Introduction
The chewing of khat leaves (Catha edulis) is
a widespread recreational custom in Eastern Africa and the Arabian
Peninsula. The plant contains the alkaloids, cathine and cathinone,
which have amphetamine-like properties and produce a variety of
pleasurable effects. A significant number of people chew khat
leaves to experience the euphoric and stimulating effects (1).
Apart from its neurological effects, khat can also
act systemically and its use has been associated with tachycardia,
hypertension, gastrointestinal disturbances and relaxation of the
bladder wall (2).
There is increasing evidence indicating that
khat-related hepatotoxicity also occurs in animals (3,4).
The use of khat has been associated with severe liver injury, such
as acute hepatitis (5,6) and chronic liver disease (7). However, studies on the toxicological
potential of khat are insufficient (8), particularly regarding the
mechanism(s) by which khat induces the reported liver lesions.
Apoptosis is a regulated form of cell death that is
distinguishable from necrosis by its distinct morphological
features, which include cytoplasmic shrinkage, plasma membrane
blebbing and nuclear chromatin condensation (9), as well as biochemical features such
as the externalization of phosphatidylserine, DNA fragmentation and
the activation of specific caspases (10,11). Many plant-derived substances
induce apoptosis in mammalian cells (12,13). Khat has been found to induce
caspase-dependent apoptotic cell death in various leukemic cells
(14), as well as in normal human
oral keratinocytes and fibroblasts (15). Reactive oxygen species (ROS) are
unstable molecules, ions or radicals that are generated through
normal cellular metabolic processes. They include free radical
species, such as superoxide anion and hydroxyl radical, as well as
non-radical species, such as hydrogen peroxide. These molecules are
involved in a number of normal cellular processes, such as gene
expression (16), and
proliferation and differentiation (17). Exogenous and endogenous stress may
generate excessive amounts of ROS that cause damage to DNA,
proteins and lipids, which induce cell cycle arrest, premature
senescence (18) and the
activation of pathways leading to cell death (19).
Khat has been found to reduce free radical
metabolizing enzymes (20) and to
induce apoptotic cell death via the regulation of oxidative stress
in primary normal oral keratinocytes and fibroblasts (15).
Given that hepatocyte toxicity is an important toxic
effect of khat, we used L02 human hepatic cells as an in
vitro model in the current study to evaluate the cytotoxic
effects of khat. Furthermore, we also examined the activation of
ROS and mitogen-activated protein kinase (MAPK) signaling in L02
cells exposed to khat.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was
obtained from HyClone Laboratories (Logan, UT, USA). Fetal bovine
serum (FBS) was provided by Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Antibodies
against caspase-8 (13423-1-AP) and caspase-9 (10380-1-AP) were
supplied from the Proteintech Group, Inc. (Chicago, IL, USA).
Antibodies specific for Fas (sc-1023), Bcl-2 (sc-7382), Bax
(sc-7480), extracellular signal-regulated kinase (ERK; sc-271270),
phospho-ERK (p-ERK; sc-81492), p38 (sc-7149), phospho-p38 (p-p38;
sc-101759), c-Jun NH2-terminal kinase (JNK; sc-1648), phospho-JNK
(p-JNK; sc-135642) and β-actin (Sc-1616r) were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other chemicals
were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless
otherwise specified.
Khat extraction
Catha edulis Forsk (khat) purchased from
Sana’a, Yemen was used in this study. Fresh khat leaves along with
soft stems were collected during the summer period, weighed, washed
with distilled water three times and allowed to dry for three days
in a clean, dry room protected from sunlight. After drying, the
plant was weighed, packed in a closed foil packet and stored at 4°C
until use. Khat was extracted from the leaves as described
previously (14,21,22). Briefly, dried khat leaves (100 g)
were swiftly chopped into small (5 mm) pieces and dissolved in 100
ml of 95% ethanol, centrifuged at 5,000 rpm for 5 min and the
supernatant then filtered with Whatman filter paper. Ethanol (100
ml) was added to the remaining leaves and the procedure was
repeated. The ethanol khat extract was concentrated using a rotary
evaporator (Labtech, Inc., Hopkinton, MA, USA) at 30°C with a
rotation speed of 70 rpm until 70% of the ethanol solvent had
evaporated. The resulting viscous solution was diluted with 100 ml
of distilled water and then stirred at 1,000 rpm with for 1 h at
room temperature. The filtrate was kept frozen at -70°C for 24 h
and then dried by lyophilization (Lyophilization Technology, Inc.,
Ivyland, PA, USA). Typically, 100 g of dried leaves yielded 8 g of
khat extract powder. As previously described, high performance
liquid chromatography analysis confirmed that the alkaloids in the
khat extract consisted of 80% cathine and 20% norephedrine; no
cathinone was detected (14,21,22). Lyophilized khat extract was
dissolved in Hank’s buffered salt solution (HBSS) without
Ca2+, Mg2+ to a final concentration of 200
μg/ml and filter (0.2 μm) sterilized before being added to the
cells.
Cell culture and treatment
L02 cells were cultured in DMEM containing 10% FBS
and maintained at 37°C in a 5% CO2 humidified incubator.
The cells were grown to 60–70% confluence before the experiments
were conducted. L02 cells were exposed to various concentrations of
khat (10, 50 and 100 μg/ml) and incubated at 37°C, 5%
CO2 for up to 24 h. The cells not treated with khat
served as the control group.
Cell growth inhibition assay
The number of viable cells was determined using the
trypan blue exclusion assay as previously described by Ahmed et
al (23). Briefly, the cells
were seeded in 6-well plates to 60–70% confluence, incubated
overnight, and then exposed to the indicated concentrations of khat
for 4, 8, 16 and 24 h. Floating and adhering cells were collected
and stained with 0.2% trypan blue for 5 min at room temperature
before they were examined under a fluorescence microscope (Olympus,
Tokyo, Japan). Following the internalization of the dye into the
cells, the cells whose nuclei were stained blue were considered
dead. The results are expressed as a percentage of the control.
Staining of apoptotic cells with Hoechst
33258
Following treatment with khat, the cells were washed
with 0.1 mol/l PBS (pH 7.2) and resuspended in the same buffer. The
cells contained in 100 μl of cell suspension (1×106
cells/ml) were incubated with 1 ml Hoechst 33258 (1 mg/ml in
distilled water) for 10 min. Apoptotic cells were evaluated under a
fluorescence microscope.
Annexin V/PI staining assay
Apoptosis was assessed by measuring the membrane
redistribution of phosphatidylserine using an Annexin V-FITC
apoptosis detection kit according to the manufacturer’s
instructions. After drug treatment, the cells were collected,
washed twice with PBS and resuspended in 500 ml of staining
solution containing FITC-conjugated Annexin V antibody (5 ml) and
PI (5 ml of 250 mg/ml stock). After incubating the cells in ice for
30 min, the cells were analyzed by flow cytometry (FACSCalibur;
Becton-Dickinson, San Jose, CA, USA). Basal apoptosis and necrosis
were also determined in the untreated cells. The percentage of
cells undergoing apoptosis was determined by three independent
experiments.
Transmission electron microscopy
The cells were fixed in 0.1 M Na-cacodylate buffer,
pH 7.4 containing 2% glutaraldehyde. The samples were rinsed with
buffer and post-fixed in 1% osmium tetroxide. The specimens were
dehydrated using graded ethanol and embedded in epoxy resin.
Ultra-thin sections were double-stained with uranyl acetate and
lead citrate. Specimens were examined under an electron microscope
(Jeol 1230; Jeol Ltd., Tokyo, Japan), and the micrographs were
processed using an Agfa Arcus II scanner and Adobe Photoshop 7.0.1
software.
Western blot analysis
The cells were harvested and lysed on ice for 30 min
in modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl,
pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 50
mM NaF, 1 mM Na3VO4, 5 mM sodium
pyrophosphate and a protease inhibitor tablet). The cell lysates
were centrifuged at 14,000 × g for 15 min, and the supernatant was
recovered. The total protein concentration was determined using the
BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The lysates
were denatured by boiling in SDS sample buffer. The proteins were
separated on SDS/PAGE 4–20% SDS-polyacrylamide gels and then
transferred onto polyvinylidene difluoride membranes (Amersham
Pharmacia Biotech, Inc., Piscataway, NJ, USA) using a semi-dry
transfer cell (Bio-Rad). After blocking the membranes, the
membranes were probed with the appropriate primary antibodies.
Membrane-bound primary antibodies were detected using secondary
antibodies that were conjugated to horseradish peroxidase. Western
blots were visualized using enhanced chemiluminescence detection
reagents (Sigma) according to the manufacturer’s instructions. The
quantification of protein bands was performed via scanning using
the Bio-Rad Gel Doc™ XR and ChemiDoc™ XRS systems and analyzed
using Quantity One 1-D analysis software version 4.6.3.
Measurement of ROS production
The generation of ROS was measured by flow
cytometric analysis using dichlorofluorescein-diacetate (DCFH-DA)
as a substrate (24). Briefly,
following treatment with khat at a concentration of 100 μg/ml for
4, 8, 16 and 24 h, the cells were harvested, washed twice with cold
PBS, and suspended in PBS (1×106 cells/ml). The cell
suspension (500 μl) was placed in a tube, loaded with DCFH-DA to a
final concentration of 5 μM, and incubated for 30 min at 37°C. ROS
production was assessed based on the DCF fluorescence intensity
from 10,000 cells that was obtained by flow cytometry.
Statistical analysis
Data are expressed as the means ± SD and analyzed
using SPSS 10.0 statistical software (SPSS Inc., Chicago, IL, USA).
The one-way ANOVA procedure followed by LSD post hoc tests was used
to determine the significance of differences among groups
(P<0.01 and P<0.05).
Results
Effect of khat on the viability of L02
cells
The inhibitory effect of khat was evaluated by
measuring the viability of L02 cells. At the indicated
concentrations of 10, 50 and 100 μg/ml, treatment with khat
inhibited the growth of the L02 cells in a time-dependent manner,
compared to the control group (P<0.05, P<0.01). In the
current study, we also found that khat significantly decreased the
viability of the L02 cells at the concentration of 100 μg/ml
following treatment for 8 h (Fig.
1). Therefore, the concentration of 100 μg/ml of khat was
selected for the remaining experiments.
Effect of khat on apoptosis of L02
cells
To investigate whether the reduction in cell
viability was due to apoptosis, cytometric analysis and
morphological observation of the cells were performed. We
double-stained the cells with Annexin V and PI and analyzed the
results using flow cytometry. Following serum starvation for 24 h,
the L02 cells were exposed to 100 μg/ml of khat for 4, 8, 16 and 24
h, yielding apoptotic rates of 4.7±1.4, 21.7±3.1, 33.4±4.4 and
39.5±4.7%, respectively (Fig. 2).
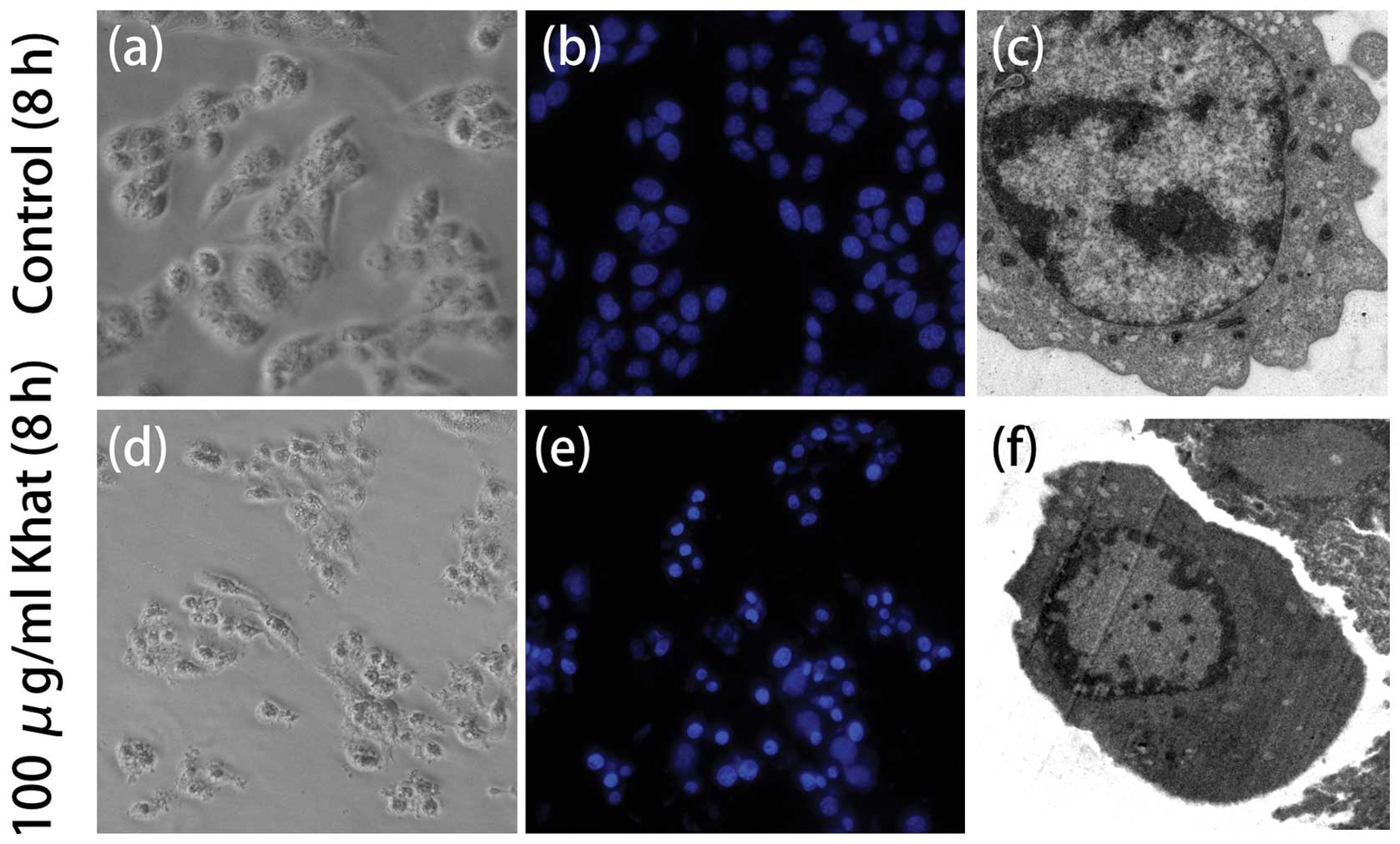
For a further assessment of apoptosis, we analyzed chromatin
condensation and apoptotic bodies. the L02 cells were treated with
the indicated concentrations of khat for 8 h. The vehicle-treated
cells exhibited regular and round-shaped nuclei. Bu contrast, the
majority of cells treated with khat (100 μg/ml) were stained with
Hoechst 33258, which is bound to chromatin. The treated cells
exhibited primary characteristics of apoptotic cells, namely the
condensation and fragmentation of nuclei. When observed under an
electron microscope, the khat-treated cells showed chromatin
condensation and nuclear shrinkage consistent with apoptosis
(Fig. 3).
Effect of khat on the expression of
apoptosis-related proteins
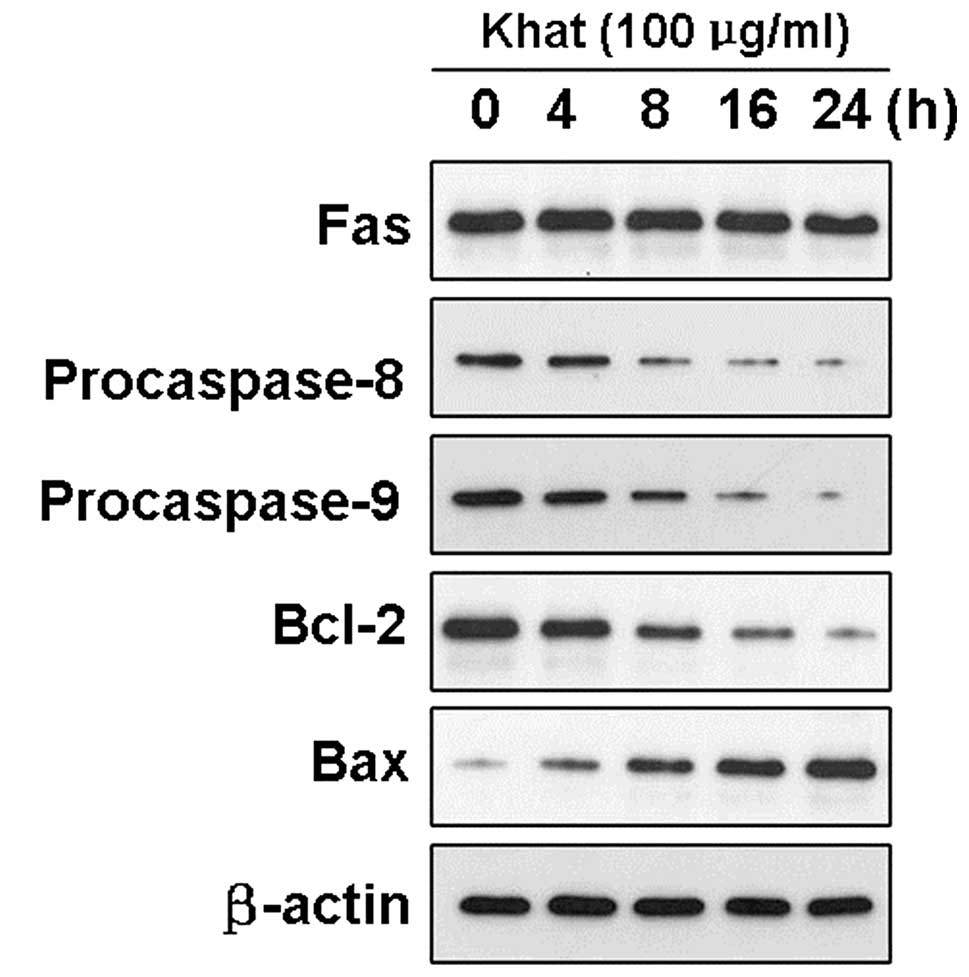
We assessed the activated caspase-8 and -9 levels in
the L02 cells before and after treatment with khat by western blot
analysis. Khat exposure significantly activated caspase-8 and -9 in
a time-dependent manner (Fig. 4).
After incubation with khat at the concentration of 100 μg/ml for
different periods of time (0, 4, 8, 16 and 24 h), the intensity of
the bands corresponding to procaspase-8 and -9 decreased. However,
under the same experimental conditions, the expression level of Fas
remained stable following treatment with khat, which suggested that
caspase activation not stimulated by Fas but by other death
signaling pathways.
In addition, Bax protein expression levels in L02
cells treated with khat demonstrated a marked increase compared to
the control protein expression levels. Conversely, the L02 cells
treated with khat showed a marked decrease in Bcl-2 protein
levels.
Effect of khat on the activation of
MAPKs
In order to clarify the involvement of MAPKs in the
khat-induced cell death of L02 cells, the levels of phosphorylated
MAPKs (p38, ERK and JNK) were investigated by western blot analysis
(Fig. 5). The phosphorylation of
JNK and ERK was significantly increased following treatment with
khat at the concentration of 100 μg/ml. However, the
phosphorylation levels of p38 were not altered under the same
conditions. The total levels of each MAPK were not altered during
the incubation period. Therefore, the ERK and JNK signaling
pathways may be involved in the response of L02 cells to khat.
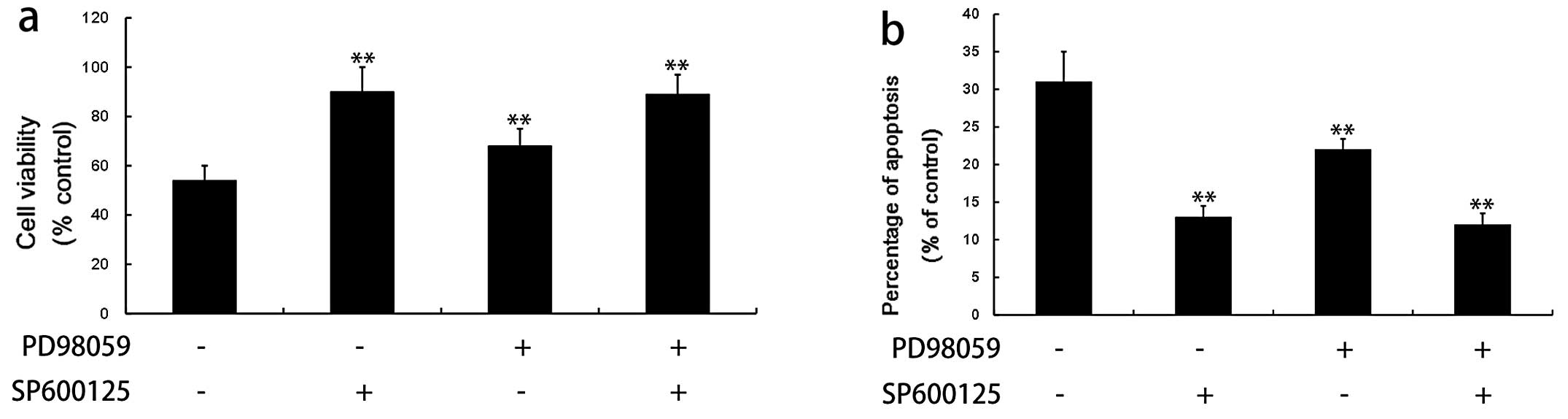
To further evaluate the possible roles of MAPKs in
khat-induced apoptosis, we examined cell viability and apoptotic
rates in the presence or absence of specific inhibitors of JNK
(SP600125) and ERK1/2 (PD98059). SP600125 and PD98059 prominently
reversed the khat (100 μg/ml)-induced cell death observed in the
typan blue exclusion assay and flow cytometric analysis (Fig. 6), which was concomitant with the
block of Bcl-2 reduction as well as caspase-8 and -9 activation
(data not shown). These results suggest that both JNK and ERK are
involved in the apoptotic progression caused by khat in L02
cells.
Effect of khat on ROS in L02 cells
ROS have been implicated as potential modulators of
apoptosis. In the L02 cells, treatment with khat caused a
dose-dependent accumulation of intracellular ROS (Fig. 7a).
To further determine the role of khat-induced ROS
generation in the activation of JNK and ERK, the L02 cells were
pre-treated with 5 mM N-acetyl-L-cysteine (NAC), a specific
inhibitor of ROS, for 2 h and then incubated with 100 μg/ml of khat
for 8 h.
NAC prominently reversed khat (100 μg/ml)-induced
cell death, which was observed using the trypan blue exclusion
assay (Fig. 7b). NAC markedly
decreased the activity of ERK and JNK induced by khat (Fig. 7c). However, NAC had no effect on
total ERK1/2 and JNK expression. These results suggest that
khat-induced ROS accumulation may contribute to the activation of
ERK and JNK in L02 cells.
Discussion
In the current study, we investigated the role of
khat in the apoptosis of L02 cells and further clarified the
underlying molecular mechanisms. To the best of our knowledge, the
current study is the first report the role of ROS generation and
MAPK signaling pathways in khat-induced hepatocytic apoptosis.
The results of the cell viability assay indicated
that treatment with khat inhibited the growth of L02 cells in a
time-dependent manner compared to the control group (Fig. 1). In agreement with these data,
flowcytometric analysis revealed that 100 μg/ml of khat induced
apoptosis in L02 cells in a time-dependent manner (Fig. 2). The khat-treated L02 cells
underwent morphological changes, such as plasma membrane blebbing,
cell shrinkage and condensation of nuclear chromatin (Fig. 3).
The induction of cell death by khat was synchronous,
and occurred in the majority of cells in a concentration-dependent
manner (Fig. 2). These results
suggest that these effects may be elicited through a specific
mechanism(s). Our results indicate that caspase-8 and -9 may be
involved in the cascade of cellular events leading to khat-induced
cell death. However, under the same experimental conditions, the
expression level of Fas remained stable following treatment with
khat, which suggests that the activation of caspases is not
stimulated by Fas, but by other death signaling pathways.
The Bcl-2 protein is a well-known suppressor of
apoptosis that homodimerizes with itself or heterodimerizes with
the homologous protein, Bax, which is a promoter of apoptosis
(25,26). These two proteins are critical
mediators of apoptosis, and their expression ratio is regulated by
an apoptotic inducer. In the current study, we found that khat
upregulated Bax protein expression and downregulated Bcl-2
expression levels, which increased the Bax/Bcl-2 ratio in relation
to apoptosis coordination. These data suggest that khat causes
hepatic apoptosis by modulating the Bax/Bcl-2 ratio.
A previous study indicated that the MAPK signaling
pathway is involved in the cytotoxicity induced by bifenthrin
(27). Therefore, it is important
to investigate the roles of MAPK signaling molecules in
khat-induced hepatocyte toxicity. The MAPK family includes the ERK,
JNK and p38 kinase, which are involved in cell survival,
proliferation and apoptosis in response to various growth or stress
stimuli.
The activation of ERK has been implicated in cell
proliferation and cell cycle progression (28), whereas JNK and p38 are more
commonly activated in response to stress and toxicants that induce
cell apoptosis (29).
Importantly, a number of studies support the concept that sustained
JNK activation leads to apoptosis (30,31). This hypothesis was also supported
by the current study. Our results demonstrate that treatment with
khat causes a sustained JNK activation and that the specific
inhibitor of JNK, SP600125, significantly blocks the khat-induced
apoptosis of L02 cells.
Although ERK1/2 was also activated by khat, the
inhibition of ERK activity only partially reversed khat-induced
hepatocyte toxicity (Fig. 6).
These results demonstrate that khat-induced hepatocyte apoptosis is
predominantly mediated by the sustained activation of the JNK
pathway and is only partially mediated by the ERK cascade.
Oxidative stress has been implicated as a mechanism
of hepatocytic toxicity from numerous toxicants (32,33). In the current study, khat induced
ROS production. This result suggests that ROS production may be
attributed to khat-induced hepatocytic apoptosis. Previous studies
have demonstrated the mechanistic involvement of alterations in
signal transduction cascades in response to ROS generation
(32,33). In the current study, we also
determined that the antioxidant, NAC, attenuated the khat-induced
activation of JNK and ERK. These results indicate that the outcome
of the challenge with khat depends on the oxidative stress-induced
activation of a series of signaling cascades that promote
hepatocyte apoptosis.
In conclusion, the data from the present study
suggest that the intracellular response in L02 cells following
exposure to khat triggers the generation of intracellular ROS and
sequentially induces the sustainable activation of JNK, which in
turn results in a decrease in cell viability and an increase in
cell apoptosis. To our knowledge, this is the first study that
estimates the possible cytotoxic effects of khat on hepatocytes at
the molecular level.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (Grant nos. 30570554,
31171027 and 31000471), the Important National Science and
Technology Specific Projects (Grant no. 2009ZX09301-014), and the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, the State Education Ministry (no. 20091341).
References
|
1
|
Kalix P, Geisshusler S, Brenneisen R,
Koelbing U and Fisch HU: Cathinone, a phenylpropylamine alkaolid
from khat leaves that has amphetamine effects in humans. NIDA Res
Monogr. 105:289–290. 1990.PubMed/NCBI
|
|
2
|
Al-Habori M: The potential adverse effects
of habitual use of Catha edulis (khat). Expert Opin Drug
Saf. 4:1145–1154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Habori M, Al-Aghbari A, Al-Mamary M and
Baker M: Toxicological evaluation of Catha edulis leaves: a
long term feeding experiment in animals. J Ethnopharmacol.
83:209–217. 2002.
|
|
4
|
Al-Mamary M, Al-Habori M, Al-Aghbari AM
and Baker MM: Investigation into the toxicological effects of
Catha edulis leaves: a short term study in animals.
Phytother Res. 16:127–132. 2002. View
Article : Google Scholar
|
|
5
|
Peevers CG, Moorghen M, Collins PL, Gordon
FH and McCune CA: Liver disease and cirrhosis because of khat
chewing in UK Somali men: a case series. Liver Int. 30:1242–1243.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roelandt P, George C, d’Heygere F, et al:
Acute liver failure secondary to khat (Catha edulis)-induced
necrotic hepatitis requiring liver transplantation: case report.
Transplant Proc. 43:3493–3495. 2011.PubMed/NCBI
|
|
7
|
Chapman MH, Kajihara M, Borges G, et al:
Severe, acute liver injury and khat leaves. N Engl J Med.
362:1642–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvalho F: The toxicological potential of
khat. J Ethnopharmacol. 87:1–2. 2003. View Article : Google Scholar
|
|
9
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, et al: Early redistribution of plasma membrane
phosphatidylserine is a general feature of apoptosis regardless of
the initiating stimulus: inhibition by overexpression of Bcl-2 and
Abl. J Exp Med. 182:1545–1556. 1995. View Article : Google Scholar
|
|
12
|
Horie N, Hirabayashi N, Takahashi Y,
Miyauchi Y, Taguchi H and Takeishi K: Synergistic effect of green
tea catechins on cell growth and apoptosis induction in gastric
carcinoma cells. Biol Pharm Bull. 28:574–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai KC and Lee TC: Genetic damage in
cultured human keratinocytes stressed by long-term exposure to
areca nut extracts. Mutat Res. 599:66–75. 2006. View Article : Google Scholar
|
|
14
|
Dimba EA, Gjertsen BT, Bredholt T, et al:
khat (Catha edulis)-induced apoptosis is inhibited by
antagonists of caspase-1 and -8 in human leukaemia cells. Br J
Cancer. 91:1726–1734. 2004.PubMed/NCBI
|
|
15
|
Lukandu OM, Costea DE, Neppelberg E,
Johannessen AC and Vintermyr OK: khat (Catha edulis) induces
reactive oxygen species and apoptosis in normal human oral
keratinocytes and fibroblasts. Toxicol Sci. 103:311–324.
2008.PubMed/NCBI
|
|
16
|
Fialkow L, Wang Y and Downey GP: Reactive
oxygen and nitrogen species as signaling molecules regulating
neutrophil function. Free Radic Biol Med. 42:153–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dumont P, Burton M, Chen QM, et al:
Induction of replicative senescence biomarkers by sublethal
oxidative stresses in normal human fibroblast. Free Radic Biol Med.
28:361–373. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macip S, Igarashi M, Fang L, et al:
Inhibition of p21-mediated ROS accumulation can rescue p21-induced
senescence. EMBO J. 21:2180–2188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang C, Zhang Z, Ding M, et al: Vanadate
induces p53 transactivation through hydrogen peroxide and causes
apoptosis. J Biol Chem. 275:32516–32522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Qirim TM, Shahwan M, Zaidi KR, Uddin Q
and Banu N: Effect of khat, its constituents and restraint stress
on free radical metabolism of rats. J Ethnopharmacol. 83:245–250.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Banjaw MY and Schmidt WJ: Behavioural
sensitisation following repeated intermittent oral administration
of Catha edulis in rats. Behav Brain Res. 156:181–189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aziz HA, Peh KK and Tan YT: Extraction and
microencapsulation of khat: effects on sexual motivation and
estradiol level in female rats. J Sex Med. 6:682–695. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed AE, Aronson J and Jacob S: Induction
of oxidative stress and TNF-alpha secretion by
dichloroacetonitrile, a water disinfectant by-product, as possible
mediators of apoptosis or necrosis in a murine macrophage cell line
(RAW). Toxicol In Vitro. 14:199–210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su YT, Chang HL, Shyue SK and Hsu SL:
Emodin induces apoptosis in human lung adenocarcinoma cells through
a reactive oxygen species-dependent mitochondrial signaling
pathway. Biochem Pharmacol. 70:229–241. 2005. View Article : Google Scholar
|
|
25
|
Chen L, Willis SN, Wei A, et al:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fletcher JI and Huang DC: Controlling the
cell death mediators Bax and Bak: puzzles and conundrums. Cell
Cycle. 7:39–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Xu L, Zhao M, Liu W, Zhang C and
Zhou S: Enantiomer-specific, bifenthrin-induced apoptosis mediated
by MAPK signalling pathway in Hep G2 cells. Toxicology.
261:119–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roos WP and Kaina B: DNA damage-induced
cell death by apoptosis. Trends Mol Med. 12:440–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wullaert A, Heyninck K and Beyaert R:
Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK
activation in hepatocytes. Biochem Pharmacol. 72:1090–1101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Czaja MJ: Cell signaling in oxidative
stress-induced liver injury. Semin Liver Dis. 27:378–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh R and Czaja MJ: Regulation of
hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol.
22(Suppl 1): S45–S48. 2007. View Article : Google Scholar
|