Introduction
As an opportunistic human pathogen,
Staphylococcus aureus (S. aureus) has become the main
cause of nosocomial infections and is one of the major pathogens in
osteomyelitis and implant-related infections (1). Novel approaches to the management
and further understanding of the pathophysiology of bone infection
are urgently required. Evidence demonstrates that bacterially
infected osteoblasts (OBs) secrete chemokines, cytokines and
antimicrobial peptides, suggesting that these cells may contribute
to appropriate host responses or progressive inflammatory damage in
combating localized infection (2–4).
Clinical antimicrobial prophylaxis, including the systemic and
local use of antibiotics, has proven to be valuable in the
prevention of infection in clinical practice (5). However, the misuse of antibiotics
has led to the evolution and rapid spread of clinical bacterial
strains resistant to one or several conventional antimicrobial
agents (5,6). As a result, an increasing number of
infections involving methicillin-resistant S. aureus (MRSA),
even ‘Superbugs’, have emerged (1,7).
Presently, the global spread of MRSA is a matter of great concern
in the treatment of staphylococcal infection, since it has rapidly
acquired resistance to all clinical antibacterial agents (8). In light of this situation,
antimicrobial peptides are attractive candidates as therapeutic
agents for bacterial infections due to their selectivity, speed of
action, relative difficulty in the selection of resistant mutants,
and inherent immunological compatibility (9).
A major family of antimicrobial proteins in mammals
comprises the β-defensins. β-defensins are small (2–6 kDa),
cationic proteins which exhibit potent antimicrobial activity at
micro- to nanomolar concentrations (10). Previous studies have shown that
β-defensins activate the innate immune system within bone and may
potentially be used for reducing the rate of peri-implant
infections (4). Human β-defensin
(HBD)-3 is characterized by its strong, broad-spectrum, and
salt-insensitive antibacterial activity against several bacteria,
including multi-resistant strains, such as MRSA and
vancomycin-resistant Enterococcus faecium (11). Previously, we demonstrated the
bactericidal effect of HBD-3 and its role in inhibiting the
formation of MRSA (ATCC 43300) biofilms (12). In a recent study, murine homologs
of HBDs, termed mouse β-defensins (MBDs) were isolated and
characterized. To date, more than 10 different mouse β-defensins
have been isolated (13). A BLAST
search of the mouse protein database with the amino acid sequence
of HBD-3 revealed great similarity to mouse β-defensin-14 (MBD-14).
Futhermore, recombinant MBD-14, similar to HBD-3, has also
exhibited similar broad-spectrum, nanomolar microbicidal activity
against a variety of microorganisms, including Gram-positive and
-negative bacteria and the yeast, Candida albicans. These
data suggest that MBD-14 is the structural and functional ortholog
of HBD-3 (14,15). Therefore, it may prove useful to
investigate the expression and regulation of MBD-14 in vitro
based on its homology to the human ortholog, HBD-3.
Several studies have reported that the expression of
MBD-14 can be induced in epithelial tissue following mechanically-
and metabolically-induced skin barrier disruption under
inflammatory conditions (16).
The p38 mitogen-activated protein kinases (p38 MAPK) and
extracellular signal-regulated kinase (ERK) signaling pathways have
been shown to regulate human β-defensin 2 mRNA expression in middle
ear epithelial cells by inflammatory stimuli (17). These data suggest that pleural
mesothelial cells contribute to host innate immune defense upon
exposure to Gram-positive bacteria or their products within the
pleural space, by increasing p38 MAPK activity and upregulating
MBD-2 expression (18). In
addition, Varoga et al demonstrated the effective induction
of HBD-3 expression via Toll-like receptor (TLR)-2 and -4 in OBs
incubated with bacterial conditioned medium using enzyme-linked
immunosorbent assay (ELISA) and real-time polymerase chain reaction
(PCR) (19). However, the
molecular mechanisms underlying inflammatory mediator-induced
MBD-14 expresssion in OBs remain unknown. Furthermore, it is
crucial to provide a theoretical basis for the use of MDB-14 as a
therapeutic agent in the treatment of intramedullary infection with
MRSA in vivo. Lipopolysaccharide (LPS), which is a major
component of the outer surface of Gram-negative bacteria,
stimulates murine OBs to induce pro-inflammatory cytokines,
including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α),
through the activation of the nuclear factor-κB (NF-κB) pathway
(20). Therefore, LPS is suitable
as a positive control for inflammatory response to evaluate whether
the model of acute inflammation and signaling pathways for OBs have
been established. In this study, we confirmed the effects of MRSA
supernatant (SAS) on the release of MBD-14 in mouse OBs, and
targeted signaling pathways to elucidate the molecular mechanisms
underlying the effects of inflammatory stimuli.
Materials and methods
Bacteria and drugs
ATCC 43300 (MRSA) was purchased in a freeze-dried
form from the American Type Culture Collection (ATCC; Rockefeller,
MD, USA). Species identification and susceptibility testing was
performed with the Vitek 2 automated system (Bio Mérieux, Marcy
I’Étoile, France). The phenotypic classification of the ATCC 43300
strain was further confirmed by methicillin-resistant determinant A
(MecA) expression, which encodes PBP2a (penicillin-binding protein
2a) and mediates methicillin resistance (21). Recombinant MBD-14 was commercially
available and purchased from EIAab Science Co., Ltd. (Wuhan,
China). Pyrrolidine dithiocarbamate (PDTC), SB203580, antibodies
against p-ERK, p-p38 MAPK, NF-κB p65, NF-κB p-P65, p-IκBα and GAPDH
were purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA).
Cultivation of mouse OBs
The murine osteoblastic cell line, MC3T3-E1, is a
non-transformed cell line established from newborn mouse calvaria.
These cells exhibit the osteoblastic phenotype, as evidenced by the
expression of ALPase (22),
synthesis of the extracellular matrix (ECM) components, such as
osteocalcin and type-1 collagen, and their ability to mineralize
the ECM (22). MC3T3-E1 cells
(ATCC, Manassas, VA, USA) were cultured in DMEM (Gibco, Grand
Island, NY, USA), supplemented with 10% heat-inactivated fetal
bovine serum, 2 mM glutamine, 100 U/ml penicillin and 100
μg/ml streptomycin, in a humidified atmosphere of 5%
CO2 in air at 37°C. Cells were trypsinized and
cultivated for an additional 2 days before use at an appropriate
density. When the mono-layers reached approximately 85% confluence,
stimulation experiments were performed in serum-free DMEM
containing ascorbic acid in a humidified atmosphere with 5%
CO2 at 37°C as previously described (23).
Stimulants
The production of bacterial culture supernatants was
carried out as previously described by Gläser et al
(24). In this method, ATCC 43300
bacteria were grown in BBL Trypticase Soy Broth (TSB; BD
Biosciences, Franklin Lakes, NJ, USA) under shaking conditions at
37°C until they reached an optical density of 1.0; 1 ml of this
culture was then added to 9 ml TSB and the resulting culture was
incubated for 24 h in 50-ml centrifuge tubes (Corning Inc., NY,
USA) without shaking. Subsequently, the bacteria were centrifuged
at 5,000 × g for 15 min and then cell-free conditioned medium was
prepared by filtering the supernatant through a 0.22-μm
pore-size filter (Tullagreen, Carrigtwohill, County Cork, Ireland)
(25). The resulting supernatants
were used for stimulation experiments. Stimulation experiments with
TSB medium were performed to analyze the potentially unexpected
effects of the medium. The concentration of SAS was determined by
the number of S. aureus bacteria in the resulting culture
incubated for 24 h as previously described (19). The number of colony-forming units
(CFUs) was counted and expressed relative to the amount of TSB for
planktonic bacteria in 50 ml centrifuge tubes (CFUs/ml). Mouse OBs
in the monolayer culture were exposed to various concentrations of
SAS (diluted 1:10, 1:20, 1:40 and 1:80, i.e., the number of OBs vs.
the concentration of bacterial exoproducts from the number of
Staphylococcus aureus).
Cell viability assay
A Cell Counting kit-8 (Beyotime, Shanghai, China)
was used to examine cell viability according to the manufacturer’s
instructions. MC3T3-E1 cells were seeded at 1×104
cells/well in 96-well culture plates (Corning Inc.) and were then
treated with SAS at various concentrations at 37°C in a humidified
incubator containing 95% air and 5% CO2. After 12, 24,
48 and 72 h, the 10 μl CCK-8 solution was added to each well
and incubated for 1 h in an incubator. The absorbance was measured
using a Synergy HT multidetection microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm as
previously described (26,27).
Real-time PCR
At various time points, the cells treated with SAS
at various concentrations were harvested and total RNA was isolated
using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to
the manufacturer’s instructions with an additional DNase I
(Fermentas)-treatment step to eliminate residual genomic DNA. Total
RNA (1 μg) was reverse transcribed using a Fermentas
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Real-time PCR was performed on
an ABI 7500 Fast machine (Applied Biosystems, Courtaboeuf, France)
using Roche FastStart Universal SYBR-Green Master (Rox) (Roche
Applied Science, Indianapolis, IN, USA). The reactions were
performed using cDNA templates and specific forward and reverse
primers (Table I). Refer to
Table I for the detailed
annealing temperature which was used according to the different
primers. The specificity of the amplification reaction was
determined by analyzing the melting curves. The relative amount of
target gene expression was normalized to β-actin as an internal
control. The quantification of gene expression was based on the
cycle threshold value of each sample, which was calculated as the
average of 3 replicate measurements for each sample analyzed as
previously described (12,28).
 | Table INucleotide sequences for the primers
used in this study. |
Table I
Nucleotide sequences for the primers
used in this study.
| Target gene | Direction | Primer sequence
(5′-3′) | Annealing
temperature (°C) | Size (bp) |
|---|
| p38 MAPK | F |
CGTGTGGCAGTTAAGAAGC | 57 | 214 |
| R |
GGCACTTCACGATGTTGTT | | |
| ERK | F |
TGCTGAACACCACTTGTGA | 57 | 219 |
| R |
GGAAGATAGGCCTGTTGGA | | |
| IκBα | F |
GCAATCATCCACGAAGAGA | 57.5 | 168 |
| R |
ATCACAGCCAGCTTTCAGA | | |
| NF-κB p65 | F |
CCCACCATCAAGATCAATG | 56.5 | 160 |
| R |
TATGGATACTGCGGTCTGG | | |
| β-actin | F |
AATGGGTCAGAAGGACTCCT | 56 | 250 |
| R |
ACGGTTGGCCTTAGGGTTCAG | | |
ELISA
The collected supernatants from the different
stimulation experiments were examined by MBD-14, DEFb14 ELISA kit
(EIAab Science Co., Ltd.) according to the manufacturer’s
instructions. ELISA was performed on each sample in trip-licate.
The optical density of each sample was measured with a Synergy HT
multidetection microplate reader (BioTek Instruments) at a
wavelength of 450 nm. The examination of TNF-α and IL-6 levels was
performed according to the standard protocols of the ELISA kit
(EIAab Science Co., Ltd.).
Western blot analysis
Cells in a 6-well plate were harvested under
different conditions and washed with PBS 3 times. Protein was
extracted using the Total protein Extraction kit according to the
manufacturer’s instructions (ProMab Biotechnologies, Inc.,
Richmond, CA, USA). Proteins were quantified using the BCA protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Samples
containing equal amounts of protein (40 μg/lane) were run on
12% polyacrylamide gels, transferred onto nitrocellulose membranes
(Whatman, Dassel, Germany) and blocked for 1 h with 5% non-fat milk
in TBST (Tris-buffered solution containing 0.1% Tween-20) at room
temperature. The membranes were then soaked with primary antibodies
overnight at 4°C followed by secondary antibody incubation for 1 h
at room temperature. Finally, the membranes were reacted with
enhanced chemiluminescence (ECL) reagents and band densities were
determined using Gel Pro 4.0 analyzer software (Media Cybernetics
Inc., Bethesda, MD, USA) and the integrated optical density (IOD)
was calculated as previously described (29).
Statistical analysis
The results are expressed as the means ± SEM derived
from at least 3 independent experiments. Statistical analysis
between groups was performed by one-way ANOVA. The level of
p<0.05 was considered to indicate a statistically significant
difference. SPSS software (version 19.0; IBM, Chicago, IL, USA) was
used for statistical analysis.
Results
Phenotype classification, minimum
inhibitory concentration (MIC) and bacterial count
ATCC 43300 exhibited a low level of susceptibility
to penicillin (MIC, 64 μg/ml) and recombinant MBD-14
exhibited a strong antimicrobial activity against ATCC 43300 (MIC,
8 μg/ml). According to a low level susceptibility to
penicillin and MecA gene expression, ATCC 43300 was the
methicillin-resistant strain (21). In addition, the amount of bacteria
in the resulting culture was calculated as approximately
3.5±0.6×109 CFUs/ml using the spread plate method.
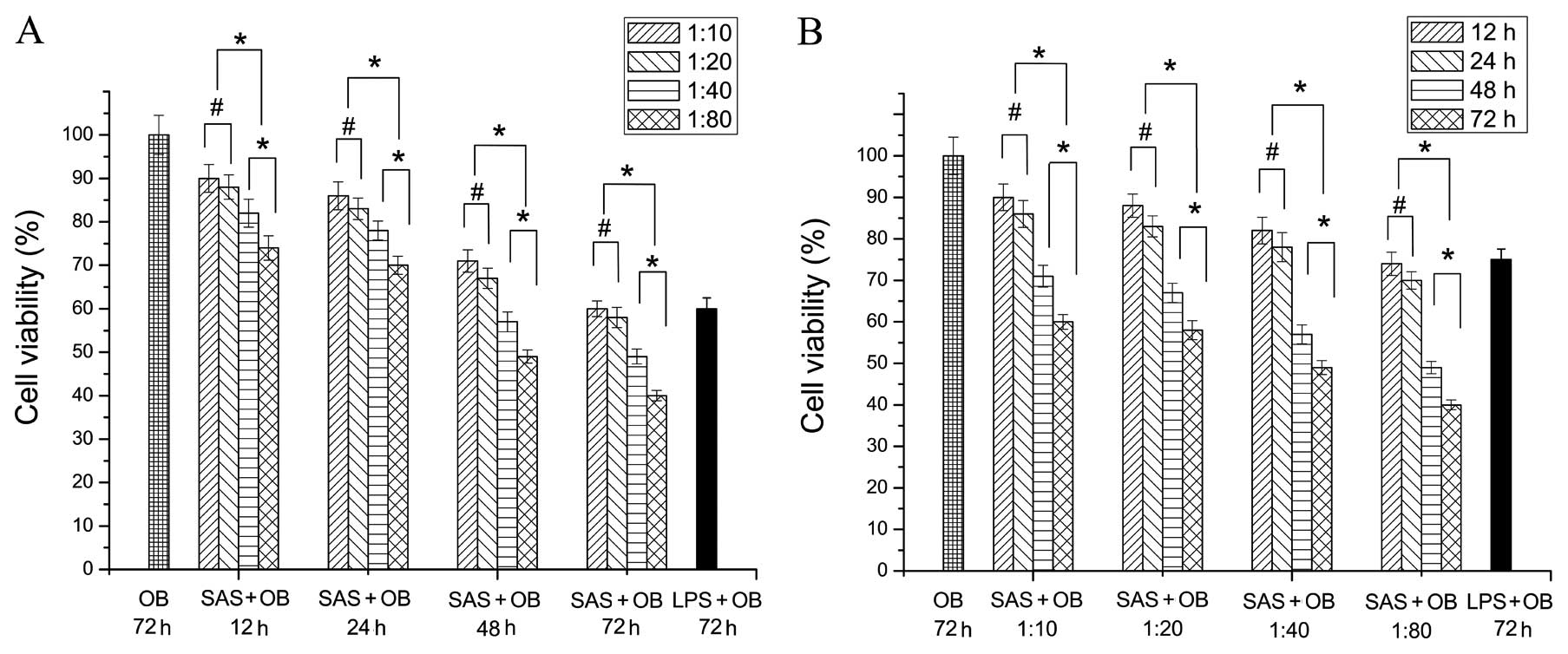
SAS inhibits the proliferation of primary
mouse OBs
In order to determine the optimal proportion and
action time of the mixed agent which could contribute to the
inflammatory reaction with low cytotoxic response, a cytotoxicity
assay was conducted to investigate the effects of culture with
various concentrations of SAS on OBs at different time points (12,
24, 48 and 72 h). We first evaluated the effect of various
concentrations of SAS on cell viability. OBs were treated with SAS
for 12, 24, 48 and 72 h, at the following concentrations: diluted
at 1:10, 1:20, 1:40 and 1:80. Throughout the incubation period,
cell proliferation was analyzed using a CCK-8 kit. At 72 h,
treatment with 10 μg/ml LPS was used as the positive control
to evaluate whether the cell proliferation was already affected.
All investigated treatment ratios from 1:20 to 1:80 induced less OB
proliferation compared to the untreated group (OB group), as shown
in Fig. 1 (p<0.05). In the OB
group in which OBs without SAS stimulation were cultured for 72 h,
the proliferation of cells measured by CCK-8 assay was highest and
normalized to 100% (negative control). At 12 h, the proliferation
of cells was the highest at the 1:10 treatment ratio, followed by
the 1:20 treatment ratio. However, no significant difference was
observed (p>0.05). A lower cell number was consistently observed
in the groups treated with higher concentrations than those treated
with the concentration of 1:10 and 1:20 (p<0.05). Moreover, the
lowest cell numbers were observed in the group treated with the
concentration of 1:80 (p<0.05), and the same findings were
observed at 24, 48 and 72 h (Fig.
1A). When the cells were cultured with the same concentrations
of bacterium-free supernatant for different periods of time, the
levels of cell proliferation positively correlated with the
duration (Fig. 1B). The
proliferation of cells was the highest at 12 h, followed by 24 h.
However, no significant difference was observed (p>0.05).
Furthermore, the lowest number of OBs was observed in the group
treated for 72 h, followed by the group treated for 48 h. The
difference between these 2 groups was significant. The above
findings regarding the different treatment times were observed at
different concentrations (Fig.
1B).
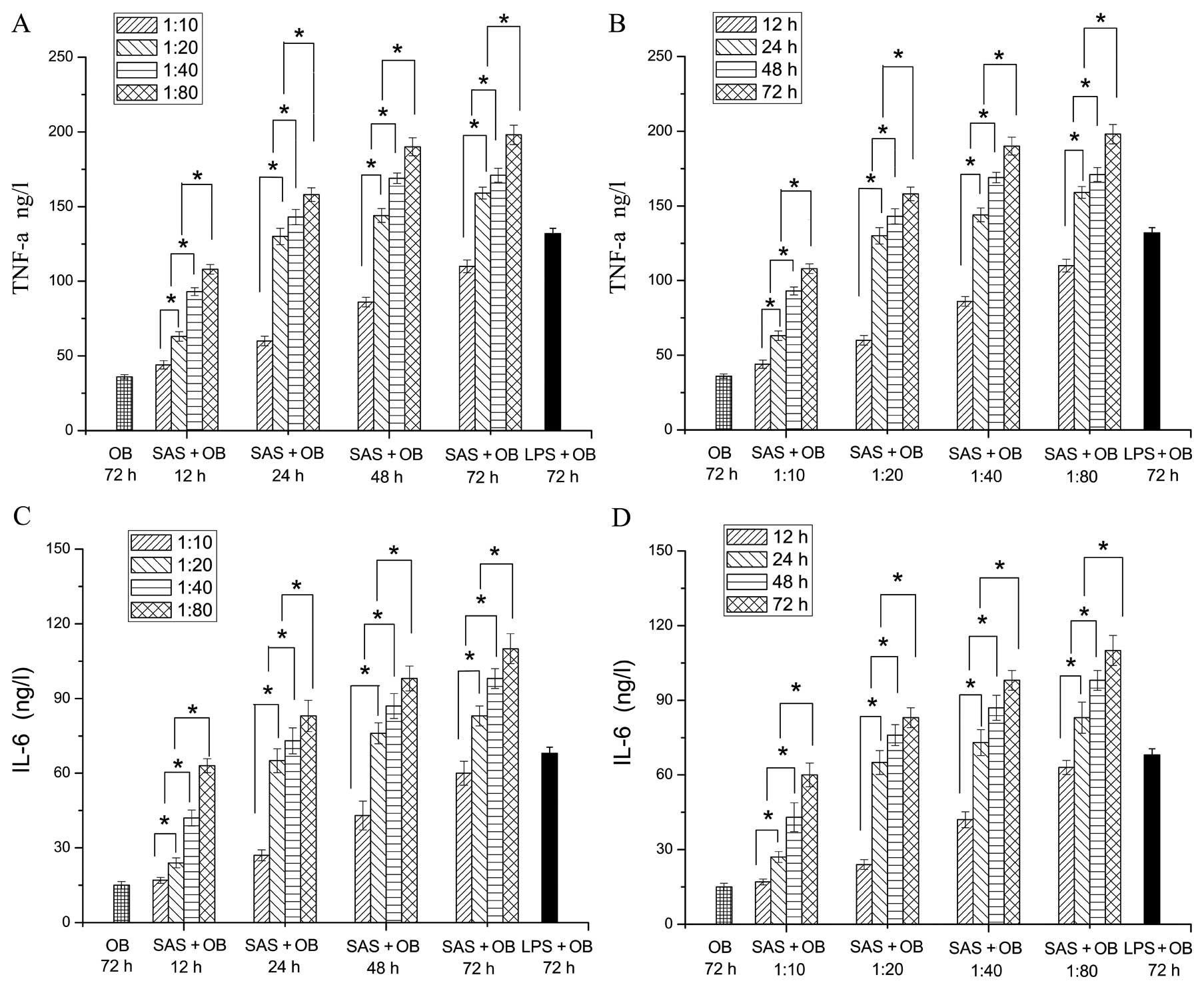
Release of the pro-inflammatory
cytokines, TNF-α and IL-6, in infected OBs
Subsequently, the level of the pro-inflammatory
cytokines, TNF-α and IL-6, was assessed by ELISA at different time
points (12, 24, 48 and 72 h) when the cells were incubated with
various concentrations of SAS. At 72 h, treatment with 10
μg/ml LPS was used as the positive control to evaluate
whether the model of acute inflammation for OBs has been
established. When the cells were cultured with different doses of
bacterium-free supernatant, the levels of TNF-α and IL-6 positively
correlated with the concentrations of bacterium-free supernatant
and the duration. The levels of TNF-α in the SAS + OB and LPS + OB
groups treated with different concentrations for different periods
of time were significantly higher than those of the OB group at 72
h (negative control). As shown in Fig. 2A, the release of TNF-α with SAS
stimulation was the lowest at the concentration of 1:10 at 12 h,
followed by the 1:20, 1:40 and 1:80 concentrations. Moreover, a
significant difference was observed between the 1:10 and 1:20
concentrations, and the highest level of inflammatory cytokines was
observed at the concentration of 1:80. At 24, 48 and 72 h, the
levels of TNF-α showed a similar tendency to those of TNF-α at 12 h
(Fig. 2A). Obviously, at the
concentration of 1:20, there was statistically significant
difference in the level of inflammatory cytokines between 12 and 24
h. In addition, no statistically significant differences were
observed between the group treated with the concentration of 1:20
at 24 h and the LPS + OB group (positive control) (Fig. 2B). The SAS + OB group treated at
the concentration of 1:20, which increased the level of TNF-α at 24
h showed similar abilities compared with the LPS + OB group,
suggesting an effective stimulation of the OBs. The variation trend
of IL-6 showed a similarity with that of TNF-α (Fig. 2C and D). In conclusion, the
results of CCK-8 assay and ELISA for TNF-α and IL-6 showed that the
optimal ratio of the mixed agent was 1:20 and the optimal treatment
time was 24 h.
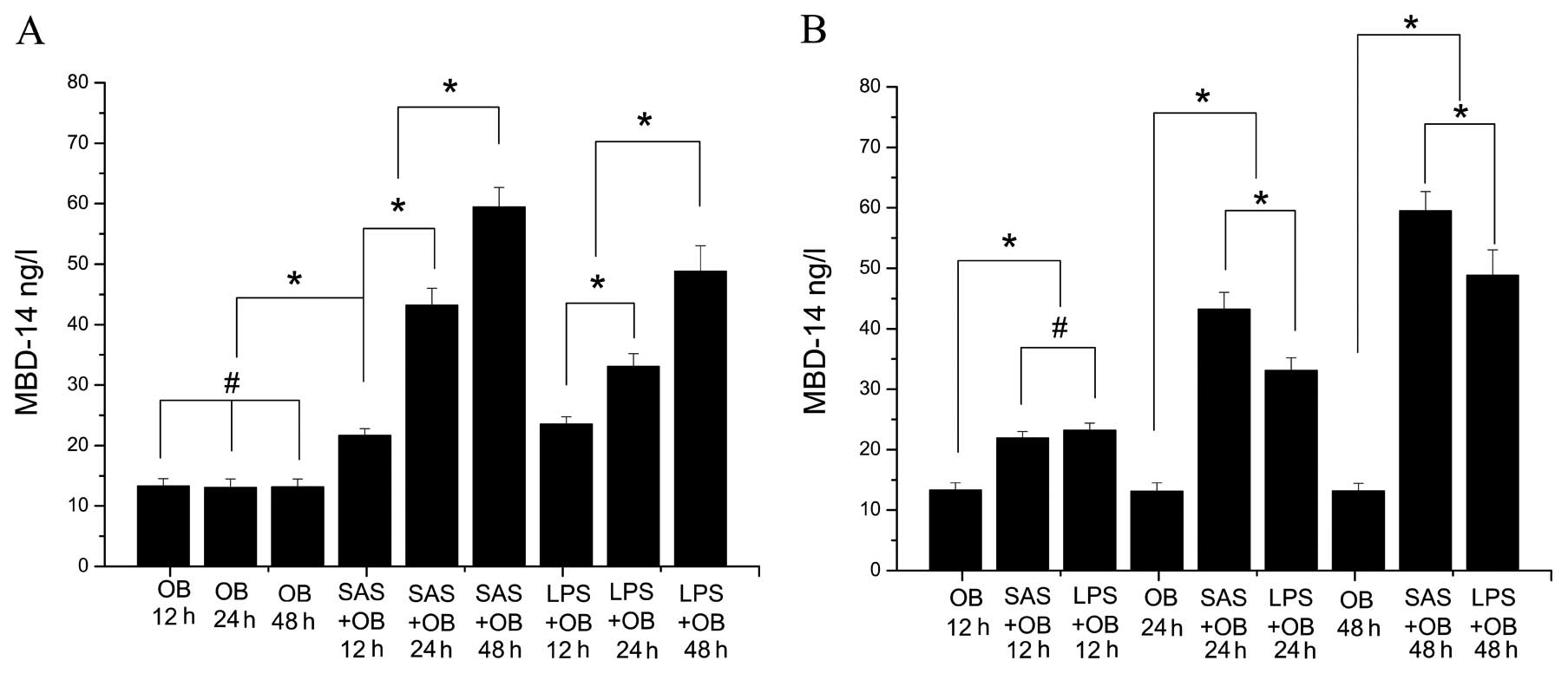
MBD-14 release is continuously induced in
SAS-challenged OBs
To confirm the constitutive expression of MBD-14 and
determine whether mouse OBs are a suitable model to examine the
induction of MBD-14 following exposure to bacterial supernatant and
LPS, the protein level of secreted MBD-14 in cell culture
supernatants was examined by ELISA. Cells stimulated with SAS
(1:20) were incubated for 12, 24 and 48 h and SAS significantly
increased the release of MBD-14 compared to the untreated controls
(OB group) (Fig. 3A) (p<0.05).
The level of MBD-14 at 12 h was the lowest at all time points
following stimulation. In addition, bacterial stimulation increased
the level of MBD-14 by up to 3-fold, similar to the OB group after
24 h (p<0.05). The SAS-induced MBD-14 secretion level peaked
within 48 h (approximately 60 ng/ml) (Fig. 3A) (p<0.05). Fig. 3B demonstrates the higher level of
MBD-14 induction following stimulation with SAS compared to
treatment with LPS at 24 and 48 h (p<0.05). This upregulation
was more pronounced when SAS was used appropriately as a
stimulus.
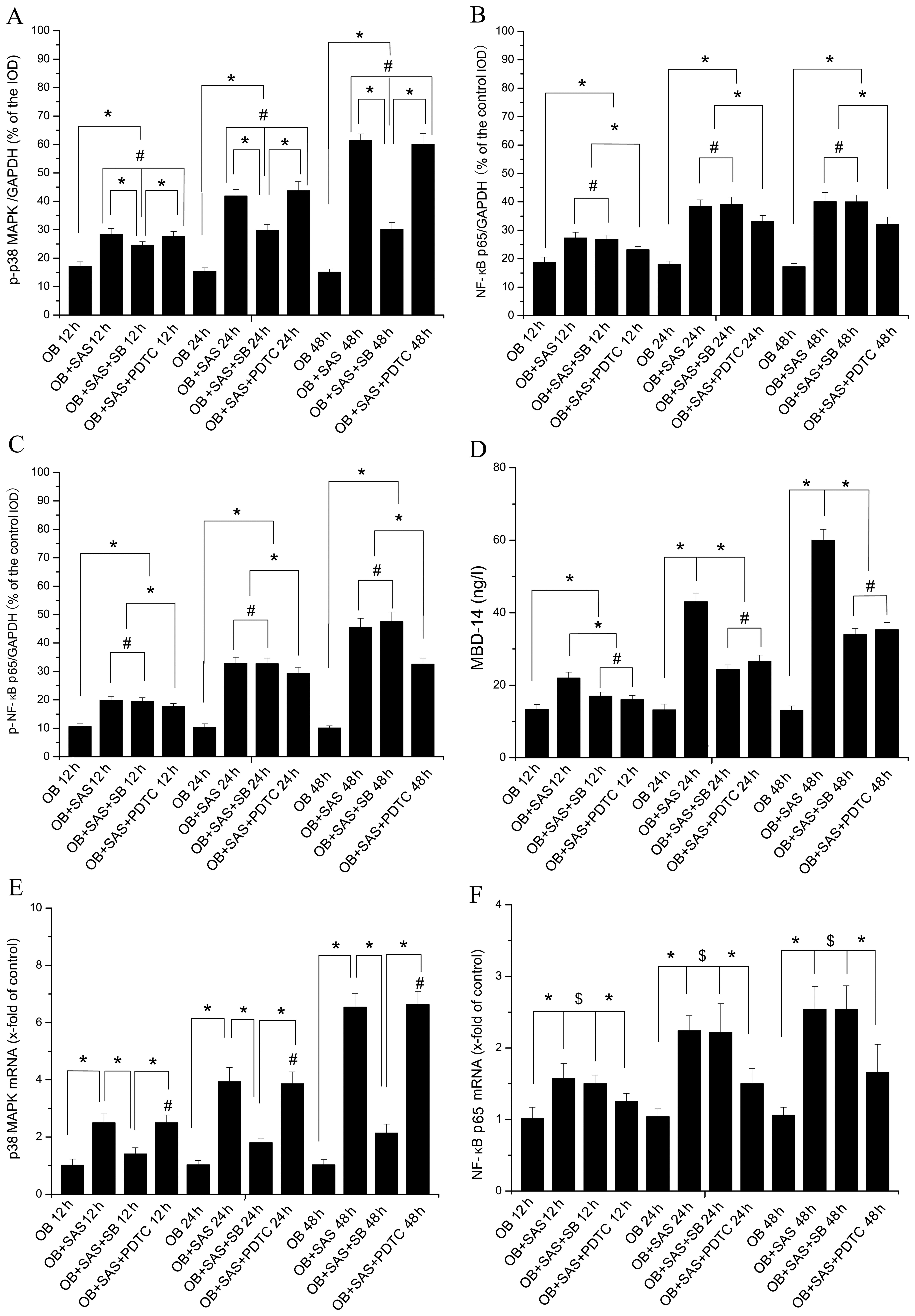
Role of p38 MAPK in the SAS-induced
release of MBD-14
The p38 and other intracellular MAPK pathways have
been implicated in the regulation of HBD-3 expression, in a number
of bacterial stimulation models in primary keratinocytes (25). To examine the overall level of p38
and ERK activation (phosphorylation) in OBs challenged with S.
aureus secreted bacterial exoproducts, the cells lysed at
various time points following exposure to SAS were used for the
detection of the phosphorylated form of p38 and ERK by immunoblot
analysis (Figs. 4 and 5A and B). Real-time PCR was used to
demonstrate the mRNA expression of p38 MAPK and ERK upon
stimulation with SAS (Fig. 5C and
D). Furthermore, the amount of MBD-14 released from OBs exposed
to bacterial supernatants was then examined by ELISA (Fig. 9D). As shown in Figs. 4 and 5, cells without SAS stimulation
contained a discernible level of activated p38 MAPK at the
baseline. However, the mRNA expression and the phosphorylation of
p38 MAPK gradually increased within 12 h and remained elevated for
at least 48 h with SAS (1:20) and 10 μg/ml LPS stimulation,
a striking contrast to that of ERK at 3 time points (Figs. 4 and 5). Moreover, the treatment of OBs with
LPS increased p38 MAPK and ERK activation within 12 h, and this
level of activation persisted for at least 48 h. Subsequently, the
contribution of p38 MAPK activation to the SAS-induced release of
MBD-14 was investigated using the selective inhibitor, SB203580,
which specifically inhibits the phosphorylation of the downstream
kinases of p38 isoforms α and β. Pre-treatment of the cells with
SB203580 for 30 min prior to exposure to SAS significantly
inhibited the SAS-induced phosphorylation and mRNA expression of
p38 MAPK, and significantly attenuated the release of MBD-14
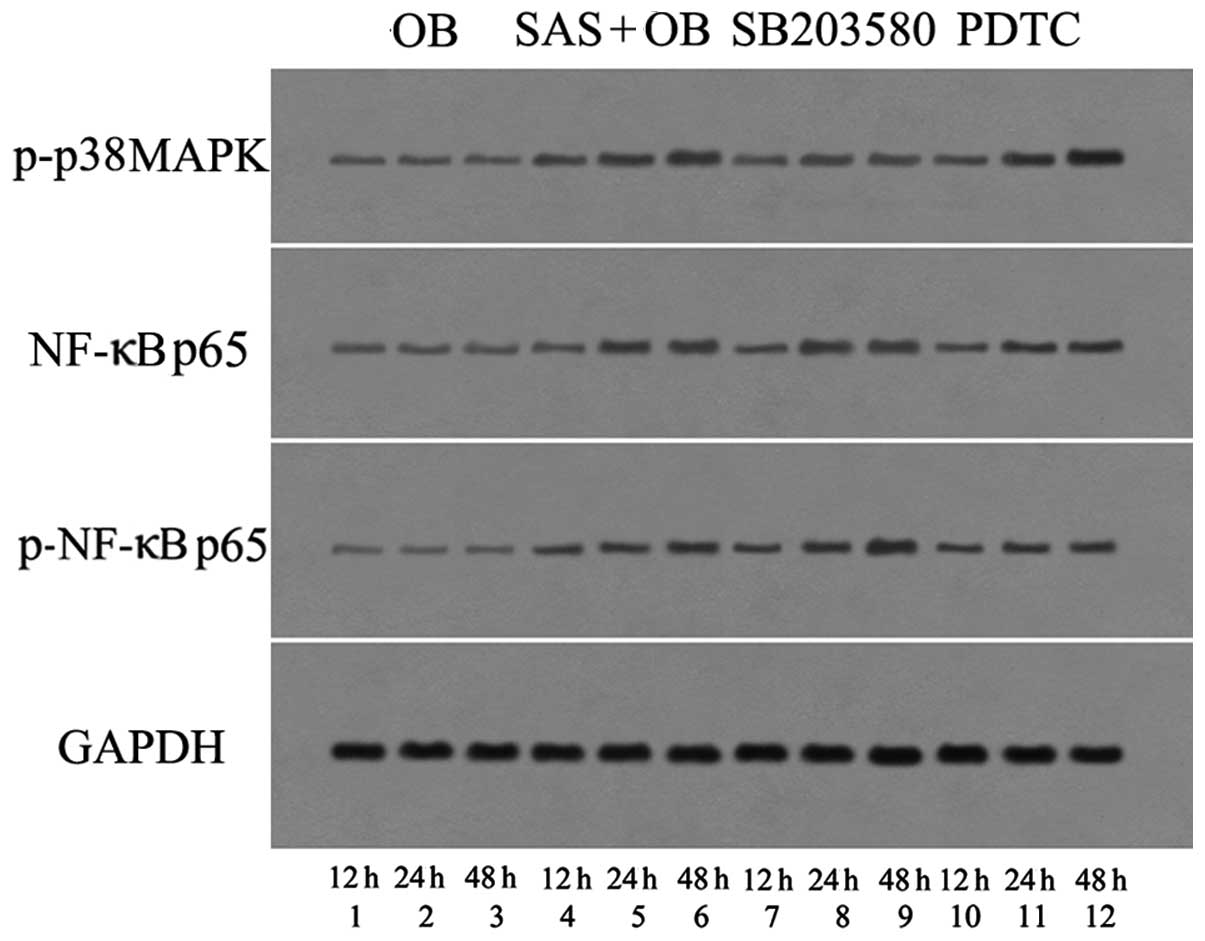
(Figs. 8 and 9A, D and E). After using SB203580, the
amount of MBD-14 released decreased to almost 20 ng/ml at 24 h and
30 ng/ml at 48 h, which was significantly lower than the levels in
the the OB + SAS group (p<0.05) (Fig. 9D). These results suggest that the
p38 MAPK signaling pathway is involved in the SAS-induced release
of MBD-14, whereas ERK is not.
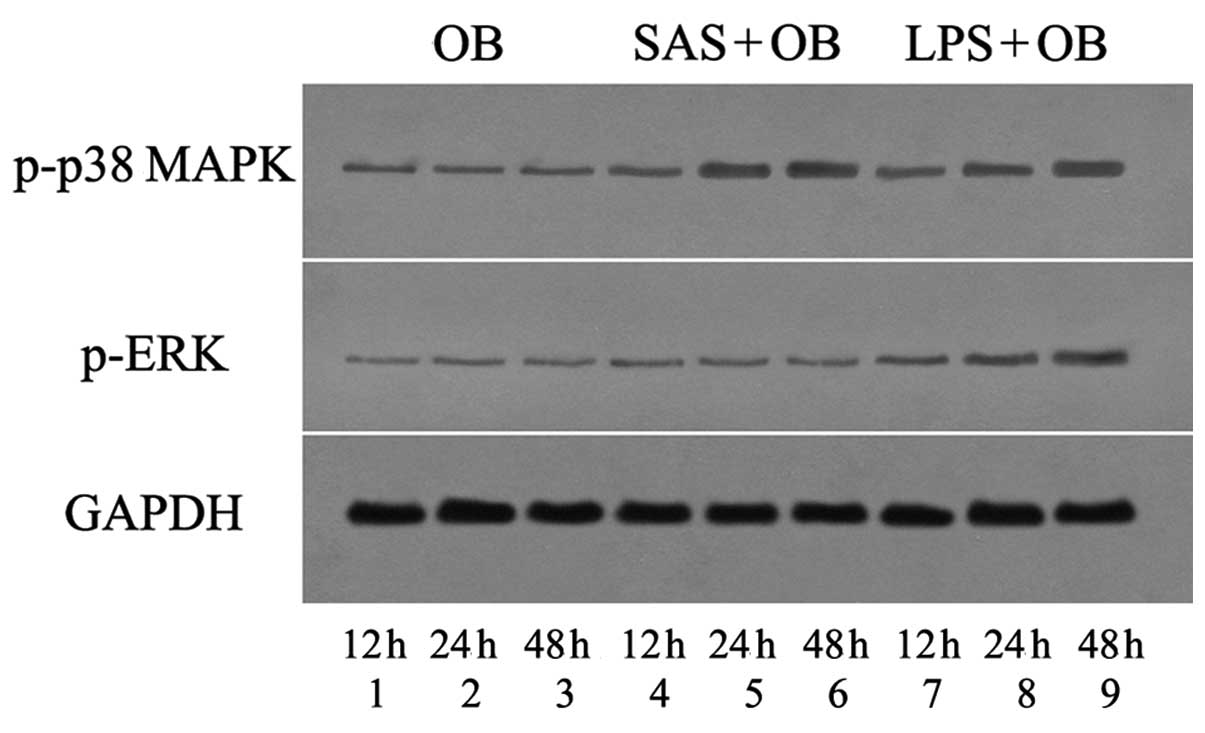 | Figure 4Western blot analysis assays verified
the effects of Staphylococcus aureus supernatant (SAS) on
p38 mitogen-activated protein kinase (MAPK) and extracellular
signal-regulated kinase (ERK) activation under different treatment
conditions. Osteoblasts (OBs) were treated without (lanes 1-3) or
with SAS (1:20, lanes 4-6) or lipopolysaccharide (LPS) (lanes 7-9)
for the indicated periods of time. Pre-incubation with specific
antibody revealed only a weak detection in the OB group (lane 1, 12
h; lane 2, 24 h; and lane 3, 48 h). Lane 4, cells stimulated with
SAS for 12 h; lane 5, cells stimulated with SAS for 24 h; and lane
6, cells stimulated with SAS for 48 h. Lane 7, cells incubated with
LPS for 12 h; lane 8, cells incubated with LPS for 24 h; and lane
9, cells incubated with LPS for 48 h. |
Role of NF-κB in the SAS-induced release
of MBD-14
NF-κB has been shown to be involved in the
transcriptional activation of pro-inflammatory cytokines, including
IL-6 and TNF-α, in murine OBs exposed to extracellular stimuli
(20). To determine whether
bacterial exoproducts affect the activity of the NF-κB pathway in
OBs, the cells lysed at various time points following exposure to
SAS were used for the detection of the phosphorylated form of the
inhibitory subunit of NF-κBα (IκBα) and NF-κB by western blot
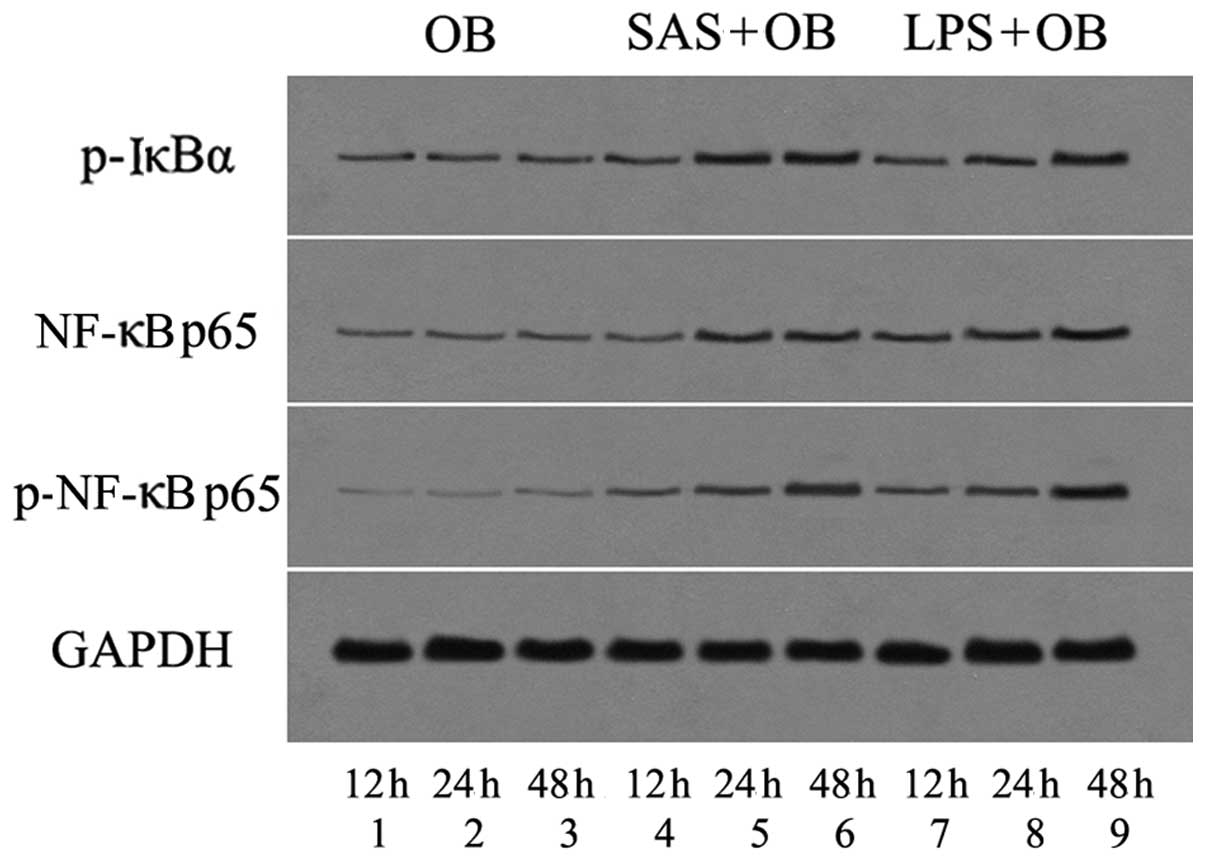
analysis (Figs. 6 and 7A–C). We examined whether SAS increased
the mRNA expression of NF-κB p65 by real-time PCR (Fig. 7D). Within 12 h following treatment
with SAS (1:20) and 10 μg/ml LPS, the mRNA expression of
NF-κB p65 and the phosphorylation of IκBα and NF-κB p65 slightly
increased to the basal level. At later time points, the SAS-induced
phosphorylation and mRNA expression of NF-κB p65 and IκBα increased
within 24 h and reached a maximal level at 48 h. Moreover,
treatment of the cells with LPS increased NF-κB activation within
12 h, and this level of activation persisted for at least 48 h
(Figs. 6 and 7A–C). Subsequently, we examined the role
of the NF-κB signaling pathway in the SAS-induced MBD-14
expression. The downregulation of the NF-κB pathway by the relative
inhibitor, PDTC, was verified by western blot analysis and
real-time PCR (Figs. 8 and
9F). The cells were pre-treated
for 30 min with 20 μM PDTC and then treated with SAS for an
additional 12, 24 and 48 h, followed by the measurement of the
phosphorylation and mRNA expression of NF-κB p65. Pre-treatment of
cells with PDTC markedly inhibited the SAS-induced phosphorylation
of NF-κB p65 at the different time points (Figs. 8 and 9B and C). Correspondingly, the amount of
MBD-14 released decreased to 24±2.1 ng/ml at 24 h and 34±2.8 ng/ml
at 48 h following pre-treatment with PDTC, which was far lower than
the level in the OB + SAS group (p<0.05) (Fig. 9D). These results suggest that
NF-κB mediates the SAS-induced MBD-14 expression.
Crosstalk between different signaling
pathways
To determine whether the SAS-induced activation of
the p38 MAPK pathway is mediated through NF-κB, the cells were
pre-treated for 30 min with the inhibitor, PDTC, and then treated
with SAS for an additional 12, 24 and 48 h, followed by the
measurement of the phosphorylation and mRNA expression of p38 MAPK.
PDTC did not significantly alter the phosphorylation and mRNA
expression of p38 MAPK in response to SAS treatment (p<0.05)
(Figs. 8 and 9E). These results suggest that the NF-κB
pathway does not regulate p38 MAPK activation. In order to
determine whether the SAS-induced activation of NF-κB is mediated
through the p38 MAPK pathway, the cells were pre-treated for 30 min
with SB203580 and then treated with SAS for different periods of
time, followed by the measurement of the phosphorylation and mRNA
expression of NF-κB p65. Subsequently, SB203580 did not inhibit the
activation of NF-κB p65 in response to SAS treatment (Figs. 8 and 9F). These results suggest that the p38
MAPK pathway does not regulate NF-κB activation.
Discussion
In this study, we demonstrate that SAS significantly
induces the release of MBD-14, a peptide with potent
anti-staphylococcal activity, in the murine osteoblastic cell line,
MC3T3-E1. To our knowledge, this is the first study demonstrating
that the molecular mechanisms leading to the enhanced release of
MBD-14 from OBs may partly involve the activation of the p38 MAPK
and NF-κB pathways in response to S. aureus secreted
bacterial exoproducts. Previous studies have focused on the primary
roles of OBs in synthesizing the components of the bone matrix and
regulating the activity of bone resorption (23). However, increasing evidence
indicates that they have additional tasks in bone infection. A
previous study provided evidence that OBs play an important role in
controlling bacterial bone infection through the constitutive
secretion of β-defensins, verified in tissue samples of cultured
OBs and infected mandibular bone (4). The constitutive expression of MBD-14
in samples of cultured OBs in our study was in accordance with
previous findings. Furthermore, previous studies have suggested the
effective induction of HBD-3 stimulated with SAS in OBs; however,
the molecular mechanisms underlying the SAS-induced β-defensin
release from OBs remain unknown (19).
In this study, the role of the p38 MAPK and NF-κB
pathways in the release of the structural and functional ortholog
of HBD-3, MBD-14 following SAS stimulation was evaluated. The p38
MAPK pathway is an important regulator of several cellular
processes. This key intracellular signal transduction pathway
controls fundamental cellular activities, such as cell growth, cell
proliferation, differentiation and migration, as well as apoptosis.
In addition, it modulates the expression of antimicrobial peptides,
such as HBD-2 in epithelial cells and HBD-3 in keratinocytes during
inflammation (25,30). In our cell culture model, p38
MAPK, as opposed to ERK, was significantly phosphorylated in the
SAS-treated OBs. We provide evidence that the p38 MAPK pathway
participates in the SAS-induced release of MBD-14. SB203580, a
well-characterized chemical inhibitor of p38, attenuated the
phosphorylation and the mRNA expression of p38 MAPK, as well as the
release of MBD-14 by SAS. The transcription factor, NF-κB, is
composed of homo- or heterodimers of NF-κB family members,
Rel-A/p65, Rel-B, c-Rel, p50 and p52. In the canonical NF-κB
pathway, SAS activates the IκB kinase, which in turn phosphorylates
NF-κB-associated IκB, leading to the ubiquitination and degradation
of IκB. The NF-κB dimers, p50 and p65, are sequentially released
and translocated into the nucleus to regulate gene transcription.
Moreover, it is well known that RelA/p65 is a critical
trans-activation subunit for NF-κB (31). Our results revealed that SAS
(diluted 1:20) significantly induced the phosphorylation of IκBα
and the degradation of IκBα, which ultimately led to the
phosphorylation of NF-κB p65 and in turn increased the
transactivation activity of NF-κB. Pre-treatment with PDTC markedly
inhibited the SAS-induced phosphorylation and mRNA expression of
NF-κB p65 at different time points (30). The amount of MBD-14 released from
the OBs pre-treated with PDTC markedly decreased compared with that
in the OB + SAS group. The reduced secretion of MBD-14 following
treatment with inhibitors indicates the role of these pathways in
sensing Gram-positive microbes and mediating anti-inflammatory
response in cultured OBs. Further studies are required to elucidate
the p38 MAPK and NF-κB binding motifs of the MBD-14 gene promoter
which contribute to the SAS-mediated transcription, as well as the
other signaling pathways responsible for MBD-14 expression in OBs
(32).
The main cause of bacterial bone infection is due to
S. aureus colonization (1). We therefore assessed the influence
of S. aureus supernatants on the release of MBD-14 from
cultured OBs. The bacterial exoproducts released by S.
aureus mainly included a large amount of toxins, such as
enterotoxins that act at very low concentrations and have
superantigenic activity to trigger a complex molecular pathway,
ultimately leading to an increased expression of pro-inflammatory
genes (33,34). Following the co-culture of OBs
with SAS (1:20), MBD-14 secretion increased to higher levels after
24 h and finally peaked after 48 h. The marked upregulation of
MBD-14 following exposure to Gram-positive bacterial exoproducts in
this study, suggests an important role of antimicrobial peptides in
host response in inflammatory bone disease. In addition, Lucke
et al speculated whether the induction of HBD-3 in cultured
hosteoblasts and bone play a dual role during immune response
(35). Their direct antimicrobial
properties contribute to the local innate immune response by
combating microbial invasions. By linking innate and adaptive
immune responses, β-defensins secreted by OBs may promote the
production of pro-inflammatory mediators, chemoattract macrophages
and monocytes, initiate the antigen-specific activation of
infiltrating cells, and facilitate the development of cell-mediated
host responses to invading pathogens (36–38). However, further studies are
required to clarify the role of MBD-14 in immune defense, and to
achieve a balance between their direct antimicrobial properties and
the recruitment capabilities contributing to the innate and
adaptive immune response.
Our data revealed an emerging framework for
understanding the SAS-induced release of MBD-14 from OBs as part of
host immunity. Of note, SAS and LPS significantly increased the
release of MBD-14. Based on our data, we propose a novel method
that OBs treated with relative agonists to activate p38 MAPK and
NF-κB can effectively improve the release of MBD-14, protecting
against microbial invasion during the early period of bone
infection.
In conclusion, the findings of the present study
demonstrate that SAS induces the release of MBD-14 in MC3T3-E1
cells which appears to be mediated at least in part through the
activation of the signaling molecules, p38 MAPK and NF-κB, but not
ERK. The regulation of the expression of MBD-14 by different
pathogens may be important in maintaining or augmenting the
endogenous antimicrobial barrier in bone in response to bacterial
invasion. The elucidation of the host signaling pathways that
contribute to the induction of MBD-14 expression may aid in the
understanding of host susceptibility to staphylococcal infection
during various disease states, such as osteomyelitis and
implant-related infection, and may provide novel therapeutic agents
for enhancing innate defense mechanims within the bone. Further
studies are required to investigate the effectiveness of the
treatment of S. aureus intramedullary infection in
vivo through a certain therapeutic agent. Our data may provide
a new direction for research into this topic.
Acknowledgements
This study was supported by the
Interdisciplinary (Engineering-Medical) Research Fund of Shanghai
Jiao Tong University (Grant no. YG2011MS30), the Shanghai Municipal
Health Bureau Science Fund for Young Scholars (Grant no.
2010QJ036A), the Opening Project of State Key Laboratory of High
Performance Ceramics and Superfine Microstructure (Grant no.
SKL201206SIC) and the National Natural Science Foundation of China
(Grant no. 81171688).
References
|
1
|
Montanaro L, Speziale P, Campoccia D, et
al: Scenery of Staphylococcus implant infections in
orthopedics. Future Microbiol. 6:1329–1349. 2011.PubMed/NCBI
|
|
2
|
Marriott I, Gray DL, Rati DM, et al:
Osteoblasts produce monocyte chemoattractant protein-1 in a murine
model of Staphylococcus aureus osteomyelitis and infected
human bone tissue. Bone. 37:504–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ning R, Zhang X, Guo X and Li Q:
Staphylococcus aureus regulates secretion of interleukin-6
and monocyte chemoattractant protein-1 through activation of
nuclear factor kappaB signaling pathway in human osteoblasts. Braz
J Infect Dis. 15:189–194. 2011. View Article : Google Scholar
|
|
4
|
Warnke PH, Springer IN, Russo PA, et al:
Innate immunity in human bone. Bone. 38:400–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campoccia D, Montanaro L, Speziale P and
Arciola CR: Antibiotic-loaded biomaterials and the risks for the
spread of antibiotic resistance following their prophylactic and
therapeutic clinical use. Biomaterials. 31:6363–6377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Z, Neoh KG, Kang ET, Poh C and Wang W:
Titanium with surface-grafted dextran and immobilized bone
morphogenetic protein-2 for inhibition of bacterial adhesion and
enhancement of osteoblast functions. Tissue Eng Part A. 15:417–426.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumarasamy KK, Toleman MA, Walsh TR, et
al: Emergence of a new antibiotic resistance mechanism in India,
Pakistan, and the UK: a molecular, biological, and epidemiological
study. Lancet Infect Dis. 10:597–602. 2010. View Article : Google Scholar
|
|
8
|
Zuo GY, An J, Han J, et al: Isojacareubin
from the Chinese Herb Hypericum japonicum: potent
antibacterial and synergistic effects on clinical
methicillin-resistant Staphylococcus aureus (MRSA). Int J
Mol Sci. 13:8210–8218. 2012.PubMed/NCBI
|
|
9
|
Hancock RE: Mechanism of action of newer
antibiotics for gram-positive pathogens. Lancet Infect Dis.
5:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pazgier M, Hoover DM, Yang D, Lu W and
Lubkowski J: Human beta-defensins. Cell Mol Life Sci. 63:1294–1313.
2006. View Article : Google Scholar
|
|
11
|
Maisetta G, Batoni G, Esin S, Florio W,
Bottai D, Favilli F and Campa M: In vitro bactericidal activity of
human beta-defensin 3 against multidrug-resistant nosocomial
strains. Antimicrob Agents Chemother. 50:806–809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu C, Tan H, Cheng T, et al: Human
β-defensin 3 inhibits antibiotic-resistant Staphylococcus
biofilm formation. J Surg Res. Dec 20–2012.(Epub ahead of
print).
|
|
13
|
Yamaguchi Y, Nagase T, Makita R, et al:
Identification of multiple novel epididymis-specific beta-defensin
isoforms in humans and mice. J Immunol. 169:2516–2523. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hinrichsen K, Podschun R, Schubert S,
Schröder JM, Harder J and Proksch E: Mouse beta-defensin-14, an
antimicrobial ortholog of human beta-defensin-3. Antimicrob Agents
Chemother. 52:1876–1879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Röhrl J, Yang D, Oppenheim JJ and Hehlgans
T: Identification and biological characterization of mouse
beta-defensin 14, the orthologue of human beta-defensin 3. J Biol
Chem. 283:5414–5419. 2008.PubMed/NCBI
|
|
16
|
Ahrens K, Schunck M, Podda GF, et al:
Mechanical and metabolic injury to the skin barrier leads to
increased expression of murine β-defensin-1, -3, and -14. J Invest
Dermatol. 131:443–452. 2011.PubMed/NCBI
|
|
17
|
Lee HY, Takeshita T, Shimada J, et al:
Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88
and IRAK-TRAF6-p38 MAPK signaling pathway in human middle ear
epithelial cells. BMC Infect Dis. 8:872008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hussain T, Nasreen N, Lai Y, Bellew BF,
Antony VB and Mohammed KA: Innate immune responses in murine
pleural mesothelial cells: toll-like receptor-2 dependent induction
of beta-defensin-2 by staphylococcal peptidoglycan. Am J Physiol
Lung Cell Mol Physiol. 295:L461–L470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varoga D, Wruck CJ, Tohidnezhad M, et al:
Osteoblasts participate in the innate immunity of the bone by
producing human beta defensin-3. Histochem Cell Biol. 131:207–218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Die L, Yan P, Jun Jiang Z, Min Hua T, Cai
W and Xing L: Glycogen synthase kinase-3 beta inhibitor suppresses
Porphyromonas gingivalis lipopolysaccharide-induced CD40
expression by inhibiting nuclear factor-kappa B activation in mouse
osteoblasts. Mol Immunol. 52:38–49. 2012.PubMed/NCBI
|
|
21
|
Nihonyanagi S, Kanoh Y, Okada K, et al:
Clinical usefulness of multiplex PCR lateral flow in MRSA
detection: a novel, rapid genetic testing method. Inflammation.
35:927–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki A, Guicheux J, Palmer G, et al:
Evidence for a role of p38 MAP kinase in expression of alkaline
phosphatase during osteoblastic cell differentiation. Bone.
30:91–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han SH, Kim KH, Han JS, et al: Response of
osteoblast-like cells cultured on zirconia to bone morphogenetic
protein-2. J Periodontal Implant Sci. 41:227–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gläser R, Harder J, Lange H, Bartels J,
Christophers E and Schröder JM: Antimicrobial psoriasin (S100A7)
protects human skin from E. coli infection. Nat Immunol.
6:57–64. 2005.PubMed/NCBI
|
|
25
|
Menzies BE and Kenoyer A: Signal
transduction and nuclear responses in Staphylococcus
aureus-induced expression of human beta-defensin 3 in skin
keratinocytes. Infect Immun. 74:6847–6854. 2006.PubMed/NCBI
|
|
26
|
An N, Rausch-fan X, Wieland M, Matejka M,
Andrukhov O and Schedle A: Initial attachment, subsequent cell
proliferation/viability and gene expression of epithelial cells
related to attachment and wound healing in response to different
titanium surfaces. Dent Mater. 28:1207–1214. 2012. View Article : Google Scholar
|
|
27
|
Li JY, Huang JY, Li M, et al: Anisomycin
induces glioma cell death via down-regulation of PP2A catalytic
subunit in vitro. Acta Pharmacol Sin. 33:935–940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan H, Peng Z, Li Q, Xu X, Guo S and Tang
T: The use of quaternised chitosan-loaded PMMA to inhibit biofilm
formation and downregulate the virulence-associated gene expression
of antibiotic-resistant Staphylococcus. Biomaterials.
33:365–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bar-Yehuda S, Luger D, Ochaion A, et al:
Inhibition of experimental auto-immune uveitis by the A3 adenosine
receptor agonist CF101. Int J Mol Med. 28:727–31. 2011.PubMed/NCBI
|
|
30
|
Jang BC, Lim KJ, Paik JH, et al:
Up-regulation of human beta-defensin 2 by interleukin-1 beta in
A549 cells: involvement of PI3K, PKC, p38 MAPK, JNK, and NF-kappaB.
Biochem Biophys Res Commun. 320:1026–1033. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shim KS, Kang JS, Lee MH and Ma JY:
Selaginella tamariscina water extract inhibits receptor
activator for the nuclear factor-κB ligand-induced osteoclast
differentiation by blocking mitogen-activated protein kinase and
NF-κB signaling. Pharmacogn Mag. 8:184–191. 2012. View Article : Google Scholar
|
|
32
|
Mineshiba J, Myokai F, Mineshiba F,
Matsuura K, Nishimura F and Takashiba S: Transcriptional regulation
of beta-defensin-2 by lipopolysaccharide in cultured human cervical
carcinoma (HeLa) cells. FEMS Immunol Med Microbiol. 45:37–44. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu DL, Omoe K, Sashinami H, Shinagawa K
and Nakane A: Immunization with a nontoxic mutant of staphylococcal
enterotoxin A, SEAD227A, protects against enterotoxin-induced
emesis in house musk shrews. J Infect Dis. 199:302–310. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rasooly R and Hernlem B: TNF as biomarker
for rapid quantification of active Staphylococcus
enterotoxin A in food. Sensors (Basel). 12:5978–5985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lucke M, Schmidmaier G, Sadoni S, et al:
Gentamicin coating of metallic implants reduces implant-related
osteomyelitis in rats. Bone. 32:521–531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Semple F, MacPherson H, Webb S, et al:
Human β-defensin 3 affects the activity of pro-inflammatory
pathways associated with MyD88 and TRIF. Eur J Immunol.
41:3291–3300. 2011.
|
|
37
|
Lai Y and Gallo RL: AMPed up immunity: how
antimicrobial peptides have multiple roles in immune defense.
Trends Immunol. 30:131–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Röhrl J, Yang D, Oppenheim JJ and Hehlgans
T: Human beta-defensin 2 and 3 and their mouse orthologs induce
chemo-taxis through interaction with CCR2. J Immunol.
184:6688–6694. 2010.PubMed/NCBI
|