Introduction
Inflammatory bowel disease (IBD), which is a term
used to describe a group of inflammatory conditions, such as
ulcerative colitis (UC) and Crohn’s disease (CD), is associated
with chronically relapsing disorders of the gastrointestinal tract
(1–3). Histologically, it is characterized
by the presence in the gut of extensive areas of ulceration,
pronounced infiltration of neutrophils and epithelial cell
necrosis. Although these conditions have been treated with
5-aminosalicylic acid derivatives, corticosteroids and
immunosuppressants, such as azathioprine and cyclosporine (1), few non-toxic therapeutic options are
currently able to modulate intestinal inflammation. Therefore, the
challenge remains to develop novel and specific therapies for IBD
(4).
Nuclear factor-κB (NF-κB) activation is one of the
most important events involved in the pathogenesis of IBD (5,6).
NF-κB can be activated by several upstream stimuli, including
Toll-like receptors (TLRs). The transmembrane TLRs are a family of
pattern-recognition receptors (PRRs) that enable the innate and
adaptive immune systems to recognize pathogen-associated molecular
patterns (PAMPs). Thus far, over 13 members of the TLR family have
been identified in mammals, of which TLR4 is the most extensively
studied (7). TLR4 can be
activated by recognizing various PAMPs in bacteria (8,9).
Upon ligand binding, TLR4 undergoes a conformational change and
dimerizes, recruiting adaptor proteins, such as myeloid
differentiation primary response gene 88 (MyD88), which in turn
activates NF-κB, transducing the immune-related signals to the
nucleus (10,11). Due to its essential role in the
pathogenesis of inflammatory diseases, including IBD (12–18), modulation of the TLR4/NF-κB
signaling pathway may be a main target for the treatment of
inflammation.
Natural products, such as those found in traditional
Chinese medicine (TCM), have received much attention due to their
anti-inflammatory potential. Qing Hua Chang Yin (QHCY) is a
well-known traditional Chinese formula consisting of a combination
of 11 herbs, including Herba et Gemma Agrimoniae, Coptis
chinensis Franch, Radix Sanguisorbae, Radix Paeoniae
Rubra, Elettaria cardamomum, Magnolia
officinalis, Artemisia capillaris Thunb., Herba
Eupaatorii Fortunei, Semen Coicis, Semen Dolichoris
Album and Poria cocos. In conjuction, these components
confer QHCY properties, such as eliminating heat and dampness, and
strengthening the spleen, thus increasing vitality (tonifying the
Spleen Qi in Chinese). According to TCM theory, the accumulation of
toxic dampness and heat is one of the major causative factors in
the pathogenesis of UC. QHCY therefore has long been used in China
to clinically treat UC (19–24). However, the precise mechanisms
behind the therapeutic effects of QHCY against UC remain largely
unknown. Thus, in the present study we evaluated the therapeutic
effects of QHCY on an established mouse model of colitis induced by
dextran sulfate sodium (DSS) and investigated the possible
molecular mechanisms involved.
Materials and methods
Materials and reagents
DSS (average molecular weight, 36,000–50,000) was
purchased from MP Biochemicals (Solon, OH, USA). The BCA Protein
assay and nuclear protein extraction kits were purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Antibodies
for western blot analysis were obtained from Cell Signaling
Technology (Beverly, MA, USA). The mouse serum amyloid A (SAA)
ELISA kit was obtained from Immunology Consultants Laboratory, Inc.
(Newberg, OR, USA). All the other chemicals, unless otherwise
stated, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of QHCY
In total, 220 g dehydrated Herba et Gemma
Agrimoniae, 33 g dehydrated Coptis chinensis Franch, 100
g dehydrated Radix Sanguisorbae, 110 g dehydrated Radix
Paeoniae Rubra, 56 g dehydrated Elettaria cardamomum,
110 g dehydrated Magnolia officinalis, 110 g dehydrated
Artemisia capillaris Thunb,, 110 g dehydrated
Herba Eupaatorii Fortunei, 220 g dehydrated Semen
Coicis, 110 g dehydrated Semen Dolichoris Album and 220
g dehydrated Poria cocos were extracted with boiling water 3
times. The extracts were then combined and concentrated by boiling
to a final volume of 1,000 ml. The final concentration of QHCY
crude drug was ~1.4 mg/ml.
Establishment of mouse model of colitis
and QHCY treatment
Male BALB/c mice (with an initial body weight of
20–22 g) were obtained from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China) and housed under pathogen-free conditions
with a 12 h light/dark cycle. Food and water were provided ad
libitum and the mice were allowed to acclimatize for 1 week
prior to the experiment. Housing conditions and all animal
experiments were approved by the Institutional Animal Care and Use
Committee of Fujian University of Traditional Chinese Medicine,
Fuzhou, China. Colitis was induced by the administration of 3% DSS
(weight to volume ratio dissolved in distilled water) in the
drinking water for 8 days. On the first day of model construction,
the animals were randomly divided into 3 groups (n=10): the normal
control group in which the mice received neither DSS stimulation
nor QHCY treatment; and the DSS-induced UC model or QHCY-treated
group in which the mice received DSS stimulation and then received
an intragastric administration of 200 μl of saline or QHCY,
respectively, daily for 12 days.
Evaluation of clinical
manifestations
The progression of DSS-induced colitis was monitored
daily in a blinded manner, including the observation of changes in
body weight, stool consistency and the presence of rectal bleeding
blood in the stool. The disease activity index (DAI) score was
calculated as the sum of scores for weight loss, stool consistency
and rectal bleeding as previously described (25) (Table
I).
 | Table IDisease activity index score. |
Table I
Disease activity index score.
| Score | Weight loss (%) | Stool
consistency | Rectal bleeding |
|---|
| 0 | 0 | Normal | Normal |
| 1 | 1–5 | | |
| 2 | 6–10 | Loose |
Hemoccult-positive |
| 3 | 11–20 | | |
| 4 | >21 | Diarrhea | Gross bleeding |
Sample collection
At the end of the experiments, the animals were
anesthetized and blood was collected via right heart ventricle
puncture in lightly heparinized syringes and kept on ice. Sera were
separated by 5 min centrifugation at 5,000 × g and stored at −80°C
prior to the analysis. The colons were excised and the length from
the cecum to the anus was measured. One portion of each distal
colon was cut and fixed in 10% formalin for histological
examination; the remainder was used for further analysis.
Histopathological examination
Small pieces of the colon tissues were fixed with
10% buffered formalin for 24 h. Samples were then
paraffin-embedded, sectioned and stained with hematoxylin and eosin
(H&E). Histopathological changes were observed under a light
microscope.
Measurement of SAA levels by ELISA
The level of SAA in the sera was measured using a
mouse SAA ELISA kit according to the manufacturer’s instructions.
All samples were assayed in triplicate. The concentrations of SAA
were determined by comparison to serial dilutions of SAA purified
standard.
Western blot analysis
Five fresh colon tissues were selected randomly from
each group, homogenized in non-denaturing lysis buffer using
homogenizer and centrifuged at 15,000 × g for 15 min. Nuclear
proteins were extracted using the Nuclear Protein Extraction kit
(Beyotime Institute of Biotechnology) according to the
manufacturer’s instructions. Protein concentrations were determined
using the BCA protein assay kit. Equal amounts of protein from each
sample were resolved on 12% Tris-glycine gels and transferred onto
PVDF membranes. The membranes were blocked for 2 h with 5% non-fat
dry milk and incubated with the desired primary antibodies against
TLR4, MyD88, IκB, p-IκB, p65 and β-actin (all in 1:1,000 dilutions)
overnight at 4°C and then with appropriate HRP-conjugated secondary
antibody followed by enhanced chemiluminescence detection.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (v11.5). Statistical analysis of the data was performed
using the Student’s t-test and one-way ANOVA. P-values <0.05
were considered to indicate statistically significant
differences.
Results
QHCY improves the clinical manifestations
in the DSS-induced UC mouse model
To evaluate the therapeutic efficacy of QHCY against
the development of mouse colitis, clinical manifestations, such as
changes in weight, stool consistency and rectal bleeding were
observed and the DAI was calculated. As shown in Fig. 1, compared with the normal group,
mice in the DSS-stimulated group displayed obvious manifestations
of UC, including body weight loss, diarrhea and rectal bleeding,
indicating the successful construction of the model. However,
treatment with QHCY significantly improved the DSS-induced
manifestations. The DAI score of the normal control, DSS-stimulated
model or QHCY-treated group was 0, 11.2±1.79 and 5.6±1.14,
respectively (P<0.05) (Fig.
1A). To verify these results, the colons of mice from each
group were harvested after sacrifice and the length from the cecum
to the anus was measured. As shown in Fig. 1B, DSS stimulation resulted in
significant colon shortening, which however was profoundly
neutralized following treatment with QHCY. The average colon length
per mouse from the normal control, model and QHCY-treated group was
6.06±0.56, 3.4±0.42 and 5.8±0.60 cm, respectively (P<0.05).
Taken together, these data demonstrate the therapeutic efficacy of
QHCY against UC.
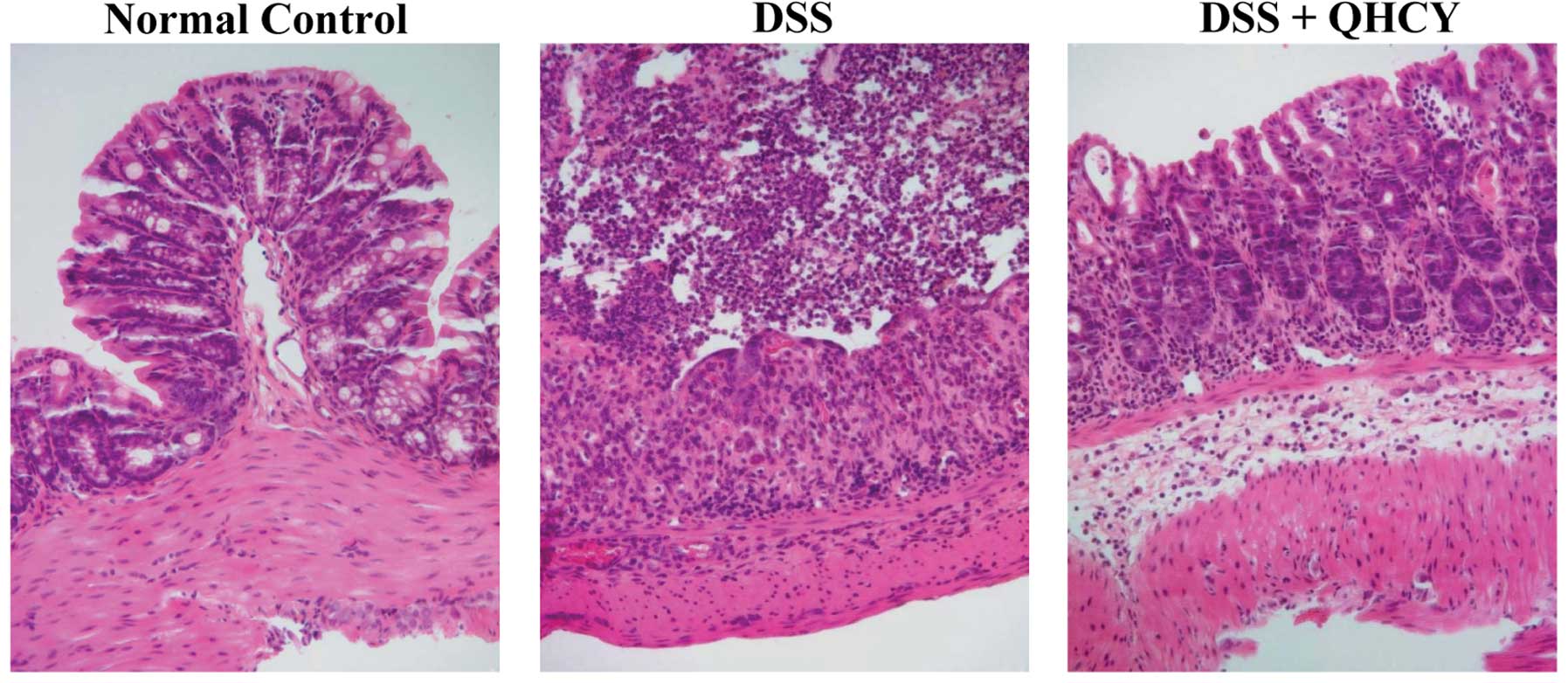
QHCY ameliorates histological damage of
colon tissue in the DSS-induced UC mouse model
The histological changes of the colonic mucosa in
the experimental mice were observed under a light microscope after
H&E staining. As shown in Fig.
2, the normal control mice displayed normal colonic histology
with an intact epithelium, well-defined gland lengths and no
leukocyte infiltration in the mucosa. DSS stimulation resulted in
mucosal ulceration, infiltration of inflammatory cells, crypt
distortion and a hyperplastic epithelium. However, the DSS-induced
histological damages in the colon tissues were significantly
ameliorated following treatment with QHCY.
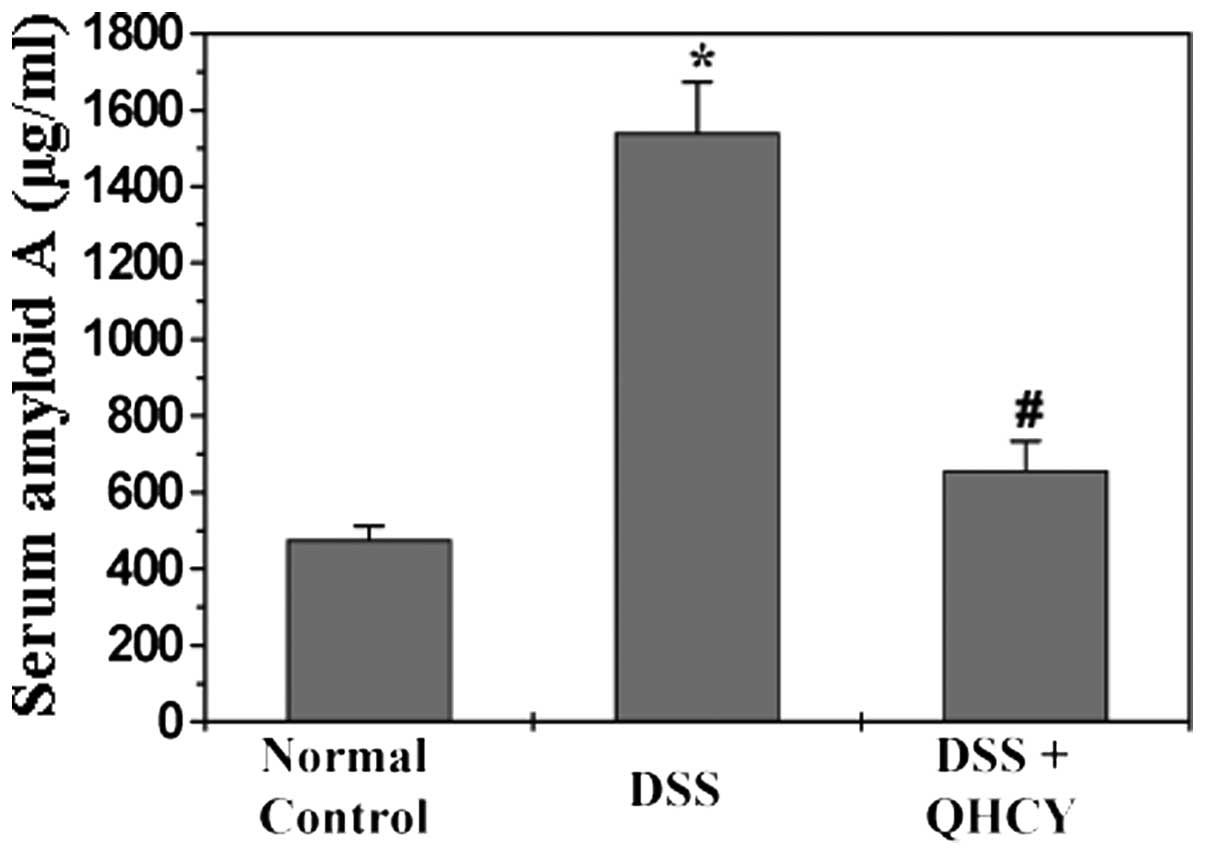
QHCY reduces the levels of SAA in the
DSS-induced UC mouse model
SAA, one of the inflammatory markers, has been shown
to be elevated in patients with UC. We therefore examined the serum
levels of SAA in the experimental mice by ELISA. As shown in
Fig. 3, the serum levels of SAA
in the UC model mice were significantly higher than those of the
mice in the normal control group (P<0.05). The administration of
QHCY significantly inhibited the DSS-induced increase in the serum
levels of SAA (P<0.05).
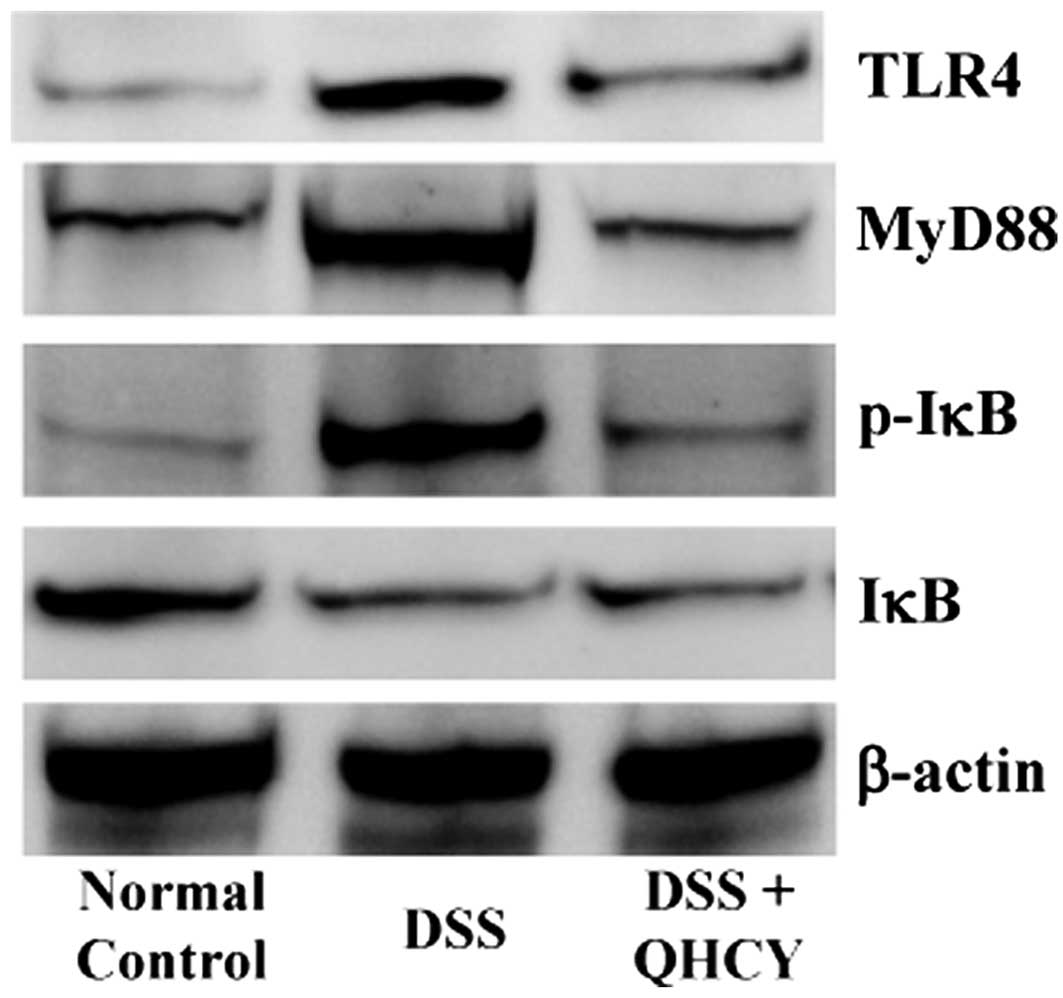
QHCY suppresses the activation of the
TLR4/NF-κB signaling pathway in the DSS-induced UC mouse model
To elucidate the mechanisms behind the therapeutic
effects of QHCY against UC, we determined its effects on the
activation of the TLR4/NF-κB pathway in colon tissues of mice with
UC. As shown in Fig. 4, the
expression of TLR4 and MyD88, as well as the phosphorylation level
of IκB were significantly increased in the DSS-induced UC model
group compared with those in the normal control group; however,
these levels were neutralized following treatment with QHCY. We
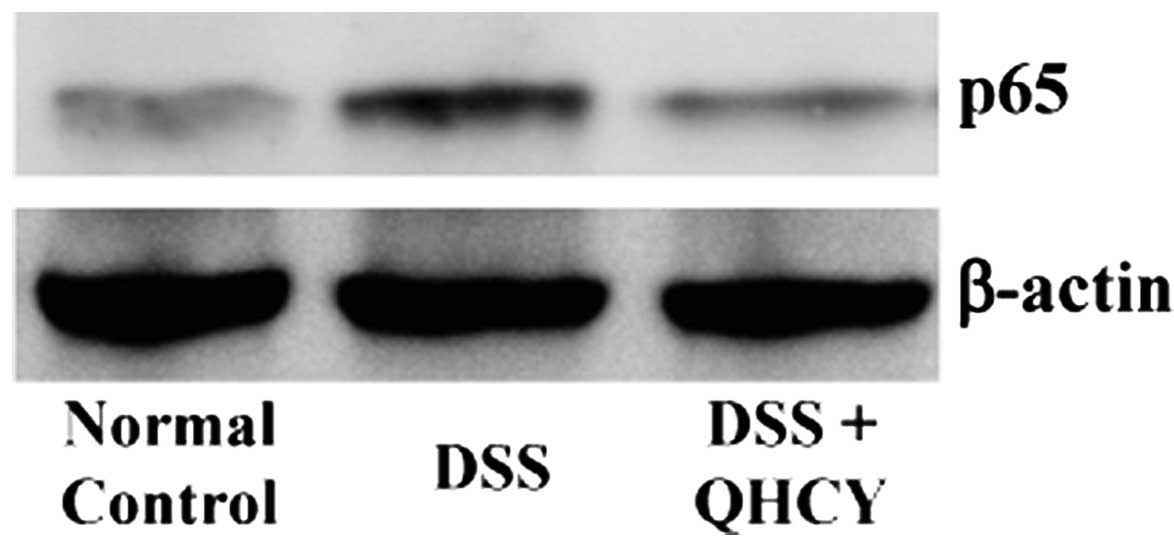
also examined alterations in the nuclear content of the NF-κB p65
subunit by western blot analysis, in order to evaluate the effects
of QHCY on NF-κB nuclear translocation, which is a critical step
for NF-κB activation. As shown in Fig. 5, QHCY significantly inhibited the
DSS-induced nuclear translocation of p65 in the colon tissues of
mice with UC.
Discussion
The use of natural anti-inflammatory products
provides an attractive and relatively non-toxic alternative remedy
to control inflammatory disorders. As a well-known traditional
Chinese formula, QHCY has long been used in China to clinically
treat UC, a major form of IBD (19–24). However, the precise mechanisms
behind the inhibitory effects of QHCY on intestinal inflammation
remain largely unclear.
In the present study, we evaluated the therapeutic
effects of QHCY against UC using an experimental mouse model of
DSS-induced colitis. We found that treatment with QHCY
significantly improved the DSS-induced clinical manifestations, as
evidenced by the prevention of body weight loss, as well as the
alleviation of diarrhea and rectal bleeding in mice with UC. In
addition, treatment with QHCY profoundly neutralized colon
shortening and ameliorated colonic histological damages in the
DSS-induced colitis mouse model. Moreover, QHCY significantly
inhibited the DSS-induced increase in serum levels of SAA, one of
the inflammatory markers which is commonly overexpressed in
patients with UC. Taken together, these data demonstrate the
therapeutic efficacy of QHCY against the development of UC.
The TLR4/NF-κB signaling pathway is one of the major
pathways mediating inflammatory responses. TLR4 is activated by
recognizing PAMPs in bacteria, which in turn triggers signaling
cascades leading to the activation of NF-κB (12). TLR4 is expressed at low levels in
normal intestines but is increased in mice with DSS-induced colitis
(13,14) and in patients with IBD (15,16), which may contribute to the
initiation or perpetuation of intestinal inflammation. Previous
studies have demonstrated that TLR4 functions as a mediator of
intestinal inflammation (17) and
that the blockade of TLR4 ameliorates DSS-induced colitis (18). Thus, the TLR4/NF-κB pathway has
become a major target for the treatment of inflammatory diseases,
including UC. The processes of TLR4/NF-κB activation include
several key links, such as MyD88 attendance,
phosphorylation/degradation of IκB and the subsequent nuclear
translocation of NF-κB. In this study, to elucidate the mechanisms
behind the therapeutic effects of QHCY against UC, we examined its
effects on the TLR4/NF-κB signaling pathway. We found that
treatment with QHCY significantly inhibited the expression of TLR4
and MyD88, as well as the phosphorylation of IκB and blocked NF-κB
nuclear translocation in the colon tissues of mice with UC,
suggesting that QHCY suppresses the activation of the NF-κB
signaling pathway.
In conclusion, to our knowledge, in this study, we
demonstrate for the first time that QHCY prevents the development
of UC in vivo through the suppression of the TLR4/NF-κB
signaling pathway. These results provide further fundamental
evidence for QHCY as a therapeutic agent for the treatment of
UC.
Acknowledgements
The present study was sponsored by a grant from the
National Natural Science Foundation of China (81173432).
Abbreviations:
|
QHCY
|
Qing Hua Chang Yin
|
|
TCM
|
traditional Chinese medicine
|
|
UC
|
ulcerative colitis
|
|
IBD
|
inflammatory bowel disease
|
|
NF-κB
|
nuclear factor-κB
|
|
TLR4
|
Toll-like receptor 4
|
References
|
1
|
Rezaie A, Parker RD and Abdollahi M:
Oxidative stress and pathogenesis of inflammatory bowel disease: an
epiphenomenon or the cause? Dig Dis Sci. 52:2015–2021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar
|
|
3
|
Odashima M, Otaka M, Jin M, et al:
Successful treatment of refractory duodenal Crohn’s disease with
infliximab. Dig Dis Sci. 52:31–32. 2007.PubMed/NCBI
|
|
4
|
Isaacs KL, Lewis JD, Sandborn WJ, et al:
State of the art: IBD therapy and clinical trials in IBD. Inflamm
Bowel Dis. 11(Suppl 1): S3–S12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber S, Nikolaus S and Hampe J:
Activation of nuclear factor kappa B inflammatory bowel disease.
Gut. 42:477–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andresen L, Jørgensen VL, Perner A, Hansen
A, Eugen-Olsen J and Rask-Madsen J: Activation of nuclear factor
kappaB in colonic mucosa from patients with collagenous and
ulcerative colitis. Gut. 54:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boone DL and Ma A: Connecting the dots
from Toll-like receptors to innate immune cells and inflammatory
bowel disease. J Clin Invest. 111:1284–1286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janeway CA Jr and Medzhitov R: Innate
immune recognition. Annu Rev Immunol. 20:197–216. 2002. View Article : Google Scholar
|
|
10
|
Bowie A and O’Neill LA: The interleukin-1
receptor/Toll-like receptor superfamily: signal generators for
pro-inflammatory interleukins and microbial products. J Leukoc
Biol. 67:508–514. 2000.PubMed/NCBI
|
|
11
|
Slack JL, Schooley K, Bonnert TP, et al:
Identification of two major sites in the type I interleukin-1
receptor cytoplasmic region responsible for coupling to
pro-inflammatory signaling pathways. J Biol Chem. 275:4670–4678.
2000. View Article : Google Scholar
|
|
12
|
Pålsson-McDermott EM and O’Neill LA:
Signal transduction by the lipopolysaccharide receptor, Toll-like
receptor-4. Immunology. 113:153–162. 2004.PubMed/NCBI
|
|
13
|
Ohkawara T, Takeda H, Miyashita K, et al:
Regulation of Toll-like receptor 4 expression in mouse colon by
macrophage migration inhibitory factor. Histochem Cell Biol.
125:575–582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortega-Cava CF, Ishihara S, Rumi MA, et
al: Strategic compartmentalization of Toll-like receptor 4 in the
mouse gut. J Immunol. 170:3977–3985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hausmann M, Kiessling S, Mestermann S, et
al: Toll-like receptors 2 and 4 are up-regulated during intestinal
inflammation. Gastroenterology. 122:1987–2000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szebeni B, Veres G, Dezsõfi A, et al:
Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the
colonic mucosa of children with inflammatory bowel disease. Clin
Exp Immunol. 151:34–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukata M, Chen A, Klepper A, et al: Cox-2
is regulated by Toll-like receptor-4 (TLR4) signaling: role in
proliferation and apoptosis in the intestine. Gastroenterology.
131:862–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ungaro R, Fukata M, Hsu D, et al: A novel
Toll-like receptor 4 antagonist antibody ameliorates inflammation
but impairs mucosal healing in murine colitis. Am J Physiol
Gastrointest Liver Physiol. 296:G1167–G1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XY and Tian DL: Etiological and
pathological characteristics of ulcerative colitis and TCM
differentiation and treatment. Beijing Zhong Yi Yao Da Xue Xue Bao.
30:554–559. 2007.(In Chinese).
|
|
20
|
Gong YP, Liu W, Ma GT, et al: Randomized
control study of ‘Qingchang Suppository’ on ulcerative colitis.
Shanghai Zhong Yi Yao Da Xue Xue Bao. 21:33–36. 2007.(In
Chinese).
|
|
21
|
Fu NL and Huang JY: Progress of clinical
research of traditional Chinese medicine for the treatment of
ulcerative colitis. Journal of Traditional Chinese Medicine.
40:501–503. 1999.(In Chinese).
|
|
22
|
Li QG: An idea about treatment of
ulcerative colitis by TCM methods. Beijing Zhong Yi. 23:149–150.
2004.(In Chinese).
|
|
23
|
Wang CH, Gao WY, Li YF, et al: Study of
Fufangkushen colon-release capsule on ulcerative colitis of
endo-retention of damp heat type. Xian Dai Zhong Xi Yi Jie He Za
Zhi. 18:13–15. 2009.(In Chinese).
|
|
24
|
Chen JT, Ke X, Fu XY, et al: The clinical
study of heat-clearing and damp-drying on the treatment of
damp-heat ulcerative colitis. Zhongguo Zhong Xi Yi Jie He Xiao Hua
Za Zhi. 17:256–257. 2009.(In Chinese).
|
|
25
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|