Introduction
White matter of the central nervous system (CNS) is
named after its glistening pale appearance visible on the cut
surface of the brain and spinal cord. This feature is attributed to
the myelin sheath, which is a multiple laminar membrane structure
wrapped around the axons and plays an important role, as an
insulate cable-coat, in maintaining the fast saltatory conduction
of action potentials along the nerve fibers (1). The lipid-rich feature of the myelin
sheath makes it very fragile to the environmental changes in the
brain, such as metabolic disorders and aging. Due to the
sensitivity of myelin sheath to senescence, it is thought to be one
of the major targets of brain aging (2). Age-related alterations in the myelin
sheath were identified in the light microscopy study of Lintl and
Braak (3), who observed a
reduction in the intensity of hematoxylin staining in the aged
myelin. Since then, increasing evidence has suggested the existence
of a progressive myelin breakdown in the aged nervous system. Many
neuroimaging studies, especially the diffusion tensor imaging and
the voxel-based morphometric analysis, showed an obvious
age-related decline in human brain white matter integrity, in other
words a reduction in the structural integrity of the myelin sheath
(4,5). Ultrastructure electron microscopy
studies demonstrated that age-related myelin breakdown could be
identified in both the central and peripheral nervous system of
humans, non-human primates, rodents and other species (6,7).
Furthermore, the alterations of the myelin sheath in an aging brain
are considered to be involved in the development of age-related
disorders, such as Alzheimer’s and Parkinson’s disease (8,9).
However, the mechanism of these age-related alterations in the
myelin sheath has not been fully understood.
Myelin-associated proteins, such as myelin basic
protein (Mbp) and proteolopid protein (Plp), are key components of
the myelin sheath and play a very important role in maintaining the
integrity of myelin multi-lamellar structure. However,
investigations concerning the expression level of myelin proteins
in aged CNS are limited, for both humans and animals. On the other
hand, there is general agreement that astrocyte and microglia
undergo activation with age in rodents, monkeys and even humans.
These two types of glial cells become hypertrophic in an aged
brain, express more senescence markers and occasionally increase
their numbers (10,11). Nevertheless, it is not fully
understood whether this activation of astrocytes and microglia
contributes to the age-related myelin breakdown. In the present
study, we investigated the age-related expression of myelin
proteins using a whole CNS mapping. The downregulation of Mbp and
myelin oligodendrocyte glycoprotein (Mog) was observed as a general
alteration of the myelin sheath in aging. Age-related activation of
astrocytes and microglia was also analyzed. The negative
correlation between the myelin protein breakdown and glial cell
activation was identified.
Materials and methods
Animals
Male Sprague-Dawley albino rats ranging in age from
postnatal month (PNM) 2–26 were used. The rats were divided into 4
groups of 7 animals each, aged PNM2, 5, 18 and 26. All the animals
were provided by the Laboratory Animal Center of the Fourth
Military Medical University (FMMU). Animals were housed in plastic
boxes at 22–26°C on a 12-h light/dark cycle. Food and water were
provided ad libitum. Drinking water was chlorinated and
acidified to pH 6.2–6.8. Cages were cleaned with autoclaving and
their environment was maintained strictly steady. The experimental
protocols were approved by the Institutional Animal Care and Use
Committee of FMMU (permit no.: SCXK2007-007). The present study was
performed in accordance with the National Institute of Health Guide
for the Care and Use of Laboratory Animals (NIH Publications no.
80-23) revised in 1996.
Immunohistochemistry
Three rats from each group were anesthetized with
pentobarbital sodium (100 mg/kg, i.p.; Sigma, St. Louis, MO, USA)
and transcardially perfused with 0.9% saline, followed by 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Whole
brain and spinal cord were dissected, post-fixed at 4°C for an
additional 90 min in the same fixative solution and transferred
into 30% sucrose 0.01 M PBS overnight. Serial 40 μm cryo-sections
(coronal brain sections and transversal spinal sections) were cut
on CM1900 freezing microtome (Leica, Mannheim, Germany), incubated
for 1 h with 0.05% Triton X-100 and then with 10% goat serum in
0.01 M PBS at room temperature, followed by incubation with the
primary antibody at 4°C overnight. The primary antibodies were
mouse anti-myelin oligodendrocyte glycoprotein (anti-Mog) (1:200,
Millipore, Billerica, MA, USA), rabbit anti-Mbp (1:200, Sigma),
rabbit anti-glial fibrillary acidic protein (anti-GFAP) (GFAP,
1:400, Millipore) and rabbit anti-ionized calcium-binding adaptor
molecule 1 (anti-Iba1) (Iba1, 1:300, Wako, Osaka, Japan).
Diamidino-phenyl-indole (DAPI, 1 μg/ml, Sigma) was administered for
at least 30 sec to stain the cell nucleus for fluorescence
labeling. Micrographic images were obtained under the microscope
(Olympus, Tokyo, Japan). GFAP or Iba1-positive cells were counted
by Image-pro Plus software in the rat cerebral cortex S1, dentate
gyrus (DG) of hippocampal formation, corpus callosum, internal
capsule, spinal cord dorsal horn and spinal cord posterior
funiculus, respectively. The number of DAPI-labeled cell nuclei in
the corpus callosum and posterior funiculus were also obtained
through Image-pro Plus software. Cell densities were calculated in
the prementioned regions.
Western blotting
Four rats from each group were anesthetized with
pentobarbital sodium (100 mg/kg, i.p.). The whole brain, without
the cerebellum, was dissected, homogenized and lysed in an ice-cold
RIPA lysis buffer (Applygen Technologies Inc., Beijing, China) of
1% NP-40, 0.1% sodium dodecyl sulphate (SDS) in 50 mM Tris-HCl, pH
7.4 and containing protease inhibitor. Protein concentrations were
determined by the BCA protein assay kit (Thermo Scientific,
Pittsburgh, PA, USA). After 10 min of incubation at 98°C with
SDS-PAGE buffer, protein samples (40 μg) were separated by 10%
SDS-PAGE gel and transferred to a polyvinylidene difluoride
membrane with a semi-dry transfer system (Bio-Rad, Hercules, CA,
USA). Membranes were blocked at room temperature for 1 h with 5%
milk in PBS containing 0.2% Tween-20, followed by incubation with
antibody. Mouse anti-Mog (1:500, Millipore), rabbit anti-Mbp
(1:500, Sigma), rabbit anti-GFAP (1:800, Millipore) and rabbit
anti-Iba1 (1:600, Wako) antibodies served as the primary
antibodies. Mouse anti-rat β-tubulin antibody (1:8,000, Sigma) was
used as an internal control. The membranes were developed with
Pierce ECL Western blotting substrate kit (Thermo Scientific) and
the signals were captured with FluorChem® FC2 (Alpha
Innotech, San Leandro, CA, USA). Scanned images were analyzed by
Quantity One 1-D analysis software (Bio-Rad).
Statistical analysis
Data are expressed as means ± SEM. Differences among
groups were compared using one-way ANOVA, followed by Bonferroni’s
or Fisher’s PLSD post-hoc analysis when appropriate. Correlation
between the expression levels of GFAP, Iba1, Mbp and Mog based on
western blot analysis results was analyzed by Pearson’s
coefficients and multiple comparisons were corrected by using
Bonferroni’s analysis. Multiple regression analyses, with stepwise
procedure, were performed to investigate the determinants of the
myelin protein decline in aged rat brains. All analyses were
performed using the SPSS statistical package. Statistical
significance was indicated by P<0.05.
Results
Age-related down-regulation of myelin
proteins
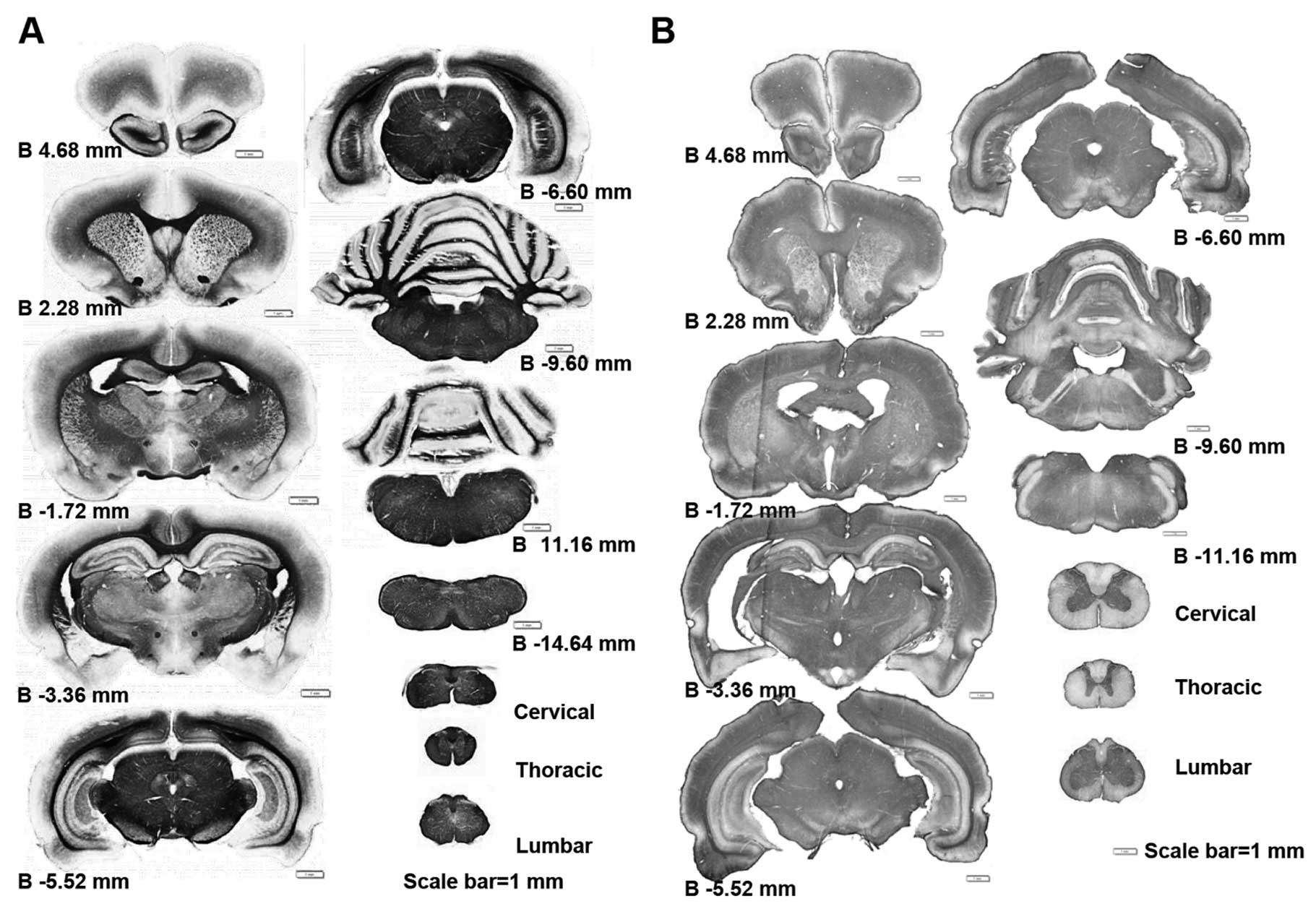
Using whole CNS immunohistochemical mapping, we
observed a marked downregulation in the expression of myelin
associated proteins, such as Mog and Mbp (Fig. 1 and data not shown). This
alteration of myelin protein expression is universal in the CNS, it
appears from the prefrontal section of the brain to the medulla and
can be identified in any segment of the spinal cord (Fig. 1). This myelin-protein
downregulation does not occur only in very old rat CNS. In fact,
this alteration was first identified in PNM5 in our study (data not
shown). As Mog and Mbp are usaully highly expressed in the white
matter, the reduction of Mog and Mbp levels was much more obvious
in the white matters of the corpus callosum in the brain and the
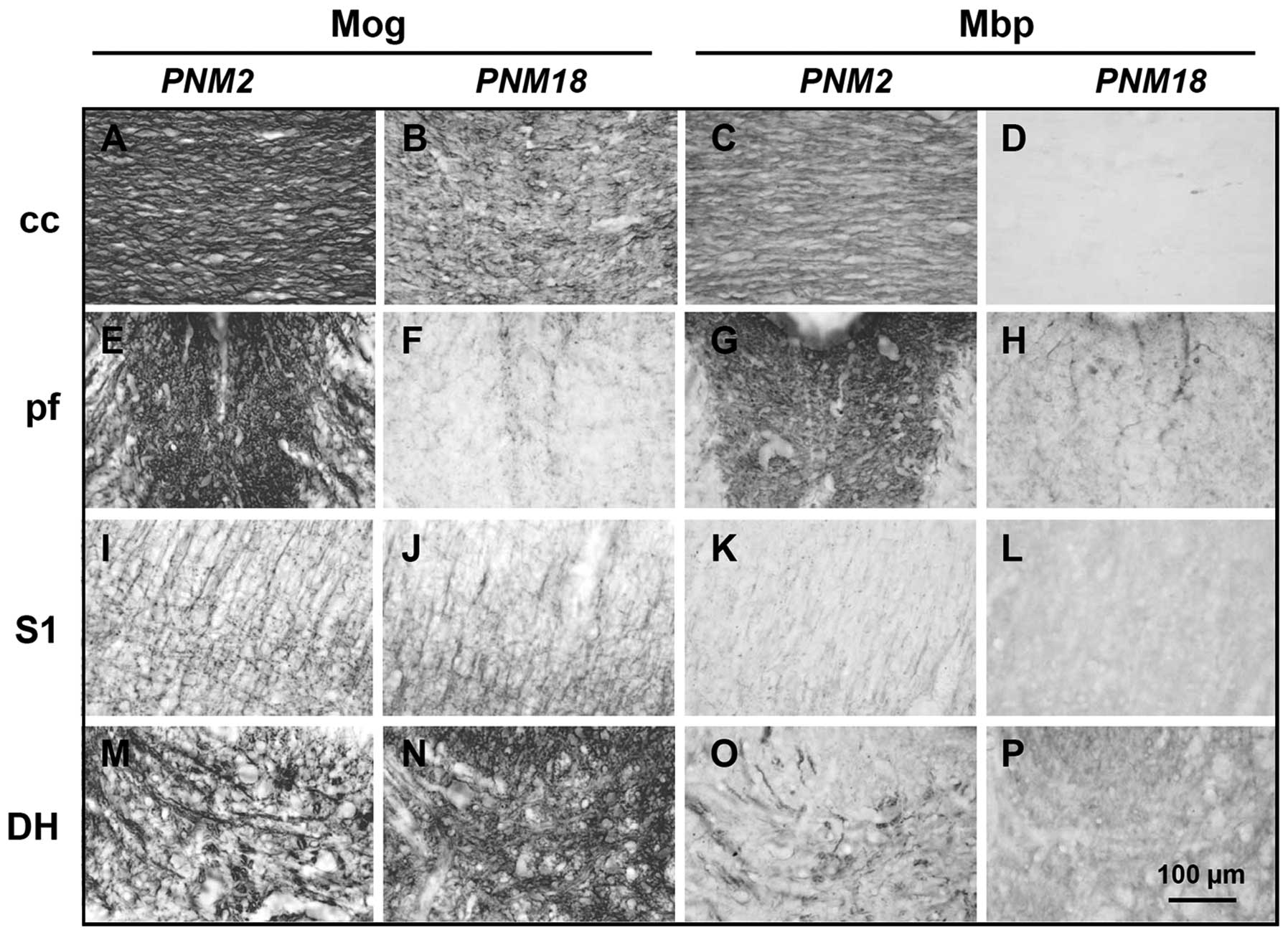
posterior funiculus in the spinal cord (Fig. 2A–H). Immunohistochemical data
showed that Mog and Mbp were highly expressed and distributed
parallel to the nerve fibers in PNM2 corpus callosum. However, the
expression of Mog markedly decreased and had a twisted and
corrugated appearance in PNM18 corpus callosum (Figs. 2A and B, and 3A and B), while Mbp was hardly
detectable (Fig. 2C and D). The
posterior funiculus of the spinal cord in PNM18 lost most response
to Mog and Mbp antibodies and only some Mog- and Mbp-positive cells
were present (Figs. 2E–H, and
3D and E). The changes of myelin
protein expression were a little complicated in the grey matters.
Compared with the PNM2 rat CNS, the amount of Mbp seems to be at
the same level in the primary somatosensory cortex (Fig. 2K and L) and spinal cord dorsal
horn (Fig. 2O and P) of PNM18
rat. Specifically, Mog was expressed even more in the aged
somatosensory cortex (Fig. 2I and
J) and dorsal horn (Fig. 2M and
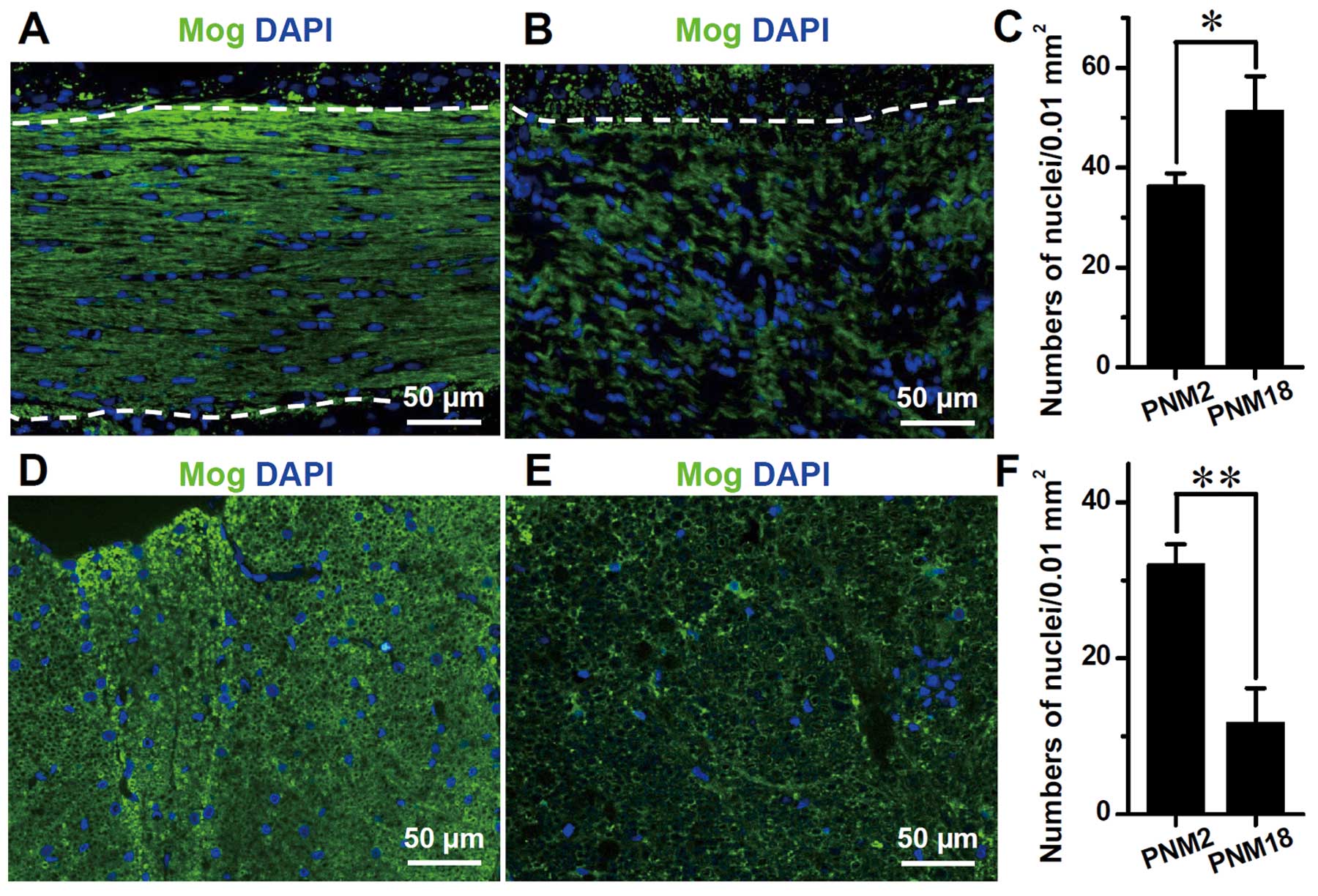
N). DAPI labeling showed that the number of glial cells,
including oligodendrocytes, astrocytes and microglia, increased
significantly in the aged corpus callosum. Contrary to the
arrangement of glial nuclei in rows between nerve fibers in PNM2,
the glial nuclei in PNM18 corpus callosum were distributed more
irregularly and some of them were grouped together as in a nest
(Fig. 3B). By contrast, the glial
nuclei decreased significantly in the posterior funiculus of aged
spinal cords (Fig. 3E and F).
However, linked nuclei could still be observed (Fig. 3E).
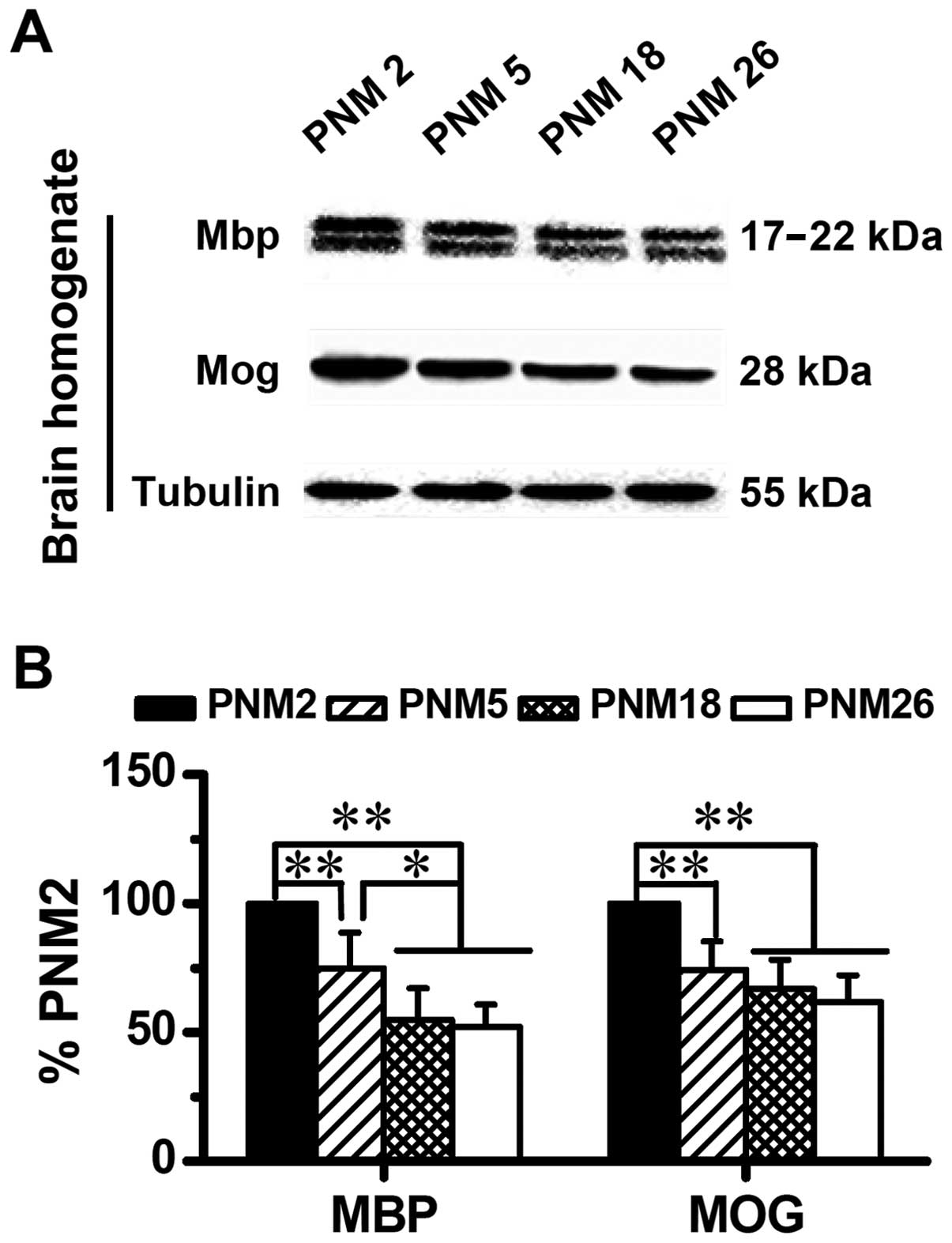
Similar to the results of immunohistochemical
mapping and immunofluorescence, results of western blotting also
showed an age-related alteration in the expression of Mog and Mbp
in the rat brain (Fig. 4). The
downregulation of Mog and Mbp is age-dependent. Compared with PNM2,
the expression level of Mog and Mbp in PNM26 decreased almost 50%
(Fig. 4B).
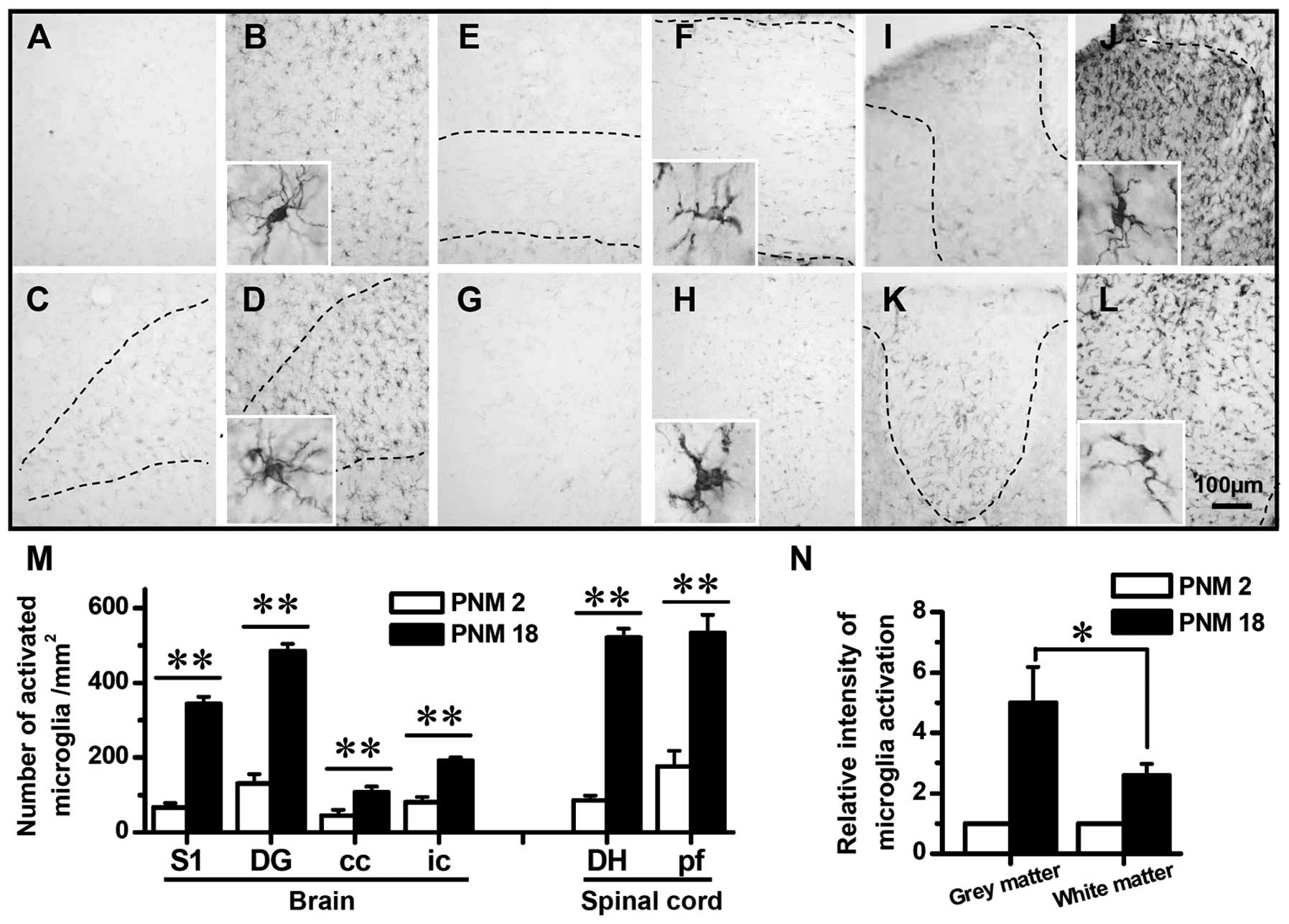
Activation of astrocytes and microglia in
aged rat CNS
The immunohistochemical mapping of the rat CNS was
conducted to investigate the activation of astrocytes and
microglia. GFAP and Iba1 were used as activation markers of
astrocytes and microglia, respectively. The mapping results showed
a significant age-related increase in the expression of GFAP and
Iba1 (Figs. 5 and 6). In PNM2 rat, the pale appearance of
tissue slides indicated the weakness in CNS response to anti-GFAP
or Iba1 antibody. The upregulation of GFAP and Iba1 was first
detected in PNM5 and was also observed in PNM18 and PNM26 rat CNS.
The number of GFAP-positive cells markedly increased in the grey
and white matters of PNM18 rat (Fig.
5A–L and N). In the primary somatosensory cortex and dentate
gyrus of hippocampal formation of PNM18, a 10-fold increase in the
density of astrocyte was identified (Fig. 5A–D and M). Similarly, the GFAP
labeling indicated that the density of astrocyte increased three to
seven times in aged corpus callosum, internal capsule and spinal
cord posterior funiculus (Fig. 5E–H
and K–M). Of note, although the number of GFAP-positive cells
increased in the white matters of PNM18 rat, the non-cell-shaped
staining was greater in PNM2 white matter (Fig. 5E–H, K and L). This dark background
of white matter in PNM2 is probably caused by the staining of a
neuropil that was formed by inactivated astrocytes. On the other
hand, the activation of microglia was also significant in the grey
and white matters (Fig. 6) and
the upregulation of Iba1 in aged grey matter was greater than that
in white matter (Fig. 6N).
Iba1-positive microglia were hardly detectable in the whole CNS of
PNM2, which yielded the pale and achromatous appearance of slides.
However, the number of Iba1-labeled microglia was markedly elevated
in PNM18 brain and spinal cord. A 2- to 6-fold increase in the
density of activated microglia was identified in the cerebral
cortex, dentate gyrus of hippocampal formation, corpus callosum,
internal capsule, spinal cord dorsal horn and spinal cord posterior
funiculus, respectively (Fig.
6A–M).
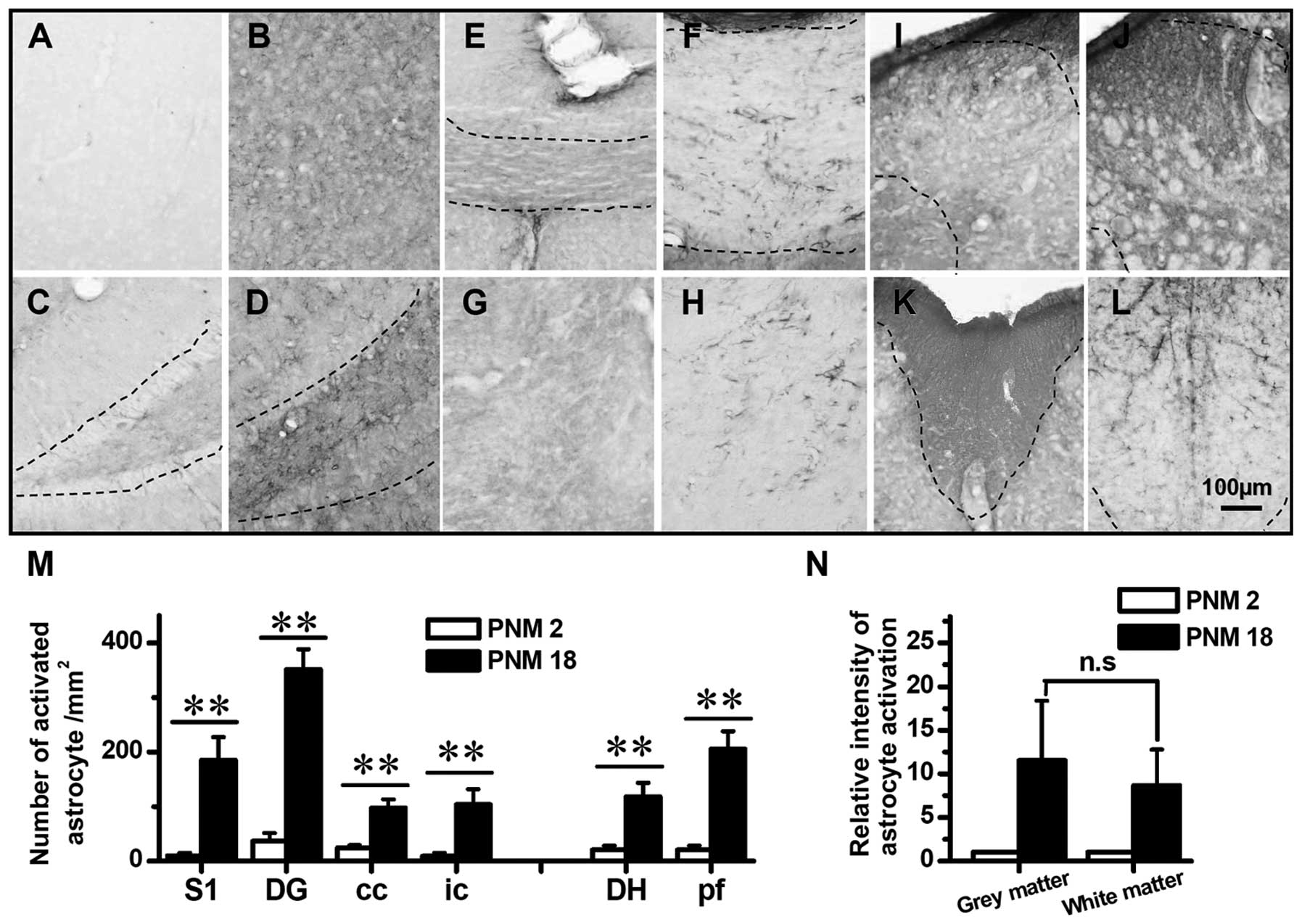 | Figure 5Astrocyte activated in aged rat brain
and spinal cord. (A), (C), (E), (G), (I) and (K) show the GFAP
immunochemistry staining in PNM2 rat cerebral cortex S1, dentate
gyrus of hippocampal formation, corpus callosum, internal capsule,
spinal cord dorsal horn and spinal cord posterior funiculus,
respectively. (B), (D), (F), (H), (J) and (L) show the astrocyte in
postnatal month 18 (PNM18) rat brain and spinal cord positions
mentioned above. The number of activated astrocyte per square
millimeter in young and aged rat brains and spinal cords are shown
in (M). (N) shows the relative intensity of astrocyte activation in
aged rats (normalized by comparing with PNM2 number of activated
astrocyte per square millimeter) within the grey and white matters,
respectively. Scale bar, 100 μm; *P<0.05;
**P<0.01 compared with PNM2; Error bars: ± SEM. |
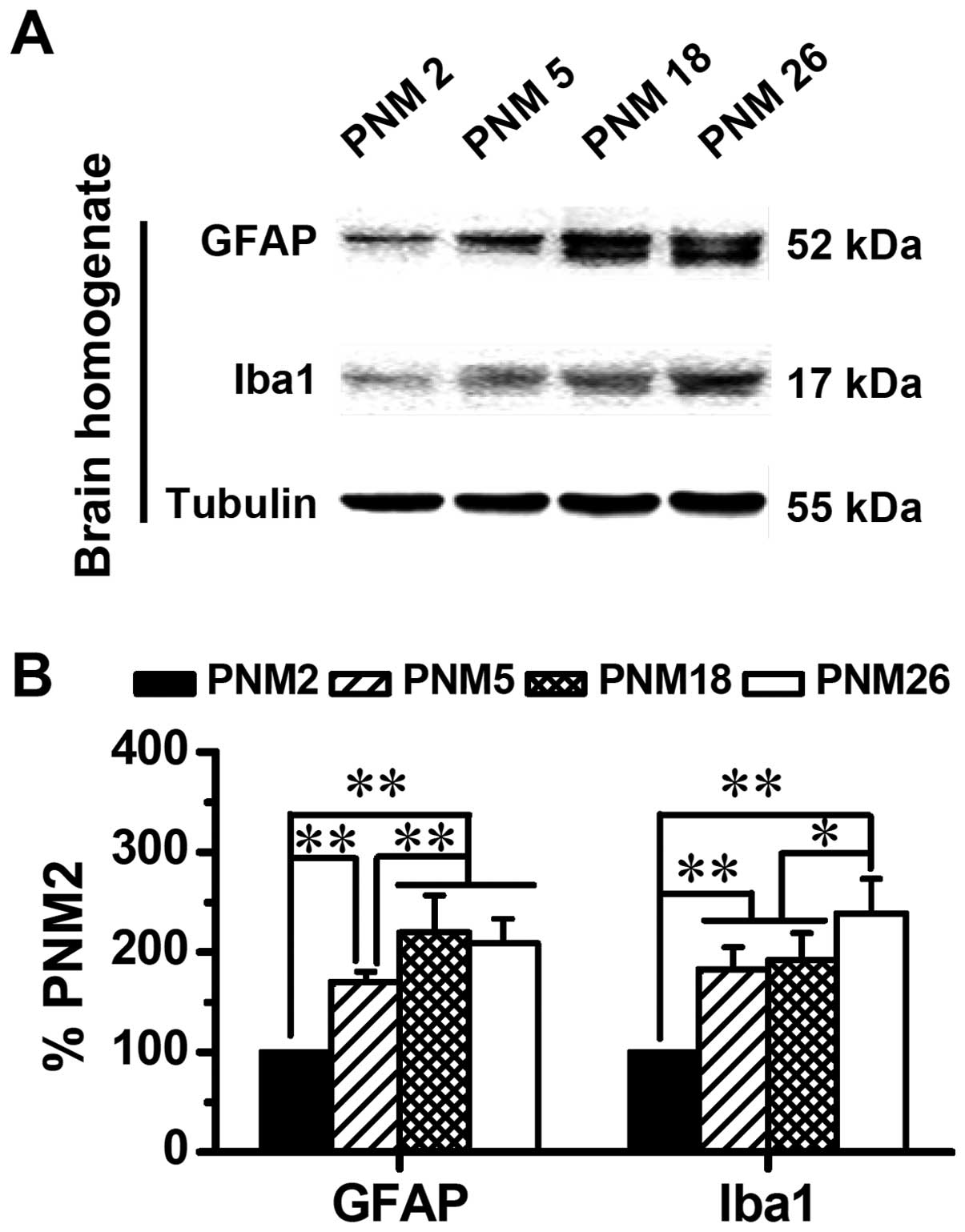
Western blotting data showed an age-related
accumulation of GFAP and Iba1 in the brain (Fig. 7). The expression of GFAP and Iba1
increased progressively with age, which doubled the levels of GFAP
and Iba1 in PNM18 and PNM26 rat brains compared with those in PNM2
(Fig. 7B).
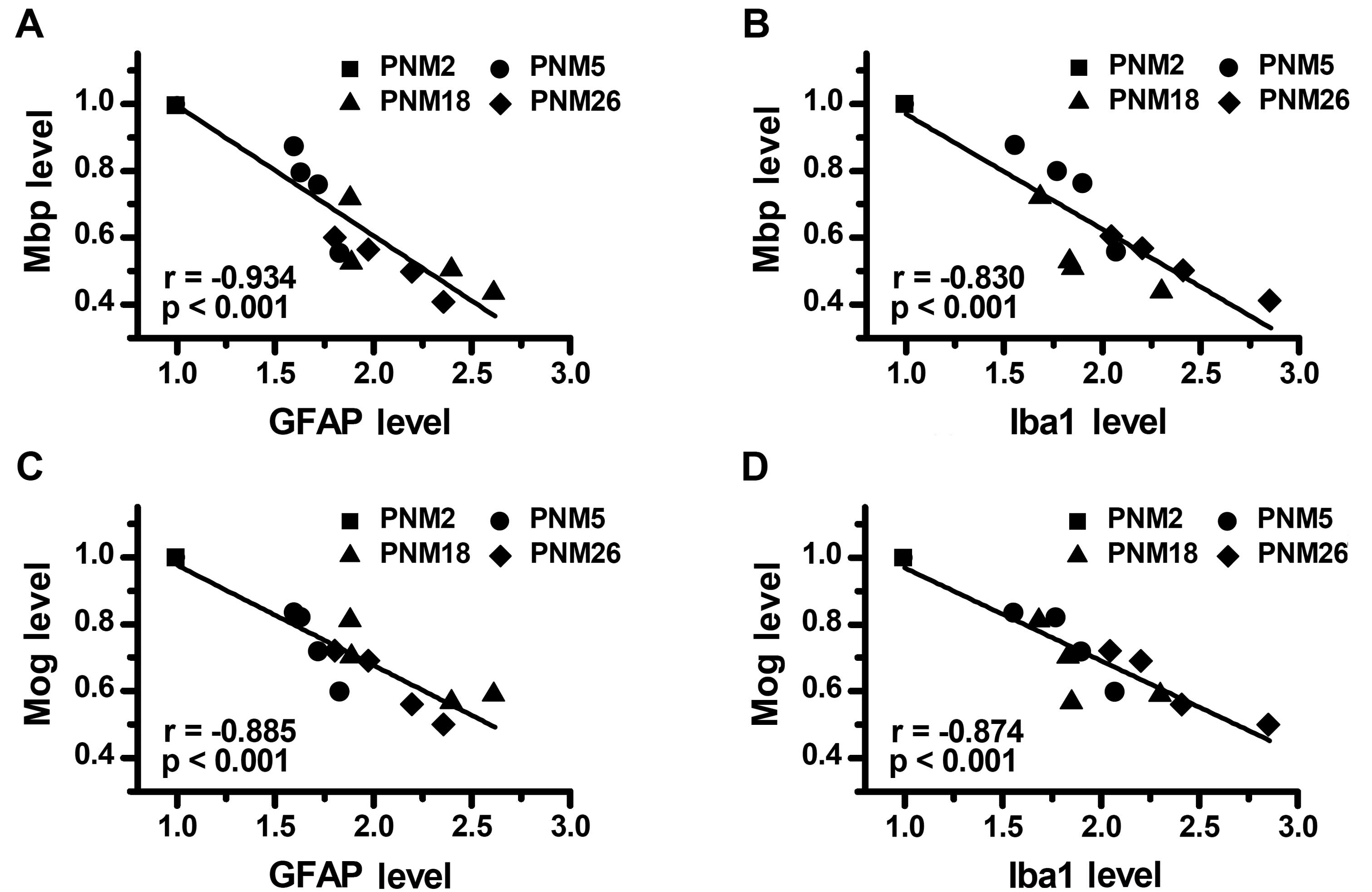
Correlation between age-related glial
activation and myelin protein decline in aging
Considering that age-dependence was present both in
the reduction of myelin proteins and the increase of glial markers,
we evaluated whether these two alterations were correlated with
each other. The main findings of the correlational analyses were
that: i) the downregulation of Mbp showed a significant negative
correlation with the expression of GFAP (Fig. 8A) and Iba1 (Fig. 8B) in the brain; ii) the level of
expression of Mog in the brain was negatively correlated with the
concentration of GFAP (Fig. 8C)
and Iba1 (Fig. 8D), respectively.
Stepwise multiple regression analysis, with the expression levels
of GFAP and Iba1 as independent variables and the expression levels
of Mog and Mbp as dependent variables, identified the concentration
of GFAP and Iba1 in the brain as independent factors for predicting
the expression level of Mog (Adj. R-square = 0.861, P<0.001) and
Mbp (Adj. R-square = 0.848, P<0.001). These data indicated that
the upregulation of GFAP and Iba1, in other words, the activation
of astrocytes and microglia, may contribute to the decline of
myelin proteins in the aging process.
Discussion
Although there is evidence to show the effects of
normal aging on the myelin sheath in human and animals,
investigations have focused on the limited age-related changes in
the expression of myelin proteins in CNS. Different experimental
methods, different animals and different regions of interest in CNS
could induce confusing and even conflicting results (12–14). Therefore, a comprehensive detailed
investigation of the expression of myelin proteins in aged CNS is
still needed. In the current study, a universal reduction in the
expression of Mog and Mbp was identified in the CNS of aged rats
through well-controlled mapping. This downregulation of myelin
proteins was consistent with Mbp, P0 decline in peripheral nerves
(15,16) and was likely to be a reason
accounting for the reduced hematoxylin staining of aged myelin,
which was identified in early studies on humans (3).
Mbp is one of the major abundant proteins in the
myelin sheath, comprising >30% of the total proteins in most
species (17). Mbp is an
extrinsic protein localized exclusively at the cytoplasmic surface
in the major dense line of myelin sheath and is believed to be the
principal protein stabilizing the major dense line of CNS myelin
(17). Splits of the lamellae at
the major dense line were found to be the most common morphological
alterations in aged myelin sheath after a series of ultrastructural
investigations (11,18) and could probably be due to the
age-related Mbp decrease observed in our study. On the other hand,
Mog is a CNS myelin protein of great neuroimmunological interest,
but its function remains to be determined (19). As yet, no other investigations
have shown this age-related decrease of Mog. However, what changes
were induced by this downregulation of Mog in aged CNS remain a
mystery and should be further examined.
DAPI labeling of the nuclei in the corpus callosum
indicated a significant increase of the glial cell number in aging
brains. Considering that oligodendrocytes were the major cell type
in corpus callosum, we assumed that elevation in the number of
DAPI-labeled nuclei was mainly attributed to the increase of
oligodendrocytes. This is consistent with the ultrastructural
studies that were administered in the cerebral and visual cortexes
of aging primates in the studies by Peters and Sethares and by
Peters et al (20,21). The aggregation of glial nuclei in
the aged corpus callosum, observed in our study (Fig. 3), and the similar pair- or
row-together of oligodendrocytes, which was found in aged monkey
cortexes using electron microscopy (20,22), suggested that oligodendrocytes
proliferate with age and this proliferation leads to an increase in
their number. However, DAPI labeling also indicated a decrease in
the number of glial cells in aged spinal cord posterior funiculus.
Further studies are needed to determine whether the increase in the
number of oligodendrocytes in aging is universal in CNS.
Results of the immunohistochemical mapping and
western blotting indicated a significant upregulation of GFAP and
Iba1 in aged rat CNS. This increase of age-related GAFP (mRNA and
protein expression levels) and hypertrophy of astrocytes in aged
rodent brains were also identified in other studies (23,24). However, previous reports have
suggested that the number of astrocytes did not appear to increase
or increased slightly in number during normal aging (11,25), which was not consistent with the
significant age-related increase in the number of astrocytes in the
present study. The dark neuropil staining in the white matter,
which was observed in PNM2 in our study (Fig. 5E–H, K and L) suggested that the
inactive astrocytes expressed low levels of GFAP that were not
easily detected by GFAP immunohistochemistry. Therefore, we assumed
that the great changes of GFAP in our study were a good marker of
astrocyte activation, but could not be used to evaluate the
proliferation of astrocytes in aging.
On the other hand, Iba1 was widely used as a
microglial activation marker, since it is greatly upregulated in
activated microglia (26). Based
on Iba1 immunohistochemical staining, age-related activation of
microglia was identified in our study, which was also detected in
the brain of aged rats (27) and
primates (28) using other
microglial activation markers.
Our correlation analysis revealed that the
age-related decrease of Mbp and Mog was highly correlated with the
activation of astrocytes and microglia in aged rat CNS. As previous
attention was mostly focused on the interaction between
astrocytes/microglia and neurons, this negative correlation is
relatively novel. A decreased neuroprotective capacity of aged
astrocyte was also found by a previous in vitro study
(29). This compromised
neuroprotection of astrocytes, during aging, was believed to be
associated with the reduced nerve growth factors in aging, such as
FGF-2 and BDNF, which are released by astrocytes (30). Considering that sufficient nerve
growth factors are also needed in maintaining the integrity of the
myelin sheath (31), we assumed
that the reduced nerve growth factors in aging astrocytes may
contribute to the decline of myelin proteins. On the other hand,
chronic activated microglia and astrocytes can induce damage by
releasing highly toxic products, such as reactive oxygen
intermediates, inflammatory cytokines and complementary factors
(32). There is evidence showing
that myelin proteolysis, in aging, is linked to calpain-1 and the
complement system expressed in microglia (33). However, the exact mechanism of the
decrease of age-related myelin proteins and the contribution of
astrocytes and microglial activation in this decrease remain
unknown. Further studies must be performed to explore the links
between the downregulation of myelin proteins and activation of
astrocytes and microglia.
In summary, a significant decline of myelin proteins
in the whole CNS of aged rats was identified by immunohistochemical
staining and western blotting. The decrease in myelin proteins was
highly correlated with the age-related activation of astrocytes and
microglia. Mog and Mbp mapping could be used as a good model to
investigate the aging effects on myelin sheath in CNS. The
correlation of myelin breakdown and glial activation in aging is
able to provide new evidence concerning the connection of
inflammation and myelin breakdown mechanism in age-related
neurodegenerative diseases.
Acknowledgements
This study was supported by grants from the Major
State Basic Research Development Program of China (973 Program)
(no. 2011CB504100, 2013BAI04B04) and the National Natural Science
Foundation of China (no. 81171049).
References
|
1
|
Waxman SG: Conduction in myelinated,
unmyelinated, and demyelinated fibers. Arch Neurol. 34:585–589.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherin JE and Bartzokis G: Human brain
myelination trajectories across the life span: implications for CNS
function and dysfunction. Handbook of the Biology of Aging. Masoro
EJ and Austa SN: 7th edition. Academic Press; San Diego, CA: pp.
333–346. 2011, View Article : Google Scholar
|
|
3
|
Lintl P and Braak H: Loss of intracortical
myelinated fibers: a distinctive age-related alteration in the
human striate area. Acta Neuropathol. 61:178–182. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madden DJ, Bennett IJ, Burzynska A, Potter
GG, Chen NK and Song AW: Diffusion tensor imaging of cerebral white
matter integrity in cognitive aging. Biochim Biophys Acta.
1822:386–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhadelia RA, Price LL, Tedesco KL, et al:
Diffusion tensor imaging, white matter lesions, the corpus
callosum, and gait in the elderly. Stroke. 40:3816–3820. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verdu E, Ceballos D, Vilches JJ and
Navarro X: Influence of aging on peripheral nerve function and
regeneration. J Peripher Nerv Syst. 5:191–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peters A: The effects of normal aging on
myelinated nerve fibers in monkey central nervous system. Front
Neuroanat. 3:112009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartzokis G: Alzheimer’s disease as
homeostatic responses to age-related myelin breakdown. Neurobiol
Aging. 32:1341–1371. 2011.
|
|
9
|
Bohnen NI and Albin RL: White matter
lesions in Parkinson disease. Nat Rev Neurol. 7:229–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blasko I, Stampfer-Kountchev M, Robatscher
P, Veerhuis R, Eikelenboom P and Grubeck-Loebenstein B: How chronic
inflammation can affect the brain and support the development of
Alzheimer’s disease in old age: the role of microglia and
astrocytes. Aging Cell. 3:169–176. 2004.
|
|
11
|
Peters A: The effects of normal aging on
nerve fibers and neuroglia in the central nervous system. Brain
Aging: Models, Methods, and Mechanisms. Riddle DR: Boca Raton, FL:
CRC Press, Taylor & Francis; pp. 97–125. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sloane JA, Hinman JD, Lubonia M, Hollander
W and Abraham CR: Age-dependent myelin degeneration and proteolysis
of oligodendrocyte proteins is associated with the activation of
calpain-1 in the rhesus monkey. J Neurochem. 84:157–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing Y, Samuvel DJ, Stevens SM, Dubno JR,
Schulte BA and Lang H: Age-related changes of myelin basic protein
in mouse and human auditory nerve. PLoS One. 7:e345002012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciftci G, Yarim GF, Yarim M, et al: The
effects of aging on central nervous system steroid prophiles and
myelin basic protein in rats. Aging Clin Exp Res. 24:117–124.
2011.
|
|
15
|
Melcangi RC, Magnaghi V, Cavarretta I,
Martini L and Piva F: Age-induced decrease of glycoprotein Po and
myelin basic protein gene expression in the rat sciatic nerve.
Repair by steroid derivatives. Neuroscience. 85:569–578. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Melcangi RC, Magnaghi V and Martini L:
Aging in peripheral nerves: regulation of myelin protein genes by
steroid hormones. Prog Neurobiol. 60:291–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campagnoni AT and Campagnoni CW: Myelin
basic protein gene. Myelin Biology and Disorders. Lazzarini RA: 1.
Elsevier Academic Press; San Diego, CA: pp. 387–400. 2004
|
|
18
|
Peters A and Sethares C: Aging and the
myelinated fibers in prefrontal cortex and corpus callosum of the
monkey. J Comp Neurol. 442:277–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pham-Dinh D, Dautigny A and Linington C:
Myelin oligodendrocyte glycoprotein gene. Myelin Biology and
Disorders. Lazzarini RA: 1. Elsevier Academic Press; San Diego, CA:
pp. 469–489. 2004
|
|
20
|
Peters A and Sethares C: Oligodendrocytes,
their progenitors and other neuroglial cells in the aging primate
cerebral cortex. Cereb Cortex. 14:995–1007. 2004. View Article : Google Scholar
|
|
21
|
Peters A, Josephson K and Vincent SL:
Effects of aging on the neuroglial cells and pericytes within area
17 of the rhesus monkey cerebral cortex. Anat Rec. 229:384–398.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peters A: Age-related changes in
oligodendrocytes in monkey cerebral cortex. J Comp Neurol.
371:153–163. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goss JR, Finch CE and Morgan DG:
Age-related changes in glial fibrillary acidic protein mRNA in the
mouse brain. Neurobiol Aging. 12:165–170. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berciano MT, Andres MA, Calle E and
Lafarga M: Age-induced hypertrophy of astrocytes in rat supraoptic
nucleus: a cytological, morphometric, and immunocytochemical study.
Anat Rec. 243:129–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long JM, Kalehua AN, Muth NJ, et al:
Stereological analysis of astrocyte and microglia in aging mouse
hippocampus. Neurobiol Aging. 19:497–503. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ito D, Imai Y, Ohsawa K, Nakajima K,
Fukuuchi Y and Kohsaka S: Microglia-specific localisation of a
novel calcium binding protein, Iba1. Brain Res Mol Brain Res.
57:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogura K, Ogawa M and Yoshida M: Effects of
ageing on microglia in the normal rat brain: immunohistochemical
observations. Neuroreport. 5:1224–1226. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sloane JA, Hollander W, Moss MB, Rosene DL
and Abraham CR: Increased microglial activation and protein
nitration in white matter of the aging monkey. Neurobiol Aging.
20:395–405. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pertusa M, Garcia-Matas S, Rodriguez-Farre
E, Sanfeliu C and Cristofol R: Astrocytes aged in vitro show a
decreased neuroprotective capacity. J Neurochem. 101:794–805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blasco I, Humpel C and Grubeck-Loebenstein
B: Glial cells: astrocytes and oligodendrocytes during normal brain
aging. Handbook of the Neuroscience of Aging. Hof PR and Mobbs CV:
Elsevier Academic Press; San Diego, CA: pp. 47–52. 2009
|
|
31
|
de Groot DM, Coenen AJ, Verhofstad A, van
Herp F and Martens GJ: In vivo induction of glial cell
proliferation and axonal outgrowth and myelination by brain-derived
neurotrophic factor. Mol Endocrinol. 20:2987–2998. 2006.PubMed/NCBI
|
|
32
|
Godbout JP and Johnson RW: Age and
neuroinflammation: a lifetime of psychoneuroimmune consequences.
Immunol Allergy Clin North Am. 29:321–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duce JA, Hollander W, Jaffe R and Abraham
CR: Activation of early components of complement targets myelin and
oligodendrocytes in the aged rhesus monkey brain. Neurobiol Aging.
27:633–644. 2006. View Article : Google Scholar
|