Introduction
The fibroblast growth factor signaling system plays
an important role in the development of most multicellular animals.
It controls the proliferation, differentiation, migration and
apoptosis of virtually all cell types. The genomes of mice and
humans code for 22 different FGF ligands (FGF1–FGF23, FGF15=FGF19)
that can interact with four different FGF receptors (FGFRs;
FGFR1–FGFR4) (1,2). The FGFs bind together with heparan
sulfate to either one of the four receptors and trigger, via
transphosphorylation, several intracellular signaling cascades,
such as the mitogen-activated protein kinase (MAPK)/Erk, the
phosphoinositide 3-kinase (PI3K)/Akt, the Jak/Stat and the
phosphoinositide phospholipase C (PLC)γ pathway.
All FGFRs contain three extracellular
immunoglobulin-like domains (Ig domains 1–3), a single
transmembrane domain and an intracellular tyrosine kinase domain
(2). Alternative splicing
contributes to the complexity of the system. Each of the receptors
FGFR1, FGFR2 and FGFR3 occurs in two different splice variants that
differ by the precise amino acid sequence of Ig domain 3. At the
genomic level, this domain is encoded by three different exons,
namely exons IIIa, IIIb and IIIc. Exon IIIa codes for the first
half of Ig domain 3 and is used for all splice variants, but exons
IIIb and IIIc are used in a mutually exclusive manner to give rise
to two different splice variants, the b and the c variants. A total
of seven different receptors can therefore be generated, FGFR1b,
FGFR1c, FGFR2b, FGFR2c, FGFR3b, FGFR3c and FGFR4 (1,2).
The b splice variants are primarily expressed in epithelial
tissues, the c variants mainly in mesenchymal tissues.
The metanephric (permanent) kidney of mammals is
formed by two different tissues, the metanephric mesenchyme and the
ureteric bud (3,4). In mice, the development of the
metanephric kidney begins on embryonic day E10.5 when the ureteric
bud invades the metanephric mesenchyme. By a series of reciprocal
interactions, the metanephric mesenchyme induces the ureteric bud
to branch in a stereotypical fashion, while the ureteric bud
induces the metanephric mesenchyme to condense around its tips and
to undergo a mesenchymal-to-epithelial conversion that leads to the
formation of renal vesicles. The renal vesicles develop further
into comma- and s-shaped bodies and finally form functional
nephrons. Gene expression profiling has revealed that the
developing mouse kidney expresses Fgfs 1, 7, 8, 9, 10, 12 and 20
(5).
In the year 2000, we discovered a fifth FGFR that we
termed FGF receptor-like protein 1 (FGFRL1) (6). This receptor contains three Ig
domains and a single transmembrane domain similar to the classical
FGFRs. However, it lacks the tyrosine kinase domain and instead
contains a short unrelated sequence at the intracellular side that
ends with a histidine-rich domain (7–10).
FGFRL1 is expressed primarily in cartilage and developing bones,
and at lower levels in many other organs including kidneys and
muscles. Mice with a targeted disruption of the novel receptor-like
gene (knockout mice) develop to term and are born alive (11,12). However, these animals die
immediately after birth and show severe kidney dysgenesis or kidney
agenesis due to the lack of renal vesicles (13). We confirmed that FgfrL1 is in fact
expressed in renal vesicles and all nephrogenic structures during
the early steps of kidney development (14,15).
The molecular mechanisms behind the involvement of
FGFRL1 in FGF signaling have not yet been elucidated. It cannot
signal by transphosphorylation as it does not contain any
intracellular tyrosine kinase domain. When mRNA for FGFRL1 was
injected into blastomers of Xenopus embryos, it interfered
with FGF signaling and led to gastrulation defects that affected
the trunk and tail of the embryos (16). This effect was overcome by the
co-injection of mRNA for FGFR1. We therefore concluded that FGFRL1
may act as a decoy receptor that binds and neutralizes FGF ligands.
However, this hypothesis was challenged by more recent findings
obtained with FgfrL1 null mice. A comparison of the mRNA profiles
from wild-type and knockout animals using gene microarrays revealed
that the lack of FgfrL1 was not compensated for by another Fgfr or
by any downstream signaling molecule (14). Furthermore, the phenotype of our
knockout animals was strikingly similar to the phenotype of animals
with a conditional deletion of Fgf8, which also lack any nephrons
in their metanephric kidneys (13,17,18). If FgfrL1 served as a simple decoy
receptor for Fgf ligands one would expect to observe more, and not
less Fgf signaling in our knockout animals and consequently an
increased, rather than a decreased, number of nephrogenic
structures and/or ureteric buds.
To gain a better understanding of the working
mechanisms of FgfrL1, we decided to compare the exact expression
pattern of FgfrL1 with that of the other Fgfrs. We hypothesized
that a particular receptor would show an expression pattern similar
to that of FgfrL1 if FgfrL1 is involved, directly or indirectly, in
its signaling cascade. We found that the FgfrL1 expression pattern
greatly resembled that of Fgfr1, but clearly differed from that of
Fgfr2–Fgfr4, suggesting that FgfrL1 may participate in Fgfr1
signaling.
Materials and methods
Animals
Kidneys were obtained from mice (strain C57BL/6)
bred at our local animal facility. For a timed pregnancy, the noon
of the day, at which a vaginal plug could be detected, was counted
as E0.5. All animal experiments were approved by the Swiss Federal
Veterinary Office (BVET) (BE84/12).
RNA preparation and northern
blotting
Kidneys were dissected from mouse embryos and
immediately placed into RNA-later buffer (Sigma, St. Louis, MO,
USA). In order to obtain enough RNA, kidney rudiments of early
developmental stages (E12.5–E14.5) were pooled from 20–30
individual embryos. RNA was prepared using the GeneElute mammalian
total RNA kit from Sigma and separated on 1% agarose gels in the
presence of 1 M formaldehyde. The resolved bands were transferred
from the gel to a nylon membrane by vacuum blotting. The membrane
was hybridized at 42°C with radioactively labeled cDNA probes in a
buffer containing 50% formamide. These probes were labeled by the
random primed oligolabeling method with [α-32P] dCTP.
After overnight hybridization, the blot was washed at regular
stringency [1X standard saline citrate (SSC)] and exposed to X-ray
film (Carestream Kodak BioMax MS; Sigma).
Hybridization probes for the canonical Fgfrs were
generated by PCR utilizing cDNA prepared from E16.5 mouse kidneys
and the primer pairs listed in Table
I. Probes for Fgfr1–Fgfr3 were selected in a manner that they
hybridized equally well with the b and the c splice variants. The
PCR fragments were inserted into the BamHI/XbaI site
of the expression vector pSPT19 (Roche Applied Science, Basel,
Switzerland). The probe for FgfrL1 corresponded to an
XbaI/BamHI fragment derived from the full-length cDNA
sequence (9), which was subcloned
into pSPT19. The final probes encompassed nucleotides 766–1554
(corresponding to amino acids 7–269) of Fgfr1 (NM_010206),
nucleotides 1336–1979 (amino acids 57–271) of Fgfr2 (NM_010207),
nucleotides 374–1093 (amino acids 23–262) of Fgfr3 (NM_008010),
nucleotides 503–1242 (amino acids 115–360) of Fgfr4 (NM_008011),
nucleotides 661–1417 (amino acids 190–441) of FgfrL1 (AJ293947) and
nucleotides 211–899 (amino acids 26–255) of calbindin (NM_009788).
The reading frame and authenticity of all constructs were verified
by DNA sequencing.
 | Table IPrimers used for preparation of
hybridization probes. |
Table I
Primers used for preparation of
hybridization probes.
| Primer | Sequence | Accession no. |
|---|
| Calb1 up | ATGGATCCGACGGAAGTGGTTACCTGGA | |
| Calb1 low | TATCTAGATAAGAGCAAGGTCTGTTCGGTA | NM_009788 |
| Fgfr1 up | GTGGATCCTCTTCTGGGCTGTGCT | |
| Fgfr1 low | ATTCTAGACCAGGGCCACTGTCTTGT | NM_010206 |
| Fgfr2 up | GAGGATCCACCAACCAAATACCAAATC | |
| Fgfr2 low | TGTCTAGACCGTTCAACGACATCGAG | NM_010207 |
| Fgfr3 up | GAGGATCCTGGTCCAGAGCAGCG | |
| Fgfr3 low | ACGTCTAGACCTAGAATGGCTGTCTGG | NM_008010 |
| Fgfr4 up | GTGGATCCTGGATGACTCCTTAACCTC | |
| Fgfr4 low | TGTCTAGAGGGGTTGCTGTTGTCCAC | NM_008011 |
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed
using E15.5 mouse kidneys following the protocol provided in the
GUDMAP gene expression database (http://www.gudmap.org/Research/Protocols/McMahon.html).
Riboprobes were generated from cDNA sequences cloned into pSPT19
(see above) by transcription in the presence of digoxigenin-labeled
UTP using the SP6/T7 DIG RNA Labeling kit from Roche as previously
described (13). Kidneys were
dissected from 15.5-day-old embryos and fixed overnight with 4%
paraformaldehyde (PFA). The tissue was dehydrated by serial
treatment with methanol/phosphate buffer (25, 50, 75 and 100%
methanol in PBS containing 0.1% Tween-20). The specimens were
rehydrated, bleached for 30 min with 6% hydrogen peroxide and
digested for 15 min with 10 μg/ml proteinase K. Subsequently, the
samples were fixed with 0.2% glutaraldehyde/4% PFA, pre-hybridized
at 68°C for 2 h in hybridization buffer (50% formamide, 5X SSC, 1%
SDS, 50 μg/ml t-RNA from yeast, 50 μg/ml heparin) and then
hybridized overnight with the riboprobes. Following washing with
50% formamide, 5X SSC, 1% SDS at 65°C, the samples were blocked for
2 h at room temperature with 3% bovine serum albumin in
Tris-buffered saline and then incubated with anti-digoxigenin
antibodies conjugated with alkaline phosphatase (Roche, 1:2,000).
After extensive washing, the hybridization signal was developed
with BM Purple (Roche) for 7–78 h.
Results
Analysis of Fgfr expression by northern
blotting
The expression of the four classical receptors was
analyzed by northern blotting with samples from embryonic kidneys
at stage E15.5. Special care was taken so that the four
hybridization probes had a similar specific radioactivity to allow
the direct comparison of the resulting hybridization signals
between the four probes. Moreover, the sequences of the probes were
selected in a manner that the probes hybridized equally well with
the b and the c splice variants of the receptors.
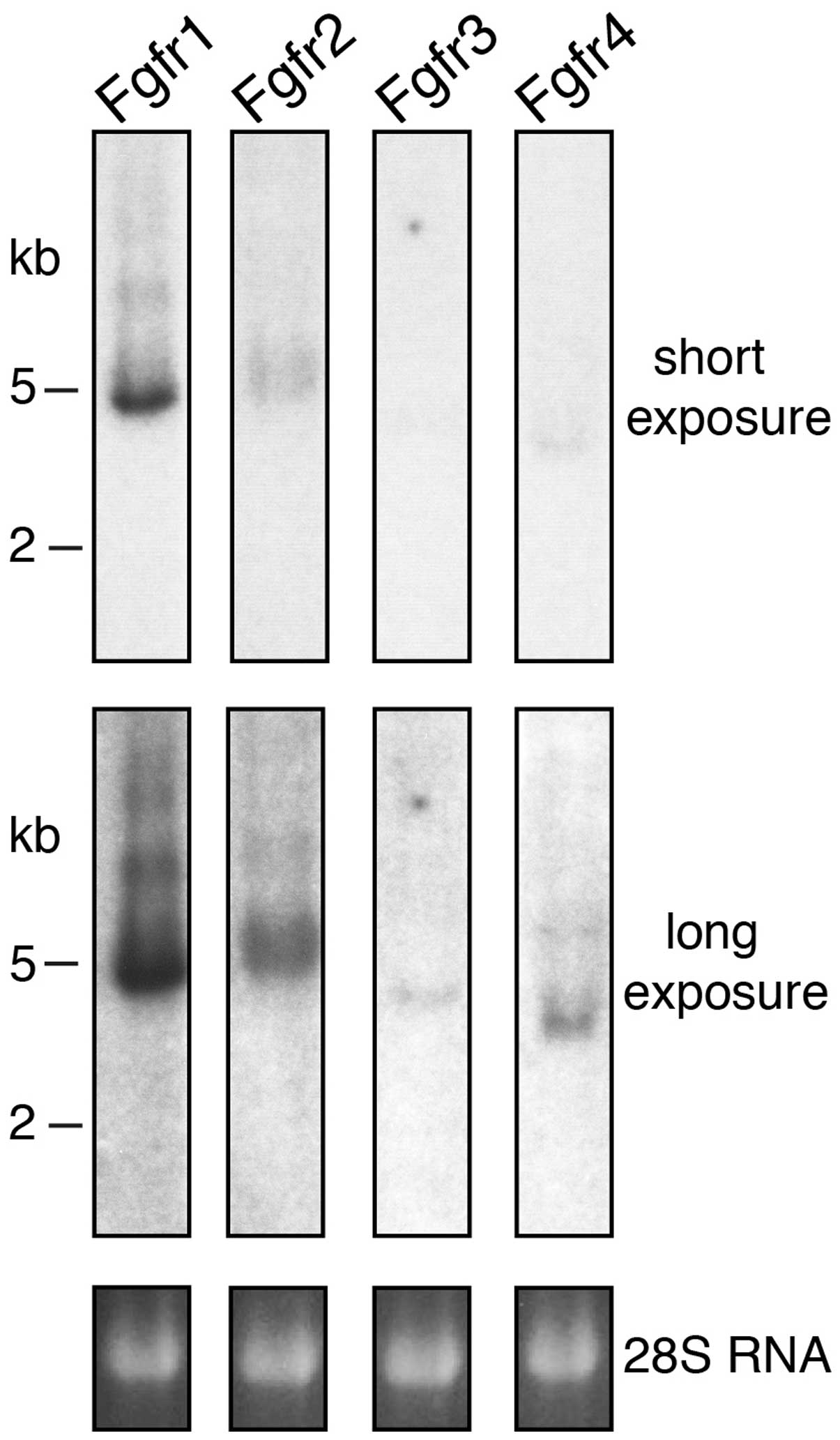
A particularly strong hybridization signal was
obtained with the probe for Fgfr1 (Fig. 1). This result suggests that Fgfr1
is the principal receptor in the developing mouse kidney at stage
E15.5. A strong signal was also observed with the probe for Fgfr2.
However, in this case the resulting band was broader and fuzzier,
possibly due to the presence of several distinct mRNA splice
variants and/or different polyadenylation sites. By contrast, the
signals for Fgfr3 and Fgfr4 were extremely weak and could be
detected only after extended exposure of the northern blots,
indicating that these receptors are expressed in the kidneys at
very low levels during early development. The electrophoretic
mobility of all the positive bands was consistent with the size of
the four mRNAs predicted from their cDNA sequences (Fgfr1 5,000 bp,
Fgfr2 5,200 bp, Fgfr3 4,500 bp and Fgfr4 3,500 bp). Our northern
blotting experiment also demonstrated that no unexpected
crossreaction occurred between each of the probes and the four
receptors.
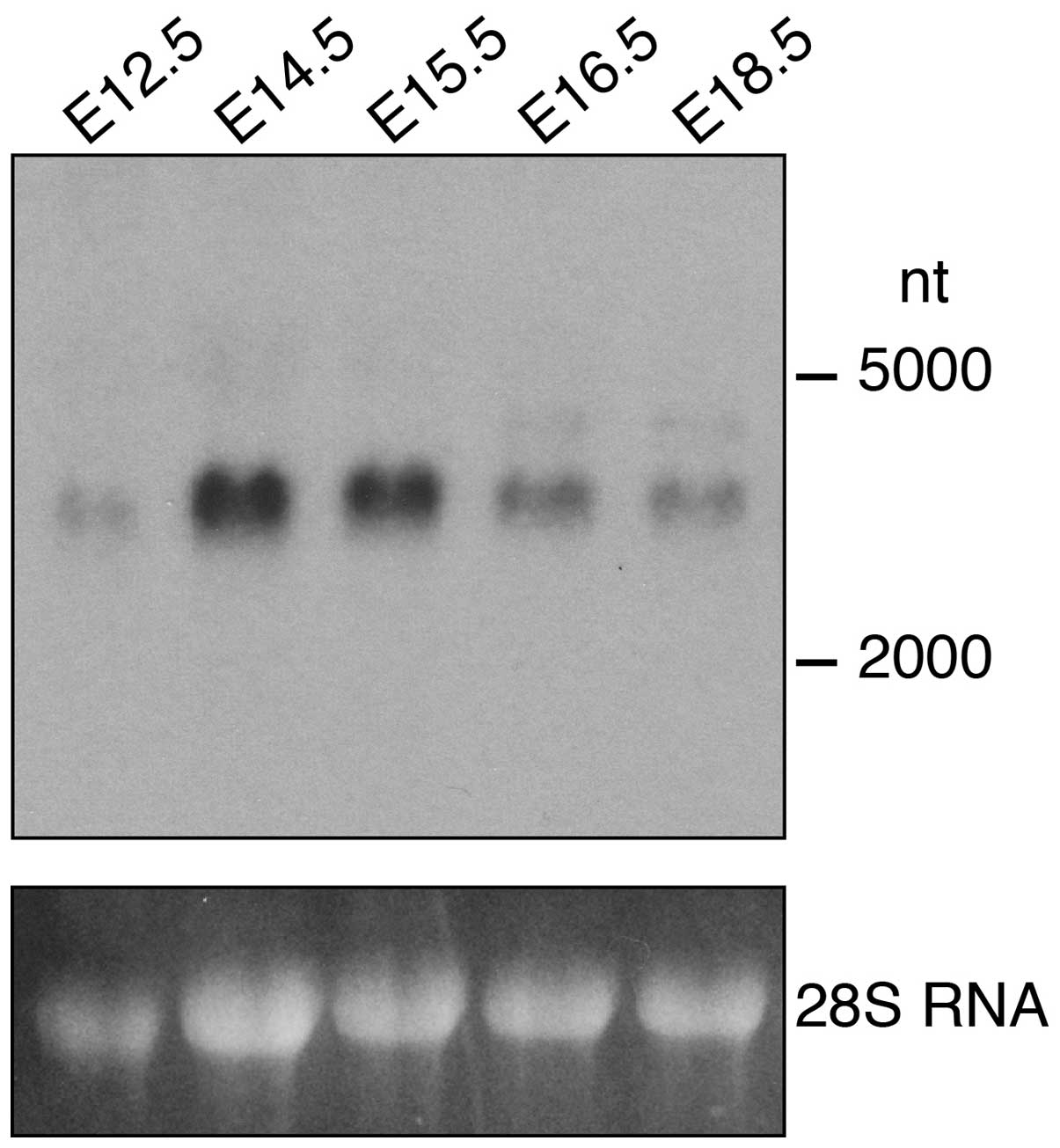
The expression of FgfrL1 was analyzed on a separate
northern blot containing RNA from kidneys at five different
developmental stages (Fig. 2). A
band of 3,000 nt was observed consistent with the published size of
the mouse FgfrL1 mRNA (8,9). This band was barely detectable at
stage E12.5 but became clearly visible at E14.5 and E15.5 and
decreased thereafter. This result suggests that FgfrL1 is required
during the early stages of kidney development when renal vesicles
and comma- and s-shaped bodies develop (3,4).
Analysis of Fgfr expression by in situ
hybridization
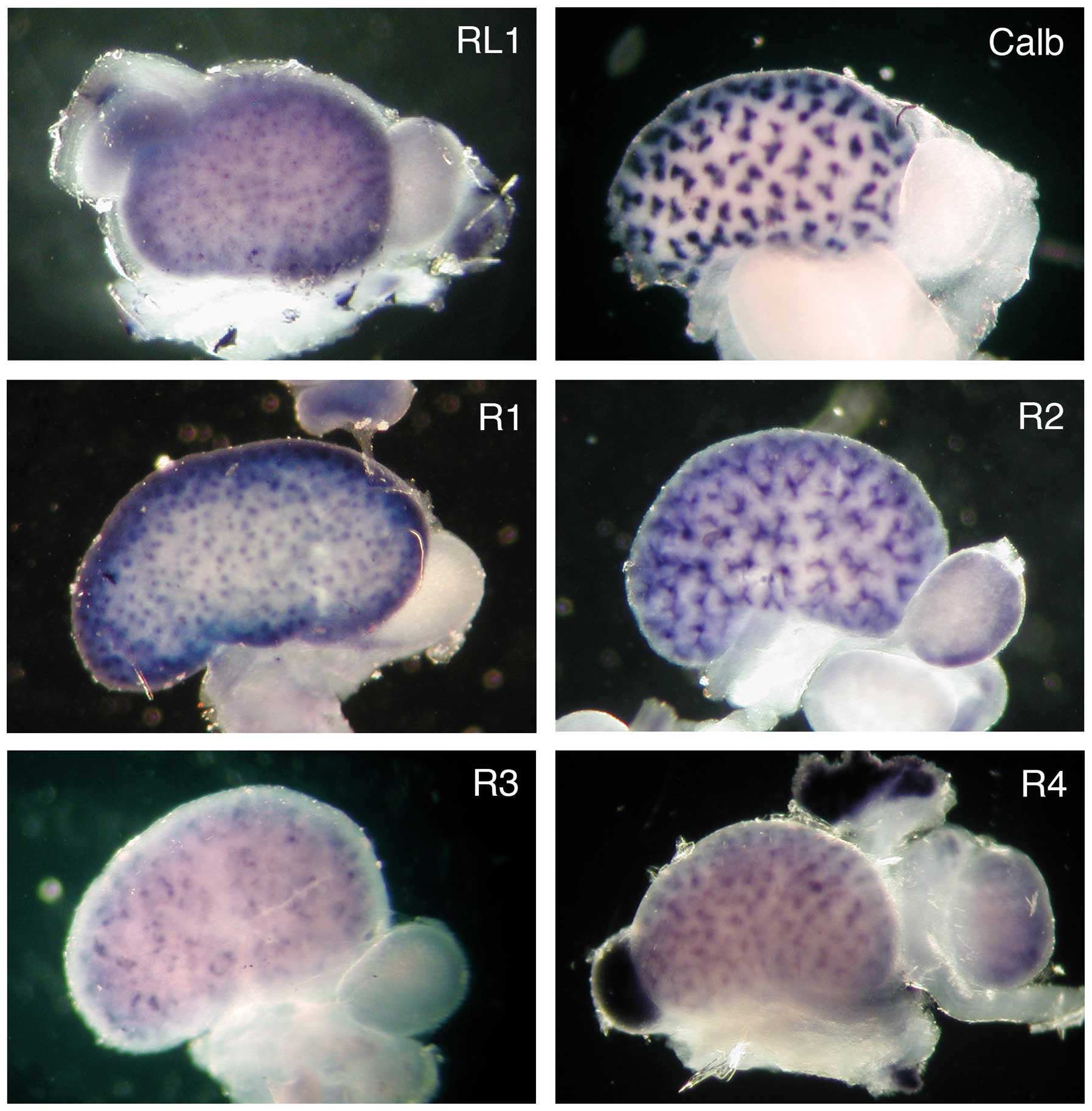
The spacial distribution of the FgfrL1 mRNA was
compared with the distribution of the four classical receptors by
whole-mount in situ hybridization of entire kidneys at stage
E15.5 (Fig. 3). The cDNA
sequences that had been used above for the northern blotting
experiment were utilized for the preparation of biotinylated
riboprobes. The hybridization of a control kidney with a probe for
calbindin yielded a pattern of clover leaf-like structures
consistent with the expression of this marker gene in all ureteric
buds. Hybridization of a kidney with a probe for FgfrL1 produced a
completely different pattern. A fine punctate or dotted
distribution was observed, which was consistent with expression of
the FgfrL1 gene in renal vesicles and nascent nephrogenic
structures. Hybridization with a probe for Fgfr1 produced a very
similar pattern, suggesting that the expression of Fgfr1 and FgfrL1
overlapped to a large extent. Hybridization with a probe for Fgfr2
revealed a pattern resembling the distribution of calbindin.
However, the signal of Fgfr2 was more diffuse, particularly at the
ureteric tips, suggesting that Fgfr2 expression was not confined
exclusively to the ureteric bud, but occurred to some extent also
in nascent nephrons. The signals obtained with the probes for Fgfr3
and Fgfr4 were extremely weak. They both revealed distributions
that were more complex than the above-mentioned expression
patterns.
The progression of the enzymatic reaction that was
used for development of the signal shown in Fig. 3 may provide a crude measure of the
relative expression levels of the four receptors. A robust signal
was obtained after 7 h with the probe for Fgfr1 and after 20 h with
that for Fgfr2, indicating that these two receptors are the major
Fgf signaling proteins in the developing kidney at E15.5. To obtain
a signal for Fgfr3 and Fgfr4, the color reaction had to proceed for
78 h, again suggesting that these receptors are expressed at
extremely low levels. The development time for FgfrL1 was 19 h,
comparable to that of Fgfr2.
Discussion
In this study, we used the whole-mount in
situ hybridization technique to show the spacial expression
pattern of five different Fgfrs in the developing mouse kidney.
This technique yields information about the three-dimensional
distribution of the corresponding mRNAs in the cortex of the
kidney, whereas in situ hybridization of thin sections would
show only a two-dimensional expression pattern. Whole-mount in
situ hybridization appears to be more sensitive than section
hybridization as it accumulates signals from the depth of the
kidney cortex, which offers an extra advantage when expression
levels are very low. In fact, section in situ hybridization
did not yield convincing data with probes for Fgfr3 and Fgfr4.
Moreover, the signal obtained with FgfrL1, the fifth Fgfr, proved
to be extremely weak by section hybridization and had to be
electronically enhanced for visualization in a previous publication
(14). By contrast, whole-mount
in situ hybridization with FgfrL1 yielded robust signals
that did not have to be enhanced.
In this study, we demonstrate that Fgfr1 and Fgfr2
are the major receptors of the Fgf signaling system expressed in
the early stages of developing kidneys. On the other hand, the
expression of Fgfr3 and Fgfr4 was very low, raising doubts about
the functional significance of these receptors during kidney
development. This observation is in accordance with the results
obtained from experiments using knockout mice (Table II). The disruption of the genes
for Fgfr3 and Fgfr4, either alone or in concert, did not produce
any altered phenotype in the mouse kidneys (19,20). Fgfr3 and Fgfr4 knockout animals
were viable and only Fgfr3-null mice showed an obvious phenotype
with abnormally long bones (19).
By contrast, the disruption of each of the receptors Fgfr1 and
Fgfr2 caused lethality at very early embryonic stages before
nephrogenesis was initiated, preventing the analysis of these genes
during kidney formation (21–24). Thus, a conditional targeting
approach had to be used for these cases. Interestingly, the
conditional disruption of Fgfr1 in the metanephric mesenchyme or in
the ureteric bud did not yield any overt phenotype (25–27). Likewise, the conditional deletion
of Fgfr2 in the metanephric mesenchyme did not produce any severe
alterations (25). Only the
conditional deletion of Fgfr2 in the ureteric bud produced animals
with abnormalities in ureteric branching, but the phenotype was
relatively mild and the animals were viable (26,27). The phenotype was more severe when
both receptors were deleted in concert. After the compound deletion
of Fgfr1 and Fgfr2, no metanephric mesenchyme formed, suggesting
that either Fgfr1 or Fgfr2 is required for nephrogenesis but that
the two receptors can substitute for one another (25,27). The observations made with the four
classical receptors are in sharp contrast with the targeted
disruption of the fifth receptor. The global deletion of FgfrL1
yielded animals that specifically lacked the metanephric kidneys
and died at birth (13). At
E10.5, the ureteric bud of these animals still invaded the
metanephric mesenchyme, but branching stopped after the T-state and
no renal vesicles were formed. It is intriguing to note that the
targeted disruption of FgfrL1, but not of any other Fgfr,
completely inhibited kidney development, although FgfrL1 is
expressed at very low levels in the kidneys.
 | Table IIPhenotype of embryos and kidneys after
the targeted inactivation of Fgfrs. |
Table II
Phenotype of embryos and kidneys after
the targeted inactivation of Fgfrs.
| Receptor | Mouse phenotype
(general KO) | Kidney phenotype
(conditional KO for Fgfr1, Fgfr2) |
|---|
| Fgfr1 | Lethal E7.5–E9.5 | Normal |
| Fgfr2 | Lethal E4–E10 | Abnormalities in
ureteric branching |
| Fgfr3 | Abnormally long
bones | Normal |
| Fgfr4 | Normal | Normal |
| FgfrL1 | Perinatally lethal
(P0–P1) | Absence of
nephrons |
In this study, we demonstrated that the spacial
distribution of FgfrL1 mRNA closely resembled that of Fgfr1, but
clearly differed from that of Fgfr2–Fgfr4. FgfrL1 cannot signal on
its own as it lacks the intracellular tyrosine kinase domain. Since
it still interacts with Fgf ligands, it is likely that it
indirectly modulates the Fgf signaling of another receptor. Thus,
we concluded that this other receptor may be Fgfr1, since only this
receptor shows a similar distribution in developing kidneys.
Originally we (7,16),
as well as others (8) have
speculated that FgfrL1 may act as a decoy receptor that binds and
neutralizes Fgf ligands. However, recent results obtained by DNA
microarray profiling suggest the opposite (14). The disruption of the FgfrL1 gene
in mice was not accompanied by the specific upregulation of any
target genes that are known to be controlled by Fgf signaling. Yet,
such an upregulation would be expected if FgfrL1 acted as a simple
decoy receptor. By contrast, we found that approximately 50 gene
products were significantly downregulated upon the disruption of
FgfrL1 expression, including wingless-type MMTV integration site
family member 4 (Wnt4), dickkopf 1 (Dkk1), early growth response 1
(Egr1), Fgf8 and LIM homeobox 1 (Lhx1) (14). It is therefore likely that FgfrL1
acts as a positive regulator of Fgf signaling, rather than as a
decoy receptor, at least in the kidneys.
It has been demonstrated that the developing kidneys
of mice express Fgfs 1, 7, 8, 9, 10, 12 and 20 (5). Of these ligands, FgfrL1 appears to
interact only with Fgf8 (16).
One mechanism of action may therefore be that FgfrL1 binds to Fgf8
and serves as a co-receptor, presenting this ligand to Fgfr1. The
activation of Fgfr1 would then lead to downstream signaling events
that ultimately allow the survival of cells in the induced
metanephric mesenchyme and the inhibition of apoptosis. In fact,
the phenotypes of FgfrL1- and Fgf8-null mice are intriguingly
similar (13,17,18). In both animal models, the
development of nephrons is inhibited and increased apoptosis is
observed in the metanephric mesenchyme. Another possibility may be
that FgfrL1 is involved in the conversion of the induced
metanephric mesenchymal cells into renal epithelial cells by
controlling the alignment of mesenchymal cells. We have previously
demonstrated that FgfrL1 can serve as an adhesion molecule if
coated on bacterial plastic dishes (28). Moreover, it promotes cell-cell
fusion if expressed at the surface of HEK293 cells and if mixed
with CHO cells (29). It is
possible that this cell fusion activity simply represents the
ultimate stage of very tight cell-cell adhesion. FgfrL1 may
therefore control the condensation of the metanephric mesenchyme
around the ureteric tips by bringing together mesenchymal cells in
an epithelial-like manner. The mechanisms by which Fgfr1 and Fgf8
are involved in this process are not yet fully elucidated. Further
experiments are required to differentiate between all the
possibilities outlined above.
Acknowledgements
This study was supported by grants from the Swiss
National Science Foundation (31003A-143350) and the Helmut Horten
Foundation.
Abbreviations:
|
FGF
|
fibroblast growth factor (human)
|
|
Fgf
|
fibroblast growth factor (mouse)
|
|
FGFR
|
fibroblast growth factor receptor
(human)
|
|
Fgfr
|
fibroblast growth factor receptor
(mouse)
|
|
FGFRL1
|
fibroblast growth factor receptor-like
protein 1
|
References
|
1
|
Itoh N: The Fgf families in humans, mice,
and zebrafish: their evolutional processes and roles in
development, metabolism, and disease. Biol Pharm Bull.
30:1819–1825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beenken A and Mohammadi M: The FGF family:
biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Costantini F and Kopan R: Patterning a
complex organ: branching morphogenesis and nephron segmentation in
kidney development. Dev Cell. 18:698–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendry C, Rumballe B, Moritz K and Little
MH: Defining and redefining the nephron progenitor population.
Pediatr Nephrol. 26:1395–1406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown AC, Adams D, de Caestecker M, Yang
X, Friesel R and Oxburgh L: FGF/EGF signaling regulates the renewal
of early nephron progenitors during embryonic development.
Development. 138:5099–5112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiedemann M and Trueb B: Characterization
of a novel protein (FGFRL1) from human cartilage related to FGF
receptors. Genomics. 69:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trueb B, Zhuang L, Taeschler S and
Wiedemann M: Characterization of FGFRL1, a novel FGF receptor
preferentially expressed in skeletal tissues. J Biol Chem.
278:33857–33865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sleeman M, Fraser J, McDonald M, Yuan S,
White D, Grandison P, Kumble K, Watson JD and Murison JG:
Identification of a new fibroblast growth factor receptor, FGFR5.
Gene. 271:171–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiedemann M and Trueb B: The mouse Fgfrl1
gene coding for a novel FGF receptor–like protein. Biochim Biophys
Acta. 1520:247–250. 2001.PubMed/NCBI
|
|
10
|
Trueb B: Biology of FGFRL1, the fifth
fibroblast growth factor receptor. Cell Mol Life Sci. 68:951–964.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baertschi S, Zhuang L and Trueb B: Mice
with a targeted disruption of the FgfrL1 gene die at birth due to
alterations in the diaphragm. FEBS J. 274:6241–6253. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Catela C, Bilbao-Cortes D, Slonimsky E,
Kratsios P, Rosenthal N and Te Welscher P: Multiple congenital
malformations of Wolf-Hirschhorn syndrome are recapitulated in
Fgfrl1 null mice. Dis Model Mech. 2:283–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerber SD, Steinberg F, Beyeler M,
Villiger PM and Trueb B: The murine FgfrL1 receptor is essential
for the development of the metanephric kidney. Dev Biol.
335:106–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerber SD, Amann R, Wyder S and Trueb B:
Comparison of the gene expression profiles from normal and FgfrL1
deficient mouse kidneys reveals downstream targets of FgfrL1
signaling. PLoS One. 7:e334572012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trueb B, Amann R and Gerber SD: Role of
FGFRL1 and other FGF signaling proteins in early kidney
development. Cell Mol Life Sci. 70:2505–2518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steinberg F, Zhuang L, Beyeler M, Kälin
RE, Mullis PE, Brändli AW and Trueb B: The FGFRL1 receptor is shed
from cell membranes, binds fibroblast growth factors (FGFs) and
antagonizes FGF signaling in Xenopus embryos. J Biol Chem.
285:2193–2202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grieshammer U, Cebrian C, Ilagan R, Meyers
E, Herzlinger D and Martin GR: FGF8 is required for cell survival
at distinct stages of nephrogenesis and for regulation of gene
expression in nascent nephrons. Development. 132:3847–3857. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perantoni AO, Timofeeva O, Naillat F,
Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF and
Lewandoski M: Inactivation of FGF8 in early mesoderm reveals an
essential role in kidney development. Development. 132:3859–3871.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colvin JS, Bohne BA, Harding GW, McEwen DG
and Ornitz DM: Skeletal overgrowth and deafness in mice lacking
fibroblast growth factor receptor 3. Nat Genet. 12:390–397. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinstein M, Xu X, Ohyama K and Deng CX:
FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in
the murine lung. Development. 125:3615–3623. 1998.PubMed/NCBI
|
|
21
|
Deng CX, Wynshaw-Boris A, Shen MM,
Daugherty C, Ornitz DM and Leder P: Murine FGFR-1 is required for
early postimplantation growth and axial organization. Genes Dev.
8:3045–3057. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi TP, Harpal K, Henkemeyer M and
Rossant J: Fgfr-1 is required for embryonic growth and mesodermal
patterning during mouse gastrulation. Genes Dev. 8:3032–3044. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Weinstein M, Li C, Naski M, Cohen
RI, Ornitz DM, Leder P and Deng C: Fibroblast growth factor
receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8
and FGF10 is essential for limb induction. Development.
125:753–765. 1998.PubMed/NCBI
|
|
24
|
Arman E, Haffner-Krausz R, Chen Y, Heath
JK and Lonai P: Targeted disruption of fibroblast growth factor
(FGF) receptor 2 suggests a role for FGF signaling in
pregastrulation mammalian development. Proc Natl Acad Sci USA.
95:5082–5087. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poladia DP, Kish K, Kutay B, Hains D, Kegg
H, Zhao H and Bates CM: Role of fibroblast growth factor receptors
1 and 2 in the metanephric mesenchyme. Dev Biol. 291:325–339. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Kegg H, Grady S, Truong HT,
Robinson ML, Baum M and Bates CM: Role of fibroblast growth factor
receptors 1 and 2 in the ureteric bud. Dev Biol. 276:403–415. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bates CM: Role of fibroblast growth factor
receptor signaling in kidney development. Am J Physiol Renal
Physiol. 301:F245–F251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rieckmann T, Kotevic I and Trueb B: The
cell surface receptor FGFRL1 forms constitutive dimers that promote
cell adhesion. Exp Cell Res. 314:1071–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steinberg F, Gerber S, Rieckmann T and
Trueb B: Rapid fusion and syncytium formation of heterologous cells
upon expression of the FGFRL1 receptor. J Biol Chem.
285:37704–37715. 2010. View Article : Google Scholar : PubMed/NCBI
|