Introduction
The formation of hypertrophic scars (HS) is a common
abnormal response to wound healing which generally occurs following
surgery, trauma and burns. Patients with HS often have severe
impairment of their quality of life, including itching, pain,
stiffness, loss of joint mobility or anatomical deformities that
finally delay the resumption of normal life and create a huge
financial burden on the healthcare system. Many treatments for HS,
such as surgical excision, corticosteroid injections, splinting and
pressure therapy, as well as novel methods, including interferon
and 5-fluorouracil therapy, are not often completely successful and
HS easily recur. There is still no consensus regarding the most
effective treatment to completely and permanently improve scars
with minimal side-effects (1).
Currently, little is known about the incidence and risk factors for
HS, although histologically, HS are characterized by excessive
fibroblast hypercellularity, an overproduction of collagen and
atypical extracellular matrix (ECM) remodeling in the scar tissue
(2). Although the exact
mechanisms of HS remain unclear, decreased fibroblast apoptosis in
the wound bed and consequently, increased proliferation are
observed during the development of HS (3).
Normal wound healing requires the orchestrated
recruitment and proliferation of various cell types in wounds
followed by their rapid disappearance. Thus, following
re-epithelialization, contraction and when sufficient ECM is
formed, the myofibroblast phenotype normally disappears mainly by
the activation of programmed cell death (4). Simultaneously, inflammatory cells
are also eliminated to allow the formation of granulation tissue.
However, in HS, myofibroblasts and inflammatory cells tend to
persist, which may represent an important element in the mechanisms
of excessive ECM deposition and scar contractures. Previous studies
have suggested that the decrease in myofibroblast sensitivity in
response to apoptosis leads to an imbalance between ECM deposition
and degradation, resulting in HS (4,5).
While apoptosis is believed to play an important role in these
processes, its upstream regulators and effectors in the wound
environment remain unclear. Certain studies have described a
decrease in apoptosis together with higher resistance to apoptotic
inducers in HS myofibroblasts (6), whereas others have found an
increased rate of apoptosis (7,8).
The focal upregulation of p53 expression, which
plays an important role in the inhibition of apoptosis, has been
reported in situations of excessive scarring (9). There is evidence that Akt mediates
this process through the phosphorylation of Bad (9), a negative regulator of Bcl-2, which
downregulates apoptosomal activation by Bax and Bak, leading to the
inhibition of the intrinsic pathway of apoptosis. In this pathway,
imbalance between pro-(Bax and Bak) and anti-apoptotic (Bcl-2 and
Bcl-xL) proteins on the mitochondrial membrane stimulates the
release of several mitochondrial proteins, including cytochrome
c, apoptosis-inducing factor, endonuclease G and second
mitochondria derived activator of caspase/direct inhibitor of
apoptosis protein (IAP)-binding protein with a low isoelectric
point (pI) (Smac/DIABLO) (10,11). In the cytosol, cytochrome
c, apoptotic peptidase activating factor-1 (Apaf-1) and
pro-caspase-9 form the apoptosome complex which activates primary
downstream targets (i.e., pro-caspase-3). Finally, active caspases
induce characteristic apoptotic changes through their ability to
cleave certain key protein substrates in the cell. These caspases
can be inhibited by IAPs through direct binding with the
baculovirus inhibitory repeat (BIR) domain of IAPs. Of note, little
is known about the wound healing effects of the Smac/DIABLO
protein, which regulates caspase inhibition by IAPs. Previous
studies have described the ability of the Smac/DIABLO protein to
downregulate cell proliferation and promote cell apoptosis
(12). The expression of Smac has
been shown to be downregulated in renal cell carcinoma (13,14), lung cancer (15), testicular germ cell tumors
(16) and hepatocellular
carcinoma (17). Moreover, an
inverse correlation has been found between the expression levels of
Smac/DIABLO and cancer progression (18).
As several studies have confirmed that Smac/DIABLO
promotes apoptosis, we hypothesized that Smac/DIABLO may be
involved in the formation of HS. In this study, we examined
Smac/DIABLO expression in HS and the effects of its overexpression
in HS fibroblasts (HSFBs).
Materials and methods
Patient specimens
HS and normal skin tissues were obtained from 25
subjects (11 males and 14 females, aged 2–34 years) who
underwent scar resection at 6–12 months following severe
empyrosis. This study was approved by the Ethics Committee of
Southwest Hospital of the Third Military Medical University
(Chongqing, China). HS tissues were erythematous, raised, pruritic
and confined to the site of injury. All samples were in the
actively hyperplastic phase, as confirmed by pathological
examination. Local infection, ulceration and treatment with
glucocorticosteroids or radiotherapy were excluded. Informed
consent was obtained from each participant.
Cell cultures
Fibroblasts were established as primary cell lines
from HS and normal skin tissue. Using sterile techniques under a
laminar flow hood, normal skin and HS tissues were washed in
triplicate in Hank’s solution, and the epidermis and subcutaneous
fat layer were then removed. The dermal specimen was minced into
pieces (sized 0.5–1.0 mm3) using a sterile
scalpel blade on a Petri dish. All specimens were washed with
phosphate-buffered saline (PBS) solution with a combination of 1%
penicillin, amphotericin B and streptomycin sulfate. In order to
allow the tissue pieces to adhere to the flask wall firmly, the
specimens were cultured in serum-containing medium (37ºC, 5%
CO2) consisting of DMEM, 10% fetal bovine serum and 1%
penicillin-streptomycin. The medium was changed every
2–3 days until the fibroblasts were grew to a monolayer and
were spread over the flask bottom when observed under a light
microscope. The tissues were then removed and the cells were
subcultured. The culture medium was changed every 4 days, and
successive subcultures were performed upon confluence. The growth
status and morphological changes of the cells were observed under
an inverted microscope. Early-passage cells (passages 3–6)
were used in this study. Normal skin fibroblasts (NSFBs) were used
as the controls for the HSFBs from the same donors, to confirm that
HSFBs acted differently from normal fibroblasts.
Immunohistochemistry of Smac
HS specimens and normal skin specimens were fixed,
embedded and cut into ~5-μm-thick sections that were placed on
Aptex-coated slides. They were then dewaxed in xylene and
rehydrated after 10 min on heat. Blocking conditions were then
performed for 30 min. The primary antibody for Smac (1:500; Bioss
Inc., Beijing, China) was then added followed by incubation for 1.5
h. The secondary antibody was then added coupled with avidin
(1:200; Bioss Inc.). We examined the tissues under an Olympus
microscope and SPOT™ digital microscope camera (Diagnostic
Instruments, Inc., Sterling Heights, MI, USA).
Western blot analysis of Smac
HSFBs and NSFBs were lysed immediately with cold
lysis buffer containing 1% phenylmethylsulfonyl fluoride, 1%
protease inhibitor cocktail and 1% sodium orthovanadate (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The supernatants were
harvested following centrifugation. Protein was collected using the
BCA protein assay kit according to the manufacturer’s instructions
(Beyotime, Shanghai, China). Proteins were separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
After blocking with 5% non-fat milk, the membranes were incubated
with an antibody against Smac (1:200; Santa Cruz Biotechnology,
Inc.) overnight at 4ºC. The membranes were washed with TBS in
triplicate and then incubated with secondary antibody for 1 h at
room temperature. The protein-antibody complex was visualized by
electrochemiluminescence (ECL) western blotting detection reagents.
β-actin was used as the control. The gray scale densities of
β-actin and Smac were assayed using ImageJ software.
Overexpression and silencing of
Smac/DIABLO
The Smac/DIABLO gene was purchased from ProteinTech
Group, Inc. (Wuhan, China). We selected Sal1 and Xba1
as the cutting sites. The primers used for the PCR amplification of
Smac/DIABLO were: Sal1, 5′-ACGCGTCGACATGGCGGCTCT
GAAGAGTTGG-3′; and Xba1, 5′-GCTCTAGATCAATCCT
CACGCAGGTAGGC-3′. Adenovirus carrying the Smac/DIABLO gene with an
enhanced green fluorescent protein was used as the overexpression
group (AD-Smac group), and adenovirus carrying green fluorescent
protein only was used as the control (control AD-Smac group).
Fibroblasts incubated with PBS only were also used as the blank
controls (HSFB group). The HSFBs were incubated with adenovirus at
various multiplicities of infection for 24 h. Three days later,
protein expression was detected by both fluorescence microscopy and
flow cytometry.
Smac siRNA was purchased from Santa Cruz
Biotechnology, Inc. The control siRNA had the same composition of
nucleotides, but there was no homology between the control siRNA
and Smac mRNA. The HSFBs were inoculated in a 24-well plate and
incubated for a whole day, then transfected with Smac siRNA (siRNA
group), or with control siRNA (control siRNA group). The blank
control was HSFBs incubated with PBS (HSFB group).
Cell proliferation analysis
We used the cell counting kit-8 (CCK-8) assay, which
is widely used to quantify cell proliferation, to assess HSFB
proliferation following transfection with AD-Smac. The detection
sensitivity of CCK-8 is higher than other tetrazolium salts, such
as 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
(MTT),
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
(XTT),
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) or water-soluble tetrazolium salt (WST-1). We inoculated the
HSFB suspension (100 μl/well) in a 96-well plate, then
pre-incubated the plate in a humidified incubator (37ºC, 5%
CO2) for 24 h. We then added 10 μl of the CCK-8 solution
to each well followed by incubation for 1 h. The absorbance was
measured at 450 nm using a microplate reader (Flash Spectrum
Biology Technology Co., Ltd.; Shanghai, China).
Detection of apoptosis and cell cycle
analysis
For the detection of apoptosis, the HSFBs
transfected with AD-Smac or the control were cultured in 6-well
plates for 24 h. Apoptosis was then detected using the terminal
deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay
kit, according to the instructions of the manufacturer (Promega,
Madison, WI, USA) and the cell cycle of the HSFBs was analyzed
using a FACSAria flow cytometer (BD Biosciences, San Jose, CA,
USA). The data were analyzed using CellQuest 3.0 software (BD
Biosciences).
mRNA levels of type I and III
pro-collagen
The levels of type I and III pro-collagen were
quantified by real-time RT-PCR. Primers used were as follows: type
I pro-collagen forward, 5′-GTTCGTCCTTCTCAGGGTAG-3′ and reverse,
5′-TTGTCGTAGCAGGGTTCTTT-3′; fragment length, 254 bp; type III
pro-collagen forward, 5′-CGAGGTAACAGAGG TGAAAGA-3′ and reverse,
5′-AACCCAGTATTCTCCACT CTT-3′; fragment length, 349 bp; human
β-actin forward, 5′-TCCCTGGAGAAGAGCTACGA-3′ and reverse, 5′-AGCA
CTGTGTTGGCGTACAG-3′. The initial denaturation was achieved at 94ºC
for 2 min, denaturation at 94ºC for 30 sec, reassociation at 55ºC
for 30 sec, extension at 72ºC for 30 sec (28 cycles) and a final
extension step at 72ºC for 10 min.
Caspase-3 and -9 activity
For caspase-3 or -9 activity assays in the HSFBs,
each reaction with a final volume of 30 μl was assembled on ice,
including caspase-3 substrate (Ac-DEVD-AFC, 1 mM) or caspase-9
substrate (Ac-LEHD-AFC, 1 mM) and 10X caspase assay buffer. The
generation of a fluorescence signal expressed in relative
fluorescent units, due to the cleavage of substrates by caspase-3
or -9, was measured using an automated spectrophotometer (Shanghai
Metash Instruments Co., Ltd., Shanghai, China) at wavelengths of
360/465 or 400/505 nm (excitation/emission).
Statistical analyses
Experiments were conducted in triplicate and data
are expressed as the means ± standard error. The Student’s t-test
was performed to evaluate group differences with SPSS 11.0
statistical software (SPSS Inc., Chicago, IL, USA). Values of
P<0.05 were considered to represent statistically significant
differences.
Results
Smac expression is lower in HS and HSFBs
than normal tissue and fibroblasts
Smac expression as detected by immunohistochemistry
in the NSFBs was stronger than in the HS, where a weak expression
was detected (Fig. 1). Following
western blot analysis of the cell extracts, the Smac protein band
at 25 kDa was more prominent in the NSFBs than in the HSFBs
(Fig. 2). Using ImageJ software,
quantitative analyses of Smac expression in 25 patients indicated
and confirmed that the relative Smac protein level corresponding to
the ratio of Smac/β-actin was significantly lower (11.03%) in the
HSFBs than in the NSFBs (25.02%) (P<0.01).
Smac overexpression induces apoptosis of
HSFBs
As Smac expression is low in HSFBs, we explored the
modifications induced by the overexpression and suppression of Smac
expression in HSFBs. The apoptotic rate of the HSFB group, as
measured by flow cytometry, was the lowest among the 5 groups
(Fig. 3). The apoptotic rates of
the control AD-Smac group and the control siRNA group were higher
than the HSFB group. Moreover, the apoptotic rate of the AD-Smac
group, which overexpressed Smac, was significantly higher than that
of the control AD-Smac group, transfected with the adenovirus only
(P<0.01). The apoptotic rate of the control siRNA group was
significantly higher than that of the siRNA group (P<0.01).
These results suggest the possible role of Smac in the induction of
apoptosis in HSFBs.
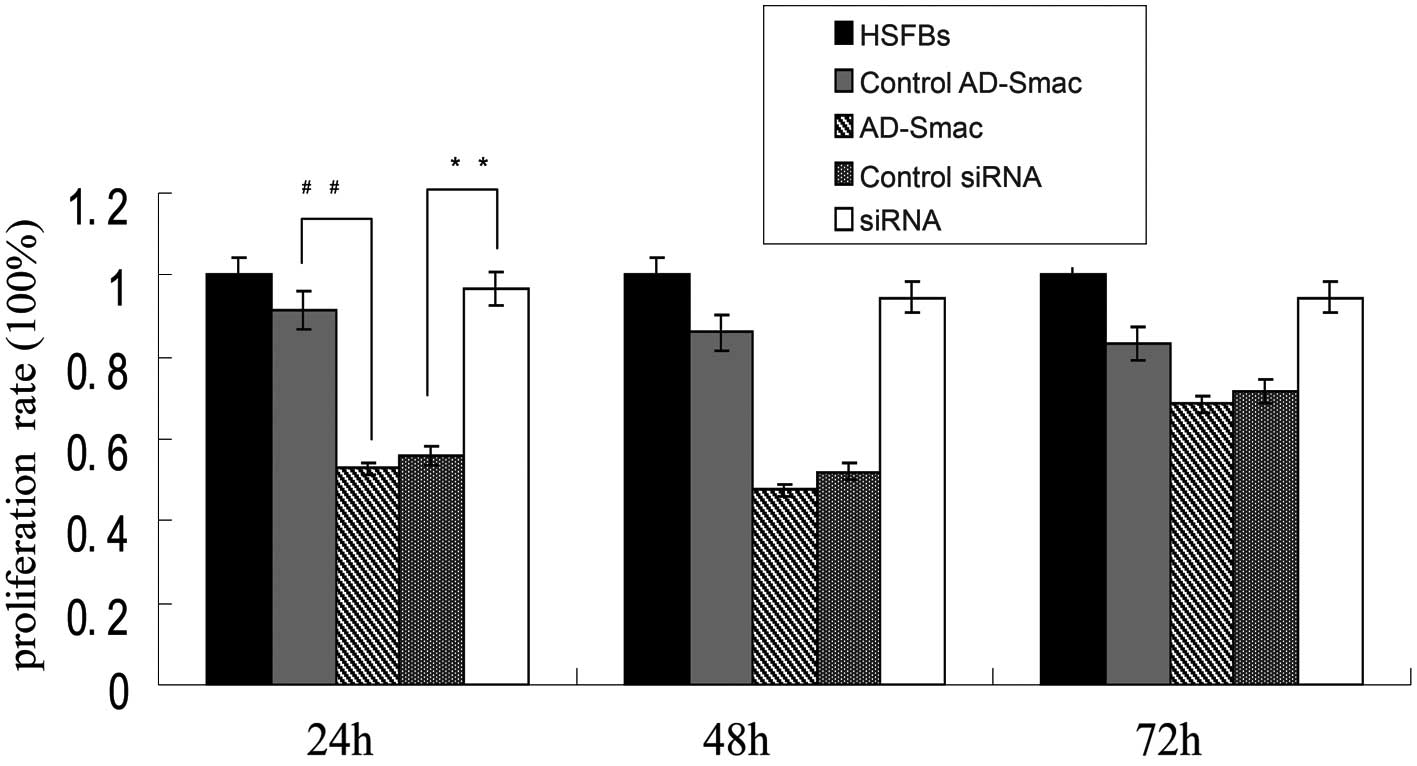
Smac overexpression significantly
inhibits HSFB proliferation
The proliferation rate of the HSFBs was regarded as
100% and the proliferation of all the other groups was lower than
that ofs the HSFBs (Fig. 4). A
significant suppressive effect on cell proliferation was observed
following the transfection of the HSFBs with AD-Smac, as shown by
CCK-8 assay. A marked decrease in proliferation was observed in the
cells overexpressing Smac (transfected with AD-Smac) (52.86%)
compared with the control AD-Smac-transfected cells (91.66%) at 24
h (P<0.01). The proliferation rate of the siRNA group was
significantly increased (96.64%) compared with that of the control
siRNA group (56.03%) at 24 h (P<0.01). The differences in the 2
proliferation rates at 24 h were greater than at 48 and 72 h.
Therefore, according to these results, it can be concluded that
Smac overexpression significantly inhibited HSFB proliferation,
while the silencing of Smac by siRNA induced HSFB
proliferation.
Smac overexpression downregulates the
mRNA levels of type I and III pro-collagen
The excessive synthesis of type I and III
pro-collagen is an important characteristic of HS. We therefore
assessed the mRNA expression levels of type I and III pro-collagen
in the HSFBs following treatment with AD-Smac or control AD-Smac
and siRNA, or control siRNA. The results revealed no significant
differences in the mRNA levels of type I and III pro-collagen among
the HSFB group, the control AD-Smac group and the control siRNA
group (P>0.05). The levels of type I and III pro-collagen were
significantly decreased in the cells overexpressing Smac (AD-Smac
group) compared with the control AD-Smac group and the HSFB group.
The levels were upregulated in the siRNA group, where Smac
expression was suppressed, compared with the control siRNA group
and the HSFB group. These results demonstrate that Smac
overexpression downregulates the mRNA levels of type I and III
pro-collagen in the HSFBs. It was also shown that Smac
preferentially affects the mRNA levels of type I pro-collagen
compared with the mRNA levels of type III pro-collagen (Fig. 5).
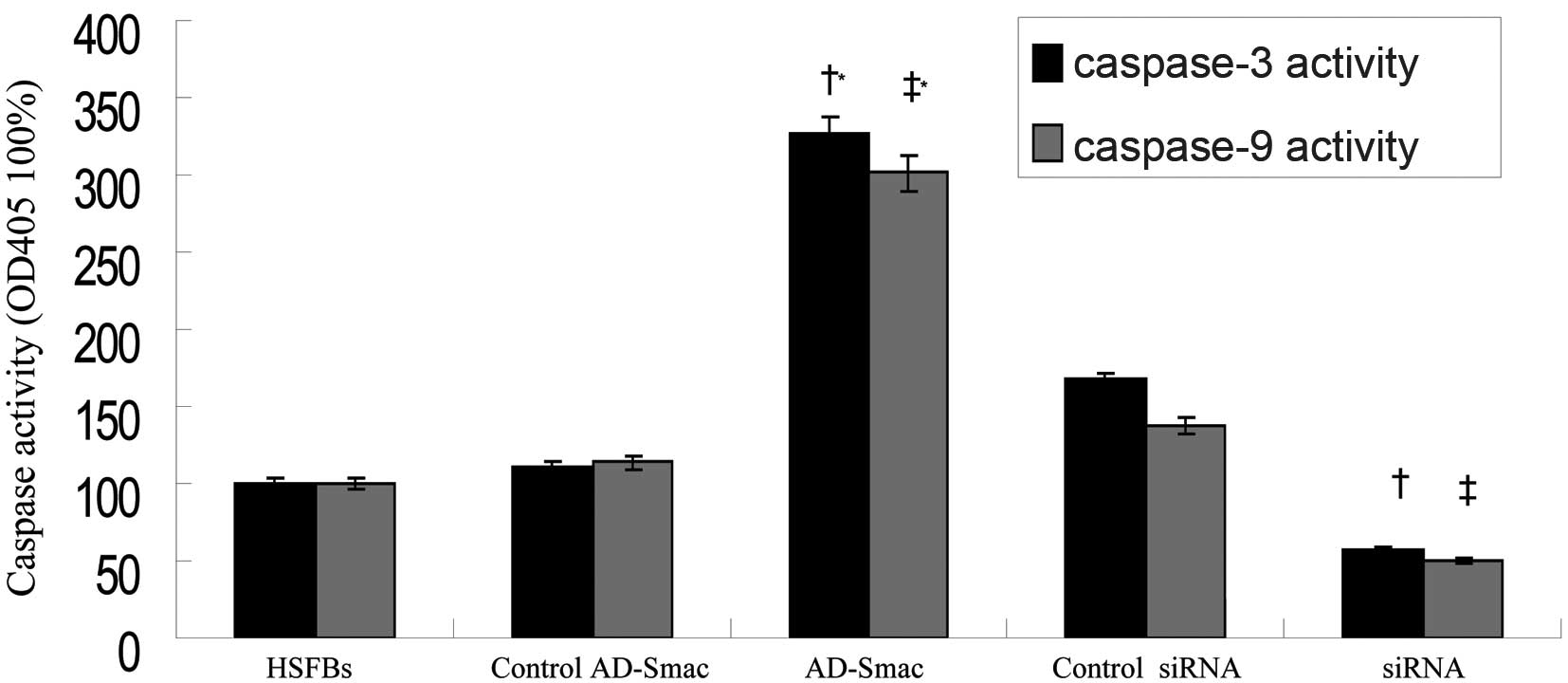
Smac overexpression enhances the activity
of caspase-3 and -9 in HSFBs
Caspase-3 and -9 are 2 important members of the
caspase family and caspase-3 activation is considered as the
executing event of apoptosis. Caspase-3 and -9 activity, as
dectected by spectrofluorimetry, was activated in the HSFBs
following transfection with AD-Smac (Fig. 6). The activity of caspase-3 and -9
was 3-fold greater in the HSFBs overexpressing Smac (AD-Smac group)
than in the control HSFBs (HSFB group) or the control
AD-Smac-transfected group. The activityof caspase-3 and -9 was
markedly reduced (by 50%) in the siRNA group, where Smac expression
was suppressed, compared with the HSFB group and control siRNA
group.
Discussion
When severe trauma or deep burns occur on the skin,
wound healing is crucial in order to restore the cutaneous barrier.
Blood platelet cells, inflammatory cells, endothelial cells,
fibroblasts and keratinocytes are recruited to prevent bleeding and
promote wound healing. These cells form new blood vessels and
produce ECM to create a new layer covering the surface of the
wound. The majority of shallow wounds heal within a few days.
However, in some individuals, the dermal fibroblasts proliferate
excessively and secrete an overabundant ECM, resulting in HS. HS
are clinically described as raised, pruritic and erythematous
fibrous lesions limited within the boundary of the original wound.
HS lesions are often bulky and inelastic scars which can severely
restrict the mobility of joints and extremities, immobilize
structures, constrict orifices and drastically compromise cosmetic
appearance (2). Increased HSFB
proliferation and decreased HSFB apoptosis have been suggested to
be the main factors in the development of HS, whereas the upstream
regulators of apoptosis in the wound environment remain unclear
(19–21). Among the numerous key factors of
wound healing (22), of all
proteins involved in the apoptotic processes (23, 24), the differences in their expression
and distribution in the wound healing process (25), a number of targets have been
suggested to improve healing without keloids or HS (22–24). However, little is known about the
effects of Smac/DIABLO on skin wound healing processes; it is
currently accepted that the expression levels of Smac/DIABLO are
downregulated in several excessive proliferative diseases or tumors
compared with normal tissues (13–18).
In this study, we investigated the expression level
of Smac/DIABLO in HSFBs and normal fibroblasts. We observed a
significant downregulation of Smac/DIABLO expression in HS compared
to normal skin tissues using both immunohistochemical labeling and
western blot analyses. Thus, our results on HS processes, similar
to other proliferative diseases or tumors (13–18), suggest the important role of
Smac/DIABLO in the process of HS, mainly through the regulation of
HSFB apoptosis and proliferation.
In order to confirm this hypothesis, we induced the
overexpression of Smac in HSFBs. The overexpression of Smac
significantly increased the apoptotic rate of the HSFBs and thus
inhibited their proliferation. Remarkably, the transfection of
these cells with Smac siRNA, suppressing Smac expression, had the
opposite effect, inducing a significant reduction in apoptosis and
a restoration of the HSFB proliferation rate. Consistent with the
data from previous studies on various proliferative diseases
(26,27), our results clearly demonstrate
that Smac/DIABLO plays an important role in the regulation of
apoptosis and proliferation of cells. However, the exact mechanism
behind the Smac regulation of proliferation of HSFBs remains
unknown.
The overexpression Smac/DIABLO by transfection of
the cells with adenovirus carrying Smac increased caspase-3 and -9
activity. Restoration of the caspase rate (to low levels) with Smac
siRNA suggests the major role played by Smac/DIABLO in the complex
regulation of HSFB proliferation during wound healing. According to
previous studies (28–30), apoptosis caused by Smac in the
HSFBs mainly depends on the caspase pathways. Following the induced
overexpression of Smac in HSFBs and its inhibition by Smac siRNA,
our results strongly suggest that the intrinsic pathway of
apoptosis is one of the main regulators of fibroblast function,
i.e., colonization and proliferation, during skin wound
healing.
However, HS is also characterized by a decrease in
collagenase content, an increased collagen synthesis mainly by
fibroblasts, leading to an abnormal collagen deposition and
atypical ECM remodeling in the scar tissue (31). The ECM in the skin is mainly
characterized by the presence of type I and III pro-collagen, whose
expression is significantly increased in HS. Remarkably, the
expression level of type III pro-collagen is lower than that of
type I pro-collagen in keloid scars. In a previous study, Oliveira
et al (32) found that
type III pro-collagen levels were significantly increased in HS
compared to non-HS, whereas no difference in type I pro-collagen
levels were observed in the same samples. Thus, the excessive
secretion of type I pro-collagen also appears to play an important
role in HS, and may be involved in the severity of HS. Thus, it
would be of importance to find exogenous factors affecting collagen
synthesis for the treatment of scarring. For example, many
treatments with growth factors (33–35), steroids (36,37) or natural products (38,39) may not only regulate the expression
of collagens, but also collagen bundle organization. In this study,
we investigated whether, in addition to its effects on HSFB
proliferation and apoptosis, the expression of Smac can influence
collagen type I and III expression. Our results demonstrated that
Smac overexpression inhibited the mRNA expression of type I and III
pro-collagen in the HSFBs. Indeed, the effects of Smac
overexpression on the mRNA levels of type I pro-collagen were more
significant than those on the mRNA levels of type III pro-collagen.
Thus, the overproduction of collagen and atypical ECM remodeling in
the scar tissue, with the modification of the ratio collagen I/III,
lead to fibrosis of the scar. These results suggest for the first
time a possible link (direct or indirect) between Smac/DIABLO and
the levels of type I and III collagen in fibroblasts, which may be
involved in the formation of HS.
In conclusion, to our knowledge, in this study, we
demonstrate for the first time that in HS tissue, Smac/DIABLO is
downregulated compared to NSFBs. This results not only in the
promotion of fibroblast proliferation or in the reduction of their
sensitivity to apoptotic signals, but also in the increase in type
I and III pro-collagen expression. As all these events were
inhibited by Smac overexpression, this suggests that Smac/DIABLO
may be a novel therapeutic target that may be used to prevent and
control HS formation. Finally, the regulation of Smac/DIABLO may
provide a potential approach for the regulation and improvement of
skin wound healing.
Acknowledgements
We are particularly grateful to Tao Wang and Lilong
Zhang for their technical assistance (College of Preventive
Medicine, Third Military Medical University, Chongqing 400038,
China). This study was supported by the National Natural Science
Foundation of China (project no. 81272102).
References
|
1
|
O’Leary R, Wood EJ and Guillou PJ:
Pathological scarring: strategic interventions. Eur J Surg.
168:523–534. 2002.
|
|
2
|
van der Veer WM, Bloemen MC, Ulrich MM, et
al: Potential cellular and molecular causes of hypertrophic scar
formation. Burns. 35:15–29. 2009.PubMed/NCBI
|
|
3
|
De Felice B, Garbi C, Santoriello M, et
al: Differential apoptosis markers in human keloids and
hypertrophic scars fibroblasts. Mol Cell Biochem. 327:191–201.
2009.PubMed/NCBI
|
|
4
|
Greenhalgh DG: The role of apoptosis in
wound healing. Int J Biochem Cell Biol. 30:1019–1030. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desmouliere A, Badid C, Bochaton-Piallat
ML and Gabbiani G: Apoptosis during wound healing, fibrocontractive
diseases and vascular wall injury. Int J Biochem Cell Biol.
29:19–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linge C, Richardson J, Vigor C, et al:
Hypertrophic scar cells fail to undergo a form of apoptosis
specific to contractile collagen-the role of tissue
transglutaminase. J Invest Dermatol. 125:72–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akasaka Y, Fujita K, Ishikawa Y, et al:
Detection of apoptosis in keloids and a comparative study on
apoptosis between keloids, hypertrophic scars, normal healed flat
scars, and dermatofibroma. Wound Repair Regen. 9:501–506. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka A, Hatoko M, Tada H, et al:
Expression of p53 family in scars. J Dermatol Sci. 34:17–24. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aarabi S, Bhatt KA, Shi Y, et al:
Mechanical load initiates hypertrophic scar formation through
decreased cellular apoptosis. FASEB J. 21:3250–3261. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du C, Fang M, Yucheng Li, et al: Smac, a
mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verhagen AM, Ekert PG, Pakusch M, et al:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao HL, Liu PS, Zheng JF, et al:
Transfection of Smac/DIABLO sensitizes drug-resistant tumor cells
to TRAIL or paclitaxel-induced apoptosis in vitro. Pharmacol Res.
56:483–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kempkensteffen C, Hinz S, Christoph F, et
al: Expression levels of the mitochondrial IAP antagonists
Smac/DIABLO and Omi/HtrA2 in clear-cell renal cell carcinomas and
their prognostic value. J Cancer Res Clin Oncol. 134:543–550. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizutani Y, Nakanishi H, Yamamoto K, et
al: Downregulation of Smac/DIABLO expression in renal cell
carcinoma and its prognostic significance. J Clin Oncol.
23:448–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sekimura A, Konishi A, Mizuno K, et al:
Expression of Smac/DIABLO is a novel prognostic marker in lung
cancer. Oncol Rep. 11:797–802. 2004.PubMed/NCBI
|
|
16
|
Kempkensteffen C, Jager T, Bub J, et al:
The equilibrium of XIAP and Smac/DIABLO expression is gradually
deranged during the development and progression of testicular germ
cell tumours. Int J Androl. 30:476–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao ST, Gui SQ and Lin MS: Relationship
between expression of Smac and Survivin and apoptosis of primary
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
5:580–583. 2006.PubMed/NCBI
|
|
18
|
Martinez-Ruiz G, Maldonado V,
Ceballos-Cancino G, et al: Role of Smac/DIABLO in cancer
progression. J Exp Clin Cancer Res. 27:482008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Z, Li s, Cao C, et al: shRNA targeting
SFRP2 promotes the apoptosis of hypertrophic scar fibroblast. Mol
Cell Biochem. 352:25–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo L, Chen L, Bi S, et al: PTEN inhibits
proliferation and functions of hypertrophic scar fibroblasts. Mol
Cell Biochem. 361:161–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao C, Li S, Dai X, et al: Genistein
inhibits proliferation and functions of hypertrophic scar
fibroblasts. Burns. 35:89–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahdavian Delavary B, van der Veer WM, van
Egmond M, et al: Macrophages in skin injury and repair.
Immunobiology. 216:753–762. 2011.PubMed/NCBI
|
|
23
|
Honardoust D, Ding J, Varkey M, et al:
Deep dermal fibroblasts refractory to migration and decorin-induced
apoptosis contribute to hypertrophic scarring. J Burn Care Res.
33:668–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akasaka Y, Ono I, Kamiya T, et al: The
mechanisms underlying fibroblast apoptosis regulated by growth
factors during wound healing. J Pathol. 221:285–299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang L, Zhang E, Yang Y, et al:
Effectiveness of apoptotic factors expressed on the wounds of
patients with stage III pressure ulcers. J Wound Ostomy Continence
Nurs. 39:391–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia L, Patwari Y, Kelsey SM, et al: Role
of Smac in human leukaemic cell apoptosis and proliferation.
Oncogene. 22:1589–1599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DJ and Huerta S: Smac mimetics as new
cancer therapeutics. Anticancer Drugs. 20:646–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chai J, Du C, Wu JW, et al: Structural and
biochemical basis of apoptotic activation by Smac/DIABLO. Nature.
406:855–862. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu G, Chai J, Suber TL, et al: Structural
basis of IAP recognition by Smac/DIABLO. Nature. 408:1008–1012.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Z, Tian Y, Wang J, et al: A dimeric
Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP.
Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol
Chem. 282:30718–30727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark JA, Cheng JC and Leung KS:
Mechanical properties of normal skin and hypertrophic scar. Burns.
22:443–446. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oliveira GV, Hawkins HK, Chinkes D, et al:
Hypertrophic vs. non hypertrophic scars compared by
immunohistochemistry and laser confocal microscopy: type I and III
collagens. Int Wound J. 6:445–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akita S, Akino K, Tanaka K, et al: A basic
fibroblast growth factor improves lower extremity wound healing
with a porcine-derived skin substitute. J Trauma. 64:809–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho JW, Kang MC and Lee KS: TGF-β1-treated
ADSCs-CM promotes expression of type I collagen and MMP-1,
migration of human skin fibroblasts, and wound healing in
vitro and in vivo. Int J Mol Med. 26:901–906. 2010.
|
|
35
|
Yan G, Sun H, Wang F, et al: Topical
application of hPDGF-A-modified porcine BMSC and keratinocytes
loaded on acellular HAM promotes the healing of combined
radiation-wound skin injury in minipigs. Int J Radiat Biol.
87:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weshahy AH and Abdel Hay R: Intralesional
cryosurgery and intralesional steroid injection: a good combination
therapy for treatment of keloids and hypertrophic scars. Dermatol
Ther. 25:273–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayashi T, Furukawa H, Oyama A, et al: A
new uniform protocol of combined corticosteroid injections and
ointment application reduces recurrence rates after surgical
keloid/hypertrophic scar excision. Dermatol Surg. 38:893–897. 2012.
View Article : Google Scholar
|
|
38
|
Wu JG, Ma L, Zhang SY, et al: Essential
oil from rhizomes of Ligusticum chuanxiong induces apoptosis
in hypertrophic scar fibroblasts. Pharm Biol. 49:86–93. 2010.
|
|
39
|
Morin C, Roumegous A, Carpentier G, et al:
Modulation of inflammation by Cicaderma ointment accelerates skin
wound healing. J Pharmacol Exp Ther. 343:115–124. 2012. View Article : Google Scholar : PubMed/NCBI
|