Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common types of head and neck cancer in Asia, particularly in
Southeast Asia and China, with a high incidence rate of
approximately 20–50 cases per 100,000 individuals per year
(1). By contrast, NPC is rare in
the United States (apart from Alaska) and Western Europe, with an
incidence of <1/100,000 individuals. The accurate detection of
NPC is not easy due to its anatomical location. In conventional
clinical therapy, radiotherapy is the primary therapeutic approach,
while using radiotherapy combined with chemotherapy is recommended
for the treatment of advanced carcinoma (2). However, the 5-year survival rate is
extremely low. Treatment failure rates remain high due to the
development of drug resistance and distant metastasis.
Drug resistance is a major complication in cancer
chemotherapy. It accounts for the ineffectiveness of chemotherapy
in the majority of cancer patients (3). Resistance occurs when tumor cells do
not respond to anticancer drugs. Cisplatin (DDP) is clinically used
as adjuvant therapy for NPC in order to induce tumor cell death.
DDP induces cytotoxicity and/or apoptosis by forming DNA adducts or
by targeting proteins/enzymes and pathways (4–6).
However, the efficacy of DDP is often accompanied by
chemoresistance. The mechanisms through which NPC cells acquire
chemoresistance are unknown. Thus, a better understanding of the
mechanisms through which cells acquire resistance and knowledge of
the molecular alterations which induce or correlate with resistance
may lead to the development of novel therapeutic strategies for NPC
(7,8).
Recent evidence suggests that epithelial-mesenchymal
transition (EMT)-type cells play critical roles in chemoresistance.
Thus, the molecular basis of chemoresistance in relation to EMT is
currently an important focus of cancer research. EMT describes a
series of marked morphological changes, characterized by a
transition from an epithelial to a mesenchymal phenotype, leading
to increased motility and invasion (9). During the acquisition of EMT
characteristics, cells lose epithelial cell-cell junctions and
undergo actin cytoskeletal reorganization. The downregulation of
epithelial molecular markers, such as E-cadherin and β-catenin is
also observed, as well as an upregulation of mesenchymal molecular
markers, such as vimentin, fibronectin, α-smooth muscle actin
(α-SMA) and N-cadherin. An increase in the production of
transcription factors that repress E-cadherin expression, including
Twist, zinc finger E-box binding homeobox 1 (ZEB1), Snail and Slug
also occurs, as well as an increase in the activity of matrix
metalloproteinases (MMPs), such as MMP-2 and MMP-9, associated with
an invasive phenotype (10). The
process of EMT has also been shown to be important in conferring
drug resistance to cancer cells against conventional therapeutics.
Several chemotherapeutic drug-resistant cell lines established
in vitro, such as tamoxifen-resistant breast cancer
(11), paclitaxel-resistant
ovarian cancer (12),
oxaliplatin-resistant colorectal cancer and gemcitabine-resistant
pancreatic cancer (13) cell
lines have shown phenotypic changes consistent with EMT. These data
clearly provide strong evidence for linking chemoresistance to EMT.
However, DDP-resistant NPC cells have not been extensively
studied.
In the present study, we isolated DDP-resistant
cells from well-characterized NPC cell lines and determined that
they have numerous EMT-like properties. We demonstrate that
DDP-resistant cells are insensitive to DDP and have a strong
invasion and migration ability. The characterization of these
stable NPC cells may provide new insight into the phenotypic
changes associated with resistance to DDP.
Materials and methods
Reagents and antibodies
DDP was purchased from Qilu Pharmaceutical Co., Ltd.
(Jinan, China). RPMI-1640 medium, fetal bovine serum (FBS) and
phosphate-buffered saline (PBS) were purchased from Gibco-BRL
(Grand Island, NY, USA). Matrigel was purchased from BD Biosciences
(Bedford, MA, USA). We purchased
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
from Sigma Chemical Co. (Castle Hill, Australia). The antibodies
against Bcl-2, Bax, Puma and myeloid cell leukemia-1 (Mcl-1; 1:100)
were purchased from Beijing Biosynthesis Biotechnology Co., Ltd.
(Beijing, China). Primary antibodies against E-cadherin, β-catenin,
vimentin, fibronectin, MMP-9, Twist, Slug, Snail, ZEB1 (1:500) and
β-actin (1:2,000) were obtained from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA).
Cell lines and culture
The human NPC cell lines, HNE1, HNE1/DDP and CNE2,
were obtained from Sun Yat-Sen University (Guangzhou, China) and
maintained in our laboratory. The HNE1, HNE1/DDP and CNE2 cells
were cultured in RPMI-1640 medium containing 10% FBS, penicillin
(100 U/ml) and streptomycin (100 U/ml), in a humidified atmosphere
of 5% CO2 at 37°C. The RPMI-1640 medium for the HNE1/DDP
cells was also supplemented with 4 μmol/l DDP. All cell lines were
tested each month for mycoplasma contamination, used only at low
passage and regularly examined under a microscope for phenotypic
changes prior to use.
Cell growth assay
To investigate cell growth, we performed a cell
proliferation assay on cells (1×104 cells/well) plated
in 24-well Costar® plates (Corning Life Sciences,
Corning, NY, USA). The experiments were carried out for 168 h, and
the number of cells was counted every 24 h. Cells from 3 samples
were trypsinized and counted using a hemocytometer at the indicated
time points.
Morphological analysis
Cells were grown to 70% confluence in DDP-free
medium appropriate for the parental cell lines and in medium with
DDP for the DDP-resistant cell lines. They were visualised under a
light microscope (CKX41; Olympus, Tokyo, Japan). Digital images
were captured using a camera mounted to the microscope (cellSens
Entry; Olympus).
Cell viability assay
Cell viability was assessed by MTT assay. MTT is a
yellow tetrazolium dye responding to metabolic activity. In living
cells, reductase enzymes alter the MTT color from a light yellow to
deep blue, corresponding to formazan crystals. Briefly,
7×103 cells/well were plated in 96-well
Costar® culture plates in triplicate. Following 24 h of
incubation, the cells were treated with various concentrations of
DDP and subsequently incubated for 24, 48 and 72 h. At the end of
the incubation period, 20 μl MTT were added to achieve a final
concentration of 0.5 mg/ml. The cells were then incubated for a
further 4 h. The medium and MTT were then removed before 150 μl
dimethylsulfoxide (DMSO) was added to each well to dissolve the
crystals. The optical density (OD) of the formazan product was
determined at a 570 nm wavelength using a microplate reader
(Bio-Tek Instruments Inc., Winooski, VT, USA). Cell viability (%) =
(ODsample - ODblank)/(ODcontrol -
ODblank) ×100. The IC50 value, defined as the
drug concentration required to reduce cell survival to 50%, was
determined by the relative absorbance of MTT. All experiments were
performed in triplicate.
Wound healing assay
Cell migration was assessed using a wound healing
assay. Cells were plated in 6-well plates (Corning Life Sciences)
at 5×105 cells/well and allowed to grow to 90%
confluence. The cells were scraped with a sterile micropipette tip
to create a gap of standard width. To remove non-adherent cells,
the plates were rinsed gently with medium twice prior to
incubation. The wound closure was monitored for 24 h at ×100
magnification. The wound areas were observed under an inverted
microscope and imaged at the appropriate fields to calculate the
healing percentages. Each experiment was performed in
triplicate.
Invasion and migration assay
The ability of the cells to pass through filters was
measured using a Transwell Boyden chamber system (Corning Life
Sciences) containing a polycarbonate filter (6.5 mm in diameter, 8
μm pore size). The membrane undersurface was coated with 50 μl of
Matrigel, mixed with RPMI-1640 serum-deprived medium at a 1:8
dilution and subsequently applied to the top side of the filter for
the cell invasion assay. By contrast, the filter was not coated for
the cell migration assay. The cells were resuspended in
serum-deprived medium. Subsequently, 200 μl of the cell suspension
(5×104 cells/well) were added to the upper chamber,
while 600 μl RPMI-1640 medium containing 10% FBS were added to the
lower chamber and served as a chemoattractant. The system was
incubated for 24 h at 37°C. The cells that did not migrate or
invade after 24 h were removed from the upper face of the filters
by scrubbing with a cotton swab. The membrane was then fixed with
4% formaldehyde for 15 min at room temperature and stained with
0.5% crystal violet for 15 min. Finally, 5 visual fields were
randomly selected from each membrane and photographed under a light
microscope at ×200 magnification. The number of migrating or
invading cells was then counted and analyzed to determine
statistically significant differences. Each condition was assayed
in triplicate. The experiments were performed independently at
least 3 times. The results were expressed as the number of cells
per field.
Quantitative reverse transcription PCR
(qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Aliquots (1 μg)
of RNA were reverse transcribed to cDNA (20 μl) using oligo(dT) and
M-MuLV reverse transcriptase (Fermentas Inc., Glen Burnie, MD, USA)
following the instructions of the manufacturer. One-fifth of the
cDNA was used as a template for PCR using the
SYBR®-Green PCR kit (Takara, Kyoto, Japan) in an ABI
StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA,
USA). The housekeeping gene, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), was selected as an internal control for each
experiment. The primers used in this study are presented in
Table I. The cycling conditions
were as follows: pre-denaturation at 95°C for 10 min, followed by
40 cycles at 95°C for 10 sec, at 57–60°C for 20 sec and at 72°C for
15 sec. The specificity of the amplification products was confirmed
by a melting curve analysis. All reactions were run in triplicate.
As a measure of relative change in expression between the parental
and resistant samples, ΔΔCt values were calculated and converted to
approximate fold change values (2−ΔΔCt).
 | Table IPrimers used for qRT-PCR. |
Table I
Primers used for qRT-PCR.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| MDR1 | F:
CCCATCATTGCAATAGCAGG
R: GTTCAAACTTCTGCTCCTGA | 157 |
| MRP | F:
AGGAGAGATCATCATCGATGG
R: GCCTTCTGCACATTCATGG | 235 |
| Bcl-2 | F:
CGACGACTTCTCCCGCCGCTACCGC
R: CCGCATGCTGGGGCCGTACAGTTCC | 319 |
| Bax | F:
TTTGCTTCAGGGTTTCATCC
R: CAGTTGAAGTTGCCGTCAGA | 246 |
| E-cadherin | F:
CATTTCCCAACTCCTCTCCTGGC
R: ATGGGCCTTTTTCATTTTCTGGG | 90 |
| β-catenin | F:
CACAAGCAGAGTGCTGAAGGTG
R: GATTCCTGAGAGTCCAAAGACAG | 146 |
| Vimentin | F:
AGATGGCCCTTGACATTGAG
R: TGGAAGAGGCAGAGAAATTC | 80 |
| Fibronectin | F:
CCCACCGTCTCAACATGCTTAG
R: CTCGGCTTCCTCCATAACAAGTAC | 264 |
| MMP-9 | F:
CGGAGTGAGTTGAACCAG
R: GTCCCAGTGGGGATTTAC | 118 |
| Snail | F:
CCAGCTCTCTGAGGCCAAGGATC
R: TGGCTTCGGATGTGCATCTTGAG | 108 |
| Slug | F:
CCCTGAAGATGCATATTCGGAC
R: CTTCTCCCCCGTTGTAGTTCTA | 116 |
| Twist | F:
TGCGGAAGATCATCCCCA
R: TCCATCCTCCAGACCGAGAA | 187 |
| ZEB1 | F:
GCACAACCAAGTGCAGAAGA
R: GCCTGGTTCAGGAGAAGATG | 141 |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG
R: AGGGGCCATCCACAGTCTTC | 258 |
Western blot analysis
The cells were plated in 6-well culture dishes
(Corning Life Sciences) at a density of 4×105
cells/well. Following 24 h of incubation, the cells were washed
with 1 ml PBS/well and harvested using trypsin. Harvested cells
were centrifuged and resuspended in lysis buffer. Following
incubation on ice for 30 min, the homogenate was centrifuged at
12,000 rpm for 30 min at 4°C. Protein concentrations were
determined using a bicinchoninic acid (BCA) assay (Beyotime
Institute of Biotechnology, Beijing, China). Subsequently, 40 μg of
protein were separated using 10–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred onto polyvinylidene difluoride (PVDF) membranes. The
blotted membranes were blocked with 5% skim milk for 2 h, and
probed with primary antibodies overnight at 4°C. The membranes were
washed and probed with secondary antibodies for 2 h. The membranes
were imaged with gel imaging equipment (Bio-Rad, Hercules, CA,
USA). β-actin was used as the loading control.
Establishment of DDP-resistant NPC
cells
The CNE2 cells were thoroughly washed with PBS and
transferred to RPMI-1640 medium, containing 10% FBS and
penicillin-streptomycin. To create stable NPC cells resistant to
DDP, the CNE2 cells were continuously exposed to DDP for >9
months. During this period, the medium was replaced every 3 days
and the cell cultures were passaged by trypsinization after 70–80%
confluency was reached. Gradually, the cells displayed resistance
to the growth-inhibitory properties of DDP. The DDP-resistant CNE2
cells were cultured in medium containing DDP for 3 additional
months prior to characterization. Through this process, we
generated a stable DDP-resistant cell line (CNE2/DDP cells).
Statistical analysis
All experiments were repeated at least 3 times. Data
are presented as the means ± SEM. The differences between mean
values were analyzed using the two-tailed Student's t-test. All
statistical analyses were performed using SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA). P-values <0.05 were considered to
indicate statistically significant differences.
Results
HNE1/DDP cells exhibit chemoresistance to
DDP
We first confirmed the resistance of NPC cells to
DDP by an MTT assay. The proliferation of NPC cells was inhibited
by various concentrations of DDP (Fig. 1A). Chemoresistance to DDP was
observed in the HNE1/DDP cells, as compared with the HNE1 cells.
The inhibitory effects of increasing DDP concentrations on
proliferation were more pronounced with HNE1 than with HNE1/DDP
cells. The IC50 values of HNE1/DDP and HNE1 cells were
29.04±2.82, 19.44±1.77 and 11.39±1.89 μmol/l and 6.84±1.59,
4.25±0.36 and 2.35±0.96 μmol/l for 24, 48 and 72 h, respectively
(Fig. 1B). Thus, DDP inhibited
the proliferation of the cells. In addition, the HNE1/DDP cells
were less sensitive than the HNE1 cells to treatment with DDP. The
HNE1/DDP cells proliferated less than the HNE1 cells under normal
culture conditions (Fig. 1C). By
western blot analysis, we then examined the protein expression
levels of multidrug resistance-associated protein (MRP), Mcl-1,
Bcl-2, Bax and Puma to further determine the chemoresistance
(Fig. 1D). The expression levels
of MRP, Mcl-1 and Bcl-2 were higher in the HNE1/DDP cells compared
with the HNE1 cells. Additionally, the expression levels of Bax and
Puma were lower in the HNE1/DDP cells. Moreover, the Bcl-2/Bax
ratio was lower in the HNE1 cells. qRT-PCR further revealed that
multidrug resistance (MDR)1, MRP and Bcl-2 were upregulated and
that Bax was downregulated in the HNE1/DDP cells (Fig. 1E). Based on these observations, we
concluded that the HNE1/DDP cells had acquired chemoresistance.
 | Figure 1HNE1/DDP cells exhibited
chemoresistance to cisplatin (DDP) compared with the parental HNE1
cells. (A) DDP inhibited the growth of nasopharyngeal carcinoma
(NPC) cells. HNE1 and HNE1/DDP cells were treated with DDP at
various concentrations (2, 4, 8, 16 and 32 μmol/l) for 24, 48 and
72 h, then 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was added to determine cell viability. Results are
expressed as a percentage of control levels. (B) IC50
values of HNE1/DDP and HNE1 cells for 24, 48 and 72 h (means ± SEM,
n=3, *P<0.05 compared with the parental HNE1 cells).
(C) Proliferation of HNE1 and HNE1/DDP cells in the normal culture
medium was examined by determination of the cell numbers at
different time points after cell seeding at the same number. (D)
Expression levels of multidrug resistance-associated protein (MRP),
Bcl-2, myeloid cell leukemia-1 (Mcl-1), Bax and Puma proteins in
the HNE1 and HNE1/DDP cells were determined by western blot
analysis. (E) Relative mRNA expression levels of MDR1, MRP, Bcl-2
and Bax genes in the HNE1 and HNE1/DDP cells were analyzed by
qRT-PCR (means ± SEM, n=3, *P<0.05 compared with
parental HNE1 cells). |
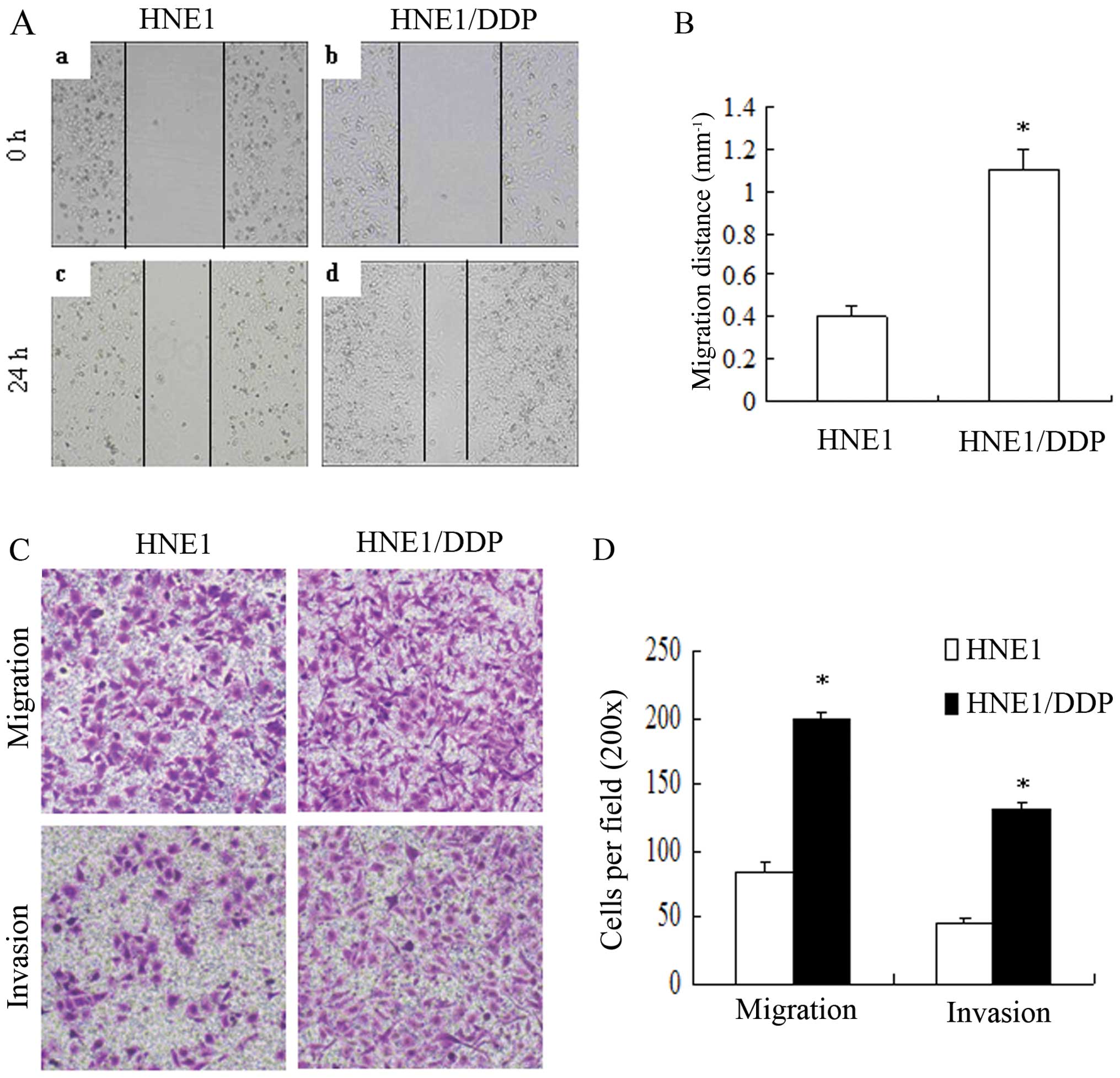
HNE1/DDP cells have an increased
migration and invasion potential in vitro
We then compared the HNE1/DDP and HNE1 cells for
various cellular functions. Since the acquisition of
chemoresistance generally correlated with an increased migration
and invasion ability in the progression of tumors, we measured the
migration and invasion ability of the HNE1/DDP and HNE1 cells using
wound healing and Transwell Boyden chamber assays. Wound healing
assays were performed to compare the migration potential of the
HNE1/DDP and the parental HNE1 cells. At 24 h, a 2.7-fold increase
in the number of HNE1/DDP cells migrating across the wound was
observed (P<0.05) (Fig. 2A and
B). In addition, we compared the migration and invasion
potential between the HNE1/DDP and HNE1 cells using Transwell
Boyden chamber assays. At 24 h, the HNE1/DDP cells showed a
2.2-fold increase in migration and a 2.8-fold increase in invasion
compared with the HNE1 cells (P<0.05) (Fig. 2C and D). Thus, the HNE1/DDP cells
had an increased migration and invasion potential as compared with
the parental HNE1 cells.
HNE1/DDP cells display morphological and
molecular changes consistent with EMT
Cell morphology was examined by microscopy at ×200
magnification (Fig. 3A). The
parental HNE1 cells showed an epithelioid, cobblestone appearance
that was rounded and contained few formations of pseudopodia. By
contrast, the phenotypic changes observed in the HNE1/DDP cells
included a loss of cell polarity, development of a spindle-shaped
morphology and increased formation of pseudopodia. To determine
whether the acquisition of resistance to DDP induced specific
molecular changes consistent with EMT, western blot analysis and
qRT-PCR were performed and revealed that the expression of
epithelial markers, such as E-cadherin and β-catenin, was reduced
in the HNE1/DDP cells compared with the HNE1 cells. The expression
of mesenchymal markers, such as vimentin and fibronectin, was
higher in the HNE1/DDP cells (P<0.05) (Fig. 3B and C). Moreover, the mRNA and
protein levels of MMP-9 were higher in the HNE1/DDP cells compared
with the HNE1 cells (P<0.05) (Fig.
3B and C). Furthermore, the mRNA and protein levels of the
EMT-related transcription factors, Snail, Slug, Twist and ZEB1,
were higher in the HNE1/DDP cells compared with the HNE1 cells
(P<0.05) (Fig. 3D and E).
Based on these observations, HNE1/DDP cells were considered to have
acquired a mesenchymal phenotype.
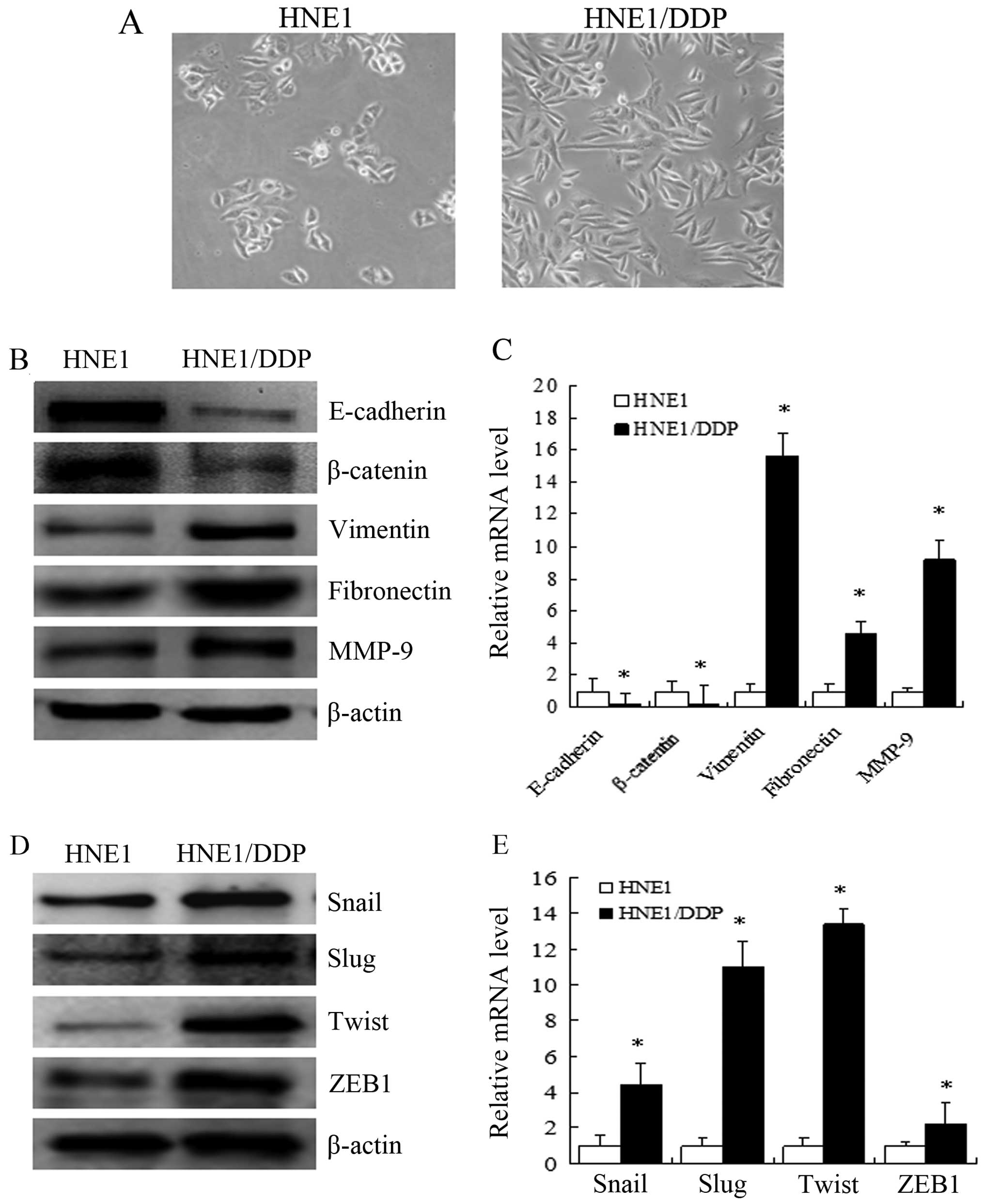 | Figure 3HNE1/DDP cells exhibit morphological
and molecular changes consistent with epithelial-mesenchymal
transition (EMT). (A) Cell morphology was observed by microscopy at
×200 magnification. The parental HNE1 cells showed an epithelioid,
cobblestone rounded appearance with limited formation of
pseudopodia. By contrast, the phenotypic changes observed in the
HNE1/DDP cells included a loss of cell polarity, causing a
spindle-shaped morphology and an increased formation of
pseudopodia. (B) Expression levels of the EMT-related proteins,
E-cadherin, β-catenin, vimentin, fibronectin and matrix
metalloproteinase (MMP)-9, were determined by western blot
analysis. The downregulation of E-cadherin and β-catenin and
upregulation of vimentin, fibronectin and MMP-9 was observed in the
HNE1/DDP cells. (C) mRNA expression levels of E-cadherin,
β-catenin, vimentin, fibronectin and MMP-9 genes were analyzed by
qRT-PCR (means ± SEM, n=3, *P<0.05 compared with the
parental HNE1 cells). (D) Increased protein levels of the
EMT-related transcription factors, Snail, Slug, Twist and zinc
finger E-box binding homeobox 1 (ZEB1), were determined by western
blot analysis. (E) mRNA expression levels of the genes coding for
the EMT-related transcription factors, Snail, Slug, Twist and ZEB1,
were analyzed by qRT-PCR (means ± SEM, n=3, *P<0.05
compared with the parental HNE1 cells). |
CNE2 cells undergo an EMT-like
transformation induced by DDP
We further examined whether the occurrence of EMT
and the enhanced expression of EMT-related molecules were observed
in other NPC cell lines resistant to DDP. For this purpose, we
established a stable DDP-resistant NPC cell line (CNE2/DDP cells).
The CNE2/DDP cells were less sensitive than the CNE2 cells to
treatment with DDP (data not shown). As shown in Fig. 4A, the parental CNE2 cells showed
an epithelioid, cobblestone appearance, similar to the HNE1 cells.
By contrast, the morphology of the CNE2/DDP cells was mixed: they
showed an epithelioid, long, spindle/fibroblastic-like shape, along
with formation of pseudopodia and an unorganized growth pattern.
Furthermore, higher levels of mesenchymal markers, such as
vimentin, fibronectin and MMP-9, and lower levels of epithelial
markers, such as E-cadherin and β-catenin, were observed in the
CNE2/DDP cells compared with the parental CNE2 cells (P<0.05)
(Fig. 4B and C). In addition, the
mRNA and protein levels of the EMT-related transcription factors,
Snail, Slug, Twist and ZEB1, were higher in the CNE2/DDP cells
compared with the CNE2 cells (P<0.05) (Fig. 4D and E).
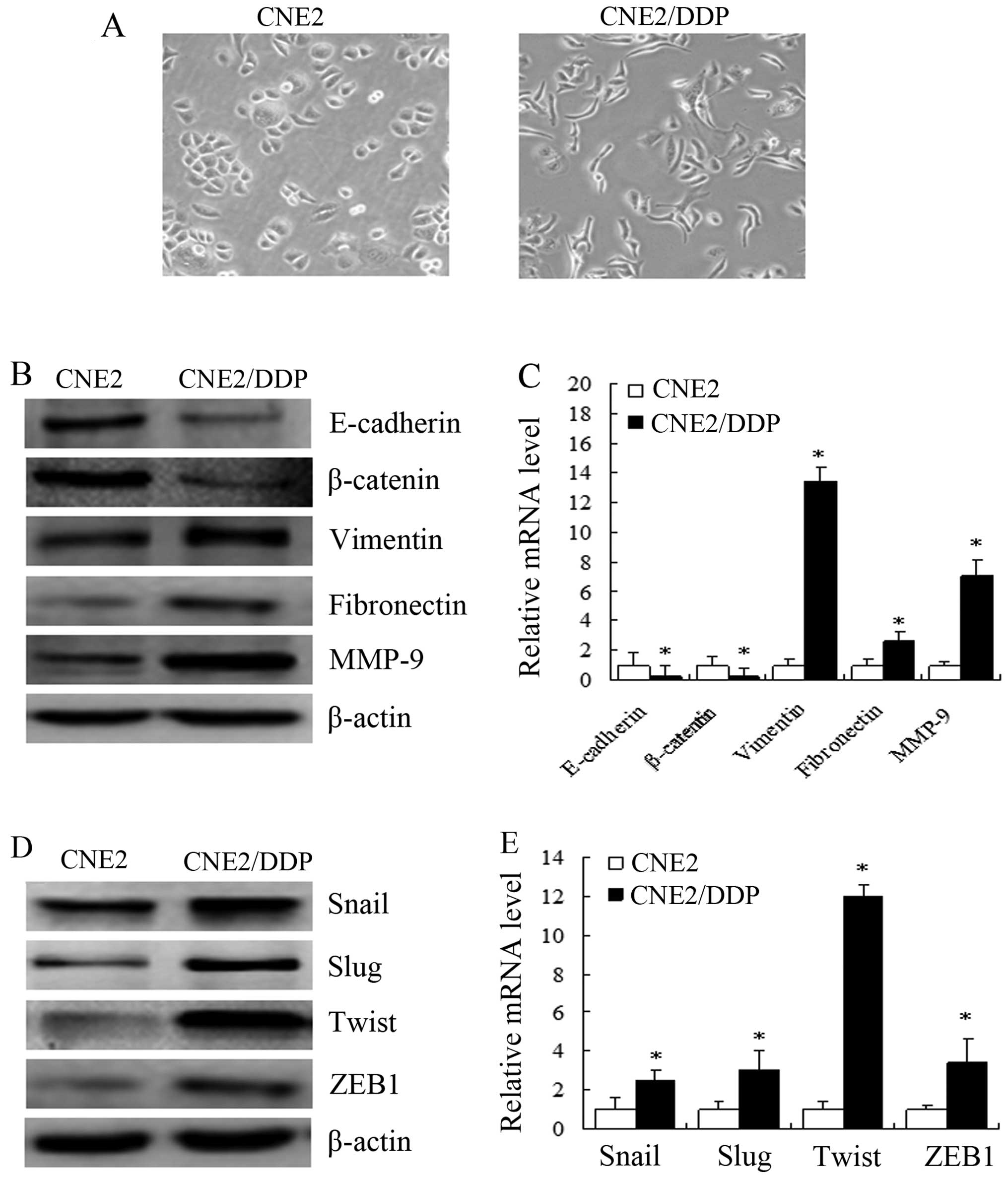 | Figure 4Cisplatin (DDP)-treated CNE2 cells
underwent an epithelial-mesenchymal transition (EMT)-like
transformation. (A) Cell morphology was observed by microscopy at
×200 magnification. Parental CNE2 cells showed an epithelioid,
cobblestone appearance, similar to HNE1 cells. By contrast, the
morphology of CNE2/DDP cells was of mixed type, showing an
epithelioid, long, spindle/fibroblastic pattern with pseudopodia
and an unorganized growth pattern. (B) Expression levels of the
EMT-related proteins, E-cadherin, β-catenin, vimentin, fibronectin
and matrix metalloproteinase (MMP)-9, were determined by western
blot analysis. The downregulation of E-cadherin and β-catenin and
upregulation of vimentin, fibronectin and MMP-9 was observed in
CNE2/DDP cells. (C) mRNA expression levels of E-cadherin,
β-catenin, vimentin, fibronectin and MMP-9 genes were analyzed by
qRT-PCR (means ± SEM, n=3, *P<0.05 compared with the
parental CNE2 cells). (D) Increased protein levels of the
EMT-related transcription factors, Snail, Slug, Twist and zinc
finger E-box binding homeobox 1 (ZEB1), were determined by western
blot analysis. (E) mRNA expression levels for genes coding for the
EMT-related transcription factors, Snail, Slug, Twist and ZEB1,
were analyzed by qRT-PCR (means ± SEM, n=3, *P<0.05
compared with parental CNE2 cells). |
Discussion
Chemotherapy with DDP is widely used in the
treatment of NPC patients; DDP as the most active and commonly used
drug is often applied for the treatment of distant metastasis or
advanced locoregional recurrence in NPC patients (14). However, the development of
resistance to DDP is a major limitation to its use in cancer
chemotherapy (15). The
biological mechanisms through which DDP leads to DDP resistance are
now beginning to be understood, and have the potential to provide
molecular targets for therapeutic intervention, improve the
prediction of response and allow the development of strategies to
overcome resistance to DDP. To further examine these mechanisms, we
selected DDP-resistant NPC cells, HNE1/DDP cells and parental HNE1
cells. Our results revealed that the HNE1/DDP cells have acquired
the characteristics of chemoresistance.
Previous studies of chemoresistance and metastasis
have been in general separately performed in the cancer research
field. Thus, little is known as to the association between
chemoresistance and cancer metastasis. Nevertheless, there are two
observations of interest: firstly, a number of tumor cells selected
for resistance to drugs have a greater metastatic potential
compared with non-resistant parental cells. Secondly, secondary
(greater metastatic potential) tumors are more resistant to
chemotherapeutic drugs than their primary counterparts in a number
of cases (16). Metastasis is a
complicated multi-step process which includes local invasion,
intravasation, survival during transport through the vasculature,
arrest at the capillaries, extravasation and, finally, outgrowth to
form macrometastatic tumors in distant organs (17,18). Clinically, an enhanced metastatic
ability and chemoresistance are frequently concurrent during the
therapeutic course of NPC, and seem to be linked in light of the
evolution towards increasingly malignant characteristics in tumors.
However, in several cases, no correlation has been observed between
chemoresistance and cancer metastasis (19). One study demonstrated that a
calcium-resistant human fibrosarcoma HI-1080 Cd-R cell variant was
developed, which was cross-resistant to DDP and more invasive than
parental HIT-1080 Cd-R cells, as shown by Transwell in vitro
assays. In addition, an increased expression of MMP-9 has been
observed in HT-1080 Cd-R cells (20). In the present study, we
demonstrated that the HNE1/DDP cells acquired an increased capacity
for migration and invasion in vitro during the development
of resistance to DDP. Furthermore, the mRNA and protein levels of
MMP-9 were increased in the HNE1/DDP cells. Therefore,
chemoresistance and metastasis are the main obstacles in the
current clinical management of NPC. Preventing, predicting and
inhibiting chemoresistance and metastasis in NPC is critical for
further improving the survival rate of patients with NPC.
Increasing evidence supports a molecular and
phenotypic association between chemoresistance and the acquisition
of the EMT-like phenotype in cancer cells. It is well established
that epithelial cells can acquire a mesenchymal phenotype through
fundamental and complex processes (21). While EMT has been widely studied
for its role in early development and cancer metastasis, it can
also influence the cellular ability to evade the effects of
platinum-based therapies (22).
EMT induces the transformation of a differentiated epithelial cell
into a mesenchymal cell with stem-like properties, and is
characterized by the loss of cell-to-cell adhesion, tight and gap
junctions, as well as the loss of cell polarity and increased
motility (23). Recent studies
have indicated that EMT may enhance the cancer development
progress, by showing that the epithelial-derived tumor cells can
change into a more preliminary mesenchymal phenotype that
facilitates motility and invasion (24,25). In this study, with a series of
experiments, we demonstrated that the acquisition of resistance to
DDP by NPC cells leads to morphological and molecular alterations
consistent with a change to a mesenchymal-like phenotype. We also
obtained molecular evidence that the EMT-like changes in the
DDP-resistant NPC cell line were associated with an increase in the
expression of the transcription factors, Snail, Slug, Twist and
ZEB1. These are the major transcription factors responsible for the
development of EMT, and bind directly to the E-boxes of the
E-cadherin promoter to repress the transcription of this gene
(26). However, the expression of
Twist in the DDP-resistant NPC cell line was significantly
increased at both the mRNA and protein levels. The increased
expression of Twist was responsible for the development of acquired
resistance to paclitaxel in NPC cells, and the ectopic expression
of Twist conferred resistance to microtubule-disrupting agents,
including paclitaxel (27).
Although details of the relevant mechanisms are still under
investigation, it is possible that the increased expression of
Twist is involved in the mechanisms underlying the occurrence of
EMT in DDP-resistant cells.
To our knowledge, chemotherapeutic agents, including
DDP, generally induce tumor regression through apoptosis, which is
modulated through a series of proto-oncogenes and tumor suppressor
genes (28,29). However, alterations in the
regulation of such apoptotic processes may lead to increased
expression levels of EMT-related molecules and result in the
failure of therapy. Although the mechanisms underlying resistance
to DDP and EMT are still under investigation (30), we argue that our finding that
DDP-resistant NPC cells undergo EMT reflects an important process
by which cancer cells may potentially acquire chemoresistance.
Blocking or reversing EMT changes may cause chemoresistant cells to
revert to chemosensitive cells.
In conclusion, we demonstrate that the development
of resistance to DDP in NPC cells is accompanied by inducible
EMT-like changes with an increased metastatic potential in
vitro. Further elucidation of the association between
resistance to DDP and EMT would allow the development of novel
therapeutic approaches for chemoresistant tumors in the future.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81372899, 81072207),
the Natural Science Foundation of Anhui Province (090413135) and
the Key Project of Natural Science Research of the Education
Department of Anhui Province, China (KJ2012A202), the innovation
project of graduate scientific research of Bengbu Medical College
of Anhui Province, China (Byycx1327).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Chen QY, Wen YF, Guo L, et al: Concurrent
chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal
carcinoma: phase III randomized trial. J Natl Cancer Inst.
103:1761–1770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabec V and Kasparkova J: Modifications
of DNA by platinum complexes. Relation to resistance of tumors to
platinum antitumor drugs. Drug Resist Updat. 8:131–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Q, Kawamura K, Yamanaka M, et al:
Upregulated p53 expression activates apoptotic pathways in
wild-type p53-bearing mesothelioma and enhances cytotoxicity of
cisplatin and pemetrexed. Cancer Gene Ther. 19:218–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Xiang S, Williams KA, et al:
Depletion of HDAC6 enhances cisplatin-induced DNA damage and
apoptosis in non-small cell lung cancer cells. PLoS One.
7:e442652012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shellard SA, Fichtinger-Schepman AM, Lazo
JS and Hill BT: Evidence of differential cisplatin-DNA adduct
formation, removal and tolerance of DNA damage in three human lung
carcinoma cell lines. Anticancer Drugs. 4:491–500. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torigoe T, Izumi H, Ishiguchi H, et al:
Cisplatin resistance and transcription factors. Curr Med Chem
Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung HW, Jin DY, Ling MT, et al: Mitotic
arrest deficient 2 expression induces chemosensitization to a
DNA-damaging agent, cisplatin, in nasopharyngeal carcinoma cells.
Cancer Res. 65:1450–1458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial - mesenchymal and mesenchymal - epithelial transitions
in carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MR, Choi HK, Cho KB, Kim HS and Kang
KW: Involvement of Pin1 induction in epithelial-mesenchymal
transition of tamoxifen-resistant breast cancer cells. Cancer Sci.
100:1834–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajiyama H, Shibata K, Terauchi M, et al:
Chemoresistance to paclitaxel induces epithelial-mesenchymal
transition and enhances metastatic potential for epithelial ovarian
carcinoma cells. Int J Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
13
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu MF, Liu LZ, He LJ, et al: Sequential
chemoradiotherapy with gemcitabine and cisplatin for locoregionally
advanced nasopharyngeal carcinoma. Int J Cancer. 132:215–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie SM, Fang WY, Liu TF, Yao KT and Zhong
XY: Association of ABCC2 and CDDP-resistance in two sublines
resistant to CDDP derived from a human nasopharyngeal carcinoma
cell line. J Oncol. 2010:9150462010.PubMed/NCBI
|
|
16
|
Wong RS and Cheong SK: Leukaemic stem
cells: drug resistance, metastasis and therapeutic implications.
Malays J Pathol. 34:77–88. 2012.PubMed/NCBI
|
|
17
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danila DC, Heller G, Gignac GA, et al:
Circulating tumor cell number and prognosis in progressive
castration-resistant prostate cancer. Clin Cancer Res.
13:7053–7058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Y, Meleady P, Cleary I, McDonnell S,
Connolly L and Clynes M: Selection with melphalan or paclitaxel
(Taxol) yields variants with different patterns of multidrug
resistance, integrin expression and in vitro invasiveness.
Eur J Cancer. 37:1041–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haga A, Nagase H, Kito H and Sato T:
Invasive properties of cadmium-resistant human fibrosarcoma HT-1080
cells. Cancer Biochem Biophys. 15:275–284. 1997.PubMed/NCBI
|
|
21
|
Wang Z, Li Y, Ahmad A, et al: Targeting
miRNAs involved in cancer stem cell and EMT regulation: An emerging
concept in overcoming drug resistance. Drug Resist Updat.
13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haslehurst AM, Koti M, Dharsee M, et al:
EMT transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pai MH, Kuo YH, Chiang EP and Tang FY:
S-Allylcysteine inhibits tumour progression and the
epithelial-mesenchymal transition in a mouse xenograft model of
oral cancer. Br J Nutr. 108:28–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing Y, Han Z, Liu Y, et al: Mesenchymal
stem cells in inflammation microenvironment accelerates
hepatocellular carcinoma metastasis by inducing
epithelial-mesenchymal transition. PLoS One. 7:e432722012.
View Article : Google Scholar
|
|
26
|
Comijn J, Berx G, Vermassen P, et al: The
two-handed E box binding zinc finger protein SIP1 downregulates
E-cadherin and induces invasion. Mol Cell. 7:1267–1278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Ling MT, Guan XY, et al:
Identification of a novel function of TWIST, a bHLH protein, in the
development of acquired taxol resistance in human cancer cells.
Oncogene. 23:474–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudin CM, Yang Z, Schumaker LM, et al:
Inhibition of glutathione synthesis reverses Bcl-2-mediated
cisplatin resistance. Cancer Res. 63:312–318. 2003.PubMed/NCBI
|
|
29
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: a cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barr MP, Gray SG, Hoffmann AC, et al:
Generation and characterisation of cisplatin-resistant non-small
cell lung cancer cell lines displaying a stem-like signature. PLoS
One. 8:e541932013. View Article : Google Scholar : PubMed/NCBI
|