Introduction
Osteoporosis is a skeletal disorder characterized by
an imbalance between bone resorption and bone formation,
culminating in fragility fractures, pain and disability (1). Although the mechanisms involved are
unclear, increasing evidence indicates that oxidative stress plays
a role in the pathogenesis of osteoporosis (2,3).
Oxidative stress is an imbalance between the free radicals and
antioxidant mechanisms in biological systems and damages cellular
macromolecules and functions. It can lead to an accumulation of
reactive oxygen species (ROS) in the body which has adverse effects
on the skeleton (4).
Advanced oxidation protein products (AOPPs) are
defined as dityrosine-containing cross-linked protein products
formed during oxidative stress by the action of chlorinated
oxidants (5). Thus far, AOPPs
have received increasing attention as novel markers of oxidative
stress. There is a close correlation between AOPPs and monocyte
activation markers in vivo, such as neopterin and
interleukin-1 receptor (IL-1R) antagonist. AOPPs trigger the
oxidative burst of human monocytes in culture (6). Evidence incidates that AOPPs
increase ROS levels in human umbilical vein endothelial cells by
activating nicotinamide adenine dinucleotide phosphate (NAD(P)H)
oxidase, extracellular-signal-regulated kinase (ERK)1/2, p38 and
nuclear factor (NF)-κB (7).
AOPPs accumulate in the body and increase with age
(8). Elevated levels of AOPPs are
detected in many types of diseases, including osteoporosis
(9–14). Certain studies have demonstrated
that oxidative stress leads to increased levels of AOPPs in
osteoblastic MC3T3-E1 cells (15,16); AOPPs have been shown to inhibit
the proliferation and differentiation of rat osteoblastic cells
through NF-κB (17); these data
suggest that AOPPs are associated with the progression of
osteoporosis. However, to our knowledge, there are no studies to
date on the effects of AOPPs on mesenchymal stem cells (MSCs).
Since impaired bone formation is an important
mechanism involved in the pathological process of osteoporosis, the
inhibition of osteoblast proliferation and the inability to form
bone may result in osteoporosis (18). Bone cells purportedly originate
from MSCs that commit to the osteogenic cell lineage, becoming
osteoprogenitor cells, pre-osteoblasts, osteoblasts and osteocytes
(19,20). MSCs in patients with osteoporosis
also have abnormal gene expression (21,22). Thus, the negative effects on MSC
osteogenic differentiation may aggravate the development of
osteoporosis. Therefore, the aim of this study was to investigate
the role of AOPPs in modulating the proliferation and osteogenic
differentiation of rat bone marrow-derived MSCs, as well as the
possible mechanisms involved.
Materials and methods
Materials
Trypsin-EDTA, 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA), the amebocyte lysate assay kit, dexamethasone,
ascorbic acid, β-glycerophosphate, alizarin red, p-nitrophenyl
phosphate and 3-(4,5-dimethylthiazozyl)-2,5-diphenyl tetrazolium
bromide (MTT) were all obtained from Sigma (St. Louis, MO, USA).
Bovine serum albumin (BSA) was from Roche Diagnostics GmbH
(Mannheim, Germany); Oil Red O was purchased from Amresco, Inc.
(Solon, OH, USA); the BCA assay kit was from CoWin Biotech
(Beijing, China); dimethyl sulfoxide (DMSO) and TRIzol reagent were
obtained from Invitrogen (Buenos Aires, Argentina); adipogenic
differentiation medium, fetal bovine serum (FBS) and Dulbecco's
modified Eagle's medium (DMEM) were all from HyClone (Logan, UT,
USA); the PrimeScript® one step RT-PCR kit and
SYBR® Premix Ex Taq™ II were from Takara Biotechnology
(Dalian, China); RIPA lysis buffer was from Beyotime Biotech
(Shanghai, China); mouse anti-receptor for advanced glycation
end-products (RAGE) antibody and phycoerythrin (PE)-conjugated
antibody against CD34 were from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA); mouse anti-GAPDH antibody was from Abcam
(Cambridge, UK); PE-conjugated antibody against CD29 was from
BioLegend Inc. (San Diego, CA, USA); the Detoxi-Gel column was from
Pierce (Rockford, IL, USA); the ECL kit was from Applygen
Technologies Inc. (Beijing, China). All other chemicals and
reagents were purchased from commercial sources and were of
analytical grade.
MSC isolation and culture conditions
All animal procedures were approved by
institutionally approved protocols and guidelines. After the rats
(Sprague-Dawley, 4 weeks of age, male) were sacrificed by the
controlled inhalation of CO2, the hind legs were
removed, the soft tissue was removed and bone marrow from the
femurs was flushed out with serum-free DMEM. The cells were then
cultured in H-DMEM with 10% FBS and 1% penicillin/streptomycin at
37°C in a humidified atmosphere containing 5% CO2. The
medium was first changed after 2 days and subsequently every 2 to 3
days. MSCs were purified by plastic adherence and expanded in
culture flasks. Third or fourth passage cells were used for all
experiments.
AOPP preparation
AOPPs-BSA was prepared as described previously
(17). Briefly, BSA (100 mg/ml)
was incubated with 200 mM hypochlorous acid (HOCl) for 30 min at
37% and dialyzed overnight against PBS to remove free HOCl.
AOPPs-BSA was passed through a Detoxi-Gel column to remove
contaminated endotoxin. Endotoxin levels in the preparation were
determined using the amebocyte lysate assay kit and were found to
be <0.25 EU/ml. AOPP content in the preparation was then
determined. Briefly, 200 μl of sample or chloramine-T were placed
in a 96-well plate, followed by 20 μl of acetic acid. The
absorbance at 340 nm was determined immediately in a microplate
reader. AOPP content was 57.6±4.9 nmol/mg protein in the AOPPs-BSA
and 0.24±0.09 nmol/mg protein in the native BSA preparation.
Flow cytometry
The MSCs were analyzed by flow cytometry to
determine the pluripotent cell characteristics. Following
trypsinization, the cells were resuspended in PBS reaching a
concentration of 3.0×106/ml. Cell suspension per sample
(100 μl) was then incubated with PE-conjugated antibodies against
CD34, and CD29. The cells were incubated in the dark for 30 min at
4°C. The control samples were incubated with PE-conjugated
isotype-matched antibodies. Following incubation, the cells were
washed with PBS and centrifuged to remove unbound antibody. The
cells were resuspended in 400 μl PBS and evaluated by flow
cytometry (Beckman-Coulter, Brea, CA, USA).
Adipogenic differentiation assay
MSCs were seeded at a density of 5×105
cells/well in 6-well plates and cultured in commercially available
adipogenic differentiation medium for 3 weeks. For Oil Red O
staining, the cells were fixed with 4% paraformaldehyde, washed in
water and stained with a 0.6% (w/v) Oil Red O solution for 1 h at
room temperature, followed by washing with water to remove unbound
dye. Oil Red O staining of lipid droplets was analyzed under a
phase-contrast inverted microscope (Olympus, Japan).
Osteogenic differentiation assay
MSCs were seeded at 5×105 cells/well in
6-well plates and were stimulated with osteogenic differentiation
medium as previously described (23) for 3 weeks. To evaluate the effects
of AOPPs on the osteogenic differentiation of MSCs, the cells were
cultured in the induction medium for 2 weeks, followed by
stimulation in 10% FBS-DMEM with the addition of BSA (200 μg/ml) or
AOPPs (200 μg/ml) for 1 week. For the detection of osteogenic
differentiation, bone nodule formation was examined by alizarin red
staining. Briefly, the cells were fixed with 95% ethanol for 10 min
and washed 3 times with PBS. Alizarin red solution (1%) was added
to the cells for 30 min at 37°C, and then washed with PBS. Bone
nodule formation was observed under a phase-contrast
microscope.
Cell proliferation assay
The MSCs were plated at 104 cells/well in
96-well plates and cultured for 2 days in 10% FBS-DMEM. Then they
were serum-deprived overnight and incubated with control medium,
BSA (200 μg/ml) or AOPPs (50–400 μg/ml) for 3 days, or with AOPPs
(200 μg/ml) for different periods of time (0, 24, 48, 72 h). MTT
assay was used to evaluate the viability of the MSCs. In brief,
MSCs in 96-well plates were washed twice with 0.01 M PBS, then
incubated in 100 μl of FBS-free DMEM supplemented with 10 μl of 5
mg/ml MTT solution at 37°C. After 4 h, the supernatant was
carefully removed, and the crystals were dissolved by incubation
with 150 μl of DMSO for 20 min. The plates were shaken for 5 min
and the absorbance at 570 nm was measured in a microplate reader.
The results are expressed as absorbance (OD at 570 nm).
Alkaline phosphatase (ALP) activity
assay
The MSCs were seeded at 105 cells/well in
24-well plates and stimulated with osteogenic differentiation
medium for 2 weeks. The cells were then serum-deprived overnight
and incubated under various culture conditions as described above
for 3 days. Cell extract was then prepared using RIPA lysis buffer.
ALP activity in the cell lysates was assayed at the end of the
incubation time with 10 mM p-nitrophenyl phosphate in 0.15 M sodium
carbonate buffer (pH 10.3) and 1 mM MgCl2 as previously
described (24). The enzyme
activity was normalized against the cellular protein concentration
and expressed as U/g protein. Protein concentrations were
determined using the BCA assay kit.
Determination of ROS generation
Intracellular ROS generation was measured by flow
cytometry with the probe DCFH-DA. Briefly, MSCs grown in 10-cm
plates were subjected to various culture conditions as described
above for 2 h. The medium was replaced by control medium with 10 μM
DCFH-DA for 30 min in the dark. Intracellular ROS generation was
visualized under a fluorescent microscope (Olympus, Tokyo, Japan).
DCF fluorescence was measured on a flow cytometer. Data were
normalized to the control values.
Gene expression by real-time polymerase
chain reaction (PCR)
MSCs for examining ALP and collagen I mRNA levels
were stimulated with osteogenic differentiation medium for 1 week;
they were then serum-deprived overnight and subjected to various
culture conditions as described above for 3 days. Cells for RAGE
were only subjected to various culture conditions as described
above for 3 days. After this period, total RNA was extracted using
TRIzol reagent according to the manufacturer's instructions. cDNA
was reverse-transcribed from 0.8 μg of total RNA using the
PrimeScript one step RT-PCR kit. The primers used were as follows:
ALP forward, 5′-AGATGGACAAGTTCCCCTTTG-3′ and reverse,
5′-ACACAAGTAGGCAGTGGCAGT-3′; collagen I forward,
5′-GTGGAAACCTGATGTATGCTTG-3′ and reverse,
5′-ATGACTTCTGCGTCTGCGTCTGGTGATA-3′; RAGE forward,
5′-ACTCACAGCCAATGTCCCTAA-3′ and reverse,
5′-CTTTGCCATCAGGAATCAGAG-3′; and β-actin forward,
5′-TTCTACAATGAGCTGCGTGTGGC-3′ and reverse,
5′-CTCATAGCTCTTCTCCAGGGAGGA-3′. Real-time PCR was carried out in a
Real-Time PCR System (Stratagene/Agilent Technologies, Wilmington,
DE, USA) using SYBR Premix Ex Taq II. The cycling conditions were
as follows: 95°C for 2 min and 40 cycles of 95°C for 5 sec, 60°C
for 30 sec. The value of 2−ΔΔCt represents the relative
level of target gene expression.
Western blot analysis of RAGE
The MSCs were stimulated with various culture
conditions as described above for 3 days following starvation
overnight in serum-free medium. Proteins were then extracted from
the MSCs using RIPA lysis buffer followed by centrifugation at 4°C,
12,000 rpm for 20 min. Protein concentration was determined by BCA
assay. Proteins were then separated by SDS-PAGE and
electrotransferred onto a PVDF membrane. The membrane was incubated
with mouse anti-RAGE (1:500) and mouse anti-GAPDH antibodies
(1:500) overnight at 4°C. The membrane was washed 3 times with TBST
and incubated with HRP-conjugated secondary IgG for 50 min. The
bands were detected using the ECL chemiluminescence detection
system.
Statistical analysis
All values are expressed as the mean ± SD. Each
experiment was repeated at least 3 times. One-way ANOVA followed by
the Scheffe's test or Dunnett's T3 (equal variances not assumed)
was performed for multiple comparisons. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and identification of MSCs
MSCs were isolated from rat femur bone marrow and
formed elongated spindle or polygon shapes. Passage 3 MSCs were
detected by flow cytometry and the results revealed that the cells
were negative for CD34 (Fig. 1A)
and positive for CD29 (Fig. 1B).
The former is a marker of hematopoietic stem cells and the latter
is a characteristic cell surface marker of MSCs. Following
stimulation in adipogenic differentiation and osteogenic
differentiation medium, lipid droplets in the cells (Fig. 1C) and bone nodules (Fig. 1D) were observed.
Effects of AOPPs on proliferation of
MSCs
MSC proliferation was evaluated by MTT assay. As
shown in Fig. 2A, AOPPs (50–400
μg/ml) significantly inhibited the proliferation of the MSCs within
3 days compared with the control medium and the unmodified BSA
preparation. Furthermore, the inhibitory effects on proliferation
were observed in the MSCs stimulated with 200 μg/ml of AOPPs for
24–72 h (Fig. 2B). These results
indicated that AOPPs inhibited MSC proliferation in a dose- and
time-dependent manner.
Effects of AOPPs on osteogenic
differentiation of MSCs
To investigate the effect of AOPPs on MSC osteogenic
differentiation, ALP activity and the mRNA expression of ALP and
collagen I were detected. The cells were stimulated as described
above. As shown in Fig. 3A, AOPPs
(100–400 μg/ml) decreased ALP activity in the MSCs; a
time-dependent decrease in ALP activity was observed in the cells
exposed to AOPPs (Fig. 3B)
compared with those cultured in the control medium and BSA. We also
found that the exposure of MSCs to AOPPs resulted in a significant
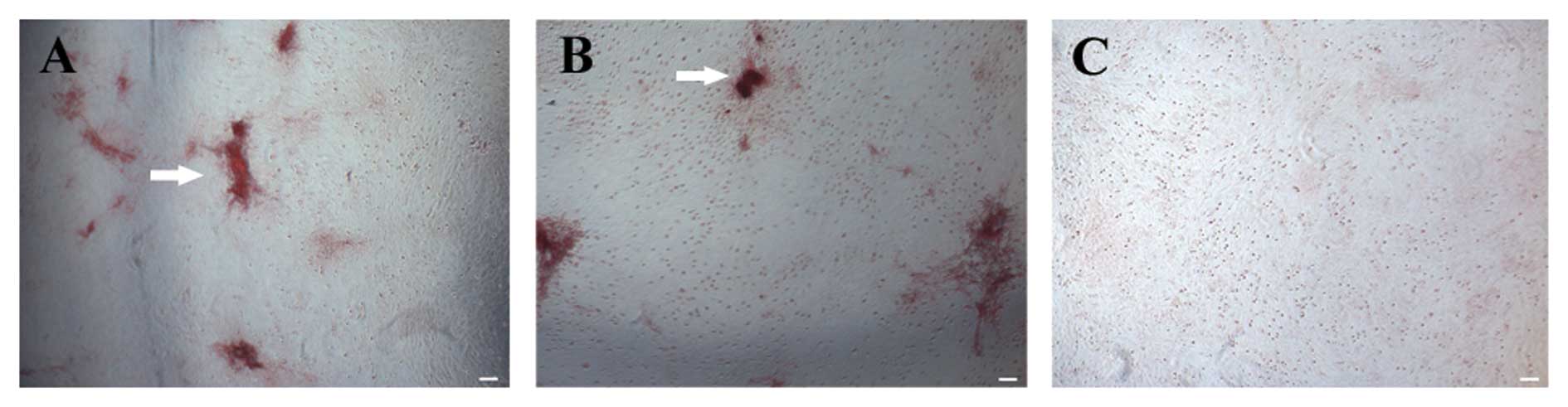
decrease in the mRNA expression of ALP (Fig. 4A) and collagen I (Fig. 4B). Although bone nodule formation
was observed in the control group, only slight bone nodule
formation was observed in the cells exposed to AOPPs (Fig. 5) after alizarin red staining.
These data indicate that AOPPs inhibit the osteogenic
differentiation of MSCs.
Effects of AOPPs on ROS generation in
MSCs
ROS generation was examined by the detection of DCF
fluorescence by flow-cytometry analysis. The increase in ROS
generation in the MSCs was observed visually in the cells exposed
to AOPPs compared with the control and BSA-treated cells (Fig. 6A-F); this increase in ROS occurred
in a dose-dependent manner (Fig.
6G).
Effects of AOPPs on RAGE mRNA and protein
expression in MSCs
RAGE is considered a receptor of AOPPs, and
oxidative stress may be activated with their binding (7). The mRNA and protein expression of
RAGE in the MSCs was measured by real-time PCR and western blot
analysis, respectively. As shown in Fig. 4C and 7, exposure to AOPPs, as opposed to
exposure to BSA, increased the mRNA and protein expression of
RAGE.
Discussion
Bone mass is maintained locally by the balance
between osteoclastic bone resorption and osteoblastic bone
formation. The latter process is carried out by osteoblasts whose
number and activity is determined by the proliferation and
differentiation of osteoblast precursors derived from MSCs
(25). Hence, MSCs play an
important role in the development of osteoporosis. The cells used
in this study were positive for the cell-surface marker, CD29, but
negative for CD34, and had the potential to differentiate into
adipose cells and osteoblasts, suggesting that these cells were
MSCs.
Increasing evidence indicates that AOPPs, as a novel
marker of oxidative stress, are involved in various diseases, such
as renal failure (5), diabetes
mellitus (9), coronary artery
disease (12) and osteoporosis
(14), and have various
biological activities. The data presented in this study demonstrate
that AOPPs inhibit the proliferation and osteogenic differentiation
of MSCs. We found that AOPPs inhibited the proliferation and ALP
activity in MSCs, as well as ALP and collagen I gene expression. In
addition, AOPPs increased the mRNA and protein expression of RAGE,
as well as the generation of ROS. These results suggest that AOPPs
exert adverse effects on MSCs through the RAGE-ROS pathway.
It has been reported that AOPPs inhibit cell
proliferation in human gingival fibroblasts (26) and osteoblasts (17); however, it also has been shown
that AOPPs (50–400 μg/ml) induce adventitial fibroblast
proliferation (27). In the
present study, we found that AOPPs inhibited MSC proliferation in a
dose- and time-dependent manner. These results indicate that AOPPs
react differently according to different cell types and doses.
The developmental sequence of osteoblast
differentiation can be characterized in 3 stages, including cell
proliferation, extracellular matrix production and mineralization.
During proliferation, the production of collagen I initially
occurs, followed by an increased in the expression of ALP and
calcium deposition (28).
Collagen I comprises approximately 90% of the organic material as
the most abundant protein component of the bone matrix. The
enhanced expression of ALP metabolizes calcium phosphate into
insoluble phosphate salts, thus mediating calcification (29). In this study, AOPPs significantly
decreased ALP and collagen I mRNA levels and inhibited ALP
secretion by MSCs in a dose- and time-dependent manner. Using
alizarin red staining, we also observed that dystrophic
mineralization occurred in the MSCs exposed to AOPPs compared with
the controls. These data indicate that AOPPs inhibit MSC osteogenic
differentiation.
AOPPs have similar biological characteristics to
those of advanced glycation end-products (AGEs), and bind to the
same receptor, RAGE (8). To
investigate the potential mechanisms behind the adverse effects
AOPPs on MSCs, we examined ROS levels and RAGE expression in the
MSCs exposed to AOPPs. ROS are products of oxidative stress, such
as O2−, H2O2 and −OH,
which have been implicated in the regulation of diverse cellular
functions, including intracellular signaling, transcriptional
activation, proliferation and apoptosis. A number of studies have
suggested that oxidative stress is involved in the progression of
osteoporosis. Oxidative stress suppresses the osteoblastic
differentiation of MSCs through ERK and NF-κB (30). Receptor activator of NF-κB ligand
(RANKL)-mediated ROS production may promote the differentiation of
MSCs into osteoclasts as an intracellular signal mediator (31). A reduction in ROS permits the
restoration of osteoblastic markers, specifically the induction of
osteoprotegerin and osteocalcin (32). Our results also demonstrated that
AOPPs increased ROS generation in the MSCs in a dose-dependent
manner, indicating that ROS production induced by AOPPs may
participate in the inhibition of proliferation and the osteogenic
differentiation of MSCs.
RAGE is capable of binding to multiple ligands,
including AGEs, AOPPs, high-mobility group box-1 and β-sheet
fibrils (33). AGEs-RAGE
interaction induces the generation of ROS through NADPH oxidase,
resulting in the apoptosis of osteoblasts/MSCs (34) and in the inhibition of the
proliferation and differentiation of osteoblasts/MSCs (35). RAGE overexpression by lentiviral
transfection has been shown to inhibit osteoblast proliferation
through the suppression of the Wnt, PI3K and ERK pathways (36). Studies using RAGE knockout mice
have also shown increased bone mass and bone biomechanical strength
and a decreased number of osteoclasts in RAGE−/− mice
compared with wild-type mice (37). These data indicate that RAGE plays
a modulatory role in the development of osteoporosis. In this
study, we also observed an increased expression of RAGE at the mRNA
and protein level as the dose of AOPPs increased. Therefore, taking
all these data into consideration, we hypothesized that AOPPs may
inhibit the proliferation and osteogenic differentiation of MSCs by
upregulating RAGE expression following an increase in ROS
production.
In conclusion, our data indicate that AOPPs inhibit
the proliferation and osteogenic differentiation of MSCs. We also
observed an increase in RAGE expression and ROS generation.
Although the association between the increased expression of RAGE
and increased ROS generation induced by AOPPs was not examined in
detail, considering the similar biological characteristics of AOPPs
and AGEs and taking into account the data from previous studies, it
can be concluded that AOPPs inhibit the proliferation and
osteogenic differentiation of MSCs through a AOPPs-RAGE-ROS
pathway. This may provide a new perspective as to the development
of osteoporosis, and further suggests that AOPPs may be an
effective clinical indicator of osteoporosis.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81270966).
References
|
1
|
Wongdee K and Charoenphandhu N:
Osteoporosis in diabetes mellitus: possible cellular and molecular
mechanisms. World J Diabetes. 2:41–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cervellati C, Bonaccorsi G, Cremonini E,
Bergamini CM, Patella A, Castaldini C, Ferrazzini S, Capatti A,
Picarelli V, Pansini FS and Massari L: Bone mass density
selectively correlates with serum markers of oxidative damage in
post-menopausal women. Clin Chem Lab Med. 51:333–338. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fatokun AA, Stone TW and Smith RA:
Responses of differentiated MC3T3-E1 osteoblast-like cells to
reactive oxygen species. Eur J Pharmacol. 587:35–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheweita SA and Khoshhal KI: Calcium
metabolism and oxidative stress in bone fractures: role of
antioxidants. Curr Drug Metab. 8:519–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witko-Sarsat V, Friedlander M,
Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers
P and Descamps-Latscha B: Advanced oxidation protein products as a
novel marker of oxidative stress in uremia. Kidney Int.
49:1304–1313. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Witko-Sarsat V, Friedlander M, Nguyen Khoa
T, Capeillere-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers
P, Drueke T and Descamps-Latscha B: Advanced oxidation protein
products as novel mediators of inflammation and monocyte activation
in chronic renal failure. J Immunol. 161:2524–2532. 1998.PubMed/NCBI
|
|
7
|
Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N,
Nagai R, Lu X, Chen BH, Shan YX, Tian JW, Nagaraj RH, Xie D and
Zhang X: Advanced oxidation protein products activate vascular
endothelial cells via a RAGE-mediated signaling pathway. Antioxid
Redox Signal. 10:1699–1712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piwowar A: Advanced oxidation protein
products. Part I. Mechanism of the formation, characteristics and
property. Pol Merkur Lekarski. 28:166–169. 2010.(In Polish).
|
|
9
|
Piwowar A, Knapik-Kordecka M and Warwas M:
AOPP and its relations with selected markers of
oxidative/antioxidative system in type 2 diabetes mellitus.
Diabetes Res Clin Pract. 77:188–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cumaoglu A, Cevik C, Rackova L, Ari N and
Karasu C: Effects of antioxidant stobadine on protein
carbonylation, advanced oxidation protein products and reductive
capacity of liver in streptozotocin-diabetic rats: role of
oxidative/nitrosative stress. BioFactors. 30:171–178. 2007.
View Article : Google Scholar
|
|
11
|
Krzystek-Korpacka M, Neubauer K, Berdowska
I, Boehm D, Zielinski B, Petryszyn P, Terlecki G, Paradowski L and
Gamian A: Enhanced formation of advanced oxidation protein products
in IBD. Inflamm Bowel Dis. 14:794–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneda H, Taguchi J, Ogasawara K, Aizawa T
and Ohno M: Increased level of advanced oxidation protein products
in patients with coronary artery disease. Atherosclerosis.
162:221–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collet C, Schiltz C, Geoffroy V, Maroteaux
L, Launay JM and de Vernejoul MC: The serotonin 5-HT2B receptor
controls bone mass via osteoblast recruitment and proliferation.
FASEB J. 22:418–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YB, Zhong ZM, Hou G, Jiang H and
Chen JT: Involvement of oxidative stress in age-related bone loss.
J Surg Res. 169:e37–e42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi EM and Kim YH: Hesperetin attenuates
the highly reducing sugar-triggered inhibition of osteoblast
differentiation. Cell Biol Toxicol. 24:225–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KH and Choi EM: Myricetin, a naturally
occurring flavonoid, prevents 2-deoxy-D-ribose induced dysfunction
and oxidative damage in osteoblastic MC3T3-E1 cells. Eur J
Pharmacol. 591:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong ZM, Bai L and Chen JT: Advanced
oxidation protein products inhibit proliferation and
differentiation of rat osteoblast-like cells via NF-kappaB pathway.
Cell Physiol Biochem. 24:105–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long MW: Osteogenesis and
bone-marrow-derived cells. Blood Cells Mol Dis. 27:677–690. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dalle Carbonare L, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009.PubMed/NCBI
|
|
22
|
Benisch P, Schilling T, Klein-Hitpass L,
Frey SP, Seefried L, Raaijmakers N, Krug M, Regensburger M, Zeck S,
Schinke T, Amling M, Ebert R and Jakob F: The transcriptional
profile of mesenchymal stem cell populations in primary
osteoporosis is distinct and shows overexpression of osteogenic
inhibitors. PloS One. 7:e451422012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kume S, Kato S, Yamagishi S, Inagaki Y,
Ueda S, Arima N, Okawa T, Kojiro M and Nagata K: Advanced glycation
end-products attenuate human mesenchymal stem cells and prevent
cognate differentiation into adipose tissue, cartilage, and bone. J
Bone Miner Res. 20:1647–1658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farley JR and Jorch UM: Differential
effects of phospholipids on skeletal alkaline phosphatase activity
in extracts, in situ and in circulation. Arch Biochem Biophys.
221:477–488. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng YQ, Fu Y, Su XP and Tang ZY: Biologic
effects of advanced oxidative protein products on the human
gingival fibroblasts. Zhonghua Kou Qiang Yi Xue Za Zhi. 44:270–273.
2009.(In Chinese).
|
|
27
|
Ouyang P, Liu S, Bei W, Lai W, Hou F and
Xu A: Effects of flavone from leaves of Diospyros kaki on
adventitial fibroblasts proliferation by advanced oxidation protein
products in vitro. Zhong Yao Cai. 27:186–188. 2004.(In
Chinese).
|
|
28
|
Siggelkow H, Rebenstorff K, Kurre W,
Niedhart C, Engel I, Schulz H, Atkinson MJ and Hufner M:
Development of the osteoblast phenotype in primary human
osteoblasts in culture: comparison with rat calvarial cells in
osteoblast differentiation. J Cell Biochem. 75:22–35. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beck GR Jr, Sullivan EC, Moran E and
Zerler B: Relationship between alkaline phosphatase levels,
osteopontin expression, and mineralization in differentiating
MC3T3-E1 osteoblasts. J Cell Biochem. 68:269–280. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM
and Luo SQ: Oxidative stress inhibits osteoblastic differentiation
of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun.
314:197–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee NK, Choi YG, Baik JY, Han SY, Jeong
DW, Bae YS, Kim N and Lee SY: A crucial role for reactive oxygen
species in RANKL-induced osteoclast differentiation. Blood.
106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barbagallo I, Vanella A, Peterson SJ, Kim
DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Di
Raimondo F, Abraham NG and Asprinio D: Overexpression of heme
oxygenase-1 increases human osteoblast stem cell differentiation. J
Bone Mineral Metab. 28:276–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan SF, Ramasamy R and Schmidt AM: The
RAGE axis: a fundamental mechanism signaling danger to the
vulnerable vasculature. Circ Res. 106:842–853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wautier MP, Chappey O, Corda S, Stern DM,
Schmidt AM and Wautier JL: Activation of NADPH oxidase by AGE links
oxidant stress to altered gene expression via RAGE. Am J Physiol
Endocrinol Metab. 280:E685–E694. 2001.PubMed/NCBI
|
|
35
|
Yamagishi S: Role of advanced glycation
end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets.
12:2096–2102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li G, Xu J and Li Z: Receptor for advanced
glycation end products inhibits proliferation in osteoblast through
suppression of Wnt, PI3K and ERK signaling. Biochem Biophys Res
Commun. 423:684–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding KH, Wang ZZ, Hamrick MW, Deng ZB,
Zhou L, Kang B, Yan SL, She JX, Stern DM, Isales CM and Mi QS:
Disordered osteoclast formation in RAGE-deficient mouse establishes
an essential role for RAGE in diabetes related bone loss. Biochem
Biophys Res Commun. 340:1091–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|