Contents
Introduction
Function of HSF1
HSF1 and cancer
HSF1 and hyperthermia
Discussion
Introduction
Hyperthermia (HT) is considered to have potential as
a cancer treatment modality (1).
HT in combination with radiotherapy and/or chemotherapy has been
used for various types of cancer, and the anticancer effects of
such combinations have been verified in many clinical trials
(1–7). One of the problems with HT therapy
is the acquisition of thermoresistance against heat stress
(8–12). In general, cells have protective
functions for various stressors that occur from outside of the
cell. Heat shock proteins (HSPs), molecular chaperones with strong
cytoprotective and antiapoptotic properties, are induced by a wide
variety of stresses, particularly heat stress (13–16); they are also induced by treatment
with HT, and, thus, it has been considered that HSPs play a role in
the acquisition of thermoresistance in cells (10–12). In mammals, the expression of HSPs
is mainly regulated by heat shock transcription factor 1 (HSF1)
(17,18). Elevation of HSF1 has been observed
in several types of human tumor tissues (19–26), and it has been shown to
participate in the initiation, proliferation and maintenance of
tumors (12,25–29). Notably, both inhibition of the
expression of HSF1 and the functional loss of HSF1 have been
suggested to enhance the sensitivity to HT under basic experimental
conditions (29–38). In the present review, the
physiological and pathological roles of HSF1 in cancer cells are
summarized, and the potential of HSF1 as a therapeutic target for
HT therapy is discussed.
Function of HSF1
The heat-shock response, which is a universal
cellular response to heat, is a critical cellular event for cell
adaptation. Heat stress elicits a wide spectrum of stress
responses, including an induction of HSPs, protein aggregation, an
imbalance of protein homeostasis, DNA and RNA damage, reactive
oxygen species production, cell growth arrest and cell death in
mammalian cells. HSPs are characterized as molecular chaperones and
they exert strong cytoprotective effects against stress-induced
proteotoxic damage (13–16). Their functions and amino acid
sequences are well conserved in a wide range of species from yeast
to humans. The expression of HSPs is primarily regulated by heat
shock transcription factors (HSFs). In humans, three HSFs (HSF1,
HSF2 and HSF4) have been identified and, among them, HSF1 plays the
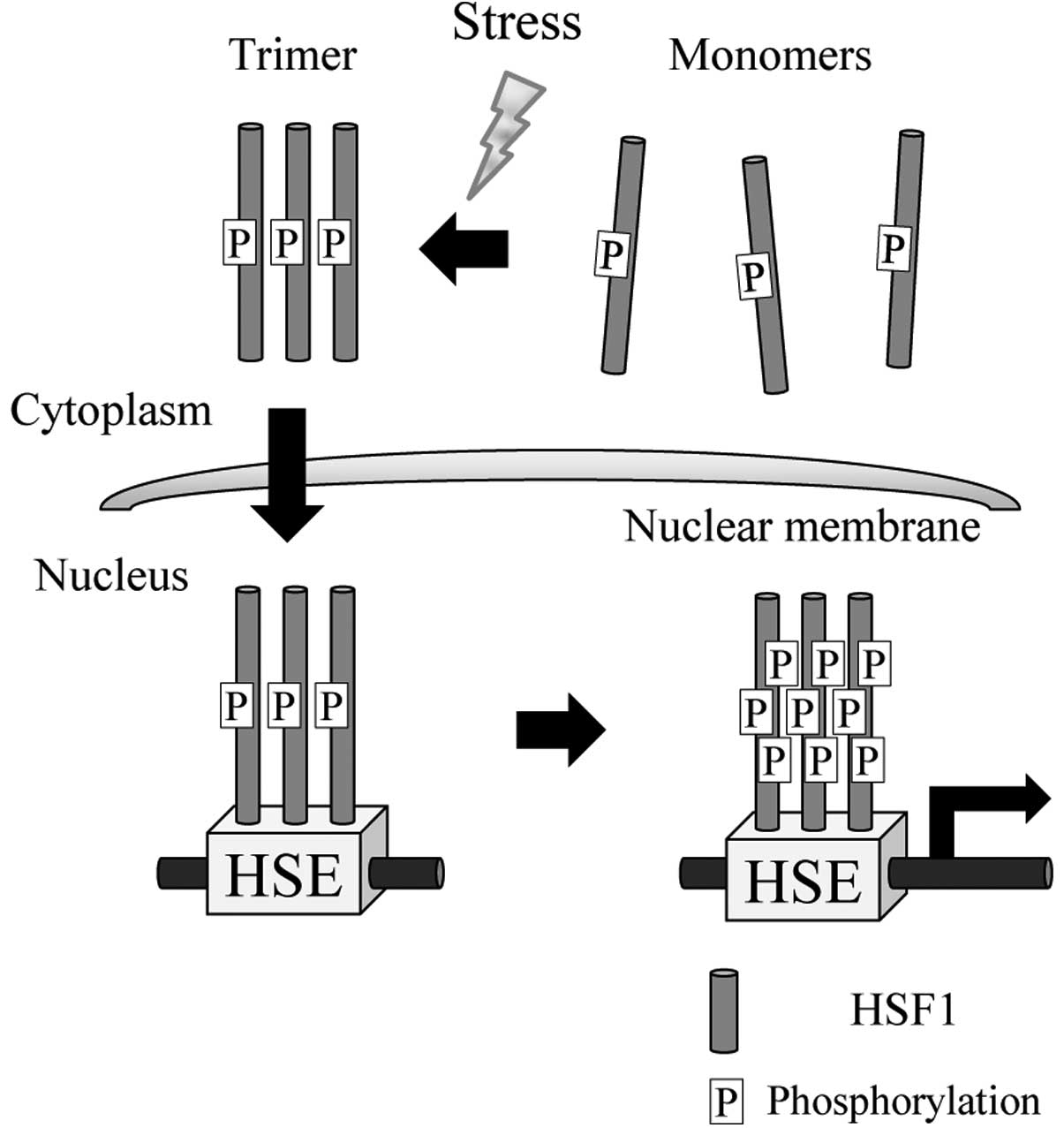
most important role against stress responses (17,18). Under nonstress conditions, HSF1 is
localized in the cytoplasm as an inactive monomer. Upon exposure to
stresses, especially heat stress, a series of events is triggered:
the HSF1 inactive monomer is converted to a DNA-binding homotrimer
that translocates from the cytoplasm to the nucleus, binds to the
heat shock elements (HSEs) that are located in the regions of
inducible HSP genes including Hsp27, Hsp70 and Hsp90, undergoes a
hyperphosphorylation reaction and activates transcription (17,18,39) (Fig.
1). In mammalian cells, heat activates the transcription of
heat responsive genes, including HSPs, coincident with a bulk
decrease in mRNA and protein syntheses, and this overall
reprogramming of gene expression permits the selective synthesis of
HSPs (40–42). In our previous studies using
global-scale microarrays, we also detected a number of genes that
were downregulated and participated in biological functions,
including post-transcriptional modification and gene expression
(43–45). This bulk decrease has been
considered to represent a form of transcriptional suppression due
to the binding of activated HSF1 to the promoter region of the gene
(40). Mariner et al
(41) recently reported that
non-corded RNA is very closely related to the mechanism by which
heat induces a decrease in gene expression.
Numerous studies targeting HSFs in gene-modified
animals have enhanced our understanding of the pleiotropic roles of
HSFs in mammals (46). A previous
study with HSF1-knockout (KO) mice demonstrated that the HSF1
protein was dispensable for the maintenance of the mice, since the
KO mice remained alive under normal environmental conditions
(30). On the other hand, HSF1 is
reported to be required for extra-embryonic development, postnatal
growth and fertility of the female in the HSF1-KO mice (47). Homma et al (48) observed hallmarks of central
nervous system disease, such as demyelination, astrogliosis and
accumulation of ubiquitinated proteins in HSF1-deficient mice.
Furthermore, previous studies with HSF1 KO animals have shown that
HSF1 is involved in the development and maintenance of several
organs, such as the heart (49),
trachea (50), nose (50) and placenta (47). It has also been demonstrated that
both HSF2 (51) and HSF4
(52) play critical roles in the
neural functions or lens development by using HSF2- and HSF4-KO
mice. Thus, accumulating evidence suggests that HSFs are closely
associated with longevity (53),
neurodegenerative diseases (54)
and cancer (12,25–29). Indeed, HSFs play a versatile role
in the organism, from homeostatic maintenance to disease.
HSF1 and cancer
In humans, tumorigenesis is a multistep development
including genome instabilities (mutation and chromosomal deletion)
and epigenetic changes (abnormal histone acetylation and DNA
methylation). During the multistep tumorigenesis, mutated nonnative
proteins are synthesized and deregulated and abnormal signal
transduction pathways exist in cancer cells. Moreover, the
conditions within the tumor microenvironment where cancer cells are
present include hypoxia, acidity, and low glucose levels, and these
differ from the conditions in normal tissue. Therefore, cancer
cells are generally exposed to more stresses compared with normal
cells (55). Several types of
tumors contain high expression levels of one or more HSPs (Hsp27,
Hsp40, Hsp60, Hsp70, Hsp90 and/or Hsp110) compared with adjacent
normal tissues (12,56,57). A significant elevation of HSF1 has
been reported in a wide variety of cancer cells or tumor tissues in
nonclinical studies (19–26) (Table
I). In human prostate carcinoma cell lines, the expression
levels of HSPs and HSF1 are elevated in cells with more highly
malignant features (19,20). An abundant expression of HSF1
protein has been observed in the tumor tissues of human prostate
(19) and pancreas (22) or oral squamous cell carcinoma
(OSCC) cells (24) compared to
their normal counterparts. In addition, Khaleque et al
(21) have shown that the protein
level of HSF1 is upregulated in breast carcinoma sections and that
HSF1 co-localizes and binds to the corepressor
metastasis-associated 1 (MTA1) protein in this cancer.
 | Table I.Examples of nonclinical and clinical
studies elevating HSF1. |
Table I.
Examples of nonclinical and clinical
studies elevating HSF1.
| Cancer type | Observations | Author/(Ref.) |
|---|
| Nonclinical
studies | | |
| Prostate cancer
specimens | Protein level is
upregulated in the malignant prostate epithelial cells | Hoang et al
(19) |
| Prostate
carcinoma cells | Protein level is
elevated in the more highly malignant carcinoma cells | Hoang et al
(19) |
| | Tang et al
(20) |
| Breast carcinoma
sections | Protein level is
upregulated and it co-localizes with MTA1 protein | Khaleque et
al (21) |
| Pancreatic cancer
tissues | Protein level is
upregulated in cancer tissues | Dudeja et al
(22) |
| OSCC cells | Protein and mRNA
levels are elevated in carcinoma cells | Ishiwata et
al (24) |
| Clinical
studies | | |
| Breast
cancer | Nuclear level is
associated with poor prognosis | Santagata et
al (23) |
| OSCC | Nuclear level is
related to tumor size | Ishiwata et
al (24) |
| Breast, colon or
lung cancer | Active HSF1
transcriptional program is strongly associated with metastasis and
mortality | Mendillo et
al (25) |
| Breast
cancer | mRNA level is
correlated with grade, metastasis and poor prognosis | Gabai et al
(26) |
In 2007, Dai et al (27) found that HSF1, a non-oncogene,
plays a major role in enabling the initiation and maintenance of
cancer in several mouse tumor models. Knockdown of HSF1 using short
hairpin RNA (shRNA) technology effectively inhibited cell viability
in several cancer cell lines, whereas it had no effect on normal
human fibroblasts. They concluded that HSF1 function helps to
maintain the growth and survival of human cancer cells (27). Similar results were reported when
other in vitro experiments were performed using melanoma
cells (29,36), OSCC cells (37), pancreatic cancer cells (22) and human epidermal growth factor
receptor 2 (HER2)-positive cancer cells (58) (Table
II). Although the molecular mechanisms by which HSF1 regulates
cellular proliferation and survival in cancer cells are less
understood, genome-wide transcriptome studies have indicated that
HSF1 regulates numerous other targets in addition to HSPs (25,59–62).
 | Table II.Examples of experimental anticancer
effects targeting HSF1. |
Table II.
Examples of experimental anticancer
effects targeting HSF1.
| Cancer type | Treatment | Effect | Author/(Ref.) |
|---|
| HSF1 silencing | | | |
| Pancreatic cancer
cells | Quercetin | Quercetin increases
the apoptosis of cells | Aghdassi et
al (64) |
| Pancreatic cancer
xenografts | Quercetin | Quercetin reduces
the growth of tumors | Aghdassi et
al (64) |
| Colon cancer
xenografts | KRIBB11 | KRIBB11 inhibits
the growth of tumors | Yoon et al
(65) |
| Pancreatic cancer
xenografts | Ly101-4B | Ly101-4B inhibits
the growth of tumors | Xia et al
(66) |
| Mammary
epithelial cell xenografts | shRNA | shRNA inhibits
HER2-induced cellular transformation and tumorigenesis | Meng et al
(59) |
| Melanoma cell
xenografts | shRNA | shRNA inhibits the
growth of melanomas | Fujimoto et
al (29) |
| Cancer cells | shRNA | shRNA inhibits the
growth of cancer cells | Dai et al
(27) |
| HER2-positive
cancer cells | shRNA | shRNA decreases
proliferation of cancer cells | Meng et al
(59) |
| Melanoma
cells | shRNA | shRNA inhibits the
growth of melanoma cells | Nakamura et
al (36) |
| | | Fujimoto et
al (29) |
| Pancreatic cancer
cells | siRNA | siRNA decreases the
viability of cancer cells | Dudeja et al
(22) |
| OSCC cells | siRNA | siRNA inhibits the
growth of OSCC cells | Tabuchi et
al (37) |
| Genetically
modified mice | | | |
| Chemical-induced
skin carcinomas | HSF1 KO mouse | KO inhibits
carcinogen-induced tumorigenesis | Dai et al
(27) |
| p53-deficient
spontaneous tumors | HSF1 KO mouse x
p53R172H mousea | KO increases
survival and alters the distribution of tumor types | Dai et al
(27) |
| p53-deficient
spontaneous tumors | HSF1 KO mouse x p53
KO mouseb | KO selectively
decreases lymphomas | Min et al
(63) |
| Chemical-induced
hepatocellular carcinomas | HSF1 KO mouse | KO inhibits
carcinogen-induced tumorigenesis | Jin et al
(28) |
| NeuT-induced
breast tumors | HSF1 KO mouse x
Her2/NeuT mousec | KO suppresses tumor
progression | Gabai et al
(26) |
Basic experiments using HSF1 KO mice clearly
demonstrate that this protein participates in the development of
skin tumors (27) and
hepatocellular carcinomas (28)
induced by 7,12-dimethylbenzanthracene (DMBA) and
diethylnitrosamine (DEN), respectively. In line with these results,
different animal experiments with genetically modified mice showed
an essential role of HSF1 in tumor development (26,27,63) (Table
II).
Interest in the role of HSF1 in cancer has grown
gradually over the last decade, and notable papers in clinical
research have recently been published (23–26) (Table
I). Santagata et al (23) indicated that nuclear localization
and increased levels of HSF1 are well associated with poor
prognosis in estrogen receptor (ER)-positive breast carcinomas.
Ishiwata et al (24)
reported that higher nuclear HSF1 expression was closely related to
tumor size and histopathologic types in OSCCs. Moreover, high
expression of HSF1 mRNA in human breast cancer was correlated with
grade, metastasis, and poor prognosis, suggesting that HSF1 is
involved in tumor progression (26). Mendillo et al (25) identified an HSF1-regulated
transcriptional program specific to highly malignant cells and
distinct from heat shock by using chromatin immunoprecipitation
coupled with massively parallel DNA sequencing. They established an
‘HSF1-cancer signature’ of 456 genes that were bound by HSF1 near
their transcription start sites, and found that this HSF1 cancer
program is active in several types of tumors and is strongly
associated with metastasis and mortality (25) (Table
I).
To date, several types of HSF1 inhibitors have been
developed, and their inhibitory effects to cancer have been
reported (35,64–68) (Table
II). Quercetin, a plant-derived flavonoid, is shown to increase
apoptosis and to reduce the growth of pancreatic cancer cells
(64). Meanwhile, this flavonoid
inhibits multiple target proteins, such as HSF1, Hsp70 and NF-κB
(67–69). In a colon cancer xenograft mouse
model, a newly synthesized KRIBB11
[N2-(1H-indazole-5-yl)-N6-methyl-3-nitropyridine-2,6-diamine]
significantly inhibited the growth of colon cancer, and its
mechanism is considered to inhibit Hsp70 synthesis through
suppression of HSF1 function by impairing the recruitment of
positive transcription elongation factor b (p-TEFb) to the Hsp70
promoter (65). Ly101-4B, a novel
triazole nucleoside analog, targets the heat shock response pathway
by downregulation of HSF1 and consequential inhibition of HSPs,
Hsp27, Hsp70 and Hsp90 (66).
This compound caused the shutdown of several oncogenic pathways and
caspase-dependent apoptosis, resulting in a potent anticancer
effect in pancreatic cancer cell xenografts (66,68). Similar results were obtained when
shRNA for HSF1 was used (29,59). These findings have helped to
elucidate the specific role of HSF1 in the pathogenesis of
cancer.
HSF1 and hyperthermia
HT has been considered a possible mode of cancer
treatment (1). HT in combination
with radiotherapy and/or chemotherapy has been used for various
types of cancer, and the anticancer effects of such combinations
have been verified in many clinical trials (1–7).
One of the problems with HT therapy is the acquisition of
thermoresistance against heat stress (8–12).
As the expression of HSPs is primarily mediated at the
transcription level by HSF1 (17,18), it is predicted that downregulation
of HSPs by the targeting of HSF1 will render tumors more sensitive
to HT. Examples of the enhancement of HT sensitivity targeting HSF1
are summarized in Table III. It
is well established that preconditioning of the cells with mild-HT
results in an increase in HSP levels and leads to the acquisition
of thermoresistance to HT. However, McMillan et al (30) were the first show that targeted
disruption of HSF1 abolished thermoresistance against lethal HT at
45°C for 40 min in preheated (43°C, 30 min) mouse embryonic
fibroblast (MEF) cells from HSF1-KO mice. In the MEF cells,
silencing HSF1 prevented induction of the expression of two
inducible HSPs, Hsp27 and Hsp70, whereas the expression of the
constitutive HSPs, Hsp60 and Hsc70, remained constant, suggesting
that inducible HSPs are involved in acquired cellular resistance to
HT (30). Consistent with this
result, functional silencing by using KO mice (31,32) or a dominant-negative (DN) mutant
(33) prevented the
thermoresistance to HT in MEF and bone marrow progenitor (BMP)
cells or breast cancer cells, respectively (Table III).
 | Table III.Examples of the enhancement of HT
sensitivity targeting HSF1. |
Table III.
Examples of the enhancement of HT
sensitivity targeting HSF1.
| Cell type
targeting | Heat treatment | Effect | Author/(Ref.) |
|---|
| HT and HSF1
silencing | | | |
| MEF cells KO
mouse | 43°C for 30 min
followed by 45°C for 40 min | KO prevents
resistance to HT | McMillan et
al (30) |
| MEF cells KO
mouse | 43°C for 30 min
followed by 45°C for 60 min | KO prevents
resistance to HT | Luft et al
(31) |
| MEF and BMP cells
KO mouse | 43°C for 20 min
followed by 44°C for 10–40 min | KO prevents
resistance to HT | Zhang et al
(32) |
| Breast cancer
cells DN mutant | 42°C for 30 min
followed by 43°C for 20–100 min | Blocking prevents
resistance to HT | Wang et al
(33) |
| Cervical cancer
cells triptolide | 42°C for 60 min
followed by 45°C for 40 min | Inhibition enhances
HT sensitivity | Westerheide et
al (35) |
| Cervical cancer
cells DN mutant | 48°C for 10
min | DN mutant increases
HT sensitivity | Xia et al
(34) |
| Melanoma cells
shRNA | 42°C or 43°C for 60
min or 45°C for 60–180 min | Silencing enhances
HT sensitivity | Nakamura et
al (36) |
| | | Fujimoto et
al (29) |
| OSCC cells
siRNA | 42°C or 44°C for 90
min | Silencing enhances
HT sensitivity | Tabuchi et
al (37) |
| Breast cancer
cell xenografts DN mutant | 43°C for 30 min
every day for a week | Blocking inhibits
growth of cancer cells | Wang et al
(33) |
| HT, compound and
HSF1 silencing | | | |
| Cervical cancer
cells siRNA and cisplatin | 43°C for 60
min | Silencing
sensitizes hyperthermochemotherapy | Rossi et al
(38) |
| Melanoma cells
shRNA and dacarbazine | 42°C for 60
min | Silencing
sensitizes hyperthermochemotherapy | Nakamura et
al (36) |
Of note, targeting of HSF1 has been suggested to
enhance the sensitivity to HT under basic experimental conditions
(29–38). We recently showed that silencing
HSF1 using small interfering RNA (siRNA) enhances the mild HT
(42°C, 90 min) and HT (44°C, 90 min) sensitivity in human OSCC
cells (37). Significant
enhancement of the HT sensitivity has also been observed in
HSF1-silenced cervical cancer (35) and melanoma cells (29,36) treated with triptolide, an
inhibitor of the human heat shock response, and shRNA for HSF1,
respectively. In addition, although HT had only a slight effect on
tumor growth in breast cancer cell xenografts, functional defect of
HSF1 by its DN mutant in combination with HT had a lower
tumorigenic capacity than the control tumors (33).
The effects of inhibition of HSH1 on the
hyperthermochemotherapy have been reported (36,38). When HSF1 of cervical cancer cells
was silenced by siRNA technology, the silencing in combination with
hyperthermochemotherapy (treatment at 43°C for 60 min plus
cisplatin) markedly increased the level of apoptosis compared to
hyperthermochemotherapy alone (38). In human melanoma cells, although
HSF1 silencing did not sensitize to chemotherapy with dacarbazine,
it significantly enhanced the sensitivity to this chemotherapy in
combination with HT (42°C, 60 min) (36). These results strongly suggest that
silencing HSF1 can enhance the sensitivity to either HT or
hyperthermochemotherapy. It is also noteworthy that combined
treatment with HT and cisplatin effectively suppresses the
activation of HSF1 in human glioblastoma cells (70).
Discussion
Although HT is considered to be a promising approach
in cancer therapy, the thermoresistance due to the increase in HSPs
in cancer cells remains a disadvantage, reducing the effects of HT
in clinical treatment. The expression of HSPs is mainly regulated
by HSF1 (17,18). HSPs (12,56,57) as well as HSF1 (19–26) are abundantly expressed in human
cancer cells of various origins, and HSPs (12,56,57) and HSF1 (12,25–29) play critical roles in the
initiation, proliferation and maintenance of cancer. Thus, the
combination of HT and HSF1-targeting may be an attractive option
and worthy of further investigation. As expected, the inhibition of
functions of HSF1 by using gene targeting or HSF1 inhibitors was
shown to reduce the acquisition of thermoresistance (30–33) and to sensitize tumors to
HT-induced cell death (29–38). In the near future, targeting HSF1
(67,68) in combination with HT may come to
be a promising approach for the treatment of cancer.
Abbreviations:
|
BMP
|
bone marrow progenitor;
|
|
DN
|
dominant-negative;
|
|
ER
|
estrogen receptor;
|
|
HER2
|
epidermal growth factor
receptor-2;
|
|
HSE
|
heat shock element;
|
|
HSP
|
heat shock protein;
|
|
HSF
|
heat shock transcription factor;
|
|
HSF1
|
heat shock transcription factor 1;
|
|
HT
|
hyperthermia;
|
|
KO
|
knockout;
|
|
MTA1
|
metastasis-associated 1;
|
|
MEF
|
mouse embryonic fibroblast;
|
|
OSCC
|
oral squamous cell carcinoma;
|
|
p-TEFb
|
positive transcription elongation
factor b;
|
|
shRNA
|
short hairpin RNA;
|
|
siRNA
|
small interfering RNA
|
References
|
1.
|
Hall EJ: Hyperthermia. Radiobiology for
the Radiologist. 5th edition. Lippincott Williams and Wilkins;
Philadelphia, PA: pp. 495–520. 2000
|
|
2.
|
van der Zee J, González González D, van
Rhoon GC, van Dijk JD, van Putten WL and Hart AA: Comparison of
radiotherapy alone with radiotherapy plus hyperthermia in locally
advanced pelvic tumours: a prospective, randomised, multicentre
trial. Dutch Deep Hyperthermia Group Lancet. 355:1119–1125.
2000.PubMed/NCBI
|
|
3.
|
Harima Y, Nagata K, Harima K, Ostapenko
VV, Tanaka Y and Sawada S: A randomized clinical trial of radiation
therapy versus thermoradiotherapy in stage IIIB cervical carcinoma.
Int J Hyperthermia. 17:97–105. 2001. View Article : Google Scholar
|
|
4.
|
Wust P, Hildebrandt B, Sreenivasa G, et
al: Hyperthermia in combined treatment of cancer. Lancet Oncol.
3:487–497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Issels RD: Hyperthermia adds to
chemotherapy. Eur J Cancer. 44:2546–2554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zagar TM, Oleson JR, Vujaskovic Z, et al:
Hyperthermia combined with radiation therapy for superficial breast
cancer and chest wall recurrence: a review of the randomised data.
Int J Hyperthermia. 26:612–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Westermann A, Mella O, Van Der Zee J, et
al: Long-term survival data of triple modality treatment of stage
IIB-III-IVA cervical cancer with the combination of radiotherapy,
chemotherapy and hyperthermia - an update. Int J Hyperthermia.
28:549–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rhee JG, Schuman VL, Song CW and Levitt
SH: Difference in the thermotolerance of mouse mammary carcinoma
cells in vivo and in vitro. Cancer Res. 47:2571–2575.
1987.PubMed/NCBI
|
|
9.
|
Dings RP, Loren ML, Zhang Y, et al: Tumour
thermotolerance, a physiological phenomenon involving vessel
normalisation. Int J Hyperthermia. 27:42–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li GC, Mivechi NF and Weitzel G: Heat
shock proteins, thermotolerance, and their relevance to clinical
hyperthermia. Int J Hyperthermia. 11:459–488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cheng L, Smith DJ, Anderson RL and Nagley
P: Human neuroblastoma SH-SY5Y cells show increased resistance to
hyperthermic stress after differentiation, associated with elevated
levels of Hsp72. Int J Hyperthermia. 27:415–426. 2011. View Article : Google Scholar
|
|
12.
|
Mosser DD and Morimoto RI: Molecular
chaperones and the stress of oncogenesis. Oncogene. 23:2907–2918.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lindquist S and Craig EA: The heat-shock
proteins. Annu Rev Genet. 22:631–677. 1988. View Article : Google Scholar
|
|
14.
|
Hartl FU: Molecular chaperones in cellular
protein folding. Nature. 381:571–579. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Beere HM: ‘The stress of dying’: the role
of heat shock proteins in the regulation of apoptosis. J Cell Sci.
117:2641–2651. 2004.
|
|
16.
|
Richter K, Haslbeck M and Buchner J: The
heat shock response: life on the verge of death. Mol Cell.
40:253–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Morimoto RI: Regulation of the heat shock
transcriptional response: cross talk between a family of heat shock
factors, molecular chaperones, and negative regulators. Genes Dev.
12:3788–3796. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Akerfelt M, Morimoto RI and Sistonen L:
Heat shock factors: integrators of cell stress, development and
lifespan. Nat Rev Mol Cell Biol. 11:545–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hoang AT, Huang J, Rudra-Ganguly N, et al:
A novel association between the human heat shock transcription
factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol.
156:857–864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tang D, Khaleque MA, Jones EL, et al:
Expression of heat shock proteins and heat shock protein messenger
ribonucleic acid in human prostate carcinoma in vitro and in tumors
in vivo. Cell Stress Chaperones. 10:46–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Khaleque MA, Bharti A, Gong J, et al: Heat
shock factor 1 represses estrogen-dependent transcription through
association with MTA1. Oncogene. 27:1886–1893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Dudeja V, Chugh RK, Sangwan V, et al:
Prosurvival role of heat shock factor 1 in the pathogenesis of
pancreatobiliary tumors. Am J Physiol Gastrointest Liver Physiol.
300:G948–G955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Santagata S, Hu R, Lin NU, et al: High
levels of nuclear heat-shock factor 1 (HSF1) are associated with
poor prognosis in breast cancer. Proc Natl Acad Sci USA.
108:18378–18383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ishiwata J, Kasamatsu A, Sakuma K, et al:
State of heat shock factor 1 expression as a putative diagnostic
marker for oral squamous cell carcinoma. Int J Oncol. 40:47–52.
2012.PubMed/NCBI
|
|
25.
|
Mendillo ML, Santagata S, Koeva M, et al:
HSF1 drives a transcriptional program distinct from heat shock to
support highly malignant human cancers. Cell. 150:549–562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gabai VL, Meng L, Kim G, Mills TA,
Benjamin IJ and Sherman MY: Heat shock transcription factor Hsf1 is
involved in tumor progression via regulation of hypoxia-inducible
factor 1 and RNA-binding protein HuR. Mol Cell Biol. 32:929–940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jin X, Moskophidis D and Mivechi NF: Heat
shock transcription factor 1 is a key determinant of HCC
development by regulating hepatic steatosis and metabolic syndrome.
Cell Metab. 14:91–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Fujimoto M, Takaki E, Takii R, et al: RPA
Assists HSF1 access to nucleosomal DNA by recruiting histone
chaperone FACT. Mol Cell. 48:182–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
McMillan DR, Xiao X, Shao L, Graves K and
Benjamin IJ: Targeted disruption of heat shock transcription factor
1 abolishes thermotolerance and protection against heat-inducible
apoptosis. J Biol Chem. 273:7523–7528. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Luft JC, Benjamin IJ, Mestril R and Dix
DJ: Heat shock factor 1-mediated thermotolerance prevents cell
death and results in G2/M cell cycle arrest. Cell Stress
Chaperones. 6:326–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhang Y, Huang L, Zhang J, Moskophidis D
and Mivechi NF: Targeted disruption of hsf1 leads to lack of
thermotolerance and defines tissue-specific regulation for
stress-inducible Hsp molecular chaperones. J Cell Biochem.
86:376–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wang JH, Yao MZ, Gu JF, Sun LY, Shen YF
and Liu XY: Blocking HSF1 by dominant-negative mutant to sensitize
tumor cells to hyperthermia. Biochem Biophys Res Commun.
290:1454–1461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Xia W, Vilaboa N, Martin JL, Mestril R,
Guo Y and Voellmy R: Modulation of tolerance by mutant heat shock
transcription factors. Cell Stress Chaperones. 4:8–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Westerheide SD, Kawahara TL, Orton K and
Morimoto RI: Triptolide, an inhibitor of the human heat shock
response that enhances stress-induced cell death. J Biol Chem.
281:9616–9622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nakamura Y, Fujimoto M, Hayashida N, Takii
R, Nakai A and Muto M: Silencing HSF1 by short hairpin RNA
decreases cell proliferation and enhances sensitivity to
hyperthermia in human melanoma cell lines. J Dermatol Sci.
60:187–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Tabuchi Y, Furusawa Y, Wada S, Ohtsuka K
and Kondo T: Silencing heat shock transcription factor 1 using
small interfering RNA enhances mild hyperthermia and hyperthermia
sensitivity in human oral squamous cell carcinoma cells. Thermal
Med. 27:99–108. 2011. View Article : Google Scholar
|
|
38.
|
Rossi A, Ciafrè S, Balsamo M, Pierimarchi
P and Santoro MG: Targeting the heat shock factor 1 by RNA
interference: a potent tool to enhance hyperthermochemotherapy
efficacy in cervical cancer. Cancer Res. 66:7678–7685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Sakurai H and Enoki Y: Novel aspects of
heat shock factors: DNA recognition, chromatin modulation and gene
expression. FEBS J. 277:4140–4149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Westwood JT, Clos J and Wu C:
Stress-induced oligomerization and chromosomal relocalization of
heat-shock factor. Nature. 353:822–827. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Mariner PD, Walters RD, Espinoza CA, et
al: Human Alu RNA is a modular transacting repressor of mRNA
transcription during heat shock. Mol Cell. 29:499–509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Spriggs KA, Bushell M and Willis AE:
Translational regulation of gene expression during conditions of
cell stress. Mol Cell. 40:228–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Furusawa Y, Tabuchi Y, Wada S, Takasaki I,
Ohtsuka K and Kondo T: Identification of biological functions and
gene networks regulated by heat stress in U937 human lymphoma
cells. Int J Mol Med. 28:143–151. 2011.PubMed/NCBI
|
|
44.
|
Tabuchi Y, Wada S, Furusawa Y, Ohtsuka K
and Kondo T: Gene networks related to the cell death elicited by
hyperthermia in human oral squamous cell carcinoma HSC-3 cells. Int
J Mol Med. 29:380–386. 2012.PubMed/NCBI
|
|
45.
|
Tabuchi Y, Furusawa Y, Kariya A, Wada S,
Ohtsuka K and Kondo T: Common gene expression patterns responsive
to mild temperature hyperthermia in normal human fibroblastic
cells. Int J Hyperthermia. 29:38–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Jin X, Eroglu B, Moskophidis D and Mivechi
NF: Targeted deletion of Hsf1, 2, and 4 genes in mice. Methods Mol
Biol. 787:1–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Xiao X, Zuo X, Davis AA, et al: HSF1 is
required for extra-embryonic development, postnatal growth and
protection during inflammatory responses in mice. EMBO J.
18:5943–5952. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Homma S, Jin X, Wang G, et al:
Demyelination, astrogliosis, and accumulation of ubiquitinated
proteins, hallmarks of CNS disease in hsf1-deficient mice. J
Neurosci. 27:7974–7986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Zou Y, Zhu W, Sakamoto M, et al: Heat
shock transcription factor 1 protects cardiomyocytes from
ischemia/reperfusion injury. Circulation. 108:3024–3030. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Takaki E, Fujimoto M, Sugahara K, et al:
Maintenance of olfactory neurogenesis requires HSF1, a major heat
shock transcription factor in mice. J Biol Chem. 281:4931–4937.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Kallio M, Chang Y, Manuel M, et al: Brain
abnormalities, defective meiotic chromosome synapsis and female
subfertility in HSF2 null mice. EMBO J. 21:2591–2601. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Fujimoto M, Izu H, Seki K, et al: HSF4 is
required for normal cell growth and differentiation during mouse
lens development. EMBO J. 23:4297–4306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Bishop NA and Guarente L: Genetic links
between diet and lifespan: Shared mechanisms from yeast to humans.
Nat Rev Genet. 8:835–844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Neef DW, Jaeger AM and Thiele DJ: Heat
shock transcription factor 1 as a therapeutic target in
neurodegenerative diseases. Nat Rev Drug Discov. 10:930–944. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Ciocca DR, Arrigo AP and Calderwood SK:
Heat shock proteins and heat shock factor 1 in carcinogenesis and
tumor development: an update. Arch Toxicol. 87:19–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Tabuchi Y, Kariya A, Yunoki T and Kondo T:
Genes involved in the cell death induced by knockdown of heat shock
transcription factor 1 in human oral squamous cell carcinoma HSC-3
cells. Thermal Med. 28:29–42. 2012. View Article : Google Scholar
|
|
59.
|
Meng L, Gabai VL and Sherman MY:
Heat-shock transcription factor HSF1 has a critical role in human
epidermal growth factor receptor-2-induced cellular transformation
and tumorigenesis. Oncogene. 29:5204–5213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Hahn JS, Hu Z, Thiele DJ and Iyer VR:
Genome-wide analysis of the biology of stress responses through
heat shock transcription factor. Mol Cell Biol. 24:5249–5256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Trinklein ND, Murray JI, Hartman SJ,
Botstein D and Myers RM: The role of heat shock transcription
factor 1 in the genome-wide regulation of the mammalian heat shock
response. Mol Biol Cell. 15:1254–1261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Page TJ, Sikder D, Yang L, et al:
Genome-wide analysis of human HSF1 signaling reveals a
transcriptional program linked to cellular adaptation and survival.
Mol Biosyst. 2:627–639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Min JN, Huang L, Zimonjic DB, Moskophidis
D and Mivechi NF: Selective suppression of lymphomas by functional
loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors.
Oncogene. 26:5086–5097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Aghdassi A, Phillips P, Dudeja V, et al:
Heat shock protein 70 increases tumorigenicity and inhibits
apoptosis in pancreatic adenocarcinoma. Cancer Res. 67:616–625.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Yoon YJ, Kim JA, Shin KD, et al: KRIBB11
inhibits HSP70 synthesis through inhibition of heat shock factor 1
function by impairing the recruitment of positive transcription
elongation factor b to the hsp70 promoter. J Biol Chem.
286:1737–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Xia Y, Liu Y, Rocchi P, et al: Targeting
heat shock factor 1 with a triazole nucleoside analog to elicit
potent anticancer activity on drug-resistant pancreatic cancer.
Cancer Lett. 318:145–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Whitesell L and Lindquist S: Inhibiting
the transcription factor HSF1 as an anticancer strategy. Expert
Opin Ther Targets. 13:469–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Xia Y, Rocchi P, Iovanna JL and Peng L:
Targeting heat shock response pathways to treat pancreatic cancer.
Drug Discov Today. 17:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Chen SS, Michael A and Butler-Manuel SA:
Advances in the treatment of ovarian cancer: a potential role of
antiinflammatory phytochemicals. Discov Med. 13:7–17.
2012.PubMed/NCBI
|
|
70.
|
Matsumoto H, Hayashi S, Shioura H, et al:
Suppression of heat-induced HSF activation by CDDP in human
glioblastoma cells. Int J Radiat Oncol Biol Phys. 41:915–920. 1998.
View Article : Google Scholar : PubMed/NCBI
|