Introduction
Human apolipoprotein A-I (apoA-I), the major
component of high-density lipoprotein (HDL), is a protein of 243
amino acids with repeated 22-residue homologous segments separated
by prolines (1). HDL plays a
critical role in reverse cholesterol transport (RCT), which is
involved in the removal of excess cholesterol from peripheral cells
and its delivery to the liver and steroidogenic cells for
catabolism (2). In vertebrates,
normal HDL exerts potent antioxidant and anti-inflammatory effects
(3) and has anti-infective
properties in plasma (4). It is
well established that there are strong correlations between apoA-I
structure and function, and the beneficial effects of HDL are
highly dependent on its apoA-I content and conformation. Lipid-free
apoA-I, as well as apoA-I in reconstituted HDL (rHDL) particles,
may stimulate insulin secretion from pancreatic β-cells (5). These data raise the possibility that
lipid-free apoA-I may play a unique role in the mammalian health
system.
As a tool for gene delivery, we previously reported
that the efficiency of viral delivery and adenovirus stability can
be significantly enhanced by applying proteoliposomes (PLs)
containing apoA-I to human cells and zebrafish models (6). Recently, a PL containing apoA-I and
its mutant (V156K) was shown to enhance the rapid tumor regression
activity of human origin oncolytic adenovirus in tumor-bearing
zebrafish and nude mice (7).
These studies raise the possibility that apoA-I may be applied to
the generation of vehicles for the delivery of pharmaceuticals,
genes and vaccines.
Over the past three decades, the characterization of
apoA-I and its mutants from a variety of sources in both the
lipid-free and lipid-bound states has been extensively attempted
with purified apoA-I from human plasma, animal plasma, and/or
various recombinant apoA-I produced in an E. coli-based
expression system (1,8). As a result, many
functional-structural domains have been elucidated, as described in
the study by Bashtovyy et al (9). Phylogenetic comparisons of apoA-I
sequences have been described in the study by Brouillette et
al (10), as they observed
that the deletion of a single residue in exon 4 of the pig and cow
did not drastically alter the straight alignment of the hydrophobic
face, whereas the rat and mouse displayed several amino acid
deletions in exon 4 that altered the straight hydrophobic face.
However, there has been no direct comparison of the
functional and structural properties of human apoA-I with those of
major vertebrates in domestic animals, such as pigs or cattle. In
the present study, we compared the functional characteristics of
human, bovine and porcine apoA-I in plasma in the lipid-free and
lipid-bound states, as regards their antioxidant and
anti-atherosclerotic activities.
Materials and methods
Materials
Dimyristoyl phosphatidylcholine (DMPC, #850345) and
palmitoyl oleoyl phosphatidylcholine (POPC, #850457) were obtained
from Avanti Polar Lipids, Inc. (Alabaster, AL, USA).
Bis(sulfosuccinimidyl) suberate (BS3, #S5799) and sodium
cholate (#C1254) were purchased from Sigma (St. Louis, MO, USA).
α-linolenic acid (all-cis-9,12,15-octadecatrienoic acid, 18:3,
#L2376) was purchased from Sigma. Bovine and porcine plasma was
obtained from the Bovine Genome Resources Bank of Korea at Yeungnam
University (Gyeongsan, Korea) and a local slaughter house
(Gyeongsan, Korea), respectively, with the addition of EDTA (final
concentration, 1 mM) as an anti-coagulant.
Purification of HDL and apoA-I
HDL and apoA-I were purified from human, bovine and
porcine plasma using ultracentrifugation and column chromatography,
according to a previously described method (11). The NH2-terminal
amino-acid sequence of apoA-I was determined using an Applied
Biosystems Procise 491 HT protein sequencer (Applied Biosystems,
Foster City, CA, USA) located in the Seoul Branch of the Korea
Basic Research Institute (Seoul, Korea).
DMPC clearance assay
The interactions of apoA-I from each species with
DMPC (3.5 mg/ml) were monitored by the method described in the
study by Pownall et al (12) with slight modifications. The mass
ratio of DMPC to protein was 2:1 (w/w) in a total reaction volume
of 0.76 ml.
Purification and oxidation of low-density
lipoprotein (LDL)
LDL (density, >1.019 to <1.063) was purified
from healthy human plasma (Blood Bank of Yeungnam University
Medical Center, Daegu, Korea) by ultracentrifugation (100,000 × g)
for 22 h at 4°C. Oxidized LDL (oxLDL) was produced by incubating
the LDL fraction with copper sulphate (CuSO4) (final
concentration, 10 μM) for 4 h at 37°C. The oxLDL was then
filtered (0.2 μm filter) and analyzed by thiobarbituric acid
reactive substances (TBARS) assay to determine the extent of
oxidation, as previously described (13).
Synthesis of reconstituted HDL
Discoidal rHDL was prepared using the sodium cholate
dialysis method (14,15) with an initial molar ratio of
POPC:cholesterol:apoA-I:sodium cholate of 95:5:1:150. The size and
hydrodynamic diameter of rHDL particles were determined by 8–25%
native polyacrylamide gradient gel electrophoresis (PAGGE;
Pharmacia PhastSystem) by comparison with standard globular
proteins (GE Healthcare, Uppsala, Sweden).
BS3-cross-linking
The number of apoA-I molecules per rHDL particle, as
well as the self-association properties of lipid-free proteins,
were determined by cross-linking with BS3 as previously
described (16), followed by the
analysis of reaction products by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on pre-cast
8–25% gradient gels (GE Healthcare).
Circular dichroism (CD)
The average α-helix contents of proteins in the
lipid-free and lipid-bound states were measured by CD spectroscopy
using a J-715 Spectropolarimeter (Jasco, Tokyo, Japan). Spectra
were obtained from 250–190 nm at 25°C in a 0.1-cm path-length
quartz cuvette at a bandwidth of 1.0 nm, a speed of 50 nm/min, and
a 4-sec response time. The protein samples were diluted to 0.07
mg/ml so that lipid-free proteins could avoid apolipoprotein
self-association, whereas lipid-bound proteins were diluted to 0.1
mg/ml. Four scans were accumulated and averaged. The α-helix
content was calculated from the molar ellipticity at 222 nm as
previously described (17).
Characterization of tryptophan (Trp)
fluorescence and isothermal denaturation
The wavelength of maximum fluorescence (WMF) of Trp
residues in apoA-I protein was determined from uncorrected spectra
obtained using an LS55 spectrofluorometer (Perkin-Elmer, Norwalk,
CT, USA) in conjunction with the WinLab software package 4.00
(Perkin-Elmer) and a 1-cm path-length Suprasil quartz cuvette
(Fisher Scientific, Pittsburgh, PA, USA). Briefly, the samples were
excited at 295 nm to avoid tyrosine fluorescence, and the emission
spectra were then scanned from 305–400 nm at room temperature. The
effects of the addition of urea on the secondary structure of
apoA-I in the lipid-bound state were monitored by observing the WMF
as described in our previous study (15).
Glycation of apoA-I in lipid-free and
lipid-bound state
In order to compare protein glycation, apoA-I from
each species in the lipid-free or lipid-bound state was incubated
with D-fructose (final concentration, 250 mM) as described our
previous study (18). apoA-I was
incubated for upto 72 h under air gas containing 5% CO2
at 37°C. The extent of advanced glycation reactions was determined
from reading the fluorometric intensity at 370 nm (excitation) and
440 nm (emission), as described previously (18).
Western blot analysis
To compare the cross-reactivity of each apoA-I
protein with antibodies specific to the human variant, equal
amounts (2.5 μg of protein) of apoA-I were loaded and
electrophoresed on 15% SDS-PAGE gels and detected by anti-human
full-length apoA-I goat antibody (ab7613; Abcam, Cambridge, UK) and
donkey anti-goat immunoglobulin G-horseradish peroxidase (HRP)
(SC2020; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as
the secondary antibody (diluted 1:2,000). The apoA-I protein
concentration in the rHDL and lipid-free states was determined
using the Lowry assay as modified by Markwell et al
(19) with bovine serum albumin
as the standard.
Inhibition of LDL oxidation
In order to determine the extent of oxidation,
purified human LDL was incubated with 10 μM CuSO4
for up to 3 h in the presence of apoA-I in the lipid-free or rHDL
state (final 2 μM of protein). During incubation, the
quantity of conjugated dienes formed was monitored by following the
absorbance at 234 nm (A234) at 37°C (20) using a Beckman DU 800
spectrophotometer (Beckman Coulter, Inc., Fullerton, CA, USA)
equipped with a MultiTemp III Thermocirculator (Amersham
Biosciences, Uppsala, Sweden).
To verify the spectroscopic data, oxidized samples
were subjected to electrophoresis on 0.5% agarose gels in order to
compare their electromobilities as previously described (21); migration of each lipoprotein is
known to depend on its intact charge and size. The gels were then
dried and the bands were stained with 0.125% Coomassie Brilliant
Blue.
Acetylation of LDL
The acetylation of LDL (acLDL) was performed using
saturated sodium acetate and acetic anhydride according to a
previously described method (22). Following acetylation and
subsequent dialysis, the acLDL protein content was determined and
filtered through a 0.22 μm filter (Millex; Millipore,
Bedford, MA, USA). To visualize the phagocytosis of acLDL, a
fluorescent cholesterol derivative
[22-(N-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino-23,24-bisnor-5-cholen-3-ol
(NBD-cholesterol), Molecular Probes N-1148; 70 μg of
NBD-cholesterol/mg of apoA-I] was added to the acLDL particles.
LDL phagocytosis assay
THP-1 cells, a human monocytic cell line, were
obtained from the American Type Culture Collection (ATCC;
#TIB-202™; Manassas, VA, USA) and maintained in RPMI-1640 medium
(HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS) until needed for experimentation. Cells that had undergone no
more than 20 passages were incubated in medium containing phorbol
12-myristate 13-acetate (PMA; final concentration, 150 nM) in
24-well plates for 24 h at 37°C in a humidified incubator (5%
CO2, 95% air) to induce differentiation into
macrophages. The differentiated and adherent macrophages were then
rinsed with warm PBS and incubated with 400 μl of fresh
RPMI-1640 medium containing 1% FBS, 50 μl of (acLDL; 1 mg of
protein/ml in PBS), and 50 μl of PBS or each protein (final
2 μM) for 48 h at 37°C in a humidified incubator. Following
incubation, the cells were washed with PBS three times and then
fixed in 4% paraformaldehyde for 10 min. The fixed cells were then
stained with oil Red O staining solution (0.67%) and then washed
with distilled water. THP-1 macrophage-derived foam cells were then
observed and photographed using a Nikon Eclipse TE2000 microscope
(Nikon, Tokyo, Japan) at ×600 magnification.
Statistical analysis
All data are expressed as the means ± SD of at least
three independent experiments with duplicate samples. Comparisons
between the results were made using the Student’s t-test with SPSS
software (version 12.0; SPSS, Inc., Chicago, IL, USA). A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of apoA-I and sequence
comparisons
Although apoA-I from all species (>95% purity)
exhibited 28–29 kDa molecular weight in the lipid-free state from
SDS-PAGE (Fig. 1A), bovine apoA-I
showed the highest position with the slowest mobility in the native
gel electrophoresis (Fig.
1B).
N-terminal amino acid sequencing confirmed the
identities of bovine (lane B) and porcine (lane P) apoA-I. In each
protein, 16 amino acids were identical to the GenBank sequences for
bovine (ID AAI02942.1) and porcine (ID CAA49234.1) apoA-I. Bovine
and porcine apoA-I differed from the human protein in their 2nd
amino acid (Asp), which corresponds to Glu; the 4th Pro in the
human sequence was deleted from the bovine and porcine proteins.
Furthermore, Pro7 in human apoA-I was replaced by Ser in bovine
apoA-I; the 14th residue, leucine, in human apoA-I was replaced by
Phe in the bovine and porcine variants (data not shown).
Immunodetection of bovine and porcine
apoA-I
The human specific antibody for apoA-I could not
detect bovine or porcine protein (2.5 μg/lane), suggesting
that the antigenic epitope was dissimilar between the species
(Fig. 1C). The comparison of the
amino acid sequences revealed the deletion of the 4th amino acid in
the human protein, Pro, in the bovine and porcine apoA-I sequences;
the loss of this residue may have disrupted the epitope. Pro4 and
Ala187 in the human protein sequence were deleted in bovine apoA-I;
Pro4, Glu120 and Asn184 were deleted in porcine apoA-I.
Characterization of rHDL
Electrophoretic analysis of POPC-rHDL-associated
apoA-I demonstrated that the major particle size ranged from 98–105
Å (Fig. 2A). The bovine
apoA-I-containing particles were the largest, at approximately 105
Å in diameter. BS3-cross-linking revealed that bovine
and porcine apoA-I rHDL contained up to five apoA-I units per
particle, whereas human apoA-I rHDL contained three apoA-I
units/particle (Fig. 2B).
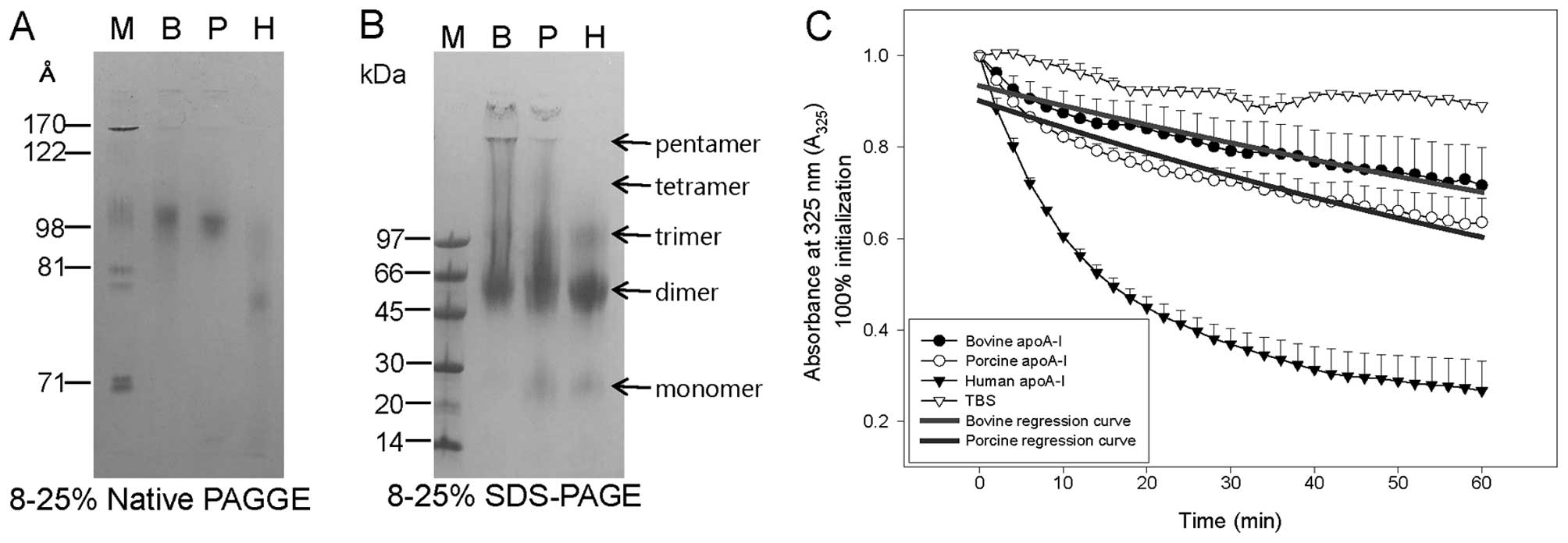 | Figure 2Electrophoretic mobility of three
mammalian apolipoprotein A-I (apoA-I) variants in the lipid-bound
state. Lane B, bovine apoA-I; lane P, porcine apoA-I; lane H, human
apoA-I. (A) Electromobility of apoA-I without sample boiling. M,
molecular weight (standard) (GE Healthcare, high-range standard).
(B) Bis-sulfosuccinimidyl suberate (BS3)-cross-linked
apoA-I in the POPC-rHDL state, as visualized by Coomassie blue
staining. M, molecular weight (standard) (Bio-Rad, low-range
standard). (C) Interaction of each apoA-I variant with dimyristyol
phosphatidylcholine (DMPC) multilamellar liposomes. The mass ratio
of DMPC to protein was 2:1 (wt/wt) in a total reaction volume of
0.76 ml. POPC, palmitoyl oleoyl phosphatidylcholine; rHDL,
reconstituted high-density lipoprotein. |
DMPC clearance
In order to compare the phospholipid and
polyunsaturated fatty acid binding capacity of the apoA-I, we
monitored the clearance speed of the lipid emulsion. Bovine and
porcine apoA-I displayed a much weaker phospholipid-binding ability
compared with human apoA-I; values for the repair half-time
(T1/2) values were calculated at around 81 and 66 min,
respectively, from the regression curve (Fig. 2C). The phospholipid-binding
ability of human apoA-I was much more pronounced as the approximate
T1/2 value was around 17 min.
Spectroscopic analyses of secondary
structure
In the lipid-free state, human and bovine apoA-I
showed a WMF of 347 and 348 nm, respectively, whereas porcine
apoA-I showed a WMF of 345 nm. Bovine and porcine proteins
contained Trp residues at the 7th, 49th, 71st and 107th positions.
In the rHDL state, the WMF apoA-I from all species demonstrated a
blue shift of 3–4 nm (Table I).
Secondary structure determination revealed α-helicity in human
apoA-I of 53 and 74% in the lipid-free and rHDL states,
respectively, which was a 21% increase. However, bovine apoA-I
showed 53 and 66% α-helicity in the lipid-free and rHDL states,
respectively, indicating the lowest increase in α-helicity
(approximately 13%). Porcine apoA-I exhibited 55 and 70% α-helicity
in the lipid-free and rHDL states, respectively, which was a 15%
increase in α-helicity.
 | Table ICompositional and spectroscopic
analyses of apoA-I from bovine, porcine and human plasma in the
lipid-bound state (rHDL)a. |
Table I
Compositional and spectroscopic
analyses of apoA-I from bovine, porcine and human plasma in the
lipid-bound state (rHDL)a.
| Bovine | Porcine | Human |
|---|
| WMFb (nm) at urea 0 M | 341±3
(348±1)c | 341±2 (346±2) | 344±2 (348±2) |
| WMF (nm) at urea 7
M | 354±2 (355±1) | 357±2 (359±2) | 355±1 (357±2) |
| α-helicityd (%) | 66±3 (53±4) | 70±4 (55±3) | 74±2 (53±3) |
| Sizee (Å) | 105 | 101 | 98, 80 |
| No. of
apoA-If/rHDL | 2–5 | 2–5 | 2, 3 |
| pIg (calculated) | 5.32 | 5.11 | 5.24 |
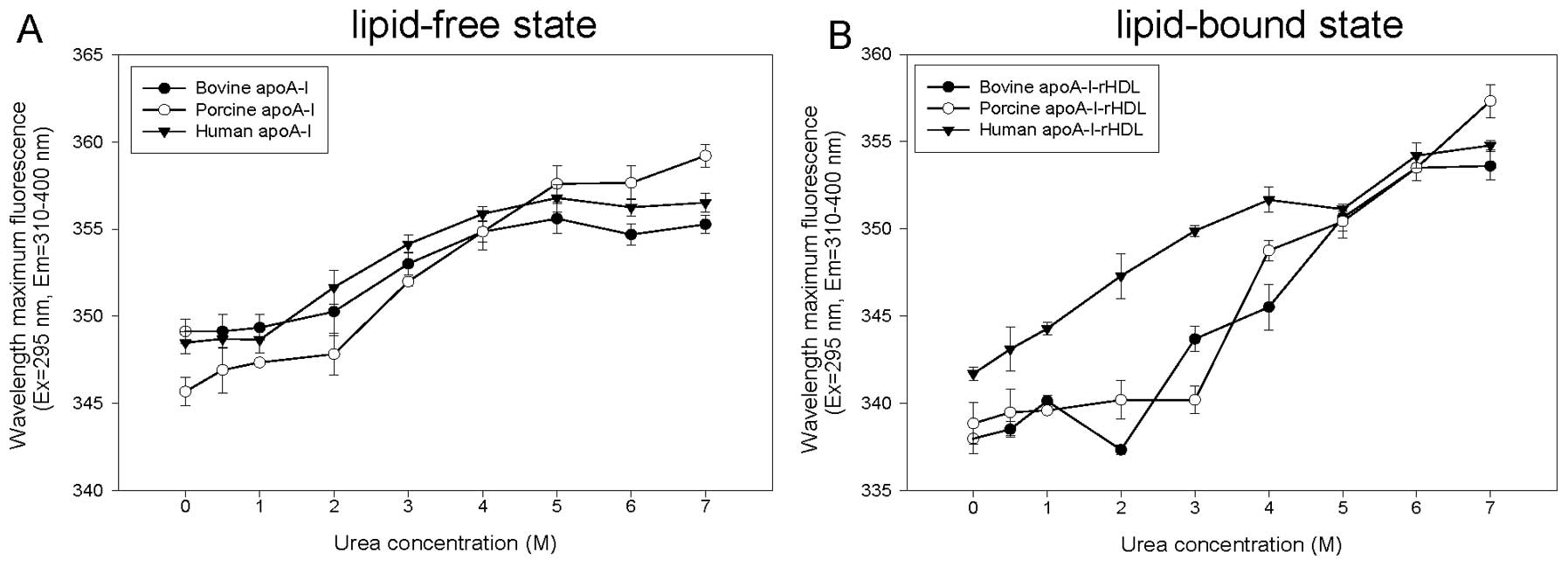
Denaturation under urea treatment
In the lipid-free state, porcine and bovine apoA-I
were resistant to denaturation at a urea concentration <2 M
(Fig. 3A). Exposure to 7 M urea
stimulated the highest WMF of porcine apoA-I, whereas bovine and
human apoA-I showed their highest WMF upon treatment with 5 M urea.
These results suggest that porcine and bovine apoA-I show variable
sensitivity upon complete Trp exposure in the aqueous phase due to
protein denaturation. In the rHDL state, porcine and bovine apoA-I
were resistant to denaturation at a urea concentration <5 M.
Human apoA-I was the most susceptible to urea-induced denaturation
(Fig. 3B).
Inhibition of LDL oxidation
In the lipid-free state, bovine apoA-I was the most
resistant to cupric ion-mediated LDL oxidation during 40 min of
incubation (Fig. 4A) (final
concentration of each apoA-I, 2 μM); bovine apoA-I
demonstrated an increase in A234 of 54%, whereas porcine
and human apoA-I were inhibited to a lesser degree (increase in
A234 of 84–89%). Agarose electrophoresis revealed that
LDL treated with bovine apoA-I (final 2 μM) migrated the
slowest. This result suggests that LDL treated with bovine apoA-I
was more resistant to the oxidation, as more oxLDL migrates faster
due to an increase in the negative charge and smaller particle size
(Fig. 4B).
Inhibition of acLDL uptake by
macrophages
We monitored the uptake of acLDL into THP-1 cells in
the presence of each apoA-I (Fig.
5) relative to the PBS-treated control (Fig. 5A) based on the detection of a
fluorescent cholesterol derivative. In the lipid-free state, bovine
apoA-I demonstrated the greatest inhibition of acLDL phagocytosis.
Specifically, in the presence of lipid-free bovine apoA-I, the
fluorescence decreased by 56% compared with the acLDL control
(Fig. 5).
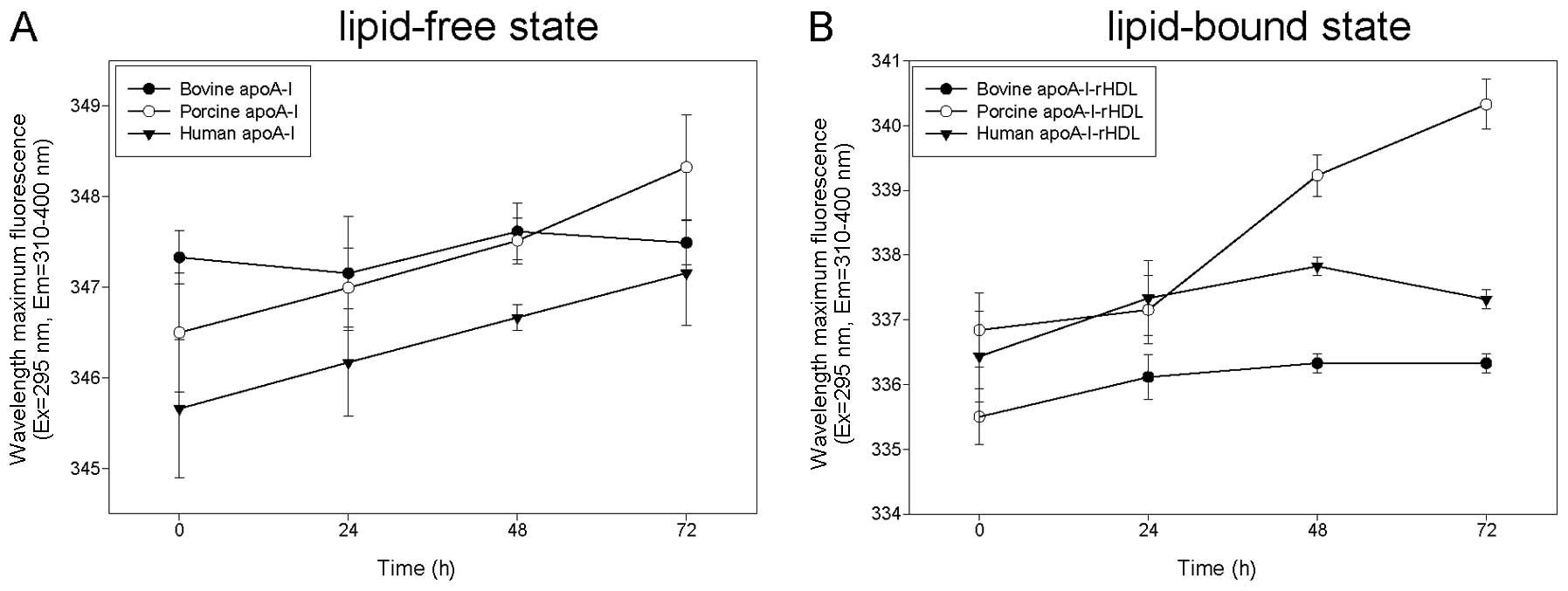
Glycation sensitivity of apoA-I
By fructosylation, apoA-I is usually prone to an
increase in the WMF by the glycation process via the modification
of its tertiary structure, resulting in the exposure of Trp in the
aqueous phase. In the lipid-free state, porcine and human apoA-I
showed an increase in WMF of approximately 2 nm during 72 h of
incubation (Fig. 6A). However,
bovine apoA-I did not show any change in WMF in the lipid-free
state (approximately 347 nm). Although apoA-I has four Trp in the
protein sequence, the sensitivity to fructosylation differed
between the species. In the rHDL state, bovine apoA-I and human
apoA-I showed the lowest increase in WMF (approximately 1 nm)
during 72 h of incubation (from 335 to 336 nm), while porcine
apoA-I showed the highest increase (approximately 3 nm; from 337 to
340 nm) (Fig. 6B). These results
suggest that bovine apoA-I is the most resistant to glycation;
however, the mechanisms invovled remain to be elucidated.
Discussion
In the current study, we found several properties
unique to bovine apoA-I compared with other mammalian apoA-I (human
and porcine). Bovine apoA-I exhibited the weakest binding ability
with DMPC (Fig. 2C) and the
weakest sensitivity to denaturation by urea (Fig. 3). Bovine apoA-I was the most
resistant to glycation with inhibition against LDL oxidation
(Fig. 4) and foam cell formation
via the phagocytosis of macrophages (Fig. 5).
apoA-I is a major component of HDL particles, which
have strong antioxidant, anti-inflammatory and anti-infective
properties (23). The
overexpression of apoA-I in mice elicits resistance to diet-induced
atherosclerosis (24). These
studies raise the possibility of protein adaptation for the
development of anti-atherosclerotic and anti-diabetic therapeutics.
Serum HDL cholesterol and apoA-I levels are inversely associated
with diabetic retinopathy, and serum apoA-I and apo-B are both
strong biomarkers of this pathology. The infusion of rHDL with
wild-type apoA-I or several mutant variants of apoA-I has been
reported as an emerging therapy for acute regression (25,26). These results suggest that apoA-I
has potent therapeutic activities for the treatment of chronic
metabolic diseases, such as diabetes and atherosclerosis.
The apoA-I amino acid sequence is conserved in
higher vertebrates, such as pigs and cattle. Bovine apoA-I (GenBank
ID AAI02942.1) and porcine apoA-I (GenBank ID CAA49234.1) consist
of 241 and 240 amino acids, respectively, in their mature
sequences. Furthermore, they exhibit 78% (bovine) or 80% (porcine)
sequence identity to the human protein (AAH05380.1). Due to these
amino acid differences, it is possible that the location of
antigenic amino acids varies according to species. Collet et
al (27) reported that
several monoclonal antibodies (mAbs) against human apoA-I are
unable to detect porcine or bovine protein, suggesting that apoA-I
immunoreactivity may differ among species. They also tested 20
different mAbs against apoA-I proteins from humans, pigs and
cattle. Between humans and pigs, only five mAbs displayed 50–100%
immunoreactivity. On the other hand, between cattle and humans,
only one mAb displayed 50–100% immunoreactivity with two
coincidental epitope regions around amino acids 23–29 and amino
acids 60–82.
Secondary structure determination revealed
α-helicity in human apoA-I of 53 and 74% in the lipid-free and rHDL
states, respectively, which was a 21% increase. However, bovine
apoA-I showed 53 and 66% α-helicity in the lipid-free and rHDL
states, respectively, indicating a 13% increase in α-helicity.
Porcine apoA-I exhibited 55 and 70% α-helicity in the lipid-free
and rHDL states, respectively, which was a 15% increase in
α-helicity. These results indicate that the α-helicity of
individual apoA-I from bovine or porcine plasma is lower than that
isolated from human plasma. The inclusion of more bovine or porcine
apoA-I molecules into rHDL (Fig.
2B) suggests that additional α-helicity is non-essential.
Another interpretation of this finding is that more bovine or
porcine apoA-I molecules are required for rHDL formation due to
their lower α-helicity.
As regards the structural and functional analysis of
apoA-I, Jonas and Krajnovich (28) reported that bovine apoA-I displays
a blue shift of approximately 10 nm in its WMF between the
lipid-free and lipid-bound (DMPC-rHDL) states. Additionally, they
observed that the α-helicity of bovine apoA-I was 58 or 72% in the
lipid-free and lipid-bound states, respectively. This is consistent
with our current results, although we used POPC as a phospholipid
in this study. The weakened DMPC clearance activity of bovine and
porcine apoA-I positively correlated with the reduced α-helicity
and the increased molecular number of apoA-I in the POPC-rHDL
state, as greater α-helicity is necessary to generate larger rHDL
particles.
The atheroprotective functions of HDL are
characterized by the potent inhibition of LDL oxidation, as well as
the expression of endothelial cell adhesion molecules and monocyte
chemoattractant protein-1. HDL promotes the efflux of cholesterol
from foam cells. In the present study, bovine apoA-I showed the
most potent inhibitory activity toward LDL oxidation and macrophage
uptake, suggesting that it may be used in nanoparticles for the
delivery of therapeutic drugs. The strong inhibition of LDL
oxidation by bovine apoA-I (Fig.
4) positively correlated with the decrease in macrophage acLDL
phagocytosis (Fig. 5).
Another major function of apoA-I is its antioxidant
activity toward LDL. It is well known that the oxidation of LDL
participates in the initiation of atherosclerosis and coronary
artery disease. Further, the uptake of oxLDL into macrophages,
which results in foam cell formation, is an early step in the
generation of atherosclerotic plaque. Based on the characterization
of rHDL (Table I), bovine apoA-I
possesses the greatest capacity to form rHDL among the species,
although its minor band size varied. Human apoA-I rHDL contained
smaller particles of approximately 80 and 98 Å, whereas rHDL
containing either bovine or porcine apoA-I exhibited particles of
105 Å (Fig. 2A). Therefore,
bovine and porcine apoA-I possibly facilitate the formation of
larger rHDL particles with the same molar ratio as greater numbers
of apoA-I molecules are required to increase rHDL. Furthermore,
bovine apoA-I cross-linking resulted in pentameric structures
without any monomeric band, indicating that the majority of bovine
apoA-I monomer participated in the cross-linking. These results
indicate the enhanced susceptibility of bovine apoA-I to
cross-linking. Bovine apoA-I has reportedly been associated with
immune function in cattle as reported in the study by Oikawa et
al (29). The serum
concentration of apoA-I was decreased in cows infected with
Salmonella typhimurium. In addition, bovine apoA-I-rHDL had the
largest particle size (approximately 105 Å) (Table I). In our previous study, higher
HDL levels were associated with a stronger antioxidant ability
compared with lower HDL levels (30). Taken together, these data suggest
that the stronger antioxidant ability of bovine apoA-I may be due
to the increased particle size. In future studies, structural and
functional correlations should be investigated in greater
detail.
In conclusion, bovine and porcine apoA-I exhibit
unique properties in the lipid-free and lipid-bound (rHDL) states
compared with human apoA-I. Specifically, they demonstrated
enhanced antioxidant abilities and were resistant to denaturation
by urea, suggesting that they may be effective delivery vehicles
for therapeutic drugs or vaccines. Future studies are required for
the development of encapsulated viral particles in HDL-like
structures and to examine their bio-availability in such
endeavors.
Acknowledgements
This study was supported by the Yeungnam University
Research grants in 2010. The authors are grateful for the BK21 plus
program of the National Research Foundation for the support of
graduate students.
References
|
1
|
Frank PG and Marcel YL: Apolipoprotein
A-I: Structure-function relationships. J Lipid Res. 41:853–872.
2000.PubMed/NCBI
|
|
2
|
Fielding PE and Fielding CJ: Plasma
membrane caveolae mediate the efflux of cellular free cholesterol.
Biochemistry. 34:14288–14292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rye KA and Barter PJ: Antiinflammatory
actions of HDL: a new insight. Arterioscler Thromb Vasc Biol.
28:1890–1891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelesidis T, Yang OO, Currier JS, Navab K,
Fogelman AM and Navab M: HIV-1 infected patients with suppressed
plasma viremia on treatment have pro-inflammatory HDL. Lipids
Health Dis. 10:352011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fryirs MA, Barter PJ, Appavoo M, Tuch BE,
Tabet F, Heather AK and Rye KA: Effects of high-density
lipoproteins on pancreatic beta-cell insulin secretion.
Arterioscler Thromb Vasc Biol. 30:1642–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KH, Yun CO, Kwon OJ, Kim CH, Kim JR
and Cho KH: Enhanced delivery of adenovirus, using proteoliposomes
containing wildtype or V156K apolipoprotein A-I and
dimyristoylphosphatidylcholine. Hum Gene Ther. 21:579–587. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo J, Yun CO, Kwon OJ, Choi EJ, Song JY,
Choi I and Cho KH: A Proteoliposome containing apolipoprotein A-I
mutant (V156K) enhances rapid tumor regression activity of human
origin oncolytic adenovirus in tumor-bearing zebrafish and mice.
Mol Cells. 34:143–148. 2012. View Article : Google Scholar
|
|
8
|
Huang R, Silva RA, Jerome WG, Kontush A,
Chapman MJ, Curtiss LK, Hodges TJ and Davidson WS: Apolipoprotein
A-I structural organization in high-density lipoproteins isolated
from human plasma. Nat Struct Mol Biol. 18:416–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bashtovyy D, Jones MK, Anantharamaiah GM
and Segrest JP: Sequence conservation of apolipoprotein A-I affords
novel insights into HDL structure-function. J Lipid Res.
52:435–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brouillette CG, Anantharamaiah GM, Engler
JA and Borhani DW: Structural models of human apolipoprotein A-I: a
critical analysis and review. Biochim Biophys Acta. 1531:4–46.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brewer HB Jr, Ronan R, Meng M and Bishop
C: Isolation and characterization of apolipoproteins A-I, A-II, and
A-IV. Methods Enzymol. 128:223–246. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pownall HJ, Massey JB, Kusserow SK and
Gotto AM Jr: Kinetics of lipid-protein interactions: interaction of
apolipoprotein A-I from human plasma high density lipoproteins with
phosphatidylcholines. Biochemistry. 17:1183–1188. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
14
|
Cho KH, Park SH, Han JM, Kim HC, Chung YJ,
Choi I and Kim JR: A point mutant of apolipoprotein A-I, V156K,
exhibited potent anti-oxidant and anti-atherosclerotic activity in
hypercholesterolemic C57BL/6 mice. Exp Mol Med. 39:160–169. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho KH: Synthesis of reconstituted high
density lipoprotein (rHDL) containing apoA-I and apoC-III: the
functional role of apoC-III in rHDL. Mol Cells. 27:291–297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staros JV: Membrane-impermeant, cleavable
cross-linkers: new probes of nearest neighbor relationships at one
face of a membrane. Biophys J. 37:21–22. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YH, Yang JT and Martinez HM:
Determination of the secondary structures of proteins by circular
dichroism and optical rotatory dispersion. Biochemistry.
11:4120–4131. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KH, Jang W, Kim KY, Kim JR and Cho
KH: Fructated apolipoprotein A-I showed severe structural
modification and loss of beneficial functions in lipid-free and
lipid-bound state with acceleration of atherosclerosis and
senescence. Biochem Biophys Res Commun. 392:295–300. 2010.
View Article : Google Scholar
|
|
19
|
Markwell MA, Haas SM, Bieber LL and
Tolbert NE: A modification of the Lowry procedure to simplify
protein determination in membrane and lipoprotein samples. Anal
Biochem. 87:206–210. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esterbauer H, Striegl G, Puhl H and
Rotheneder M: Continuous monitoring of in vitro oxidation of human
low density lipoprotein. Free Radic Res Commun. 6:67–75. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noble RP: Electrophoretic separation of
plasma lipoproteins in agarose gel. J Lipid Res. 9:693–700.
1968.PubMed/NCBI
|
|
22
|
Fraenkel-Conrat H: Methods for
investigating the essential groups for enzyme activity. Methods
Enzymol. 4:247–269. 1957. View Article : Google Scholar
|
|
23
|
Cho KH: Biomedicinal implications of
high-density lipoprotein: its composition, structure, functions,
and clinical applications. BMB Rep. 42:393–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubin EM, Krauss RM, Spangler EA,
Verstuyft JG and Clift SM: Inhibition of early atherogenesis in
transgenic mice by human apolipoprotein AI. Nature. 353:265–267.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newton RS and Krause BR: HDL therapy for
the acute treatment of atherosclerosis. Atheroscler Suppl. 3:31–38.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholls SJ, Uno K, Kataoka Y and Nissen
SE: ETC-216 for coronary artery disease. Expert Opin Biol Ther.
11:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Collet X, Marcel YL, Tremblay N, Lazure C,
Milne RW, Perret B and Weech PK: Evolution of mammalian
apolipoprotein A-I and conservation of antigenicity: correlation
with primary and secondary structure. J Lipid Res. 38:634–644.
1997.PubMed/NCBI
|
|
28
|
Jonas A and Krajnovich DJ: Effect of
cholesterol on the formation of micellar complexes between bovine
A-I apolipoprotein and L-alpha-dimyristoylphosphatidylcholine. J
Biol Chem. 253:5758–5763. 1978.
|
|
29
|
Oikawa S, Katoh N, Itoh H, Miyamoto T,
Konno M and Kajita T: Decreased serum apolipoprotein A-I
concentrations in cows infected with Salmonella typhimurium. Can J
Vet Res. 61:182–186. 1997.PubMed/NCBI
|
|
30
|
Lee H, Park JE, Choi I and Cho KH:
Enhanced functional and structural properties of high-density
lipoproteins from runners and wrestlers compared to throwers and
lifters. BMB Rep. 42:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|