Introduction
Aseptic implant loosening is the most common cause
of revision of total hip endoprosthesis (1). It is mainly caused by wear particles
generated at the articulating surfaces of implant components and at
the interface between implant and bone or implant and bone cement
(2). The biological effects of
wear particles depend on the implant material, as well as on the
amount, shape and composition of the particles (3). Generally, elongated particles
generate more pro-inflammatory responses than round particles, and
there is a growing consensus that metallic particles generate more
pro-inflammatory responses than polymers or ceramics (3). They have to be <10 μm in
diameter to be phagocytosable, leading to specific cytokine release
and a decrease in osteoblast function and an increase in osteoclast
activity (3). It is well known
that metallic wear particles influence the production of cytokines,
such as interleukin (IL)-6 and IL-8, monocyte chemotactic protein-1
(MCP-1), fibroblast growth factor (FGF) and vascular endothelial
growth factor (VEGF) (4–7). IL-6 is important in bone metabolism,
having a positive effect on the differentiation of progenitor cells
into osteoblasts and an anti-apoptotic effect, additionally
(8). Through another signalling
pathway, IL-6 can also stimulate the differentiation of osteoclasts
(8); in combination with the
macrophage colony-stimulating factor (MCSF), the differentiation
and activity of osteoclasts is increased (9).
Furthermore, it is also known that ultrahigh
molecular weight polyethylene (UHMWPE) particles inhibit the
proliferation and differentiation of pre-osteoblasts and bone
marrow cells in vitro (10). Nowadays, the age of the patients
is also considered to play a role in the effects of wear particles
(11). Hereby, many attempts to
reduce the incidence of implant loosening aim at optimising the
material and surface of implants, as well as their fixation in the
bone stock. Therefore, a variety of bone cements are available for
the cemented fixation of total hip endoprosthesis. To date, little
attention has been paid to the biological effects of bone cement
wear particles produced by abrasive wear or relative motion at bone
cement interfaces (12). In this
context, only a few studies have compared the biological effects of
clinically relevant wear particles of different bone cements on
human histiocytes and macrophages (4,12).
Polymethylmethacrylate (PMMA) is the main component
of bone cement and does not induce apoptosis in osteoclasts
(13). Particles of
hydroxyapatite, however, can induce apoptosis in osteoclasts
(14). Zambonin et al
(15) examined the effect of PMMA
on human osteoblasts. It was found that PMMA inhibits cell
proliferation and collagen synthesis. They further observed an
increased release of IL-6 and osteocalcin, which in turn increases
the activity of osteoclasts and thus complicates the integration of
implants into the bone stock (15). In addition, Gough and Downes
(16) demonstrated that,
depending on the concentration, methacrylate polymers exert a
pro-apoptotic effect on osteoblasts. Willert et al (17) observed the necrosis of bone tissue
surrounding cemented implants. Ciapetti et al (18,19) observed necrosis and apoptosis in
HL-60 cells, a human promyelocytic cell line, as well as in MG-63
cells, a human osteosarcoma cell line, following incubation with
different bone cements.
The effect of wear particles generated at the
interface between bone cement (Palacos® R) containing
zirconium dioxide (ZrO2) and total hip stems made of
alloys of cobalt-chromium-molybdenum and titanium was examined in
our previous study [Lenz et al (20)]. Here, human osteoblasts showed
decreased viability and procollagen type I synthesis, as well as a
material-and time-dependent release of IL-6 and IL-8. Additionally,
an inhibited release of cytokine and procollagen type I synthesis
depending on the concentration of the cement was observed. In
particular, wear particles generated from cobalt-chromium stems had
a negative effect on the viability and proliferation of human
osteoblasts, whereas ZrO2 particles did not have a
significant effect when compared to the control sample (20).
However, cell viability and cytokine release by
primary osteoblasts following incubation with wear particles
generated at the interface between total hip stems and different
bone cements have not been investigated thus far. Hence, the aim of
this experimental study was to determine the viability and
proliferation of human osteoblasts following incubation with wear
particles generated with 3 commercially available bone cements of
different compositions. In our previous studies [Lochner et
al (21) and Lenz et
al (20)], we investigated
the effect of several metallic wear debris on primary osteoblasts.
In our present in vitro study, we evaluated the effect of
bone cement particles on cytokine expression, particularly IL-6,
IL-8, FGF, VEGF and MCP-1, on the synthesis of procollagen type I
and on the occurrence of apoptosis and necrosis in osteoblasts
(20,21).
Materials and methods
Isolation and cultivation of human
primary osteoblasts
Human femoral heads were obtained from 3 patients (2
male donors, mean age, 69.5±4.5 years; 1 female donor, 47 years)
undergoing primary total hip replacement due to primary
osteoarthritis. The bone samples were collected after patient
consent was obtained and following approval by the local ethics
committee (A 2010-10).
For the isolation and cultivation of human
osteoblasts, cancellous bone was harvested from the inside of the
femoral heads, washed 3 times with phosphate-buffered saline (PBS)
(PAA Laboratories GmbH, Coelbe, Germany) and cut into small pieces.
Cells from different donors were kept separately.
Subsequently, the cancellous bone was treated with
Dulbecco’s modified Eagle’s medium (DMEM; Biochrom AG, Berlin,
Germany) containing collagenase A and dispase (both from Roche
Diagnostics GmbH, Mannheim, Germany) at a ratio of 1:2:1 at 37°C
for 3 h. The cell suspension was filtered through a cell strainer
(pore size, 70 μm; Nunc, Wiesbaden, Germany) and centrifuged
at 118 × g for 10 min. The resulting cell pellet was resuspended in
complete medium containing 10% fetal bovine serum (FBS), 1%
amphotericin B, 1% penicillin-streptomycin and 1% HEPES buffer (all
from Gibco®-Invitrogen, Darmstadt, Germany) and
transferred into 25 cm2 culture flasks with 8 ml of
complete medium supplemented with ascorbic acid (final
concentration, 50 μg/ml), β-glycerophosphate (final
concentration, 10 mM) and dexamethasone (final concentration, 100
nM) (all from Sigma, Seelze, Germany). Cells were incubated in a
humidified atmosphere of 5% CO2 and 37°C. The cell
culture medium was changed every second day to remove non-adherent
cells. Cell proliferation was tracked microscopically. As cells
reached 90% confluency, they were trypsinised and split at a ratio
of 1:6.
Cell seeding and particle
incubation
For all analyses, human osteoblasts
(3×103 cells) were transferred to 96-well culture plates
and incubated in complete medium as mentioned above at 37°C and 5%
CO2. Subsequently, osteoblasts were incubated with
different bone cement particles at 2 different concentrations (0.1
and 0.01 mg/ml). The particles dropped to the bottom of the wells
and came into contact with the adherent osteoblasts. After 2 and 4
days of incubation, supernatants were collected and stored at
−20°C.
Generation and preparation of wear
particles
Particles of three different commercially available
bone cement products were used in this experimental setting. Wear
particles were generated using a special test apparatus as
previously described (2). With
cyclic loading of 5 Hz, a surface pressure of 2 MPa and 3 million
cycles, smoothly polished hip stems made of 316L stainless steel
(type Exeter; Stryker GmbH, Duisburg, Germany) were rubbed against
the cement mantles. The following cements were used in the
production of cement mantles: Palacos R (Heraeus, Wehrheim,
Germany), Cemex® Genta (Exactech, Kiel, Germany) and
Simplex™ P (Stryker GmbH). The composition of each type of cement
is shown in Table I.
 | Table I.Content of used bone cements as
described in a previous study (24). |
Table I.
Content of used bone cements as
described in a previous study (24).
| Cement | Polymer and
monomer | Radiopaque
agent | Antibiotic
agent | Other
components |
|---|
| Palacos R | 33.55 g PMMA | 6.13 g
zirconium | - | 0.32 g
benzoyl-peroxide (BPO) |
| 18.4 g MMA | dioxide | | 1.4 mg
chlorophyll |
| | | 0.38 g
N,N-dimethyl-p-toluidine |
| Simplex P | 29.4 g
MMA-styrene | 4 g barium
sulphate | - | 0.6 g BPO |
| copolymer | | | 80 ppm
hydroquinone |
| 6 g PMMA, 18.31 g
MMA | | | 0.48 g
N,N-dimethyl-p-toluidine |
| Cemex Genta | 33.11 g PMMA | 4 g barium
sulphate | 1.69 g | 1.2 g BPO |
| (3% styrene) | | gentamicin
sulphate | 75 ppm
hydroquinone |
| 13.06 g MMA | | | 0.24 g
N,N-dimethyl-p-toluidine |
To determine the metal content of the generated
debris of cement and metal particles, we treated 5 mg of wear
debris with 5 ml nitric acid in an industrial microwave oven
(MDS-2000; CEM; Kamp-Linfort, Germany) for 1 h to break down
organic compounds. After cooling down at room temperature for 1 h,
the mixture was dissolved in 50 ml distilled water in order to
determine the metallic content through atomic absorption
spectroscopy (AAS; SpectrAA-30 Zeeman; Varian, Darmstadt, Germany).
AAS indicated the following average content of metal (iron) in
μg for every 1 g of wear particles: 350.00 μg/g for
Simplex P; 90.75 μg/g for Cemex Genta; and 2180.83
μg/g for Palacos R.
Pure PMMA polymer powder (Palacos R, referred to
below as PMMA) and ZrO2 particles (Heraeus) were used as
the reference particles. Five milligrams of each generated wear
particles and commercially available reference particles were
sterilized by the use of γ radiation. Subsequently, a stock
solution (10 mg/ml) was prepared by suspending the particles in 0.5
ml sterile PBS (PAA Laboratories GmbH). Further dilutions with cell
culture medium created the final particle concentrations of 0.1 and
0.01 mg/ml (21).
Procollagen type I (CICP)
quantification
Procollagen type I (Metra™ CICP EIA kit; Quidel,
Marburg, Germany) was detected in the supernatants using an
enzyme-linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions. Absorbance was measured at 405 nm
using an Opsys MR™ microplate reader (Dynex Technologies,
Denkendorf, Germany).
Quantification of cytokines
Osteoblast culture supernatants were analysed using
a Bio-Plex Pro™ assay (Bio-Plex Pro Human Cytokines Group I 5-plex
1×96; Bio-Rad, Munich, Germany) in order to determine the
expression of cytokines (IL-6, IL-8, FGF, VEGF and MCP-1). The
Bio-Plex system permits the simultaneous detection of up to 100
different types of molecules in a single well of a 96-well
microplate. The principle of the bead-based assay is similar to a
sandwich immunoassay. The assay was performed according to the
manufacturer’s instructions. An antibody directed towards the
desired cytokine target is covalently coupled to internally dyed
beads. These coupled beads react with the sample, which contains
the target biomolecule. Subsequently, a biotinylated detection
antibody specific to an epitope different from that of the capture
antibody is added to the assay, resulting in the formation of a
sandwich of antibodies around the cytokine target. A
streptavidin-phycoerythrin reporter complex is added to bind the
biotinylated detection antibodies to the bead surface. The
intensity of fluorescence was analysed in a Bio-Plex 200 System
using Bio-Plex Manager software 4.1.1 (both from Bio-Rad).
Analysis of apoptosis and
necrosis
Apoptosis and necrosis of the primary osteoblasts
were determined after 2 days using the Cell Death Detection ELISA
kit (Roche Diagnostics GmbH) according to the manufacturer’s
instructions. For necrosis, osteoblast culture supernatants were
analysed. Apoptosis was detected in the cell lysates. Absorbance
was measured at 490 nm using an Opsys MR™ microplate reader (Dynex
Technologies).
Statistical analysis
Descriptive statistics [mean value, standard error
of the mean (SEM)] were calculated for each data set. Statistical
analysis was performed using one-way ANOVA (post hoc LSD).
Significances of the respective protein data are based on the
unstimulated control on day 2. To compare day 2 with day 4,
statistical analysis was performed using the Wilcoxon test for
dependent variables. All P-values were the result of two-tailed
statistical tests, with values of P<0.05 considered to indicate
a statistically significant difference. All data were stored and
analysed using the statistics program SPSS version 15.0 (SPSS Inc.,
Chicago, IL, USA).
Results
Procollagen type I synthesis
The concentration of procollagen type I in the cell
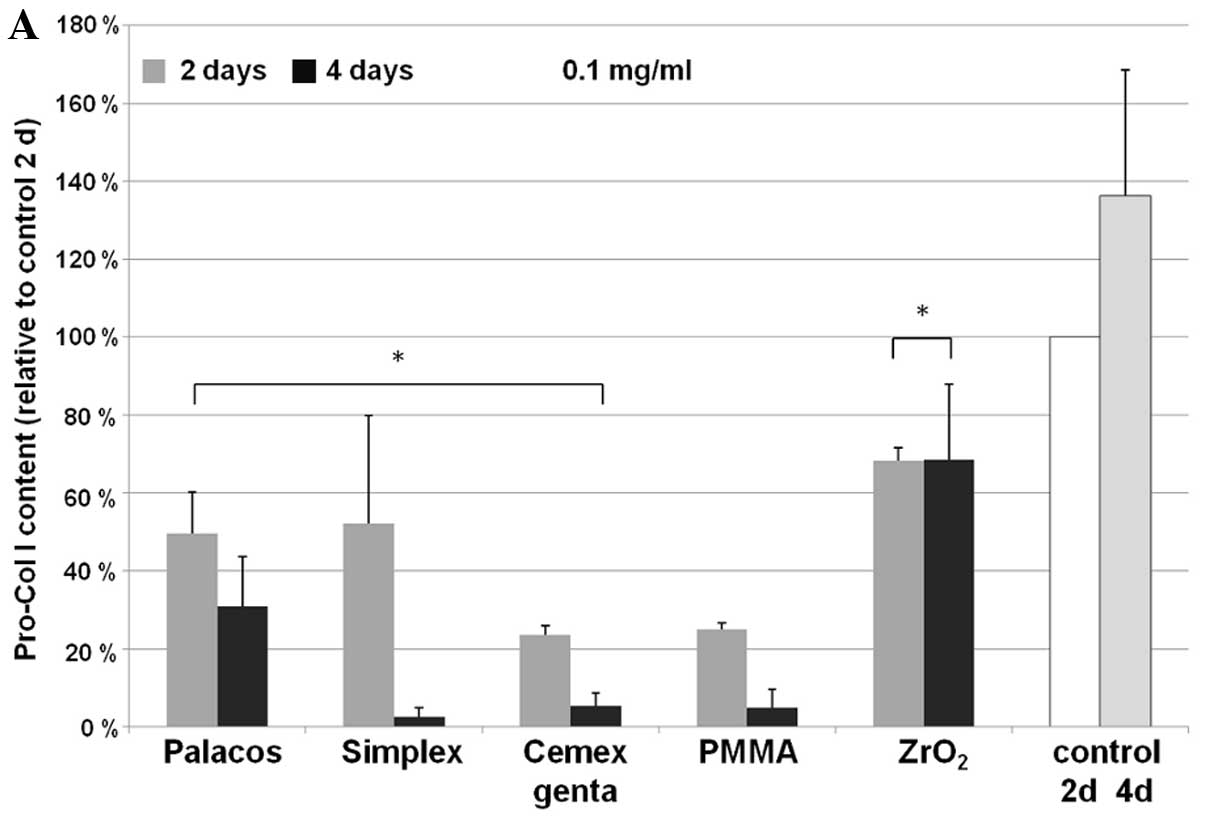
culture supernatants was determined (Fig. 1) in order to assess the synthesis
activity of the human osteoblasts. Following incubation with bone
cement particles, time- and concentration-dependent differences
were observed. For all bone cements examined, a particle
concentration of 0.1 mg/ml led to a significant decline in collagen
synthesis after 2 days when compared to the control sample (Palacos
R, P=0.013; Simplex P, P=0.017; Cemex Genta, P<0.001). For pure
ZrO2 particles, this effect was less pronounced
(P<0.001). After 4 days of incubation at a particle
concentration of 0.1 mg/ml, a significant reduction in procollagen
type I concentration (P<0.001) was observed with all the cement
particles. In particular, a pronounced reduction was observed with
Simplex P. Between days 2 and 4, Palacos R induced a less
significant decline in the synthesis rate compared to the other
bone cements (P<0.001). After 4 days of incubation with
ZrO2 particles, the synthesis of procollagen type I in
the osteoblasts did not differ significantly from that at 2 days of
incubation. Nevertheless, the total concentration was still lower
than the concentration of the day 2 control sample (P=0.005)
(Fig. 1).
Even low concentrations of cement particles (0.01
mg/ml) had a negative effect on collagen type I synthesis, although
this effect was not as pronounced as that induced by higher
particle concentrations. After 2 days of incubation with 0.01 mg of
particles/ml, there were small cement-specific differences. The
effects after 4 days of incubation with 0.01 mg/ml were similar to
those observed at 0.1 mg/ml.
Determination of cytokine release by
human osteoblasts
IL-6 and IL-8
Bone cement particles induced an increased
production of IL-6 and IL-8 by osteoblasts during the first 2 days
(Tables II and III). A time-dependent effect was
observed for all analysed bone cements, whereas differences in
particle concentrations were only observed with Cemex Genta. IL-6
concentrations were decreased in all the particle samples after 4
days compared to the control on day 2, which were significant with
the 0.1 mg/ml particle treatments. Additionally, the IL-6
concentration of the control on day 4 was significantly reduced
compared to that observed on day 2.
 | Table II.IL-6 synthesis in human osteoblasts
following treatment with particles (0.1 and 0.01 mg/ml; n=3) on
days 2 and 4. |
Table II.
IL-6 synthesis in human osteoblasts
following treatment with particles (0.1 and 0.01 mg/ml; n=3) on
days 2 and 4.
| Particle | Day 2 IL-6 content
(relative to control on day 2)
| Day 4 IL-6 content
(relative to control on day 2)
|
|---|
| 0.1 mg/ml (%) | 0.01 mg/ml (%) | 0.1 mg/ml (%) | 0.01 mg/ml (%) |
|---|
| Palacos | 143±19 | 101±3 | 56±18a | 53±9 |
| Simplex | 153±28 | 148±19 | 31±7a | 77±30 |
| Cemex Genta | 269±115 | 127±7 | 23±4a | 84±36 |
| PMMA | 413±258 | 149±67 | 32±9a | 79±28 |
|
ZrO2 | 125±15 | 145±21 | 58±17a | 52±11 |
| Control | 100±0 | 57±14a |
 | Table III.IL-8 synthesis in human osteoblasts
following treatment with particles (0.1 and 0.01 mg/ml; n=3) on
days 2 and 4. |
Table III.
IL-8 synthesis in human osteoblasts
following treatment with particles (0.1 and 0.01 mg/ml; n=3) on
days 2 and 4.
| Particle | Day 2 IL-8 content
(relative to control on day 2)
| Day 4 IL-8 content
(relative to control on day 2)
|
|---|
| 0.1 mg/ml (%) | 0.01 mg/ml (%) | 0.1 mg/ml (%) | 0.01 mg/ml (%) |
|---|
| Palacos | 417±282 | 97±11 | 55±9 | 51±7 |
| Simplex | 116±71 | 114±3 | 73±29 | 71±16 |
| Cemex Genta | 155±44 | 100±11 | 42±13 | 96±34 |
| PMMA | 354±217 | 109±13 | 67±30 | 95±33 |
|
ZrO2 | 136±23 | 200±92 | 60±16a | 60±11 |
| Control | 100±0 | 55±9 |
IL-8 expression showed an increase in the samples
incubated with 0.1 mg/ml Palacos R and PMMA after 2 days. Following
prolonged particle incubation, all samples demonstrated a decrease
in IL-8 expression. At the lower Cemex Genta and PMMA
concentrations (0.01 mg/ml), no significant differences were
observed compared to the control sample on day 2. For
ZrO2, an increased IL-8 synthesis was initially observed
after 2 days, which was even higher with the lower particle
concentration. After 4 days of incubation, a significant decrease
in IL-8 expression was observed in the samples incubated with
ZrO2 at a concentration of only 0.1 mg/ml.
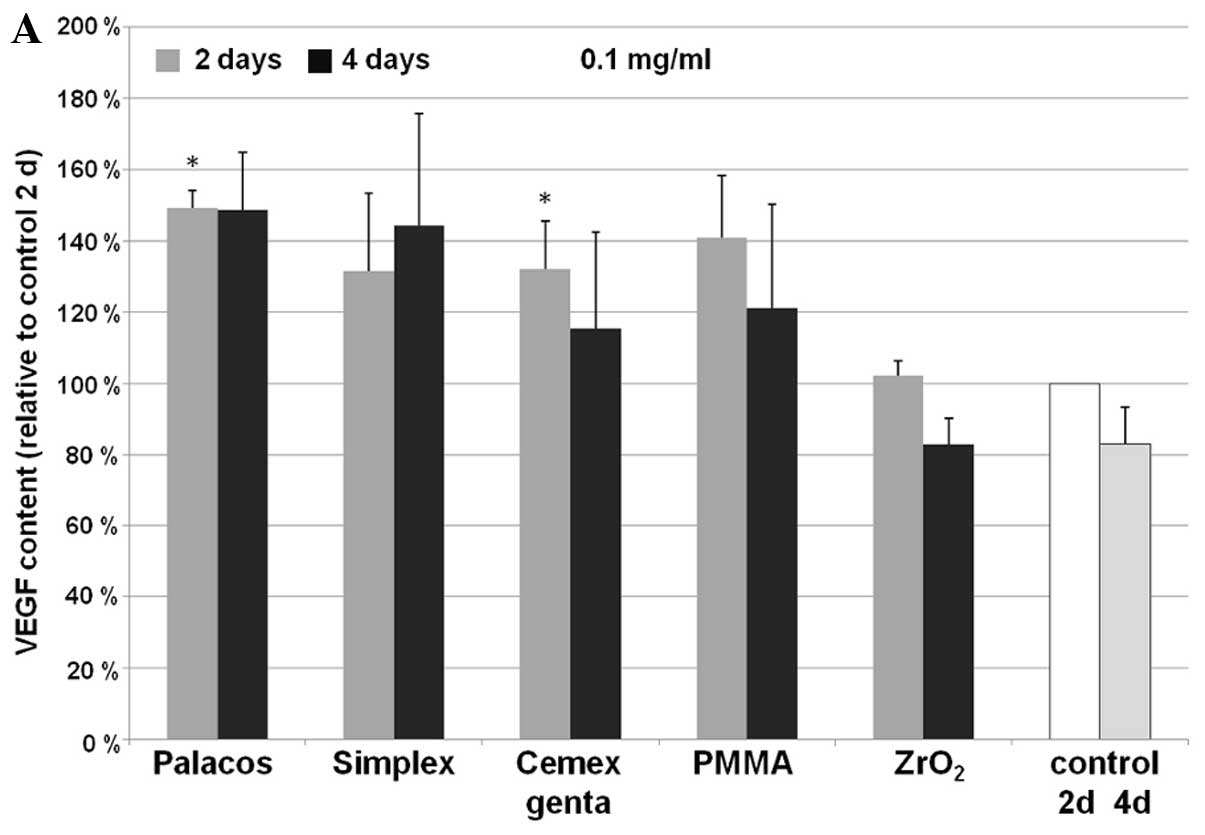
VEGF and FGF
Following treatment with all bone cement particles,
an increase in VEGF expression was observed. This increase was
significant in the samples incubated with Palacos (P=0.02) and
Cemex Genta (P=0.046) (both, 0.1 mg/ml), as well as in those
incubated with Simplex P (0.01 mg/ml; P=0.017) after 2 days of
incubation. After 4 days, concentration-dependent differences were
observed with all used particles (Fig. 2). In the approach described, no
expression of FGF was detected in the human osteoblasts.
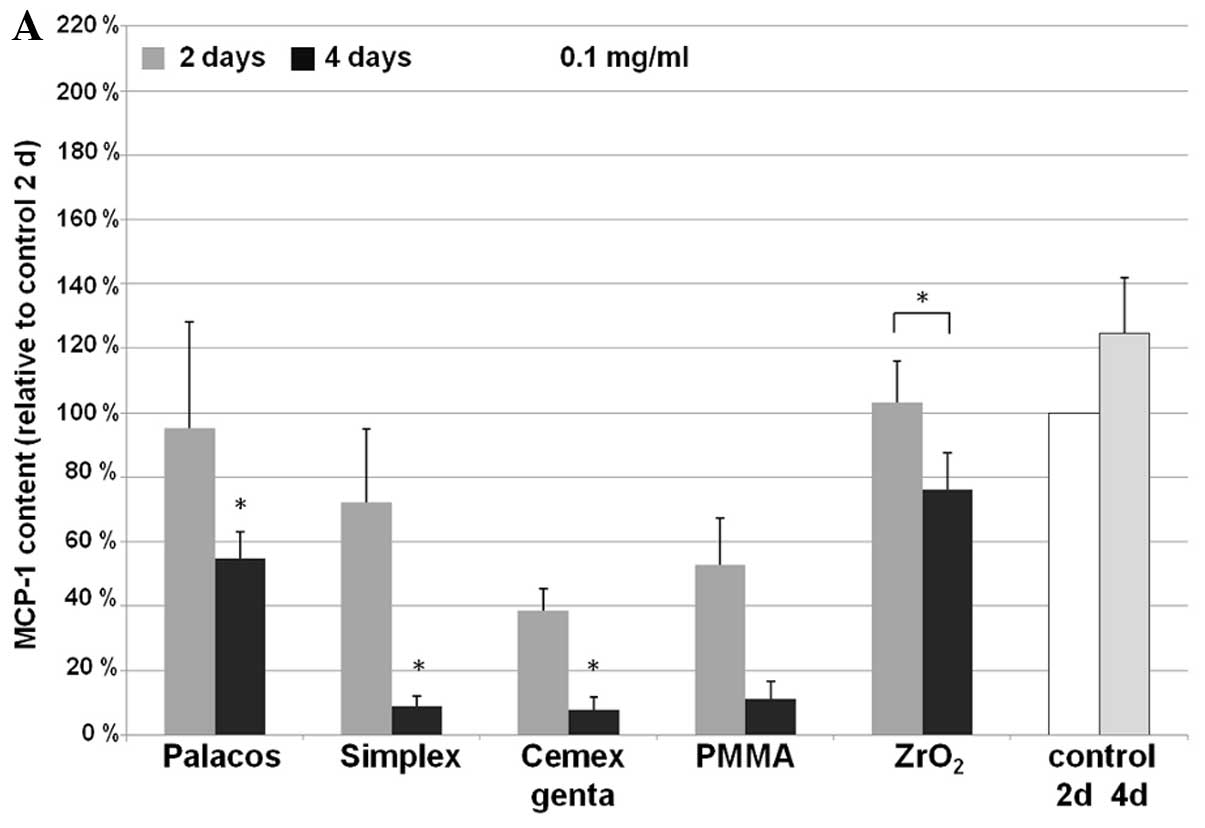
MCP-1
For the expression of MCP-1, cement-specific
differences were observed (Fig.
3). A significant, time-dependent decrease in MCP-1
concentration was observed with all bone cements used (Palacos R,
P=0.003; Simplex P, P<0.001; Cemex Genta, P<0.001) at higher
particle rates compared to the untreated control. Osteoblast
cultures treated with 0.01 mg/ml Simplex P (P=0.014) and Cemex
Genta (P=0.012) expressed significantly lower MCP-1 levels after 4
days of incubation. Compared to the control, MCP-1 synthesis was
not considerably increased following treatment with the
particles.
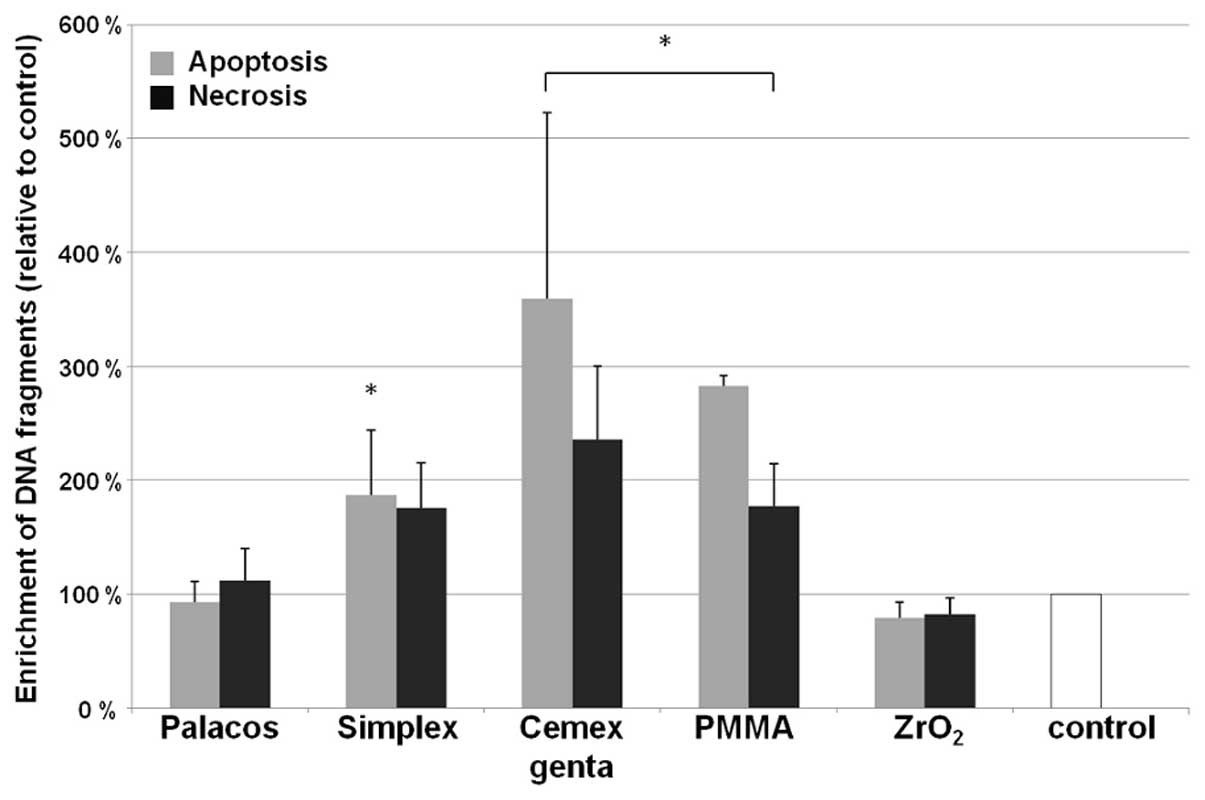
Apoptosis and necrosis
The incidence of apoptosis and necrosis also
differed depending on the type of cement and its concentration in
the sample (Fig. 4). A high rate
of apoptosis of human osteoblasts only occurred with high
concentrations (0.1 mg/ml) of wear particles of Simplex P, Cemex
Genta and PMMA powder (P<0.001). Cement-dependent differences
were significant; Cemex Genta induced the highest rate of
apoptosis, followed by PMMA and Simplex P (all, P<0.001). No
significant difference in apoptosis were observed in the samples
treated with all particle types at lower concentrations (0.01
mg/ml) compared to the control sample.
Apart from apoptosis, necrosis in the osteoblasts
was detected following incubation with bone cement particles. This
was particularly evident following incubation with Cemex Genta and
PMMA powder (both, P<0.001). No significant change in the rate
of necrosis was observed in the samples treated with Simplex P,
Palacos R and ZrO2 compared to the control sample. A
comparison of absolute values of apoptosis and necrosis showed that
human osteoblasts were more prone to apoptosis than necrosis
following incubation with particles of Simplex P, PMMA and Cemex
Genta.
Discussion
In the present experimental study, reactions
specific to different bone cement particles were observed with
respect to viability, collagen synthesis and cytokine expression in
osteoblast cultures. According to Gough and Downes (16) bone cement particles can cause
apoptosis in osteoblasts. Our research data underline these
effects, whereas apoptosis following incubation with lower particle
concentrations showed no effects. At higher concentrations,
however, differences depending on the type of cement used were
observed. It has been shown that higher concentrations of such
particles in areas surrounding hip implants affect osteolysis
(22). Palacos R particles showed
the least pro-apoptotic potential. Our data were similar as regards
the necrosis of osteoblasts; i.e., Palacos R revealed the least
variation from the control sample. The other cement particle types
showed increased necrosis at higher particle concentrations and
affected osteoblast function more severely. If the capacity for
collagen synthesis is also taken into account as an indicator of
osteoblast viability, it becomes evident that cement particles have
a particularly strong effect at higher concentrations. The
concentration of cement particles is thus extremely important for
cell reactions. A high particle concentration tends to be conducive
to cellular processes that have a negative influence on osteoblast
function and thus on the implant fixation in the bone stock. These
aspects confirmed the findings of Lenz et al (20) who analysed the effects of metallic
wear particles on the viability of osteoblasts. They found that
ZrO2 ceramic particles did not negatively affect cell
viability. This suggests that other components, such as bone cement
particles have stronger effects on osteoblasts. Our experiments, as
well as the findings of other research groups have demonstrated
that PMMA exerts a negative effect on the viability of osteoblasts
(15,16). However, the cytotoxic effect is
reduced following polymerisation (23). The PMMA content in polymerised
bone cements varies insignificantly, and thus other factors must be
taken into account (24).
Analyses of the cements have revealed that the toxic residual
monomer content in Simplex P and Cemex Genta is similar, but much
higher than in Palacos R (24).
This could be a cause of the observed cement-specific effects. The
relevance of the radiopaque agent used must be taken into account
as well. As regards the incidence of necrosis and apoptosis, in
this study, no difference compared to the control sample was
observed with cements containing ZrO2 as a radiopaque
agent. However, we found elevated levels in cements containing
barium sulphate (BaSO4) as a radiopaque agent.
BaSO4-containing cements showed a negative effect on
procollagen type I synthesis in comparison with ZrO2. As
regards cytokine expression, this difference only became apparent
after a longer incubation time (4 days). In this case, a decline in
cytokine release was observed with cements containing
BaSO4 as a radiopaque agent. Gillani et al
(25) demonstrated that
ZrO2 has a more favourable effect on osteoblast cell
density than BaSO4. The cytotoxic effect of the
antibiotic, gentamicin, on osteoblasts is less relevant (26). Only in combination with
BaSO4 does it amplify the triggered effect.
PMMA has been shown to exert a negative effect on
osteoblasts (15,16). In our study, this was only
observed with higher particle concentrations. Since such
concentrations may occur in situ after longer periods of
time, it may be assumed that this effect does not substantially
affect initial fixation but may well influence implant loosening at
a later stage.
Cytokine-mediated osteolytic processes are relevant
to aseptic implant loosening. The disruption of osteoblast
viability causes a decreased capability of repair. An important
aspect is cytokine expression. It is known that IL-6 positively
affects the differentiation of osteoblast progenitor cells, has an
anti-apoptotic effect and can stimulate the differentiation of
osteoclasts (8). Following the
addition of cement particles, the secretion of IL-6 is initially
increased. The above-mentioned anti-apoptotic effect on osteoblasts
can occur. Since there is a reduction in IL-6 after longer periods
of time, particularly at higher particle concentrations, the
anti-apoptotic effect may be negatively affected. Another
signalling pathway may be involved in the activation of osteoclasts
(8). A stronger expression of
IL-6 has been observed with wear particles with a lower metal
content (Simplex P and Cemex Genta). This suggests that the other
components (e.g., BaSO4 and PMMA) have a greater
influence on IL-6 expression.
Another factor that influences aseptic implant
loosening is an increased expression of MCP-1 and IL-8. Both
cytokines can stimulate the migration of macrophages and
neutrophile granulocytes (4). In
our study, for MCP-1, a decreased or rather the same synthesis rate
in osteoblasts following treatment with the particles was observed
compared to the untreated cells. These results are in agreement
with those by Lochner et al (21) who analysed the expression rate of
MCP-1 following treatment with metallic wear particles. Since
osteoblasts are able to express MCP-1, and MCP-1 induction was not
observed following treatment, they are therefore not associated
with osteoclast differentiation by macrophage attraction (21). By contrast, IL-8 synthesis rates
were initially increased following treatment with particles at
higher concentrations. Hereby, an initially induced secretion by
osteoblasts stimulated osteoclasts to migrate into the
periprosthetic tissue (21). In
agreement with the study by Lochner et al (21), it has been shown that IL-8
expression decreases with incubation time, as shown in the study by
Fritz et al (27). These
results suggest that osteoclast stimulation is associated with the
early stages of aseptic implant loosening.
If the metal content of the cement wear debris is
taken into account, it is evident that wear particles with a high
metal content per unit of quantity cause a higher rate of cell
death than those with a lower metal content (20). The relevance of the type of
metallic wear components and the influence on viability and
cytokine expression has been described in detail in our previous
studies [Lenz et al (20)
and Lochner et al (21)].
However, in our present study, a higher metal content in wear
debris using Palacos R cement did not lead to an increased
expression of cytokines or chemokines compared to the other bone
cements examined. Furthermore, wear debris with a high metal
content from Palacos R cement caused a lower rate of cell death
compared to the other cements with a lower metal content (e.g.,
Cemex Genta, Simplex P). We assume that this effect is due to the
fact that ZrO2 is the radiopaque agent in Palacos R
cement. Hence, the effect of these agents (ZrO2 vs.
BaSO4) may be of greater importance than the metal content of the
bone cement debris. The concentration and type of metal particles,
however, is of significant relevance for the expression of
procollagen and cytokines, even when identical bone cement is used.
Lenz et al (20)
demonstrated that wear particles with a high content of
cobalt-chromium have a stronger negative impact on expression than
wear particles containing titanium. The release of VEGF, which is
necessary for angiogenesis, was generally increased as a reaction
to the wear particles, with the exception of ZrO2.
Angiogenesis helps by quickly bringing cellular defence to the
region of interest (7,28). Cement particles can also trigger
this reaction. FGF, which is important in fracture healing and is
usually produced by chondrocytes, is not synthesised by osteoblasts
(7). Accordingly, we did not
determine any FGF levels following treatment with the
particles.
As previously described in the study by Ciapetti
et al (18), cement
particles lead to specific differences in terms of cell viability
and cytokine production. Our data show that the content of PMMA,
radiopaque agents and metallic particles are all relevant. The use
of the antibiotic, gentamicin, intensified the observed effects of
bone cement containing BaSO4. The content of PMMA in
wear particles can thus be relevant to the biocompatibility of the
bone cement used. A limitation of our present study was that the
influence of residual monomer content and antibiotics was not
examined separately. Hence, the effects of specific bone cement
components should be the subject of further studies. It is known
that the size and form of wear debris affects cellular reactions
(3). Nevertheless, particle size
and form were not analysed in the present study. Thus, no statement
to a potential effect caused by an imbalance of particle size and
form can be given. Moreover, we investigated the effect of the wear
debris as a whole on osteoblasts. The different components within
the wear debris (metal vs. bone cement) may have an effect on cell
behaviour; therefore, an investigation of separate particles is
recommended in future studies.
In conclusion, the type of wear particles is of
great relevance for adverse biological and tissue reactions in the
human body. Since particle concentration plays an important role,
the amount of wear debris generated by cemented implants and the
composition of wear particles are relevant influencing factors for
the occurrence of osteolysis and subsequent aseptic loosening
following total hip endoprosthesis.
Acknowledgements
We thank Ms. Susanne Finze for
statistical analysis and Mr. Andreas Fritsche for the preparation
of bone cement particles.
References
|
1.
|
Herberts P and Malchau H: Long-term
registration has improved the quality of hip replacement: a review
of the Swedish THR Register comparing 160,000 cases. Acta Orthop
Scand. 71:111–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bader R, Steinhauser E, Holzwarth U,
Schmitt M and Mittelmeier W: A novel test method for evaluation of
the abrasive wear behavior of total hip stems at the interface
between implant surface and bone cement. Proc Inst Mech Eng H.
218:223–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hallab NJ and Jacobs JJ: Biologic effects
of implant debris. Bull NYU Hosp Jt Dis. 67:182–188. 2004.
|
|
4.
|
Ingham E and Fisher J: Biological
reactions to wear debris in total joint replacement. Proc Inst Mech
Eng H. 214:21–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Queally JM, Devitt BM, Butler JS, Malizia
AP, Murray D, Doran PP and O’Byrne JM: Cobalt ions induce chemokine
secretion in primary human osteoblasts. J Orthop Res. 27:855–864.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Emes Y, Aybar B, Vural P, Saral NY, Atalay
B, Kaya AS, Issever H, Yalcin S and Bilir A: Effects of bone
morphogenetic proteins on osteoblast cells: vascular endothelial
growth factor, calcium, inorganic phosphate, and nitric oxide
levels. Implant Dent. 19:419–427. 2010. View Article : Google Scholar
|
|
7.
|
Schmid GJ, Kobayashi C, Sandell LJ and
Ornitz DM: Fibroblast growth factor expression during skeletal
fracture healing in mice. Dev Dyn. 238:766–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004.
|
|
9.
|
Neale SD, Sabokbar A, Howie DW, Murray DW
and Athanasou NA: Macrophage colony-stimulating factor and
Interleukin-6 release by periprosthetic cells stimulates osteoclast
formation and bone resorption. J Orthop Res. 17:686–694. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chiu R, Ma T, Smith RL and Goodman SB:
Ultrahigh molecular weight polyethylene wear debris inhibits
osteoprogenitor proliferation and differentiation in vitro. J
Biomed Mater Res A. 89:242–247. 2009.PubMed/NCBI
|
|
11.
|
Olivares-Navarrete R, Raines AL, Hyzy SL,
Park JH, Hutton DL, Cochran DL, Boyan BD and Schwartz Z: Osteoblast
maturation and new bone formation in response to titanium implant
surface features are reduced with age. J Bone Miner Res.
27:1773–1783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Mitchell W, Bridget Matthews J, Stone MH,
Fisher J and Ingham E: Comparison of the response of human
peripheral blood mononuclear cells to challenge with particles of
three bone cements in vitro. Biomaterials. 24:737–748. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
MacQuarrie RA, Fang Chen Y, Coles C and
Anderson GI: Wear-particle-induced osteoclast osteolysis: the role
of particulates and mechanical strain. J Biomed Mater Res B Appl
Biomater. 69:104–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sun J, Lin F, Hung T, Tsuang Y, Chang WH
and Liu HC: The influence of hydroxyapatite particles on osteoclast
cell activities. J Biomed Mater Res. 45:311–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zambonin G, Colucci S, Cantatore F and
Grano M: Response of human osteoblasts to polymetylmetacrylate in
vitro. Calcif Tissue Int. 62:362–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gough JE and Downes S: Osteoblast cell
death on methacrylate polymers involves apoptosis. J Biomed Mater
Res. 57:497–505. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Willert HG, Ludwig J and Semlitsch M:
Reaction of bone to methacrylate after hip arthroplasty: a
long-term gross, light microscopic, and scanning electron
microscopic study. J Bone Joint Surg Am. 56:1368–1382.
1974.PubMed/NCBI
|
|
18.
|
Ciapetti G, Granchi D, Cenni E, Savarino
L, Cavedagna D and Pizzoferrato A: Cytotoxic effect of bone cements
in HL-60 cells: distinction between apoptosis and necrosis. J
Biomed Mater Res. 52:338–345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ciapetti G, Granchi D, Savarino L, Cenni
E, Magrini E, Baldini N and Giunti A: In vitro testing of the
potential for orthopedic bone cements to cause apoptosis of
osteoblast-like cells. Biomaterials. 23:617–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lenz R, Mittelmeier W, Hansmann D, Brem R,
Diehl P, Fritsche A and Bader R: Response of human osteoblasts
exposed to wear particles generated at the interface of total hip
stems and bone cement. J Biomed Mater Res A. 89:370–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lochner K, Fritsche A, Jonitz A, Hansmann
D, Mueller P, Mueller-Hilke B and Bader R: The potential role of
human osteoblasts for periprosthetic osteolysis following exposure
to wear particles. Int J Mol Med. 28:1055–1063. 2011.PubMed/NCBI
|
|
22.
|
Hirakawa K, Bauer TW, Stulberg BN, Wilde
AH and Secic M: Characterization and comparison of wear debris from
failed total hip implants of different types. J Bone Joint Surg Am.
78:1235–1243. 1996.PubMed/NCBI
|
|
23.
|
Ciapetti G, Stea S, Granchi D, Cavedagna
D, Gamberini S and Pizzoferrato A: The effects of orthopaedic
cements on osteoblastic cells cultured in vitro. Chir Organi Mov.
80:409–415. 1995.PubMed/NCBI
|
|
24.
|
Kühn KD: Knochenzemente für die
Endoprothetik. Springer-Verlag; Berlin: 2001
|
|
25.
|
Gillani R, Ercan B, Qiao A and Webster TJ:
Nanofunctionalized zirconia and barium sulfate particles as bone
cement additives. Int J Nanomedicine. 5:1–11. 2010.PubMed/NCBI
|
|
26.
|
Rathbone CR, Cross JD, Brown KV, Murray CK
and Wenke JC: Effect of various concentrations of antibiotics on
osteogenic cell viability and activity. J Orthop Res. 29:1070–1074.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Fritz EA, Glant TT, Vermes C, Jacobs JJ
and Roebuck KA: Titanium particles induce the immediate early
stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J
Orthop Res. 20:490–498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bernardini G, Ribatti D, Spinetti G,
Morbidelli L, Ziche M, Santoni A, Capogrossi MC and Napolitano M:
Analysis of the role of chemokines in angiogenesis. J Immunol
Methods. 273:83–101. 2003. View Article : Google Scholar : PubMed/NCBI
|