity of TES
isolated from I. dentata.
Materials and methods
Plant material
Whole plants of I. dentata were collected in
May 2006 from the herbarium at the Korea Research Institute of
Chemical Technology (KRICT) and were authenticated by Dr Young Sup
Kim. A voucher specimen (KR0472) was deposited at the herbarium at
KRICT (24).
Extraction and isolation
The air-dried whole plants (6 kg) of I.
dentata were soaked in methanol (MeOH) (2×40 liters) at room
temperature for 7 days. The MeOH extract was filtered and
evaporated to dryness under reduced pressure. The concentrated
extract (840 g) was suspended in 20 liters of water and then
extracted successively with an equal volume of dichloromethane
(MC), ethyl acetate (EtOAc), and n-butanol (n-BuOH), which yielded
160 g of the MC fraction, 15 g of the EtOAc fraction, and 60 g of
the n-BuOH fraction, respectively (24). The detailed purification
procedures for TES from the fraction are shown in Fig. 1.
Reagents
RPMI-1640, penicillin, and streptomycin were
obtained from HyClone Laboratories, Inc. (Logan, UT, USA). Bovine
serum albumin and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies for extracellular signal-regulated kinase (ERK)1/2,
phosphorylated ERK1/2, c-Jun NH2-terminal kinase (JNK),
phosphorylated JNK, and β-actin, and peroxidase-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Antibodies for human interleukin (IL)-6
and IL-8 and biotinylated antibodies for human IL-6 and IL-8 were
purchased from BD Biosciences (San Jose, CA, USA). The RNeasy Mini
Kit and QuantiTect Reverse Transcription kit were purchased from
Qiagen (Hilden, Germany). IL-6, IL-8, cyclooxygenase (COX)-2, and
β-actin oligonucleotide primers were purchased from Bioneer Corp.
(Daejeon, Korea).
Cell culture
HaCaT cells were grown in RPMI-1640 medium
containing 5% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin sulfate. The cells were incubated in a
humidified 5% CO2 atmosphere at 37°C. To stimulate the
cells, the medium was replaced with fresh RPMI-1640 medium, and
exposed to UVB in the presence or absence of TES for the indicated
periods.
UVB source
UVB irradiation was delivered by a closely spaced
array of 5 sunlamps (G9T5E lamps; Sankyo Denki Co., Hiratsuka,
Japan). The distance between the sunlamps and the surface of the
cell cultures was fixed at 7.5 cm, and the distance between the
sunlamps and the surface of the cage was fixed at 30 cm. The energy
output of the UVB (290–320 nm) lamps was measured using a UV
radiometer (UVX; UVP Inc., Upland, CA, USA).
Cell viability assay
Cell viability was determined by the MTS assay.
HaCaT cells were plated at a density of 3×104 cells/well
in 96-well plates (Nunc, Copenhagen, Denmark). Each experiment
included a non-treated group as the control. To determine the
non-toxic concentration for the cells, TES (2.5, 5, 10 and 20
μM) was added to each well. The plates were incubated for 24
h at 37°C under 5% CO2. The MTS solution (5 mg/ml) was
added to each well, and the cells were cultured for another 2 h,
after which the optical density was read at 490 nm. Cytotoxicity
was then calculated using the formula: 1 − (mean absorbance value
of treated cells/mean absorbance value of untreated cells).
Enzyme-linked immunosorbent assay
(ELISA)
Cells were seeded at a density of 3×104
cells/well in 48-well tissue culture plates and pretreated with 2
concentrations of TES (5 and 10 μM) for 24 h prior to UVB
(100 mJ/cm2) stimulation. ELISA plates (Nunc) were
coated overnight at 4°C with anti-human IL-6 and IL-8 antibodies
diluted in coating buffer (0.1 M carbonate, pH 9.5), and then
washed 3 times with phosphate-buffered saline (PBS) containing
0.05% Tween-20. Nonspecific protein-binding sites were blocked with
an assay diluent (PBS containing 10% FBS, pH 7.0) for at least 1 h.
Immediately, each sample or the IL-6 or IL-8 standard was added to
the wells. Following incubation for 2 h, a working detector was
added and incubated for 1 h. Accordingly, the substrate solution
(tetramethylbenzidine) was added to the wells and incubated for 30
min in the dark before the reaction was stopped with 2 N
H3PO4. Absorbance was read at 450 nm. All
subsequent steps were performed at room temperature, and all
standards and samples were assayed in duplicate.
Western blot analysis
Protein expression was assessed by western blot
analysis according to standard procedures. The HaCaT cells were
cultured in 60-mm-diameter culture dishes (4×106
cells/well) and pretreated with 2 concentrations of TES (5 and 10
μM). After 30 min, 2 or 24 h, the cells were UVB-irradiated
(100 mJ/cm2) and then incubated at 37°C. Following
incubation, the cells were washed twice in ice-cold PBS (pH 7.4).
The cell pellets were resuspended in lysis buffer on ice for 20
min, and the cell debris was removed by centrifugation. Protein
concentrations were determined using a Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Hercules, CA, USA) according to the
manufacturer’s instructions. Equal amounts of protein (20
μg) were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then transferred onto a polyvinylidene
membrane (Millipore, Bedford, MA, USA).
The membrane was blocked with 5% nonfat milk in
Tris-buffered saline with Tween-20 buffer (150 mM NaCl, 20 mM
Tris-HCl, and 0.05% Tween-20, pH 7.4). After blocking, the membrane
was incubated with primary antibodies for 18 h, washed with
Tris-buffered saline with Tween-20, and incubated again with
anti-mouse or anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies. Immunoreactivity was
detected using enhanced chemiluminescence (Amersham, Milan,
Italy).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
HaCaT cells were cultured in 6-well tissue culture
plates (8×105 cells/well) and pretreated with 2
concentrations of TES (5 and 10 μM). After 30 min, the cells
were irradiated with UVB (100 mJ/cm2) and incubated at
37°C. Following incubation, the cells were washed twice in ice-cold
PBS (pH 7.4). Total cellular RNA was isolated using the RNA Mini
Kit (Qiagen), and 1 μg of total RNA was reverse transcribed
using the QuantiTect Reverse Transcription Kit (Qiagen) according
to the manufacturer’s instructions. The total RNA (2 μg) was
converted to cDNA by treating it with 200 units of reverse
transcriptase and 500 ng of oligo(dT) primer in 50 mM Tris-HCl (pH
8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, and 1
mM deoxynucleotide triphosphates at 42°C for 1 h. The reaction was
stopped by heating at 70°C for 15 min, and the cDNA mixture (3
μl) was used for enzymatic amplification. PCR was performed
using 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2,
0.2 mM deoxynucleotide triphosphates, 2.5 units of TaqDNA
polymerase, and 0.1 μM each of the IL-6, IL-8, COX-2, or
β-actin primers.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance (ANOVA) or Student’s t-test for single
comparisons. All data are presented as the means ± standard error
(SE), and the number of individual experiments conducted is
mentioned in each figure legend.
Results
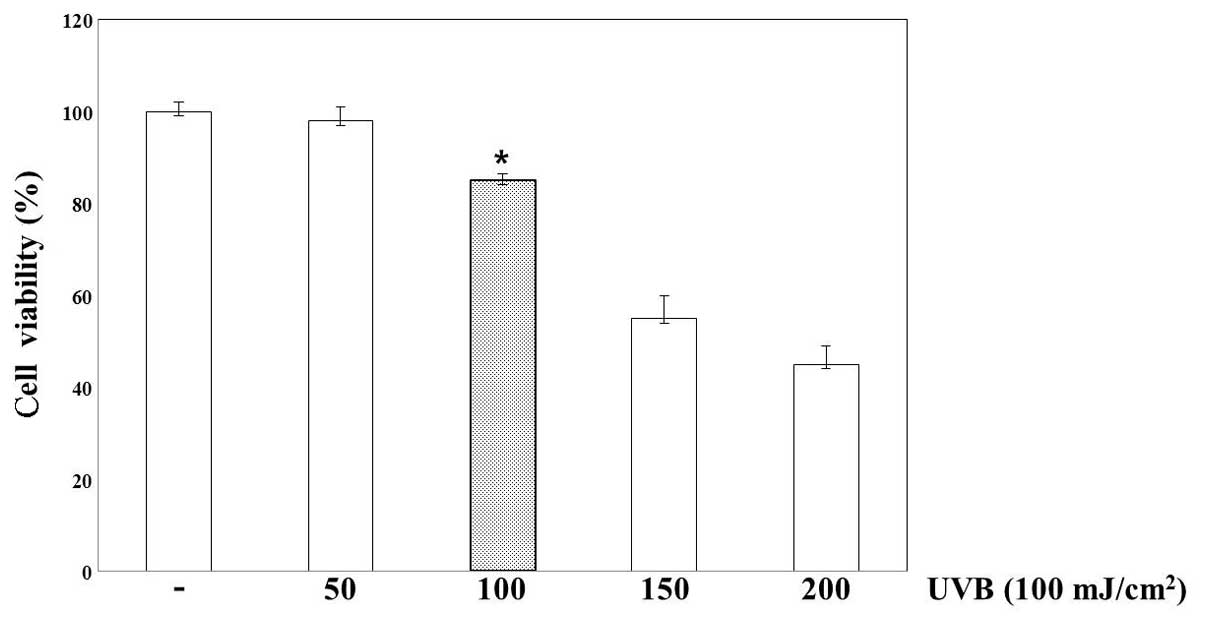
Cell viability of UVB-irradiated HaCaT
cells
The effect of TES on cell viability following UVB
irradiation was tested on HaCaT cells. Cell viability was evaluated
using an MTS assay (Fig. 2). When
the cultures were incubated after UVB irradiation, UVB-induced
toxicity increased compared to that in non-irradiated cells. Cell
viability declined, depending on the dose of UVB irradiation, and
sharply reduced at 24 h after UVB irradiation of 150
mJ/cm2. Accordingly, we selected an exposure dose of 100
mJ/cm2 for studying cellular toxicity in HaCaT cells
treated with TES 24 h after UVB irradiation (Fig. 3).
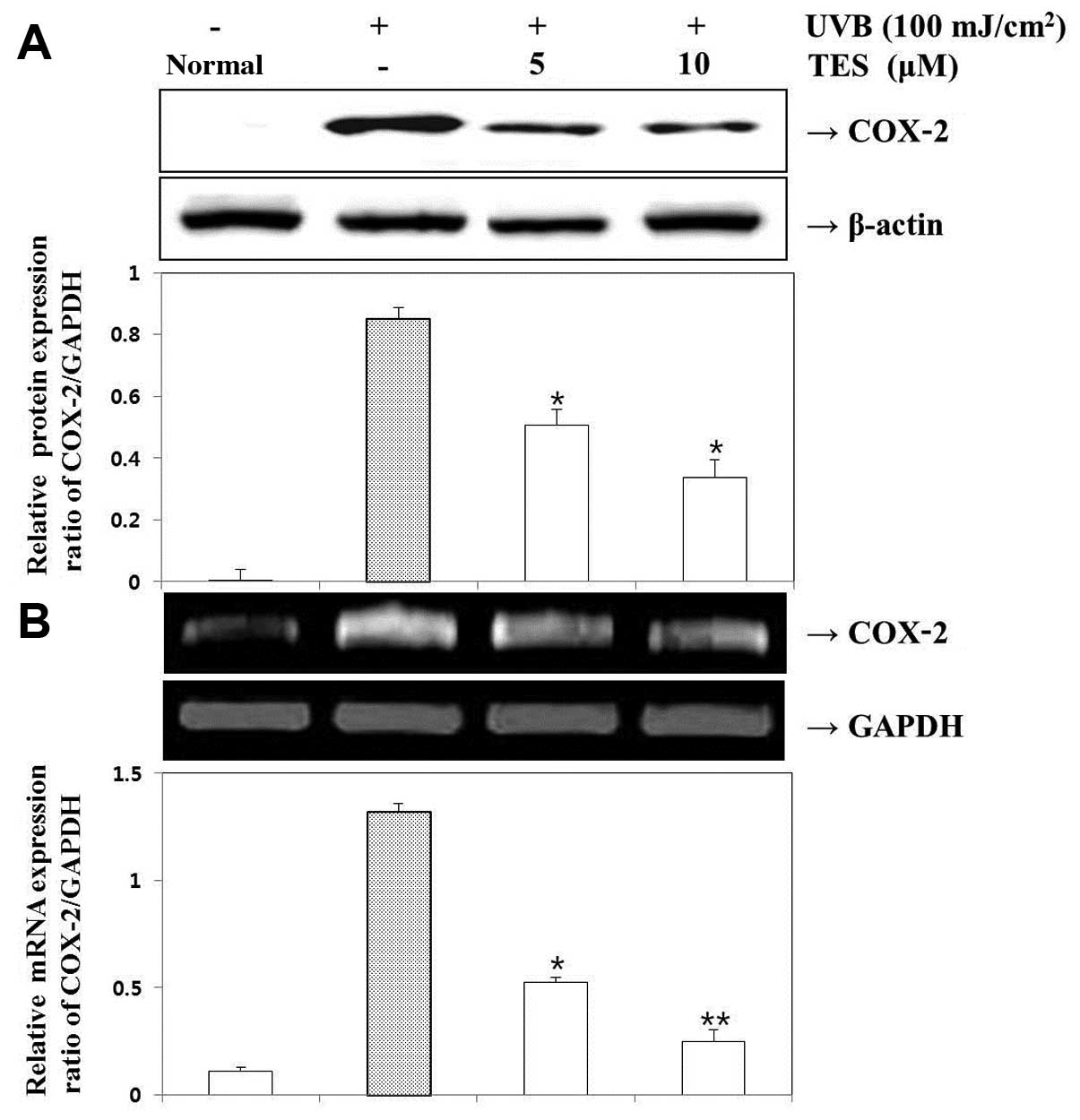
Effect of TES on UVB-induced COX-2 mRNA
expression
We next examined the effects of TES on COX-2
expression in UVB-irradiated HaCaT cells. The expression levels of
COX-2 protein and COX-2 mRNA were measured in HaCaT cells exposed
to UVB (100 mJ/cm2) for 24 h. TES effectively suppressed
UVB-induced COX-2 expression. UVB (100 mJ/cm2) also
increased COX-2 mRNA expression, which was inhibited in the
presence of TES (Fig. 4). Hence,
TES suppressed the expression of genes that are implicated in the
pathogenesis of inflammatory responses.
Effect of TES on IL-6 and IL-8 production
in UVB-irradiated cells
Since TES inhibited the production of
pro-inflammatory mediators in HaCaT cells, we further investigated
its effects on UVB-induced IL-6 and IL-8 production by performing
ELISA and RT-PCR. We found that, depending on its concentration,
TES inhibited UVB-stimulated IL-6 (Fig. 5) and IL-8 (Fig. 6) expression at both the protein
and mRNA levels.
Effect of TES on the phosphorylation of
MAPKs in UVB-induced HaCaT cells
MAPKs are essential for UVB-induced inflammation in
HaCaT cells; therefore, we evaluated the effects of TES on the
activation of MAPKs in these cells. TES markedly inhibited the
phosphorylation of JNK1/2 and ERK1/2 MAPK; these results indicate
that MAPK phosphorylation was inhibited by TES pretreatment
(Fig. 7).
Discussion
I. dentata is a perennial medicinal herb
indigenous to Korea. It was reported that intraperitoneal
administration of the herb extract resulted in decreased blood
glucose in alloxan diabetic mice (25) and prevented neurodegenerative
diseases (19,26). Young I. dentata sprouts are
commonly used as a bitter appetizing in Korea. Chemical components
including triterpenes, sesquiterpene glycosides, and flavonoids
have been isolated from the genus Ixeris, which comprises
approximately 20 species (27).
I. dentata is characterized by the presence
of guaiane sesquiterpene lactones, which are chemosystematic
markers. As a continuation of our effort to purify minor
sesquiterpenes from I. dentata amino acid-sesquiterpene
lactones, ixerisamine A and ixerisamine B were isolated together
with 12 related sesquiterpene lactones (8-epi-desacylcynaropicrin
glucoside; ixerisoside A; ixerisoside A 6′-O acetate; ixerin N;
ixerin N 6′-O acetate; ixerin M; TES; 4,8-epiisolipidiol;
8-epi-isolipidiol; 11βH-11,13-dihydrointegrifolin;
8β-hydroxy-4β,15-dihydrozaluzanin C and integrifolin) (24).
The isolation and structure determination of
compounds, as well as the inhibitory effects of isolated
sesquiterpenes on the proliferation of 4 cultured human tumor cell
lines: MES-SA (human uterine carcinoma cell line), MES-SA/DX5
(multidrug-resistant subline of MES-SA), HCT-15 (human colorectal
adenocarcinoma cell line), and HCT-15/CL02 (multidrug-resistant
subline of HCT-15) were evaluated (24).
UVB irradiation induces skin damage and inflammation
by causing the secretion of various cytokines, which are immune
regulators produced by cells. To prevent the initiation of skin
inflammation, keratinocytes that have been irreversibly damaged by
UVB must be removed through apoptosis. UVB crosses the epidermis
and reaches the upper dermis. Compared with UVA, it is more active
in terms of causing cutaneous carcinogenesis and alterations of the
cell-cycle-control signaling pathways (5).
Keratinocytes are the major target of UVB and play a
central role in inflammatory and immune modulatory changes observed
after UV exposure, at least partly through the UV-induced release
of cytokines (IL-1, IL-6, IL-8, IL-10, GM-CSF and TNF-α) (28) and cyclooxygenase products (PGE2)
(29).
Inappropriate expression and/or activity of COX-2, a
rate-limiting enzyme involved in the biosynthesis of
prostaglandins, has been implicated in UVB-induced skin
carcinogenesis (30). MAPKs are a
family of proline-directed Ser/Thr kinases comprising ERK, JNK and
p38 MAPK. Recent studies have shown that activation of ERK, JNK and
p38 MAPK is strongly correlated with acute inflammation and
development of skin cancer through increased expression of COX-2
(31–33).
In the present study, TES was isolated from I.
dentata. We examined the effect of TES on UVB-induced
pro-inflammatory cytokine production in HaCaT cells by evaluating
cells that were stimulated with UVB in the presence or absence of
TES. Pro-inflammatory cytokine production was measured by ELISA and
RT-PCR, and the activation of MAPKs was determined by western blot
analysis.
In particular, we investigated whether TES inhibits
the UVB-induced production of IL-6 and IL-8 by inhibiting the
expression of MAPK and COX-2 at the protein and mRNA levels. We
found that the inhibitory effects of TES on the production of
inflammatory mediators were accompanied by concentration-dependent
decreases in the protein and mRNA expression levels of IL-6, IL-8
and COX-2. These data demonstrate that IL-6, IL-8 and COX-2
expression in HaCaT cells is suppressed by TES; UVB-induced
phosphorylation of JNK1/2 and ERK1/2 in HaCaT cells. Therefore, it
is likely that TES acts as an antiphotoinflammatory agent by mainly
inhibiting COX-2 expression in vivo.
Inhibition of COX-2 expression has been shown to be
an important anti-inflammatory mechanism of some compounds,
including epigallocatechin gallate (34), resveratrol (35) and curcumin (36) similar to TES. Furthermore, these
effects are mediated by the inhibition of COX-2 expression and
JNK1/2 and ERK1/2 phosphorylation. In practice, the whole I.
dentata plant, a typical Oriental herb, has been used for the
treatment of indigestion, pneumonia, diabetes, hepatitis,
contusions and tumors (16). It
has also been used in Korean folk medicine for the treatment of
inflammatory diseases; therefore, this represents a potent
anti-inflammatory effect of TES accomplished by blocking
inflammatory mediators. Our data suggest that TES represents a new
source of potential drugs for the treatment of inflammatory
diseases.
In conclusion, we evaluated the effect of TES on
skin inflammation in vitro and found that TES has potential
to attenuate UVB-induced skin inflammation by suppressing MAPK
activation. Our findings provide new insight into the application
of TES as well as its nutraceutical value. Further studies on TES
are required to confirm its medicinal use.
Acknowledgements
This study was supported by Wonkwang
University in 2011.
References
|
1
|
Murphy GF: Structure, function and
reaction patterns. Dermatopathology. W.B. Saunders; Philadelphia,
PA: pp. 251995
|
|
2
|
Yager J: The skin as an immune organ.
Advances in Veterinary Dermatology. Ihrke PJ, Mason IS and White
SD: Pergamon Press; Oxford: pp. 311993
|
|
3
|
de Gruijl FR, Sterenborg HJ, Forbes PD,
Davies RE, Cole C, Kelfkens G, van Weelden H, Slaper H and van der
Leun JC: Wavelength dependence of skin cancer induction by
ultraviolet irradiation of albino hairless mice. Cancer Res.
53:53–60. 1993.PubMed/NCBI
|
|
4
|
van der Leun J and de Gruijl F: Influence
of ozone depletion on human and animal health. UV-B Radiation and
Ozone Depletion: Effects on Humans, Animals, Plants, Microorganisms
and Materials. Tevini M: Lewis Publishers; Boca Raton, FL: pp.
95–123. 1993
|
|
5
|
Rosette C and Karin M: Ultraviolet light
and osmotic stress: activation of the JNK cascade through multiple
growth factor and cytokine receptors. Science. 274:1194–1197. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Afaq F, Adhami VM and Mukhtar H:
Photochemoprevention of ultraviolet B signaling and
photocarcinogenesis. Mutat Res. 571:153–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yaar M and Gilchrest BA: Photoageing:
mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malorni W, Donelli G, Straface E, Santini
MT, Paradisi S and Giacomoni PU: Both UVA and UVB induce
cytoskeleton-dependent surface blebbing in epidermoid cells. J
Photochem Photobiol B. 26:265–270. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zamansky GB, Perrino BA and Chou IN:
Disruption of cytoplasmic microtubules by ultraviolet radiation.
Exp Cell Res. 195:269–273. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zamansky GB and Chou IN: Disruption of
keratin intermediate filaments by ultraviolet radiation in cultured
human keratinocytes. Photochem Photobiol. 52:903–906.
1990.PubMed/NCBI
|
|
11
|
Zamansky GB and Chou IN: Environmental
wavelengths of ultraviolet light induce cytoskeletal damage. J
Invest Dermatol. 89:603–606. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moll I, Bohnert E, Treib U and Jung EG:
Effects of ultraviolet B radiation on cytoskeletal and adhesion
molecules in human epidermis. Photodermatol Photoimmunol Photomed.
10:26–32. 1994.PubMed/NCBI
|
|
13
|
Godar DE: Preprogrammed and programmed
cell death mechanisms of apoptosis: UV-induced immediate and
delayed apoptosis. Photochem Photobiol. 63:825–830. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shindo Y and Hashimoto T: Ultraviolet
B-induced cell death in four cutaneous cell lines exhibiting
different enzymatic antioxidant defences: involvement of apoptosis.
J Dermatol Sci. 17:140–150. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SH: Inhibitory effects of Ixeris
dentata on the mutagenicity of aflatoxin B1,
N-methyl-N’-nitro-N-nitrosoguanidine and the growth of MG-63 human
osteosarcoma cells. J Korean Soc Food Sci Nutr. 24:305–312.
1995.
|
|
16
|
Kim M and Lee M: Volatile flavor
components of Ixeris dentata and Amaranthus
mangostanus. Han’guk Nonghwa Hakhoe chi. 31:394–399. 1988.(In
Korean).
|
|
17
|
Lee E: Effects of Ixeris dentata
ext. on lowering lipid and antioxidation. Korean J Plant Res.
24:55–60. 2011.(In Korean).
|
|
18
|
Park EK, Sung JH, Trinh HT, Bae EA, Yun
HK, Hong SS and Kim DH: Lactic acid bacterial fermentation
increases the antiallergic effects of Ixeris dentata. J
Microbiol Biotechnol. 18:308–313. 2008.PubMed/NCBI
|
|
19
|
Chung HS: Inhibition of monamine oxidase
by a flavone and its glycoside from Ixeris dentata Nakai.
Nutraceut Food. 8:141–144. 2003. View Article : Google Scholar
|
|
20
|
Chung HS, Jeong HJ, Han MJ, Park ST, Seong
KK, Baek SH, Jeong DM, Kim MJ and Kim HM: Nitric oxide and tumor
necrosis factor-alpha production by Ixeris dentata in mouse
peritoneal macrophages. J Ethnopharmacol. 82:217–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SB, Kang OH, Keum JH, Mun SH, An HJ,
Jung HJ, Hong SH, Jeong DM, Kweon KT and Kwon DY: Anti-inflammatory
effect of Ixeris dentata on ultraviolet B-induced HaCaT
keratinocytes. Nat Prod Sci. 18:60–66. 2012.(In Korean).
|
|
22
|
Kim MJ, Kim JS, Jeong DM, Han SS and Yu
CY: Effect of antioxidant, antimutagenicity and anticancer of root
extract from Ixeris dentata Nakai. Korean J Med Crop Sci.
10:222–229. 2002.
|
|
23
|
Kim JH, Lim HS, Ha H, Seo CS and Shin HK:
Inulae flos and its compounds inhibit TNF-α- and IFN-γ-induced
chemokine production in HaCaT human keratinocytes. Evid Based
Complement Alternat Med. 2012:2803512012.PubMed/NCBI
|
|
24
|
Cha MR, Choi YH, Choi CW, Yoo DS, Kim YS,
Choi SU, Kim YH and Ryu SY: New guaiane sesquiterpene lactones from
Ixeris dentata. Planta Med. 77:380–382. 2011. View Article : Google Scholar
|
|
25
|
Choi JS, Young HS and Kim BW: Hypoglycemic
and hypolipemic effects of Ixeris dentata in diabetic rats.
Arch Pharm Res. 13:269–273. 1990. View Article : Google Scholar
|
|
26
|
Oh SH, Sung TH and Kim MR: Ixeris
dentata extract maintains glutathione concentrations in mouse
brain tissue under oxidative stress induced by kainic acid. J Med
Food. 6:353–358. 2003. View Article : Google Scholar
|
|
27
|
Zidorn C: Sesquiterpene lactones and their
precursors as chemosystematic markers in the tribe Cichorieae of
the Asteraceae. Phytochemistry. 69:2270–2296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takashima A and Bergstresser PR: Impact of
UVB radiation on the epidermal cytokine network. Photochem
Photobiol. 63:397–400. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grewe M, Trefzer U, Ballhorn A, Gyufko K,
Henninger H and Krutmann J: Analysis of the mechanism of
ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by
human epidermoid carcinoma cells. J Invest Dermatol. 101:528–531.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An KP, Athar M, Tang X, Katiyar SK, Russo
J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH Jr, Mukhtar H
and Bickers DR: Cyclooxygenase-2 expression in murine and human
nonmelanoma skin cancers: implications for therapeutic approaches.
Photochem Photobiol. 76:73–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Tang Q, Gonzales MS and Bowden GT:
Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced
cyclooxygenase-2 gene expression in human keratinocytes. Oncogene.
20:3921–3926. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin SK, Kok SH, Yeh FTC, Kuo MYP, Lin CC,
Wang CC, Goldring SR and Hong CY: MEK/ERK and signal transducer and
activator of transcription signaling pathways modulate oncostatin
M-stimulated CCL2 expression in human osteoblasts through a common
transcription factor. Arthritis Rheum. 50:785–793. 2004. View Article : Google Scholar
|
|
33
|
Mahns A, Wolber R, Stäb F, Klotz LO and
Sies H: Contribution of UVB and UVA to UV-dependent stimulation of
cyclooxygenase-2 expression in artificial epidermis. Photochem
Photobiol Sci. 3:257–262. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vayalil PK, Elmets CA and Katiyar SK:
Treatment of green tea polyphenols in hydrophilic cream prevents
UVB-induced oxidation of lipids and proteins, depletion of
antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1
hairless mouse skin. Carcinogenesis. 24:927–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Afaq F, Adhami VM and Ahmad N: Prevention
of short-term ultraviolet B radiation-mediated damages by
resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol.
186:28–37. 2003.PubMed/NCBI
|
|
36
|
Cho JW, Lee KS and Kim CW: Curcumin
attenuates the expression of IL-1β, IL-6, and TNF-α as well as
cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential
upstream targets. Int J Mol Med. 19:469–474. 2007.
|