Introduction
Acute intestinal ischemia is a serious abdominal
emergency which is commonly observed in patients affected by
trauma, burns and shock, as well as in those undergoing
cardiovascular surgery and organ transplantation, resulting in a
mortality rate as high as 60 to 80% (1–3).
Transient ischemia results in biological and chemical changes which
lead to intestinal mucosal damage and barrier dysfunction (4). Reperfusion can magnify the damage
and even induce remote organ injuries and dysfunction through the
generation of numerous pro-inflammatory cytokines and the
activation of immune cells (5,6).
The mammalian target of rapamycin (mTOR), a type of
atypical serine/threonine kinase, integrates a variety of
extracellular and intracellular signals, including growth factors,
nutrients, energy depletion and stress (7). The activation of the 2 mTOR
complexes, mTORC1 and mTORC2, regulates diverse functions, such as
cell growth, proliferation, development, memory, longevity,
angiogenesis, autophagy and innate, as well as adaptive immune
responses (8–10). mTOR controls protein synthesis
through the direct phosphorylation and inactivation of a repressor
of mRNA translation, eukaryotic initiation factor 4E-binding
protein 1 (4E-BP1), and through the phosphorylation and activation
of p70 ribosomal S6 kinase (p70S6K), which in turn phosphorylates
the ribosomal protein S6 (8,11).
Cytokines, growth factors, amino acids, insulin, or Toll-like
receptor (TLR) ligands activate mTOR and increase the
phosphorylation status of 4E-BP1 and p70S6K in a
rapamycin-sensitive manner (8,11).
Certain studies have shown that the activation of the mTOR/p70S6K
signaling pathway exerts a protective effect against
ischemia/reperfusion (I/R) injury (12–15).
Ghrelin, a 28-amino acid acylated peptide produced
predominantly by the stomach, is an endogenous ligand for the
growth hormone secretagogue receptor-1a (GHSR-1a) (16). Ghrelin has been shown to possess
growth hormone-releasing properties and other endocrine and
non-endocrine activities, reflecting central and peripheral GHSR-1a
distribution (17–19). Previous studies have shown that
ghrelin inhibits leptin-induced pro-inflammatory cytokine
[interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6]
expression by human T cells and monocytes (20) and suppresses nuclear factor
(NF)-κB activation in human endothelial cells (21). It has been reported that ghrelin
attenuates the formation of I/R-induced gastric lesions and other
types of organ damage (22–25), with a decrease in TNF-α and IL-6
expression (26). mTOR activation
has also been shown to inhibit the production of pro-inflammatory
cytokines and to enhance the release of anti-inflammatory cytokines
by blocking NF-κB activation and increasing signal transducer and
activator of transcription 3 (STAT3) activity (27). Thus, in this study, we aimed to
determine whether ghrelin ameliorates organ damage in a mouse model
of intestinal I/R injury and to further elucidate the possible
mechanisms behind its protective effects.
Materials and methods
Experimental model
Intestinal ischemia was induced in male C57BL/6J
wild-type (WT) mice (16–22 g; Experimental Animal Center of Wuhan
University, Wuhan, China) by clamping the superior mesenteric
artery (SMA) for 45 min under general anesthesia using 0.5%
pentobarbital sodium (0.2 ml/10 g) administered intraperitoneally
(i.p.). The vascular clamp was released after 45 min to allow
reperfusion. Upon the initiation of reperfusion, mice were
resuscitated with 0.5 ml saline (administered i.p.), after which
treatment commenced. The mice were randomly divided into 4
experimental groups as follows: i) ghrelin group (n=10): mice were
administered (i.p.) recombinant murine ghrelin (no. 031-31, 12
nmol/kg in 0.5 ml normal saline; Phoenix Pharmaceuticals, Inc.
Belmont, CA, USA); ii) vehicle group (n=10): mice were administered
(i.p.) 0.5 ml normal saline; iii) ghrelin/D-Lys3-GHRP6 group
(n=10): mice were administered (i.p.) a mixture of ghrelin (12
nmol/kg in 0.25 ml normal saline) and D-Lys3-GHRP6 (no. 031-22, 12
nmol/kg in 0.25 ml normal saline; Phoenix Pharmaceuticals, Inc.);
and iv) control (sham-operated) group (n=10): the animals underwent
the same surgical procedure with the exception of SMA clamping.
Four hours following reperfusion, the animals were anesthetized and
blood (for serum) and tissue samples were harvested, stored
immediately at −80°C or soaked in 4% paraformaldehyde at 4°C until
measurements were made. In addition, experiments for the
observation of survival over the course of 24 h were performed
(n=11/group). All experiments were carried out in accordance with
the Guide for the Care and Use of Laboratory Animals (Institute of
Laboratory Animal Resources) and were approved by the Institutional
Animal Care and Use Committee of Wuhan No. 1 Hospital, Tongji
Medical College (Wuhan, China).
Organ injury variables
Alanine aminotransferase (ALT), aspartate
aminotransferase (AST), lactate dehydrogenase (LDH), creatinine and
urea nitrogen levels in blood serum were determined using
commercial assay kits (CicaLiquid ALT, YZB/JAP 1578-2009;
CicaLiquid AST, YZB/JAP 1579-2009; CicaLiquid LDH J, YZB/JAP
1593-2009; CicaLiquid-N CRE, YZB/JAP 1599-2009; CicaLiquid-N UN,
YZB/JAP 1590-2009; Kanto Chemical Co., Inc., Tokyo, Japan) and
measured using a Biochemical Analyzer (ADVIA 2400; Siemens AG,
Tarrytown, NY, USA).
Serum and tissue pro-inflammatory
cytokines
TNF-α, IL-1β and IL-6 levels were measured using
specific mouse ELISA kits (nos. 88-7324-88, 88-7013-88 and
88-7064-88; eBioscience, Inc., San Diego, CA, USA) in serum, tissue
from the small intestine and pulmonary tissue.
Tissue myeloperoxidase (MPO) assay
Small intestine and pulmonary MPO activity reflects
the extent of neutrophil infiltration in the small intestine and
lungs. MPO activity was evaluated using the MPO Colorimetric
Activity Assay kit (Beijing Homa Biological Engineering Co., Ltd.,
Beijing, China) normalized to nanograms of protein and levels were
measured using a Biochemical Analyzer (ADVIA 2400; Siemens).
Protein concentration was measured using the BCA Protein assay kit
(no. 23225; Pierce Protein Research Products; Thermo Fisher
Scientific, Rockford, IL, USA).
Histopathological analysis
Samples from the small intestine (impaired areas)
and lungs were fixed in 4% paraformaldehyde and embedded in
paraffin. Tissues were sectioned and stained with hematoxylin and
eosin. Histological scoring of the depth of tissue injury was
performed according to the method described in the study by Chiu
et al (28) with certain
modifications: score 0, no damage; score 1, subepithelial space at
villous tip; score 2, loss of mucosal lining of the villous tip;
score 3, loss of less than half of the villous structure; score 4,
loss of more than half of the villous structure; and score 5,
transmural necrosis. Sections were evaluated blindly. Lung injury
was analyzed for absent, mild, moderate, or severe injury (score
0–3) based on the presence of exudates, hyperemia/congestion,
neutrophilic infiltrates, intra-alveolar hemorrhage/debris and
cellular hyperplasia (29).
GHS-R and mTOR/p70S6K western blot
analysis
One hundred micrograms of protein from pulmonary and
intestinal samples was fractionated on a 5–10% SDS-PAGE gel and
electrotransferred onto a 0.45-μm polyvinylidene fluoride membrane.
The blots were blocked with 5% skimmed milk powder in Tris-buffered
saline containing 0.1% v/v Tween-20 and incubated with β-actin
(1–19) (1:800; sc-1616R; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA),
phospho-mTOR(Ser2448) mAb (1:1,000; no. 5536), mTOR
(7C10) mAb (1:1,000; no. 2983), phospho-p70S6K(Thr389)
antibody (1:1,000; no. 9234) all from Cell Signaling Technology,
Inc. (Danvers, MA, USA) and anti-p70S6K mAb (1:1,000; 05-781R;
Millipore, Billerica, MA, USA) in 5% bovine serum
albumin-Tris-buffered saline with Tween-20 at 4°C overnight. The
polyvinylidene fluoride membrane was then washed with Tris-buffered
saline with Tween-20. Following incubation with anti-rabbit IgG
HRP-conjugated antibody (1:2,000; no. 7074; Cell Signaling
Technology) in 5% bovine serum albumin-Tris-buffered saline with
Tween-20 and washing with Tris-buffered saline with Tween-20, bands
were detected using a chemiluminescent peroxidase substrate (no.
34079; ECL Plus; Thermo Fisher Scientific) and exposed on an image
station (Kodak Image Station 4000MM).
Statistical analysis
Data were expressed as the means ± SEM and compared
by analysis of variance using one-way ANOVA and the
Student-Newman-Keuls test. Survival analysis was carried out using
the Kaplan-Meier log-rank test. A P-value <0.05 was considered
to indicate a statistically signficant difference.
Results
Ghrelin improves survival following
intestinal I/R injury
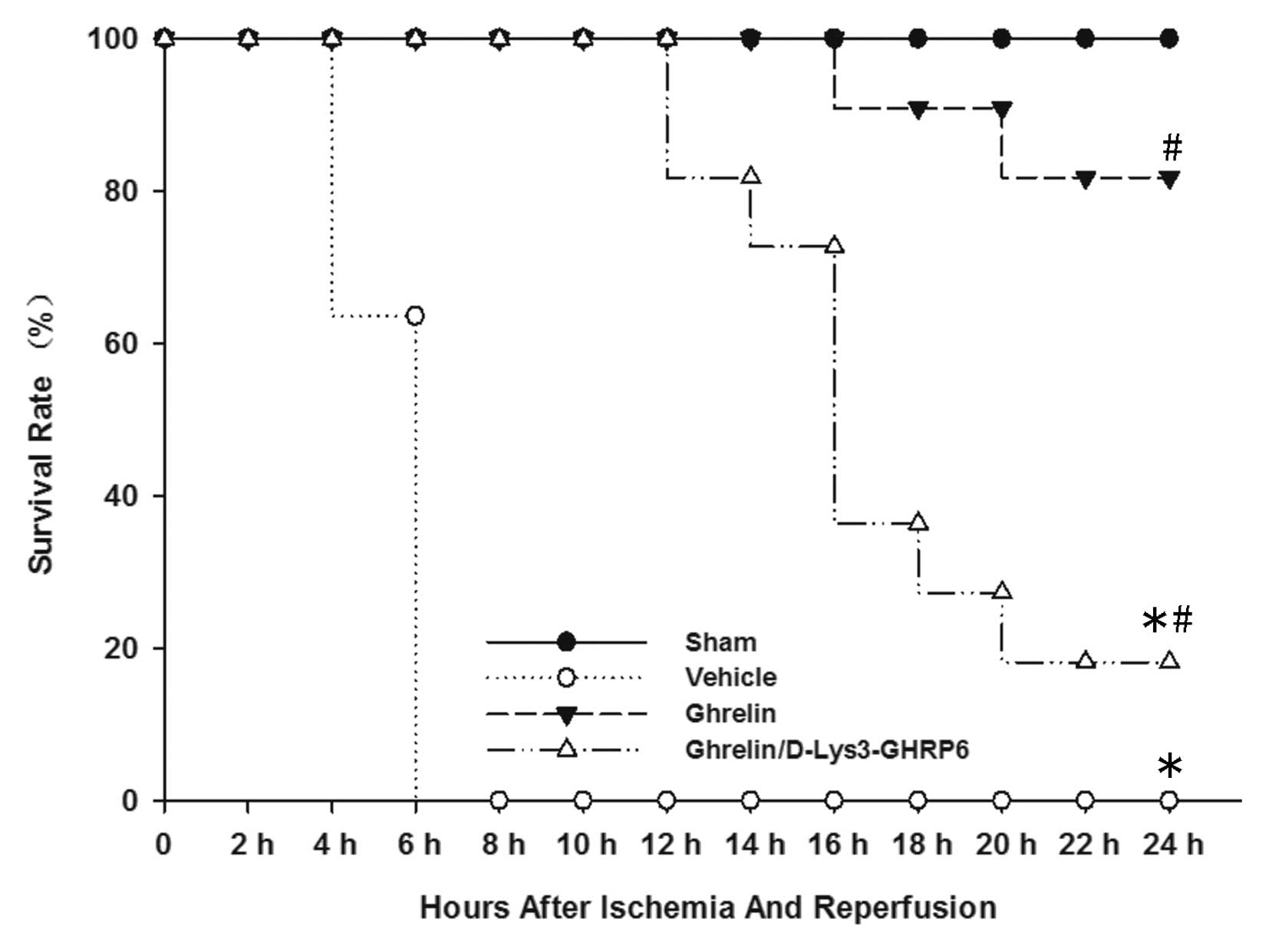
We performed survival analysis in mice receiving
ghrelin at the onset of reperfusion and compared them with control
mice treated with normal saline. We also established a group which
was administered ghrelin and D-Lys3-GHRP-6. All the control mice
died within 24 h (median survival time, 5.9 h; 95% confidence
interval, 5.2–6.6) (Fig. 1). Nine
of the 11 mice treated with ghrelin, however, remained alive 24 h
following gut I/R injury (median survival time, 22.9 h; 95%
confidence interval, 21.2–24.7) (Fig.
1). Among these, 7 mice survived for >1 week (data not
shown). Two of the 11 animals administered ghrelin and
D-Lys3-GHRP-6 remained alive 24 h following gut I/R injury (median
survival time, 17.3 h; 95% confidence interval, 14.6–20) (Fig. 1).
Ghrelin attenuates multiple organ damage
following intestinal I/R injury
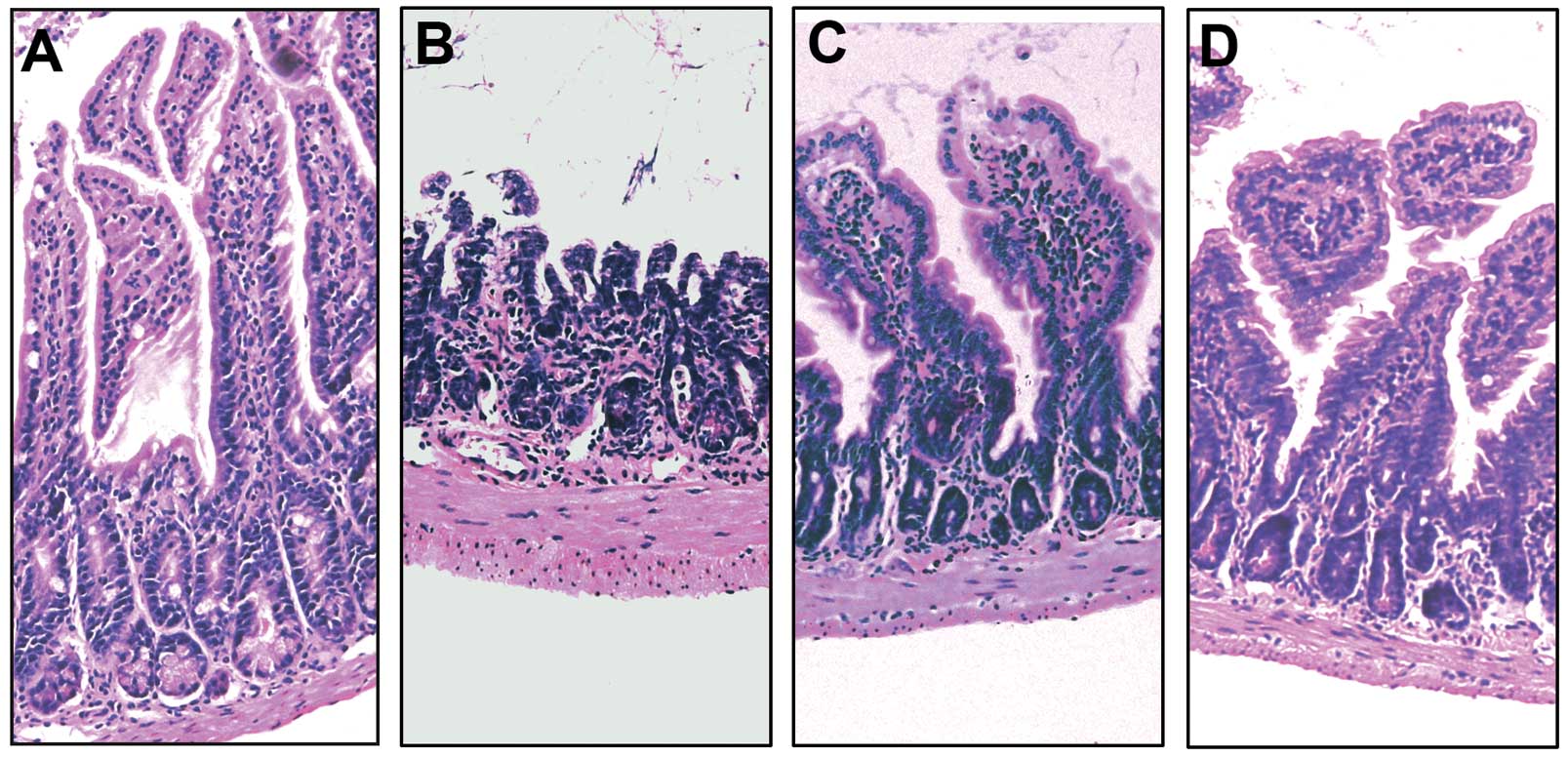
Gut I/R caused microscopic intestinal damage, such
as mucosal destruction, loss of villi and epithelial cell
apoptosis, hemorrhaging and infiltration of inflammatory cells
(Fig. 2B). Treatment with ghrelin
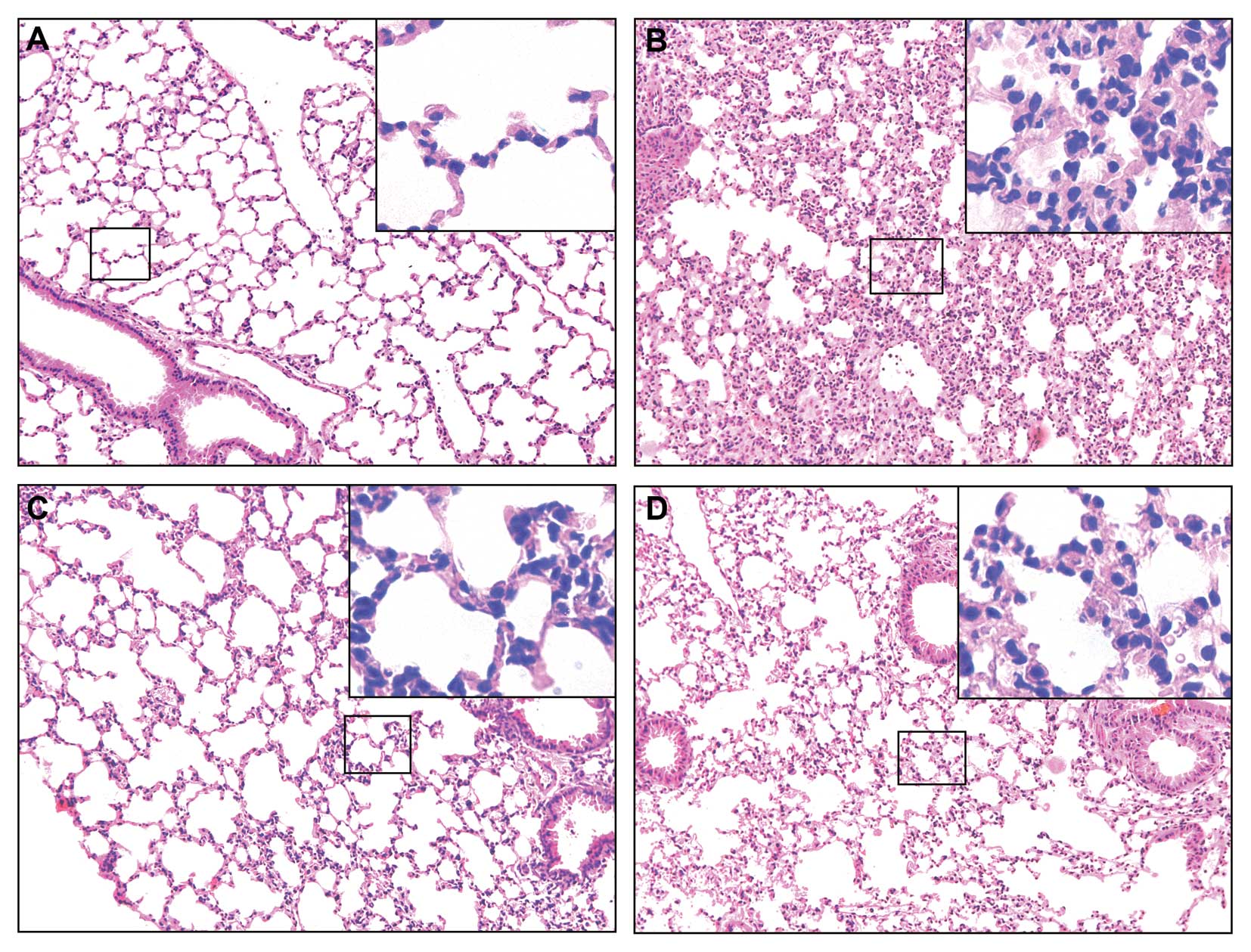
significantly improved these microscopic alterations (Fig. 2C). Similarly, lung injury
characterized by the disruption of lung architecture, extravasation
of red blood cells, and accumulation of inflammatory cells was
present in the I/R-vehicle treated animals (Fig. 3B). The ghrelin-treated mice
displayed a marked reduction in the number of infiltrating
inflammatory cells and an improvement in lung architecture
(Fig. 3C). In the gut and lungs,
tissue injury was slightly ameliorated in the mice treated with
ghrelin and D-Lys3-GHRP6 (Figs.
2D and 3D). To evaluate
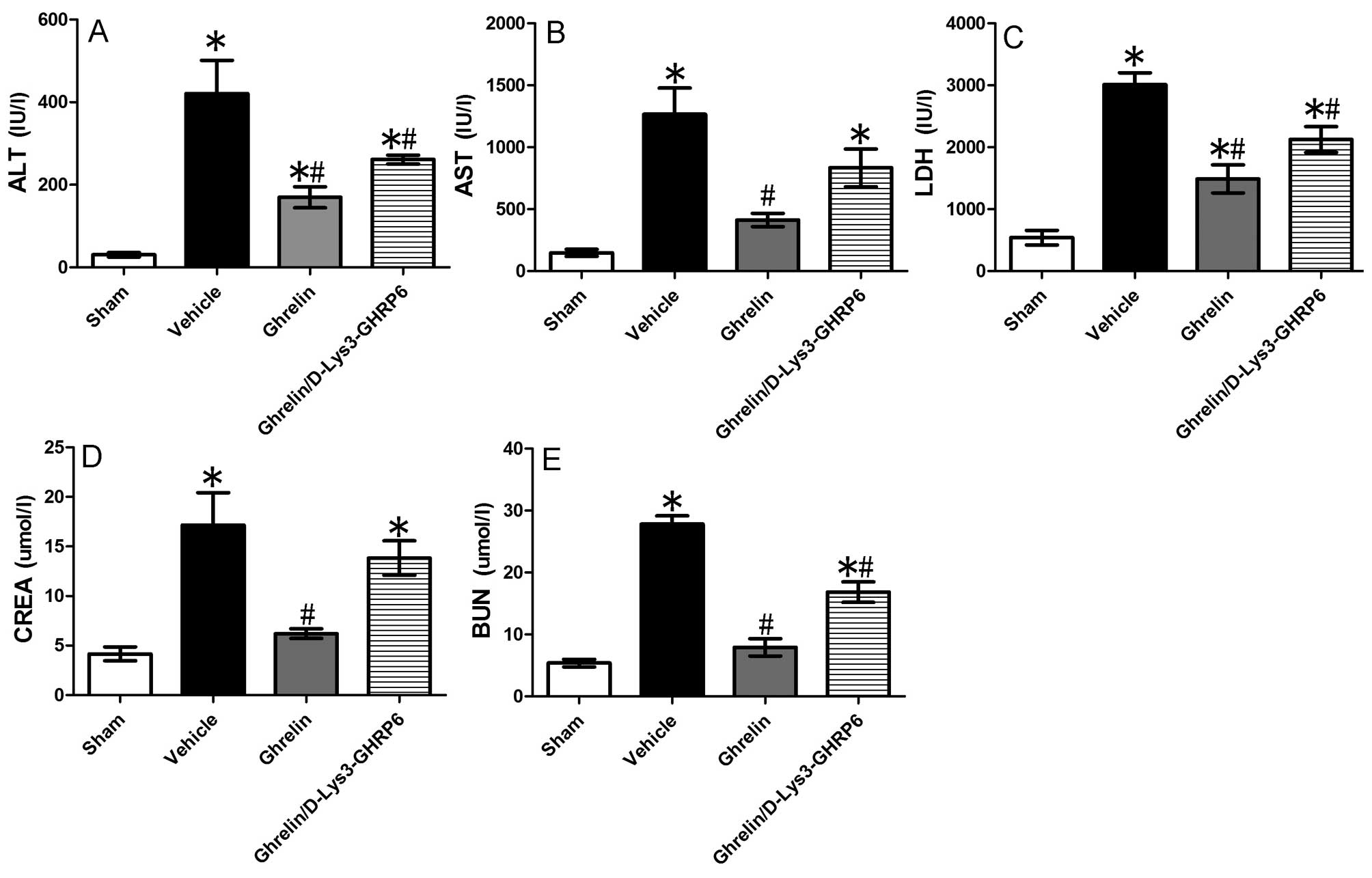
remote organ injury at the biochemical level, we assayed the serum
activities of AST, ALT, LDH, creatinine and urea nitrogen. Compared
with the sham-operated animals, serum ALT, AST, LDH, creatinine and
urea nitrogen levels in the animals with I/R injury in the
vehicle-treated group increased by 13.79-, 8.58-, 5.57-, 4.12- and
5.19-fold, respectively (P<0.05) (Fig. 4). The administration of ghrelin
decreased ALT, AST, LDH, creatinine and urea nitrogen levels by
59.7, 67.4, 50.6, 63.9 and 71.6%, respectively (P<0.05)
(Fig. 4) compared with the
vehicle-treated group. When the animals with I/R injury were
simultaneously administered ghrelin and its antagonist,
D-Lys3-GHRP6, the plasma levels of ALT, AST, LDH, creatinine and
urea nitrogen decreased by 48, 34.1, 29.6, 19.5 and 39.6%,
respectively (P<0.05) (Fig.
4).
MPO activity is an indicator of neutrophil
infiltration. As demonstrated in Fig.
5, gut I/R induced a significant increase in intestinal and
pulmonary MPO activity in the vehicle-treated mice as compared with
the sham-operated animals. Treatment with ghrelin significantly
inhibited the increase in intestinal (Fig. 5A) and pulmonary (Fig. 5B) MPO activity following gut I/R.
These results demonstrate that ghrelin attenuates the influx of
neutrophils into the gut and lungs following gut I/R injury.
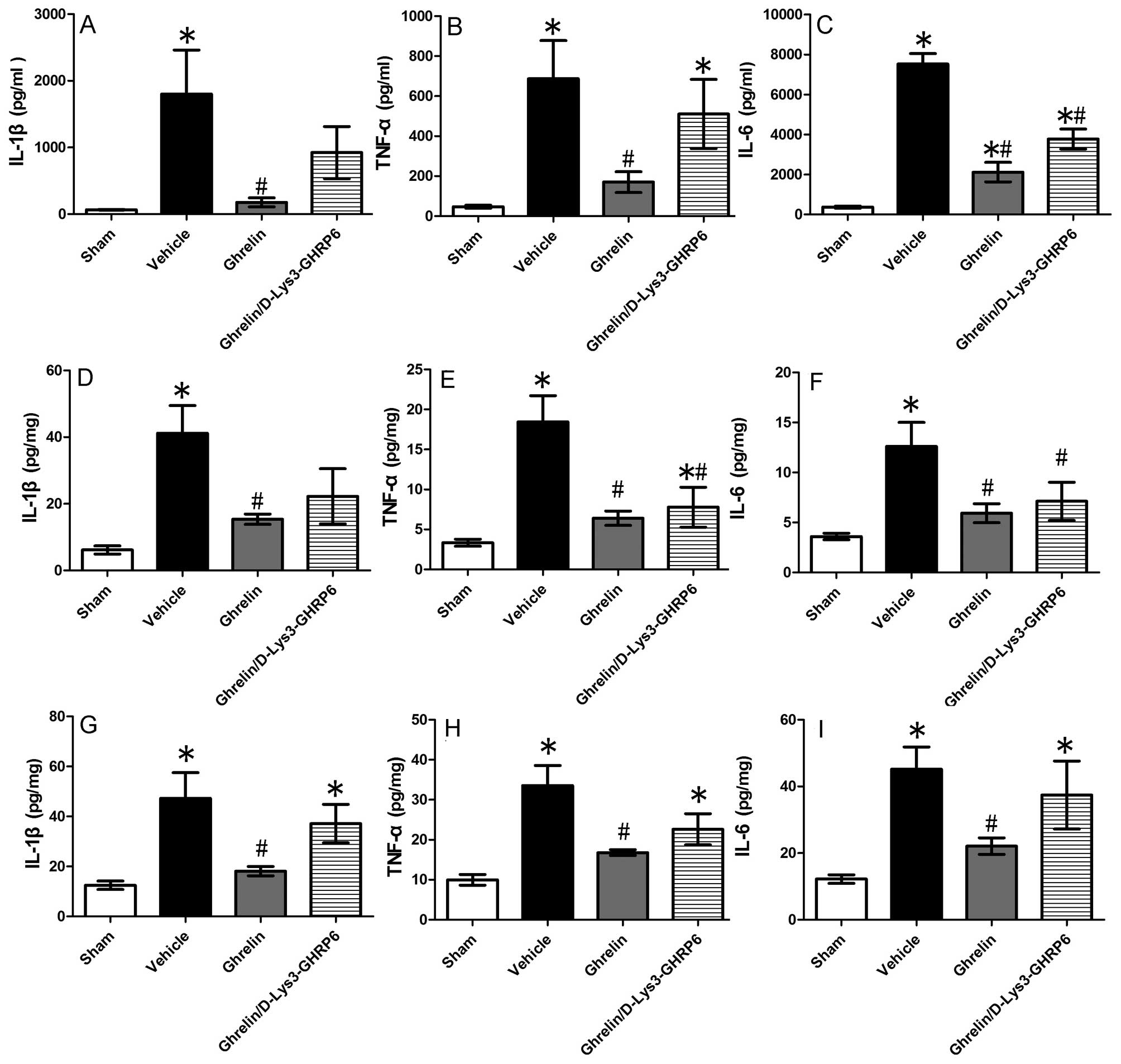
Ghrelin suppresses pro-inflammatory
responses following gut I/R injury
Pro-inflammatory cytokines play an important role in
the injury of remote organs following gut I/R. To investigate
whether the cytokines, TNF-α, IL-1β and IL-6, were affected by
treatment with ghrelin, we determined their expression levels in
the blood, intestine and lungs. We found that the cytokine levels
in the serum increased significantly following intestinal I/R
injury (TNF-α by 14.5-fold, IL-1β by 27.1-fold and IL-6 by
20.4-fold), while the administration of ghrelin markedly reduced
the pro-inflammatory response (by 75.3, 90.2 and 71.9%,
respectively) (Fig. 6A–C). We
also measured TNF-α, IL-1β and IL-6 production in the small
intestine and lungs using ELISA. We observed similar suppressive
effects of ghrelin on cytokine levels in tissue from the small
intestine (Fig. 6D–F), as well as
in pulmonary tissue (Fig. 6G–I).
As shown by our results, the levels of TNF-α, IL-1β and IL-6
significantly decreased in the tissue from the small intestine (by
65.3, 62.7 and 53%) (Fig. 6D–F)
and lung tissue (by 50, 61.7 and 51.1%) (Fig. 6G–I) following treatment with
ghrelin.
Antagonistic effect of D-Lys3-GHRP-6 on
ghrelin-mediated tissue protection following intestinal I/R
injury
D-Lys3-GHRP-6 is a ghrelin receptor antagonist. It
was used for further confirmation of the protective effects of
ghrelin in our experimental model. We observed that the levels of
the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, in the lungs
of the animals treated with D-Lys3-GHRP6 did not differ
significantly from those in the animals in the vehicle-treated
group. The levels of TNF-α, IL-1β and IL-6 in pulmonary tissue
decreased by 32.6, 21.5 and 17.2%, respectively in the group
treated with D-Lys3-GHRP6 compared with the vehicle-treated group
(Fig. 6G–I). As shown in Fig. 6A–C, the levels of TNF-α, IL-1β and
IL-6 in the serum decreased by 25.7, 48.7 and 49.9%, respectively
in the group treated with D-Lys3-GHRP6 compared with the
vehicle-treated group. In the tissue from the small intestine, the
TNF-α, IL-1β and IL-6 levels were reduced by 57.9, 46 and 43.5%,
respectively in the group treated with D-Lys3-GHRP6 compared with
the vehicle-treated group (Fig.
6D–F). However, the levels of IL-6 in the serum and tissue from
the small intestine, as well as the TNF-α levels in the tissue from
the small intestine markedly decreased. We hypothesized that
D-Lys3-GHRP6 has a partial inhibitory effect on GHSR-1a in
organisms.
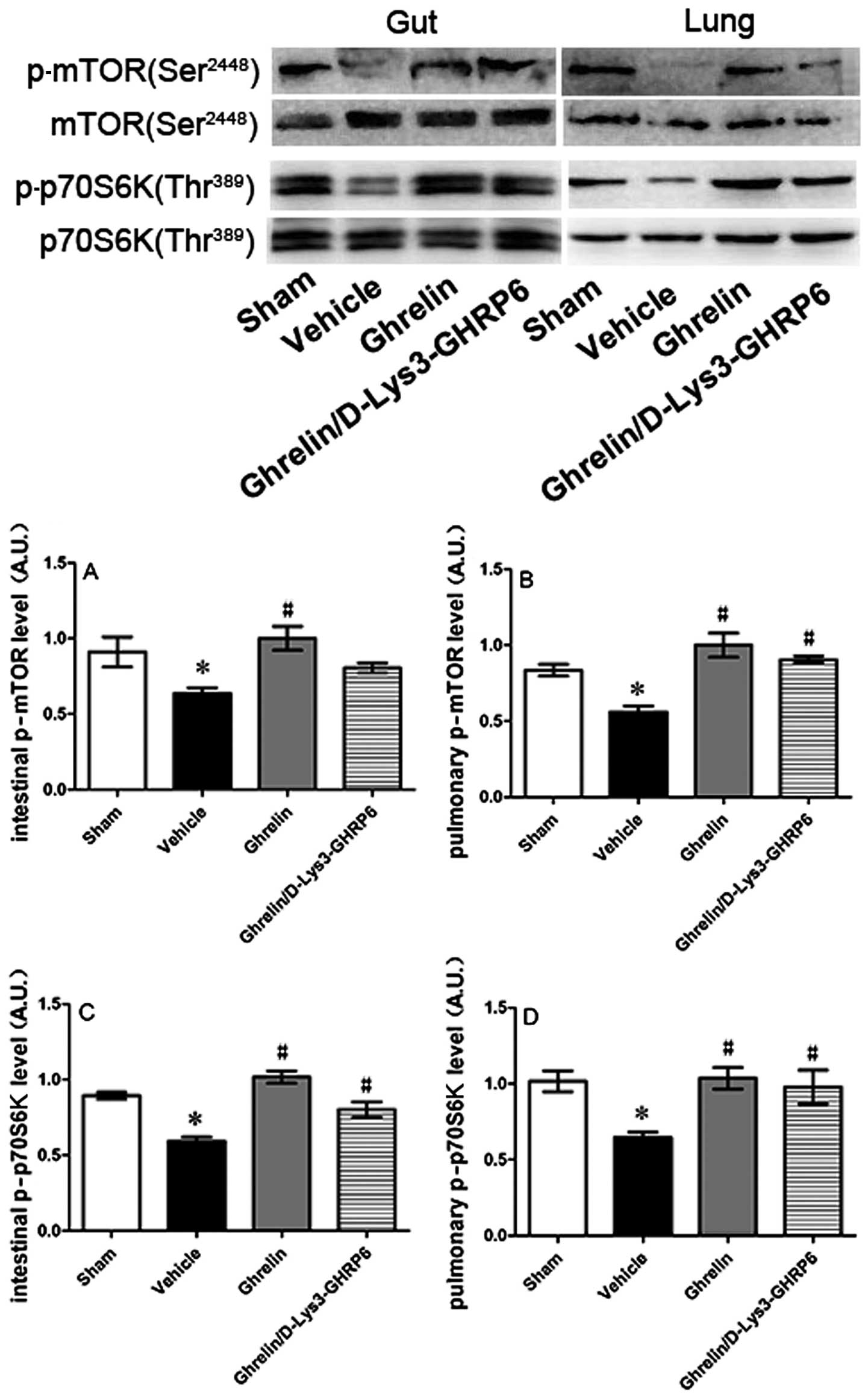
Ghrelin promotes the activation of the
mTOR signaling pathway in the small intestine and lungs
In order to determine whether ghrelin mediates the
activation of the mTOR signaling pathway, we extracted protein from
the injured small intestine and lungs and detected phosphorylated
mTOR and p70S6K levels by western blot analysis. mTOR and p70S6K
phosphorylation increased in the tissue from the small intestine
and pulmonary tissue in the animals treated with ghrelin compared
with the vehicle-treated group (Fig.
7).
Discussion
Intestinal I/R injury is a serious complication in a
variety of pathological conditions and surgical procedures,
including sepsis, strangulated bowel, vascular surgery and
hemorrhagic shock (30). The
activation of immune cells adjacent to the huge endothelial cell
surface area of the intestinal microvasculature produces initially
local and then systemic inflammatory responses, which lead to
severe tissue damage (26).
Ghrelin is an endogenous ligand for GHSR-1a
(16), and GHSR-1a expression is
present in the brain stem, pituitary gland, hypothalamus, heart,
blood vessels, lungs, stomach, pancreas, intestines, kidneys and
adipose tissue (31–33). Therefore, ghrelin plays various
physiological roles in both the central nervous system and the
periphery (19), such as the
regulation of pituitary hormone secretion, feeding, energy
homeostasis, gastrointestinal function, as well as in the
cardiovascular and immune system (34–36). In a previous study, Wu et
al (26) showed that plasma
levels of ghrelin were significantly reduced and that the
administration of exogenous ghrelin attenuated local and remote
organ injury following gut I/R in rats. In the present study, we
established a mouse model of intestinal I/R (i.e., superior
mesenteric artery occlusion) and found that the indicators of
tissue injury (MPO activity) and organ dysfunction (ALT, AST, LDH,
creatinine and urea nitrogen levels) were significantly decreased
in the ghrelin-treated mice following gut I/R. The levels of the
pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, were also
decreased in the ghrelin-treated group. When the mice were
administered D-Lys3-GHRP-6 (a ghrelin receptor antagonist), the
decrease in the levels of some of the abovementioned indicators was
insignificant; no statistically significant difference was observed
in the levels of some of these indicators between the group treated
with D-Lys3-GHRP-6 and the vehicle-treated group. These differences
were also observed during histological and and survival analysis.
Therefore, our results further confirm the protective role of
ghrelin during intestinal I/R injury in mice.
mTOR is a type of atypical serine/threonine kinase
which integrates various extracellular and intracellular signals,
including metabolism, growth, survival, aging, synaptic plasticity,
immunity and memory (10). The
activation of the mTOR/p70S6K pathway is a critical event in the
modulation of protein synthesis and the regulation of cell growth
(37–39). However, certain studies have shown
that phosphorylated forms of Akt and p70S6K are significantly
decreased in transient focal cerebral ischemia, resulting in the
suppression of the initiation step of protein synthesis and cell
growth (41,42). Thus, the activation of the
mTOR/p70S6K pathway exerts a protective effect against ischemic
brain injury (12,13,43). In our study, the same phenomenon
was observed in a mouse model of gut I/R; the phosphorylation
levels of mTOR and p70S6K decreased in the tissue from the small
intestine and pulmonary tissue following I/R. Recently, Aoyagi
et al (46) used
transgenic (Tg) mice with cardiac-specific overexpression of mTOR
(mTOR-Tg mice) to examine I/R injury and found that mTOR
overexpression inhibited necrosis during I/R injury. In our study,
we observed that mTOR and p70S6K phosphorylation levels increased
and that mucosal destruction, loss of villi, epithelial cell
apoptosis in the small intestine and the disruption of lung
architecture were alleviated in the animals administered ghrelin
following gut I/R. These findings suggest that the activation of
the mTOR/p70S6K signaling pathway inhibits cellular necrosis, and
thus protects tissue architecture from destruction during gut
I/R.
In addition to cell damage caused by ischemia and
hypoxia, excessive and sustained inflammatory responses during
reperfusion also play an important role in tissue damage. The
expression of pro-inflammatory genes is regulated by
transcriptional mechanisms. NF-κB is a critical transcription
factor required for the maximal expression of several cytokines
involved in the pathogenesis of acute lung injury and gut I/R
injury (47,48). Moreover, mTOR negatively regulates
the NF-κB pathway (27,49). In monocytes and macrophages, mTOR
inhibits NF-κB-dependent pro-inflammatory cytokine IL-12 production
and activates STAT3-dependent anti-inflammatory IL-10 production
(27,44,45). Previous studies have shown that
ghrelin inhibits leptin-induced pro-inflammatory cytokine (IL-1β,
TNF-α and IL-6) expression by human T cells and monocytes (20) and suppresses NF-κB activation in
human endothelial cells (21).
Recently, Aoyagi et al (46) reported that the expression of IL-6
and TNF-α in mTOR-Tg mouse hearts was lower than that in WT mouse
hearts following cardiac I/R (46). These data indicate that the
activation of the mTOR signaling pathway plays an anti-inflammatory
role. Additionally, it has been reported that central ghrelin
administration promotes a marked increase in the phosphorylated
(active) form of mTOR and its downstream targets, pS6K1 and p6 in
the arcuate nucleus of the hypothalamus (50). The data from the present study
demonstrate that the intraperitoneal administration of ghrelin
promotes the activity of the mTOR signaling pathway, inhibits some
pro-inflammatory cytokine release and reduces neutrophil
infiltration following gut I/R. Based on the above findings, it can
be concluded that ghrelin promotes the activation of the
mTOR/p70S6K signaling pathway and inhibits the NF-κB pathway in
during inflammation; therefore, ghrelin reduces inflammatory
responses, ameliorates organ damage and reduces mortality in the
intestinal I/R injury model. Thus, ghrelin may be a potential
therapeutic agent in various inflammatory disorders induced by gut
I/R.
Acknowledgements
This study received funding from Wuhan Municipal
Human Resources and Social Security Bureau (no. 2009-97). We thank
Professor Feili Gong from the Department of Immunology, Tongji
Medical College, Wuhan, China for his thoughtful scientific
discussions. We also thank Professor Ping Wang from the Laboratory
of Surgical Research, Feinstein Institute for Medical Research,
Manhasset, NY, USA, for providing valuable advice in writing this
manuscript.
References
|
1
|
Oldenburg WA, Lau LL, Rodenberg TJ,
Edmonds HJ and Burger CD: Acute mesentericischemia: a clinical
review. Arch Intern Med. 164:1054–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berlanga J, Prats P, Remirez D, Gonzalez
R, Lopez-Saura P, et al: Prophylactic use of epidermal growth
factor reduces ischemia/reperfusion intestinal damage. Am J Pathol.
161:373–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tendler DA: Acute intestinal ischemia and
infarction. Semin Gastrointest Dis. 14:66–76. 2003.PubMed/NCBI
|
|
4
|
Zhang M and Carroll MC: Natural antibody
mediated innate autoimmune response. Mol Immunol. 44:103–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leaphart CL and Tepas JJ III: The gut is a
motor of organ system dysfunction. Surgery. 141:563–569. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rotstein OD: Pathogenesis of multiple
organ dysfunction syndrome: gut origin, protection, and
decontamination. Surg Infect (Larchmt). 1:217–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsang CK, Qi H, Liu LF and Zheng XF:
Targeting mammalian target of rapamycin (mTOR) for health and
diseases. Drug Discov Today. 12:112–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weichhart T: Mammalian target of
rapamycin: a signaling kinase for every aspect of cellular life.
Methods Mol Biol. 821:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2012. View Article : Google Scholar
|
|
10
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
from growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
12
|
Koh PO: Melatonin prevents ischemic brain
injury through activation of the mTOR/p70S6 kinase signaling
pathway. Neurosci Lett. 444:74–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koh PO: Ginkgo biloba extract (EGb
761) prevents cerebral ischemia-induced p70S6 kinase and S6
phosphorylation. Am J Chin Med. 38:727–734. 2010. View Article : Google Scholar
|
|
14
|
Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W
and Jia LS: PTEN deletion prevents ischemic brain injury by
activating the mTOR signaling pathway. Biochem Biophys Res Commun.
404:941–945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vigneron F, Dos Santos P, Lemoine S,
Bonnet M, Tariosse L, Couffinhal T, Duplaà C and Jaspard-Vinassa B:
GSK-3β at the crossroads in the signalling of heart
preconditioning: implication of mTOR and Wnt pathways. Cardiovasc
Res. 90:49–56. 2011.
|
|
16
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arvat E, Di Vito L, Broglio F, Papotti M,
Muccioli G, et al: Preliminary evidence that Ghrelin, the natural
GH secretagogue (GHS)-receptor ligand, strongly stimulates GH
secretion in humans. J Endocrinol Invest. 23:493–495. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu JT and Kral JG: Ghrelin: integrative
neuroendocrine peptide in health and disease. Ann Surg.
239:464–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cowley MA and Grove KL: Ghrelin -
satisfying a hunger for the mechanism. Endocrinology.
145:2604–2606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin- and activation-induced pro-inflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WG, Gavrila D, Liu X, Wang L,
Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C and
Weintraub NL: Ghrelin inhibits pro-inflammatory responses and
nuclear factor-kappaB activation in human endothelial cells.
Circulation. 109:2221–2226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konturek PC, Brzozowski T, Walter B,
Burnat G, Hess T, Hahn EG and Konturek SJ: Ghrelin-induced
gastroprotection against ischemia-reperfusion injury involves an
activation of sensory afferent nerves and hyperemia mediated by
nitric oxide. Eur J Pharmacol. 536:171–181. 2006. View Article : Google Scholar
|
|
23
|
Brzozowski T, Konturek PC, Sliwowski Z,
Pajdo R, Drozdowicz D, Kwiecien S, Burnat G, Konturek S and Pawlik
WW: Prostaglandin/cyclooxygenase pathway in ghrelin-induced
gastroprotection against ischemia-reperfusion injury. J Pharmacol
Exp Ther. 319:477–487. 2006. View Article : Google Scholar
|
|
24
|
Brzozowski T, Konturek PC, Sliwowski Z,
Drozdowicz D, Kwiecien S, Pawlik M, Pajdo R, Konturek SJ, Pawlik WW
and Hahn EG: Neural aspects of ghrelin-induced gastroprotection
against mucosal injury induced by noxious agents. J Physiol
Pharmacol. 57(Suppl 6): S63–S76. 2006.PubMed/NCBI
|
|
25
|
Pawlik MW, Obuchowicz R, Biernat J,
Szczepanski W, Pajdo R, Kwiecień S, Brzozowski T, Konturek SJ and
Pawlik WW: Effects of peripherally and centrally applied ghrelin in
the pathogenesis of ischemia-reperfusion induced injury of the
small intestine. J Physiol Pharmacol. 62:429–439. 2011.PubMed/NCBI
|
|
26
|
Wu R, Dong W, Ji Y, Zhou M, Marini CP,
Ravikumar TS and Wang P: Orexigenic hormone ghrelin attenuates
local and remote organ injury after intestinal
ischemia-reperfusion. PLoS One. 3:e20262008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weichhart T, Costantino G, Poglitsch M,
Rosner M, Zeyda M, et al: The TSC-mTOR signaling pathway regulates
the innate inflammatory response. Immunity. 29:565–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bachofen M and Weibel ER: Structural
alterations of lung parenchyma in the adult respiratory distress
syndrome. Clin Chest Med. 3:35–56. 1982.PubMed/NCBI
|
|
30
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hattori N, Saito T, Yagyu T, Jiang BH,
Kitagawa K, et al: GH, GH receptor, GH secretagogue receptor, and
ghrelin expression in human T cells, B cells, and neutrophils. J
Clin Endocrinol Metab. 86:4284–4291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papotti M, Ghe C, Cassoni P, Catapano F,
Deghenghi R, et al: Growth hormone secretagogue binding sites in
peripheral human tissues. J Clin Endocrinol Metab. 85:3803–3807.
2000.PubMed/NCBI
|
|
33
|
Shuto Y, Shibasaki T, Wada K, Parhar I,
Kamegai J, et al: Generation of polyclonal antiserum against the
growth hormone secretagogue receptor (GHS-R): evidence that the
GHS-R exists in the hypothalamus, pituitary and stomach of rats.
Life Sci. 68:991–996. 2001. View Article : Google Scholar
|
|
34
|
Wu R, Dong W, Cui X, Zhou M, Simms HH, et
al: Ghrelin down-regulates pro-inflammatory cytokines in sepsis
through activation of the vagus nerve. Ann Surg. 245:480–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kojima M and Kangawa K: Ghrelin: structure
and function. Physiol Rev. 85:495–522. 2005. View Article : Google Scholar
|
|
36
|
Wang G, Lee HM, Englander E and Greeley GH
Jr: Ghrelin-not just another stomach hormone. Regul Pept.
105:75–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim DH and Sabatini DM: Raptor and mTOR:
subunits of a nutrient-sensitive complex. Curr Top Microbiol
Immunol. 279:259–270. 2004.PubMed/NCBI
|
|
38
|
Montagne J, Stewart MJ, Stocker H, Hafen
E, Kozma SC and Thomas G: Drosophila S6 kinase: a regulator
of cell size. Science. 285:2126–2119. 1999. View Article : Google Scholar
|
|
39
|
Shima H, Pende M, Chen Y, Fumagalli S,
Thomas G and Kozma SC: Disruption of the p70(s6k)/p85(s6k) gene
reveals a small mouse phenotype and a new functional S6 kinase.
EMBO J. 17:6649–6659. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrence JC Jr and Brunn GJ: Insulin
signaling and the control of PHAS-I phosphorylation. Prog Mol
Subcell Biol. 26:1–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Janelidze S, Hu BR, Siesjö P and Siesjö
BK: Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal
ischemia. Neurobiol Dis. 8:147–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mengesdorf T, Proud CG, Mies G and Paschen
W: Mechanisms underlying suppression of protein synthesis induced
by transient focal cerebral ischemia in mouse brain. Exp Neurol.
177:538–546. 2002. View Article : Google Scholar
|
|
43
|
Koh PO, Cho JH, Won CK, Lee HJ, Sung JH
and Kim MO: Estradiol attenuates the focal cerebral ischemic injury
through mTOR/p70S6 kinase signaling pathway. Neurosci Lett.
436:62–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weichhart T and Säemann MD: The multiple
facets of mTOR in immunity. Trends Immunol. 30:218–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baker AK, Wang R, Mackman N and Luyendyk
JP: Rapamycin enhances LPS induction of tissue factor and tumor
necrosis factor-alpha expression in macrophages by reducing IL-10
expression. Mol Immunol. 46:2249–2255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aoyagi T, Kusakari Y, Xiao CY, Inouye BT,
Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K and Matsui T:
Cardiac mTOR protects the heart against ischemia-reperfusion
injury. Am J Physiol Heart Circ Physiol. 303:H75–H85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sato N, Moore FA, Smith MA, Zou L,
Moore-Olufemi S, et al: Immune-enhancing enteral nutrients
differentially modulate the early pro-inflammatory transcription
factors mediating gut ischemia/reperfusion. J Trauma. 58:455–461.
2005. View Article : Google Scholar
|
|
49
|
dos Mendes SS, Candi A, Vansteenbrugge M,
Pignon MR, Bult H, Boudjeltia KZ, Munaut C and Raes M: Microarray
analyses of the effects of NF-kappaB or PI3K pathway inhibitors on
the LPS-induced gene expression profile in RAW264.7 cells:
synergistic effects of rapamycin on LPS-induced
MMP9-overexpression. Cell Signal. 21:1109–1122. 2009.PubMed/NCBI
|
|
50
|
Martins L, Fernández-Mallo D, Novelle MG,
Vázquez MJ, Tena-Sempere M, Nogueiras R, López M and Diéguez C:
Hypothalamic mTOR signaling mediates the orexigenic action of
ghrelin. PLoS One. 7:e469232012. View Article : Google Scholar : PubMed/NCBI
|