Introduction
The literature pertaining to the
Hohenbuehelia species is limited and confined to the
following reports. Vafina and Molodtsov described the synthesis of
some p-nitrophenyl 2-acylamino-2-deoxy-D-glucosides and their
hydrolysis using Hohenbuehelia serotina β-D-hexosaminidase
(1). The culture filtrate of
Hohenbuehelia geogenius inhibited the growth of two rapidly
growing grafted tumors (Ehrlich ascites carcinoma and L1210
lymphoid leukemia) and a slow-growing spontaneous mammary tumor in
mice. An active substance was isolated by solid-liquid extraction
and column chromatography and its chemical structure was elucidated
(2).
Anti-A agglutinins, the reaction of which has been
shown to be strongly inhibited by N-acetyl-D-galactosamine, have
been detected in Hohenbuehelia serotina extracts (3). Two strains of Hohenbuehelia
atrocaerulea (Pleurotaceae) that produce pleurotin, a
naphthoquinone antibiotic originally obtained from Pleurotus
griseus, have been identified. Solid substrate fermentation for
two months yielded 1–2 mg/l of pleurotin. Shipley et al
(4) detailed the developmental
process, which depended on inclusion in the medium of an aqueous
extract of alder woodm leading to a yield of pleurotin exceeding
300 mg/l from liquid fermentation. Bala et al reported that
water, ethanol and hexane extracts of the Hohenbuehelia
species inhibited growth of Gram-positive Staphylococcus
aureus and Gram-negative Escherichia coli (5). Bala et al (6) further demonstrated that a water
extract of Hohenbuehelia species inhibited six pathogens
each comprising of two Gram-positive and -negative bacteria
together with two fungi.
Polysaccharides extracted from the fruiting bodies
of Hohenbuehelia serotina have exhibited antitumor activity
in sarcoma 180-bearing mice (7).
The molecular weights of these polysaccharides ranged from 1.19×103
to 1.55×104 Da and composed of ribose, arabinose, mannose, glucose
and galactose at a ratio of 0.65:0.69:9.35:14.24:5.47; they were
isolated by Li et al (8).
However, there is a dearth of information on the proteinaceous
constituents of the Hohenbuehelia species.
Ribonucleases (RNases) have been isolated and
characterized from a multitude of organisms, including parasites,
bacteria, fungi, plants and a variety of tissues from mammals
(9–17) RNases display different activities,
such as antitumor (18–25), immunosuppressive (26), antifungal (27), and antiviral (28,29) activities. Due to the array of
potentially exploitable activities, RNases have drawn the attention
of many researchers.
RNases have been purified from the fruiting bodies
or mycelia of a diversity of mushroom species. These mushrooms
include some common edible and medicinal species as follows:
Pleurotus ostreatus (30,31), Irpex lacteus (32), Volvariella volvacea
(33), Pleurotus
tuber-regium (34),
Pleurotus pulmonarius (35), Agrocybe cylindracea
(36), Russula virescens
(37), Termitomyces
globules (38),
Cantharellus cibarius (39), Pleurotus sajor-caju
(40), Ganoderma lucidum
(41), Clitocybe maxima
(42), Thelephora ganbajun
(43), Boletus griseus
(44), Hypsizigus
marmoreus (45), Russula
delica (16,46) and Lyophyllum shimeiji
(47). The aim of this study was
to isolate and characterize an RNase isolated from the dried
fruiting bodies of the edible fungus, Hohenbuehelia
serotina, and to compare its characteristics and N-terminal
sequence with those of RNases isolated from the aforementioned
species. The comparison would reveal any differences between RNases
from different species and our findings may expand the knowledge
and provide further information on this fungus.
Materials and methods
Isolation of RNase from Hohenbuehelia
serotina
The dried fruiting bodies (200 g) of the edible
mushroom, Hohenbuehelia serotina, obtained from North China
were extracted with distilled water (2 ml/g) using a Waring blender
(Waring Laboratory Supplies, Torrington, CT, USA). Tris-HCl buffer
(pH 7.2, 1 M) was added to the supernatant, obtained by
centrifugation of the homogenate, until the concentration of Tris
reached 10 mM. The supernatant was subjected to ion exchange
chromatography on a 5×15 cm column of DEAE-cellulose (Sigma, St.
Louis, MO, USA) in 10 mM Tris-HCl buffer (pH 7.2). Following
elution of the unadsorbed proteins (fraction D1) with the same
buffer, the adsorbed proteins were desorbed sequentially with 0.2 M
NaCl and 1 M NaCl in Tris-HCl buffer to form fractions D2 and D3,
respectively. Fraction D1 was then directly chromatographed on a
5×15 cm Affi-gel blue gel column (Bio-Rad, Hercules, CA, USA) in 10
mM Tris-HCl buffer (pH 7.2). The unadsorbed proteins were eluted as
fraction B1. The adsorbed proteins were eluted sequentially with
0.2 M NaCl and 1 M NaCl in Tris-HCl buffer and collected as
fractions B2 and B3, respectively. Fraction B2 was dialyzed against
10 mM NH4OAc buffer (pH 5) subjected to ion exchange
chromatography on a 2.5×20 cm CM-cellulose (Sigma) column in 10 mM
NH4OAc buffer (pH 5). Following the removal of the
unadsorbed proteins (fraction CM1), the adsorbed proteins were
eluted with 10 mM NH4OAc buffer (pH 5) containing a
linear gradient of 0–1 M NaCl, and collected as fractions CM2 and
CM3. Fraction CM3 was dialyzed, lyophilized and further purified on
a Superdex 75 HR 10/30 column in 0.2 M
NH4HCO3 buffer (pH 8.5) using an AKTA
Purifier (GE Healthcare, Piscataway, NJ, USA).
Molecular mass determination by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
by fast protein liquid chromatography (FPLC)-gel filtration
SDS-PAGE was conducted following the protocol of
Laemmli and Favre (48), using a
12% resolving gel and a 5% stacking gel. At the conclusion of
electrophoresis, staining of the gel with Coomassie brilliant blue
was carried out. FPLC-gel filtration was performed using a Superdex
75 HR 10/30 column that had been calibrated with molecular mass
standards using an AKTA Purifier (GE Healthcare).
Analysis of N-terminal amino acid
sequence
The amino-acid sequence of the purified protein was
determined by means of automated Edman degradation. The amino acid
sequence was determined using an HP G1000A Edman degradation unit
and an HP 1000 HPLC system (Agilent Technologies, Santa Clara, CA,
USA).
Assay for RNase activity
The activity of the purified RNase toward yeast tRNA
(Sigma) was assayed by determining the generation of acid-soluble
UV-absorbing species (reaction products) with the method of Wang
and Ng (52). The RNase was
incubated with 200 μg tRNA in 150 μg of 100 mM MES buffer (pH 6.0)
at 37°C for 1 h. The reaction was terminated by the addition of 350
μl of ice-cold 3.4% perchloric acid. After remaining on ice for 15
min, the sample was centrifuged (15,000 × g) at 4°C for 15 min. The
OD260 of the supernatant was read after appropriate
dilution. One unit of enzymatic activity is defined as the amount
of enzyme that induces an increase in OD260 of one per
minute in the acid-soluble fraction per milliliter of reaction
mixture under the specified conditions.
Activity of RNase toward
polyhomoribonucleotides
The ribonucleolytic activity of the purified RNase
toward various polyhomoribonucleotides as substrates was determined
with a modification of a previously described method (33). The incubation of RNase with 100 μg
poly(A), poly(C), poly(G) or poly(U) was carried out at 37°C for 1
h in 250 μl of 100 mM sodium acetate buffer (pH 5.0), prior to the
addition of 250 μl of ice-cold 1.2 N perchloric acid containing 20
mM lanthanum nitrate to terminate the reaction. The reaction
mixture was left on ice for 15 min prior to centrifugation at
15,000 × g for 15 min at 4°C. After appropriate dilution, the
absorbance of the supernatant was read at 260 nm [for substrates
poly(A), poly(G) and poly(U)] or at 280 nm [for substrate
poly(C)].
Assay for ability to inhibit human
immunodeficiency virus type 1 reverse transcriptase (HIV-1 RT)
The assay to determine the HIV-1 RT inhibitory
activity of Hohenbuehelia serotina RNase was executed using
a non-radioactive reverse transcriptase ELISA kit (Sigma-Aldrich,
Trading Co., Ltd., Shanghai, China) as previously described
(49).
Assay for anti-proliferative activity of
RNase isolated from Hohenbuehelia serotina
The anti-proliferative activity of the purified
RNase was determined as follows: the L1210 and MBL2 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and 100 mg/l
streptomycin and 100 IU/ml penicillin, at 37°C in a humidified
atmosphere of 5% CO2. Cells (1×104) in their
exponential growth phase were seeded into each well of a 96-well
culture plate and incubated for 3 h prior to the addition of the
purified RNase followed by incubation for a further 48 h. The
radioactive precursor, 1 μCi
[3H-methyl]-thymidine, was added to each well followed
by incubation for 6 h before the cultures were harvested by means
of a cell harvester. The incorporated radioactivity was determined
by liquid scintillation counting, as previously described (40).
Results
Isolation of RNase
Ion exchange chromatography of the extract on
DEAE-cellulose yielded three fractions D1, D2 and D3 containing
similar amounts of proteins. RNase activity was found only in the
unadsorbed fraction D1. D1 was separated on Affi-gel blue gel into
one unadsorbed and also the largest fraction (B1), together with
two adsorbed fractions (B2 and B3). Fraction B2 was the only
fraction with strong RNase activity. This fraction was resolved on
CM-cellulose into an unadsorbed fraction CM1 devoid of RNase
activity, an adsorbed fraction CM2 with weak RNase activity, and
the most strongly adsorbed fraction CM3 containing the bulk of
RNase activity (Fig. 1). Fraction
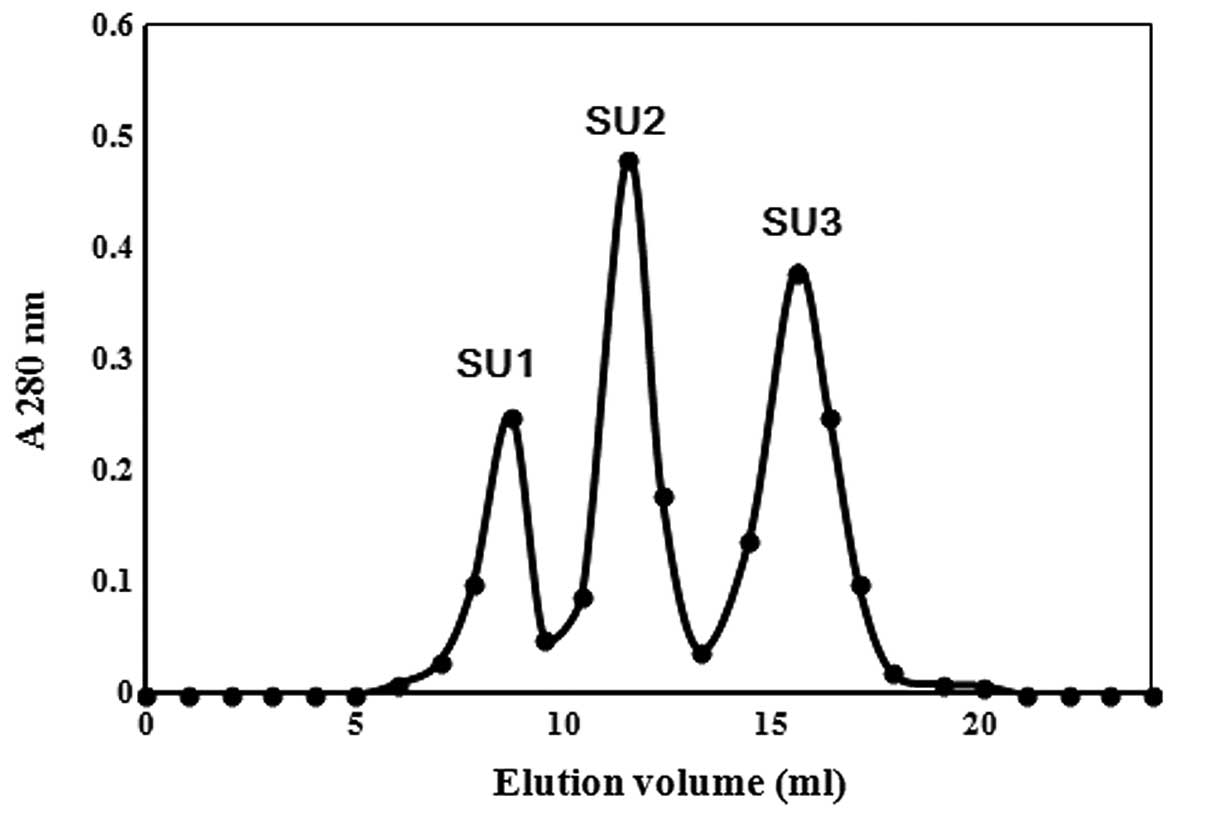
CM3 was resolved into three fractions, SU1, SU2 and SU3 upon gel
filtration on Superdex 75. RNase activity resided in the second
fraction SU2 (Fig. 2 and Table I).
 | Table IYields (from 650 g fresh
Hohenbuehelia serotina fruiting bodies) and RNase activity
(determined in 0.1 M MES buffer, pH 6.0, 37°C) of various
chromatographic fractions. |
Table I
Yields (from 650 g fresh
Hohenbuehelia serotina fruiting bodies) and RNase activity
(determined in 0.1 M MES buffer, pH 6.0, 37°C) of various
chromatographic fractions.
| Fraction | Yield (mg) | RNase activity
(U/mg) | Fraction | Yield (mg) | RNase activity
(U/mg) |
|---|
| Extract | 2018 | 35.7 | CM1 | 19.0 | <1 |
| D1 | 459 | 98.6 | CM2 | 13.4 | 51.8 |
| D2 | 403 | 12.5 | CM3 | 23.5 | 983.0 |
| D3 | 529 | <1 | SU1 | 3.6 | 102.8 |
| B1 | 165 | <1 | SU2 | 6.9 | 2705.3 |
| B2 | 79.3 | 369.5 | SU3 | 5.9 | 86.1 |
| B3 | 81.2 | 15.7 | | | |
Determination of molecular mass
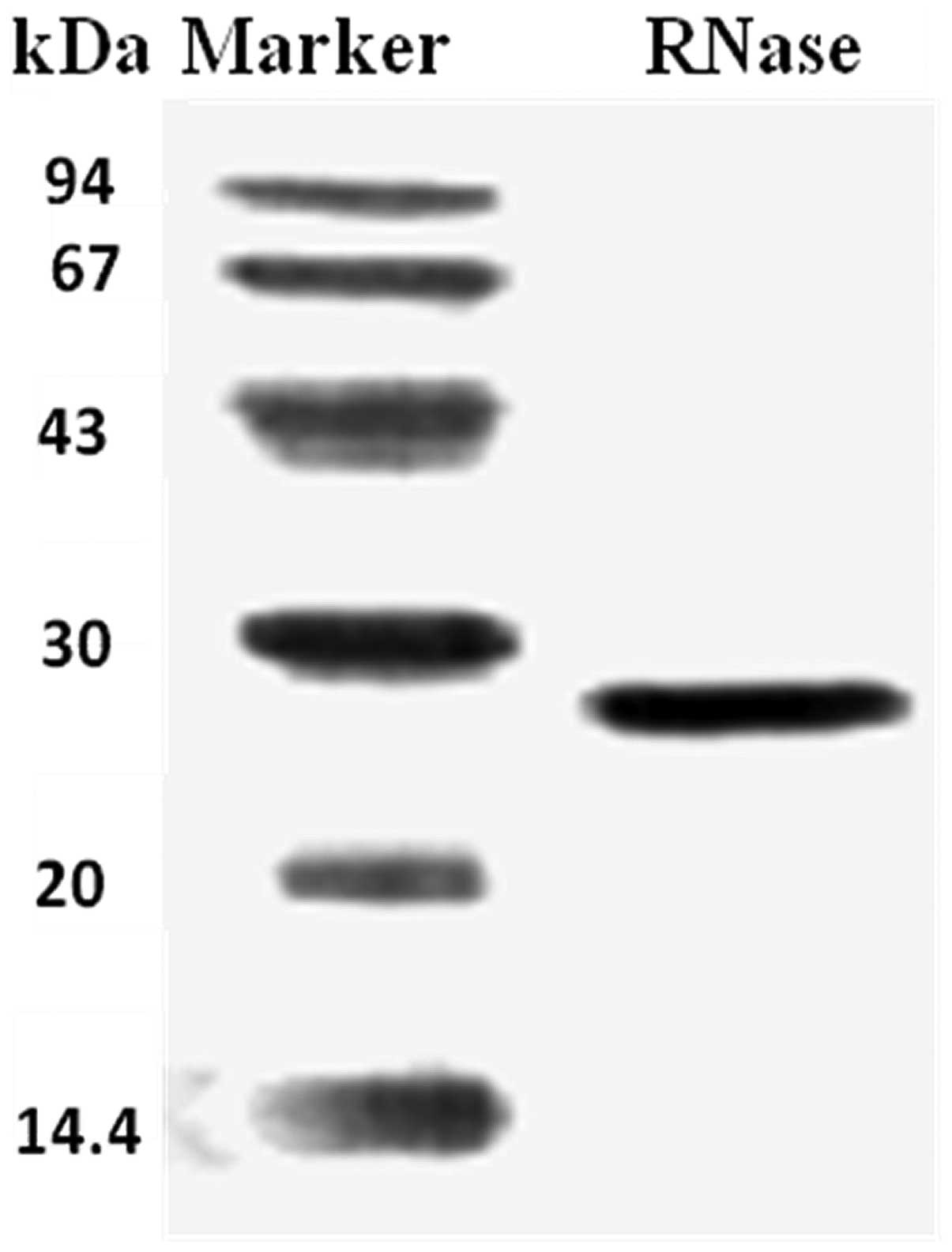
Fraction SU2 appeared as a single band with a
molecular mass of 27 kDa, as shown by SDS-PAGE (Fig. 3).
Analysis of N-terminal amino acid
sequence
The amino acid sequence was abtained by an HP 1000
HPLC system depending on Edman degradation. The N-terminal sequence
was as follows: TVGGSLAEKGN, which showed homology to other fungal
RNases to a certain degree (Table
II).
 | Table IIN-terminal sequence of
Hohenbuehelia serotina (HS) ribonuclease (RNase) in
comparison with RNases isolated from other mushrooms. |
Table II
N-terminal sequence of
Hohenbuehelia serotina (HS) ribonuclease (RNase) in
comparison with RNases isolated from other mushrooms.
| RNase | N-terminal
sequence |
|---|
| HS: | TVGGSLAEKGN |
| TG: |
DADIAVWAPPVNAQN |
| CM: |
ETAHTHAGIQYSTVDVNNSIMKAVGGGAGN |
| PP: |
AISANNERKGVNQQSVQNTYQENDV |
| VV: |
APYVQLFRPLIQPQVLATFAIANNMAQY |
| LE: |
ISSGCGTTGALSCSSNAKGTCCFEAPGGLI |
| IL: |
VNSGCGTSGAESCSNSDDGTCCFEAPGGLL |
| DI: | GQPRQPQPQLLV |
| PE: | GEVVQYYP |
| PS: | DNGEAGRAAR |
| GL: |
HLPBVPSFAYGSIKVYIN |
| RV: |
TDHTLDTMMTHTLRD |
| PO: |
ETGVRSCNCAGRSFTGTDVTNAIRSARAGGSGN |
| PT: |
ALTAQDNRVRVGNRIVGNNFNFAAVQAAYY |
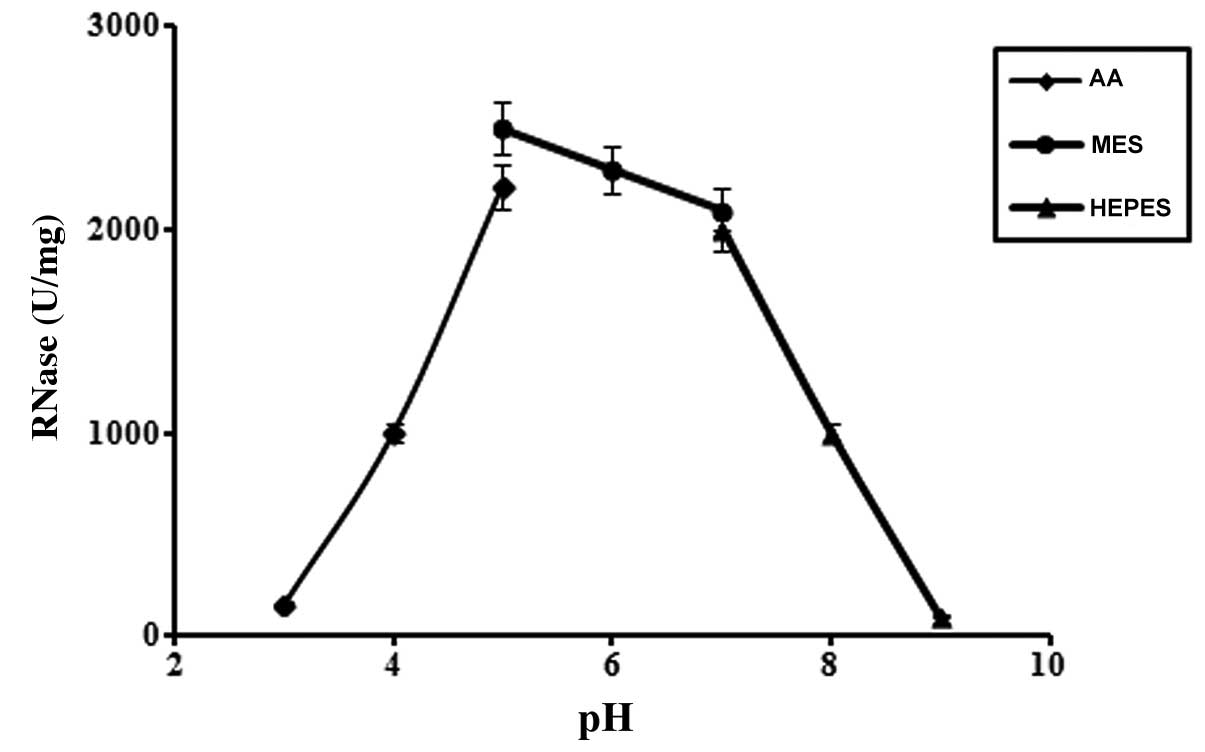
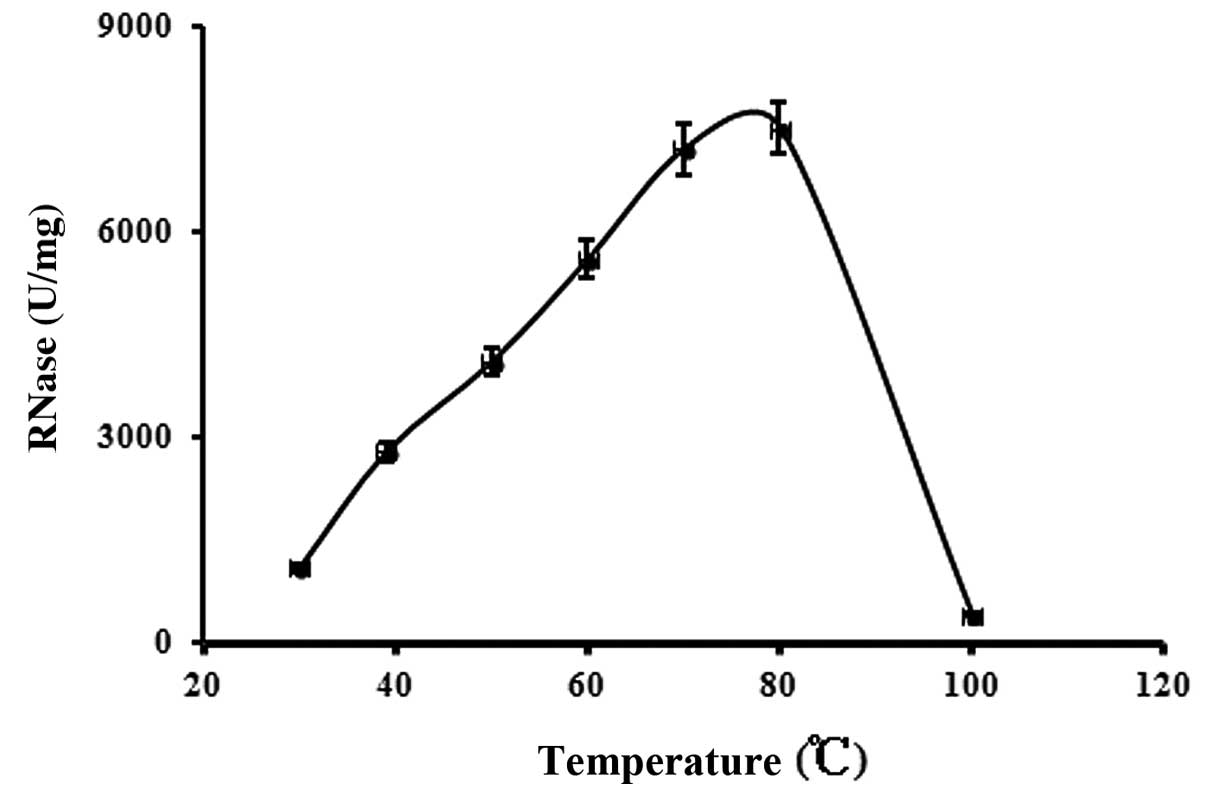
Determination of optimum pH and optimum
temperature
The optimum pH (Fig.
4) and temperature (Fig. 5)
for the purified RNase were pH 5.0 and 80°C, respectively.
Determination of polyhomoribonucleotide
specificity
The RNase exerted a ribonucleolytic activity of
455.1, 311.2, 119.4 and 105 U/mg toward poly(C), poly(U), poly(A)
and poly(G), respectively.
HIV-1 RT inhibitory activity
The RNase inhibited HIV-1 RT with an IC50
of 50 μM (Table III).
 | Table IIIInhibition rates (%) of RNase on
growth of MBL2 cells, L1210 cells, and HIV-1 RT. |
Table III
Inhibition rates (%) of RNase on
growth of MBL2 cells, L1210 cells, and HIV-1 RT.
| Inhibition rates
(%) |
|---|
|
|
|---|
| Dose (μM) | HIV-1 RT | L1210 | MBL2 |
|---|
| 10 | 13.5±0.9 | 24.6±1.8 | 18.7±1.1 |
| 20 | 21.4±1.6 | 40.9±2.7 | 31.4±2.3 |
| 40 | 42.1±2.7 | 72.1±5.2 | 49.7±3.8 |
| 80 | 73.4±4.3 | 94.3±5.5 | 73.6±5.9 |
| IC50
(μM) | 49.9 | 24.8 | 40.3 |
Anti-proliferative activity toward tumor
cells
The RNase inhibited [3H-methyl]-thymidine
uptake by L1210 cells and MBL2 cells with an IC50 of 25
and 40 μM, respectively (Table
III).
Discussion
RNase isolated from Hohenbuehelia serotina is
characterized by an N-terminal sequence distinctly different from
that of previously reported mushroom and non-mushroom RNases. The
molecular weight of previously reported mushroom RNases ranges from
9 to 42.5 kDa. The molecular mass of Hohenbuehelia serotina
RNase (27 kDa) lies within this range. It is larger than the mass
of RNases isolated from Clitocybe maxima (42), Hypsizigus marmoreus
(45), Lyophyllum shimeiji
(47), Pleurotus djamor
(50), Pleurotus eryngii
(51), Pleurotus ostreatus
(31), Pleurotus
pulmonarius (35),
Pleurotus sajor-caju (40), Russula delica (46) and Thelephora ganbajun
(43), but smaller than the mass
of RNases isolated from Boletus griseus (44), Dictyophora indusiata
(52), Ganoderma lucidum
(43), Pleurotus
tuber-regium (34),
Russulus virescens (37)
and Volvariella volvacea (33). Its mass is close to that of
Dictyophora indusiata RNase (28 kDa) and Russulus
virescens RNase (28 kDa). All these RNases are monomeric, and
all of them are acid RNases.
RNases isolated from different mushrooms may have
different optimum pH values and temperatures for RNase activity.
The optimum pH of Hohenbuehelia serotina RNase (pH 5.0) is
similar to that of Hypsizigus marmoreus RNase (pH 5.0) [Guan
et al (45)] and
Russula delica RNase (pH 5.0) [Zhao et al (46)]; however, Hohenbuehelia
serotina RNase is much more thermostable. The optimum
temperature for the purified Hohenbuehelia serotina RNase
was found to be 80°C, which is the highest optimum temperature for
RNases purified from mushrooms.
Some RNases display antiviral activity. Zinc-finger
antiviral protein inhibits HIV-1 infection by selectively targeting
multiply spliced viral mRNAs for degradation [Zhu et al
(53)]. Rana catesbeiana
RNase inhibits Japanese encephalitis virus (JEV) replication and
enhances apoptosis of JEV-infected BHK-21 cells.
Li et al (54) suggested that the targeted RNase is
an alternative anti-hepatitis B virus (HBV) agent. The HIV-1 RT
inhibitory activity of Hohenbuehelia serotina RNase has been
shown to be similar to that of RNases isolated from other
mushrooms, such as Lyophyllum shimeiji (47) and Thelephora ganbajun
(43). The mechanisms responsible
for the inhibitory effects on HIV-1 RT may involve protein-protein
interaction, as in the case of the inhibition of HIV-1 RT by the
homologous protease (55) and the
inhibition of HIV-1 protease by cathelicidin [Wong et al
(56)].
Some RNases exert inhibitory effects on tumor cells.
The binding of AS RNase and CM-AS RNase to leukaemic cells from
patients with chronic lymphatic leukaemia has been demonstrated by
indirect immunofluorescence, while no binding to normal leucocytes
and leucocytes from patients with other hemoblastoses was observed
(24). Bovine seminal RNase
(BS-RNase) manifests specific cytotoxic effects on tumor cells, and
non-malignant cells are not affected. In view of the finding that
success was met only when BS-RNase was applied intratumorally, the
properties of BS-RNase were improved by attachment to polylactic
acid nanoparticles. The nanoparticle preparation and pure BS-RNase
showed no difference when tested against leukemia (MOLT-4) and
lymphoma (H9) cell lines sensitive and resistant to cytarabine
in vitro. The aspermatogenic and anti-embryonal activities
were augmented in the nanoparticle preparation of BS-RNase. It
remains to be seen how well BS-RNase attached to polylactic acid
nanoparticles performs as an antitumoral agent in vivo
[Michaelis et al (57)].
The anticancer effect of the amphibian RNase Onconase as
demonstrated experimentally and in clinical trials has been
reported (58). Plant RNases have
also been shown to exhibit antitumor effects in a number of studies
(18,20,22).
Mushroom RNases have been shown to be effective
against hepatoma and breast cancer cells (46). Zhao et al (16) noted that Schizophyllum
commune RNase had no effect on the proliferation of leukemia
and lymphoma cells. Only a few mushroom RNases have been shown to
inhibit the growth of leukemia cells (36,40,59,60). We found that the
anti-proliferative activities of Hohenbuehelia serotina
RNase toward L1210 cells are not as remarkable as those of
Pleurotus sajor-caju RNase (40), but more effective than those of
Hypsizigus marmoreus RNase (45). To our knowledge, we also
demonstrated for the first time that mushroom RNases exhibit
anti-proliferative activity toward MBL2 cells.
Some plant RNases have antifungal activity (27,34,61) and have been classified as one
family of pathogenesis-related proteins. It is interesting to note
that Hohenbuehelia serotina RNase, similar to all previously
reported mushroom RNases, is devoid of antifungal activity.
However, its antiproliferative activity against cancer cells and
its inhibitory activity toward HIV-I RT signify that it is a
defense protein. Its RNase activity can be deployed against
invaders.
In conlcusion, the RNase isolated from
Hohenbuehelia serotina in the present study is a novel
RNase, as evidenced by a novel N-terminal sequence and a high
optimum pH. It manifests potent anti-proliferative activity toward
cancer cells and inhibitory activity toward HIV-1 RT. These
biological activities are potentially exploitable. In this regard
it is noteworthy that not all previously reported mushroom RNases
were assayed for or demonstrate anti-proliferative and HIV-1 RT
inhibitory activities.
Acknowledgements
This study was financially supported by the National
Grants of China (no. 2010CB732202) and the Special Fund for
Agro-scientific Research in the Public Interest (no.
201303080).
References
|
1
|
Vafina MG and Molodtsov NV: Synthesis of
some p-nitrophenyl 2-acylamino-2-deoxy-D-glucosides and their
hydrolysis with the beta-D-hexosaminidase from Hohenbuehelia
serotina. Carbohyd Res. 47:188–194. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riondel J, Beriel H, Dardas A, Carraz G
and Oddoux L: Studies of antitumor activity of the culture filtrate
of Hohenbuehelia geogenius (D.C. ex Fr.) Sing
(basidiomycete). Arzneimittelforschung. 31:293–299. 1981.PubMed/NCBI
|
|
3
|
Furukawa K, Ying R, Nakajima T and Matsuki
T: Hemagglutinins in fungus extracts and their blood group
specificity. Exp Clin Immunogenet. 12:223–231. 1995.PubMed/NCBI
|
|
4
|
Shipley SM, Barr AL, Graf SJ, Collins RP,
McCloud TG and Newman DJ: Development of a process for the
production of the anticancer lead compound pleurotin by
fermentation of Hohenbuehelia atrocaerulea. J Ind Microbiol
Biotechnol. 33:463–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bala N, Aitken EA, Fechner N, Cusack A and
Steadman KJ: Evaluation of antibacterial activity of Australian
basidiomycetous macrofungi using a high-throughput 96-well plate
assay. Pharm Biol. 49:492–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bala N, Aitken EA, Cusack A and Steadman
KJ: Antimicrobial potential of Australian macrofungi extracts
against foodborne and other pathogens. Phytother Res. 26:465–469.
2012.PubMed/NCBI
|
|
7
|
Ma Y, Mizuno T and Ito H: Antitumor
activity of some polysaccharides isolated from a Chinese mushroom,
‘huangmo’, the fruiting body of Hohenbuehelia serotina.
Agric Biol Chem. 55:2701–2710. 1991.
|
|
8
|
Li X, Wang Z, Wang L, Walid E and Zhang H:
Ultrasonic-assisted extraction of polysaccharides from
Hohenbuehelia serotina by response surface methodology. Int
J Biol Macromol. 51:523–530. 2012. View Article : Google Scholar
|
|
9
|
Kikovska E, Wu S, Mao G and Kirsebom LA:
Cleavage mediated by the P15 domain of bacterial RNase P RNA.
Nucleic Acids Res. 40:2224–2233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daoud R, Forget L and Lang BF: Yeast
mitochondrial RNase P, RNase Z and the RNA degradosome are part of
a stable supercomplex. Nucleic Acids Res. 40:1728–1736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gardner AF, Prangishvili D and Jack WE:
Characterization of Sulfolobus islandicus rod-shaped virus 2
gp19, a single-strand specific endonuclease. Extremophiles.
15:619–624. 2011.
|
|
12
|
Hillwig MS, Kanobe C, Thornburg RW and
Macintosh GC: Identification of S-RNase and peroxidase in petunia
nectar. J Plant Physiol. 168:734–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai LB, Chan PP, Cozen AE, Bernick DL,
Brown JW, Gopalan V and Lowe TM: Discovery of a minimal form of
RNase P in Pyrobaculum. Proc Natl Acad Sci USA.
107:22493–22498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee OR, Pulla RK, Kim YJ, Balusamy SR and
Yang DC: Expression and stress tolerance of PR10 genes from Panax
ginseng C. A. Meyer. Mol Biol Rep. 39:2365–2374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schroeder J: Purification of antimicrobial
peptides from human skin. Methods Mol Biol. 618:15–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao YC, Zhang GQ, Ng TB and Wang HX: A
novel ribonuclease with potent HIV-1 reverse transcriptase
inhibitory activity from cultured mushroom Schizophyllum
commune. J Microbiol. 49:803–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teng PK, Anderson NJ, Goldschmidt L,
Sawaya MR, Sambashivan S and Eisenberg D: Ribonuclease A suggests
how proteins self-chaperone against amyloid fiber formation.
Protein Sci. 21:262012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Fei Fang EF, Zhang CZ, Zhang L, Fong
WP and Ng TB: In vitro and in vivo anticarcinogenic effects of
RNase MC2, a ribonuclease isolated from dietary bitter gourd,
toward human liver cancer cells. Int J Biochem Cell Biol.
44:1351–1360. 2012.PubMed/NCBI
|
|
19
|
D’Errico G, Ercole C, Lista M, Pizzo E,
Falanga A, Galdiero S, Spadaccini R and Picone D: Enforcing the
positive charge of N-termini enhances membrane interaction and
antitumor activity of bovine seminal ribonuclease. Biochim Biophys
Acta. 1808:3007–3015. 2011.PubMed/NCBI
|
|
20
|
Fang EF, Zhang CZ, Fong WP and Ng TB:
RNase MC2: a new Momordica charantia ribonuclease that induces
apoptosis in breast cancer cells associated with activation of
MAPKs and induction of caspase pathways. Apoptosis. 17:377–387.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang EF and Ng TB: Ribonucleases of
different origins with a wide spectrum of medicinal applications.
Biochim Biophys Acta. 1815:65–74. 2011.PubMed/NCBI
|
|
22
|
Matousek J and Matousek J: Plant
ribonucleases and nucleases as antiproliferative agens targeting
human tumors growing in mice. Recent Pat DNA Gene Seq. 4:29–39.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michaelis M, Matousek J, Vogel JU, Slavik
T, Langer K and Cinatl J, Kreuter J, Schwabe D and Cinatl J: Bovine
seminal ribonuclease attached to nanoparticles made of polylactic
acid kills leukemia and lymphoma cell lines in vitro. Anticancer
Drugs. 11:369–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soucek J and Matousek J: The binding of
bull seminal ribonuclease and its carboxymethylated derivative to
human leukaemic cells. Folia Biol (Praha). 25:142–144.
1979.PubMed/NCBI
|
|
25
|
Lomax JE, Eller CH and Raines RT: Rational
design and evaluation of mammalian ribonuclease cytotoxins. Methods
Enzymol. 273–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matousek J, Soucek J, Riha J, Zankel TR
and Benner SA: Immunosuppressive activity of angiogenin in
comparison with bovine seminal ribonuclease and pancreatic
ribonuclease. Comp Biochem Phys B Biochem Mol Biol. 112:235–241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gómez-Gómez L, Rubio-Moraga A and Ahrazem
O: Molecular cloning and characterisation of a pathogenesis-related
protein CsPR10 from Crocus sativus. Plant Biol. 13:297–303.
2011.PubMed/NCBI
|
|
28
|
Choudhary NL, Yadav OP and Lodha ML:
Ribonuclease, deoxyribonuclease, and antiviral activity of
Escherichia coli-expressed Bougainvillea xbuttiana antiviral
protein 1. Biochemistry (Mosc). 73:273–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon YC, Kang JI, Hwang SB and Ahn BY: The
ribonuclease L-dependent antiviral roles of human
2′,5′-oligoadenylate synthetase family members against hepatitis C
virus. FEBS Lett. 587:156–164. 2013.
|
|
30
|
Nomura H, Inokuchi N, Kobayashi H, Koyama
T, Iwama M, Ohgi K and Irie M: Purification and primary structure
of a new guanylic acid specific ribonuclease from Pleurotus
ostreatus. J Biochem. 116:26–33. 1994.PubMed/NCBI
|
|
31
|
Ye XY and Ng TB: Purification and
characterization of a new ribonuclease from fruiting bodies of the
oyster mushroom Pleurotus ostreatus. J Pept Sci. 9:120–124.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe H, Fauzi H, Iwama M, Onda T, Ohgi
K and Irie M: Base non-specific acid ribonuclease from Irpex
lacteus, primary structure and phylogenetic relationships in
RNase T2 family enzyme. Biosci Biotechnol Biochem. 59:2097–2103.
1995.PubMed/NCBI
|
|
33
|
Wang H and Ng TB: Isolation of a new
ribonuclease from fresh fruiting bodies of the straw mushroom.
Biochem Biophys Res Commun. 264:714–718. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HX and Ng TB: Purification and
characterization of a potent homodimeric guanine-specific
ribonuclease from fresh mushroom (Pleurotus tuber-regium)
sclerotia. Int J Biochem Cell Biol. 33:483–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye XY and Ng TB: A novel and potent
ribonuclease from fruiting bodies of the mushroom Pleurotus
pulmonarius. Biochem Biophys Res Commun. 293:857–861. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngai P, Wang HX and Ng TB: Purification
and characterization of a ubiquitin-like peptide with macrophage
stimulating, antiproliferative and ribonuclease activities from the
mushroom Agrocybe cylindracea. Peptides. 24:639–645. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H and Ng TB: A ribonuclease with
distinctive features from the wild green-headed mushroom
Russulus virescens. Biochem Biophys Res Commun. 312:965–968.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang HX and Ng TB: Isolation of a
ribonuclease from fruiting bodies of the wild mushroom
Termitomyces globulus. Peptides. 24:973–977. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang HX, Ngai HK and Ng TB: A
ubiquitin-like peptide with ribonuclease activity against various
polyhomoribonucleotides from the yellow mushroom Cantharellus
cibarius. Peptides. 24:509–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ngai P and Ng TB: A ribonuclease with
antimicrobial, antimitogenic and antiproliferative activities from
the edible mushroom Pleurotus sajor-caju. Peptides.
25:11–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang HX, Ng TB and Chiu SW: A distinctive
ribonuclease from fresh fruiting bodies of the medicinal mushroom
Ganoderma lucidum. Biochem Bioph Res Commun. 314:519–522.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H and Ng TB: Isolation of a new
ribonuclease from fruiting bodies of the silver plate mushroom
Clitocybe maxima. Peptides. 25:935–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang HX and Ng TB: Purification of a novel
ribonuclease from dried fruiting bodies of the edible wild mushroom
Thelephora ganbajun. Biochem Bioph Res Commun. 324:855–859.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang HX and Ng TB: A ribonuclease from the
wild mushroom Boletus griseus. Appl Microbiol Biotechnol.
72:912–916. 2006. View Article : Google Scholar
|
|
45
|
Guan GP, Wang HX and Ng TB: A novel
ribonuclease with antiproliferative activity from fresh fruiting
bodies of the edible mushroom Hypsizigus marmoreus. Biochim
Biophys Acta. 1770:1593–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao S, Zhao Y, Li S, Zhang G, Wang H and
Ng TB: Zhao S, Zhao YC, Li SH, Zhang GQ, Wang HX and Ng TB: An
antiproliferative ribonuclease from fruiting bodies of the wild
mushroom Russula delica. J Microbiol Biotechnol. 20:693–699.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang RY, Zhang GQ, Hu DD, Wang HX and Ng
TB: A novel ribonuclease with antiproliferative activity from fresh
fruiting bodies of the edible mushroom Lyophyllum shimeiji.
Biochem Genet. 48:658–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Laemmli UK and Favre M: Maturation of the
head of bacteriophage T4. IDNA packaging events. J Mol Biol.
80:575–599. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Collins RA, Ng TB, Fong WP, Wan CC and
Yeung HW: A comparison of human immunodeficiency virus type 1
inhibition by partially purified aqueous extracts of Chinese
medicinal herbs. Life Sci. 60:PL345–351. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu X, Zheng SY, Cui L, Wang H and Ng TB:
Isolation and characterization of a novel ribonuclease from the
pink oyster mushroom Pleurotus djamor. J Gen Appl Microbiol.
56:231–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ng TB and Wang HX: A novel ribonuclease
from fruiting bodies of the common edible mushroom Pleurotus
eryngii. Peptides. 25:1365–1368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang HX and Ng TB: A novel ribonuclease
from the veiled lady mushroom Dictyophora indusiata. Biochem
Cell Biol. 81:373–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu Y, Chen G, Lv F, Wang X, Ji X, Xu Y,
Sun J, Wu L, Zheng YT and Gao G: Zinc-finger antiviral protein
inhibits HIV-1 infection by selectively targeting multiply spliced
viral mRNAs for degradation. Proc Natl Acad Sci USA.
38:15834–15839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li HC, Huang EY, Su PY, Wu SY, Yang CC,
Lin YS, Chang WC and Shih C: Nuclear export and import of human
hepatitis B virus capsid protein and particles. PLoS Pathog.
6:e10011622010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Böttcher M and Grosse F: HIV-1 protease
inhibits its homologous reverse transcriptase by protein-protein
interaction. Nucleic Acids Res. 25:1709–1714. 1997.PubMed/NCBI
|
|
56
|
Wong JH, Legowska A, Rolka K, Ng TB, Hui
M, Cho CH, Lam WW, Au SW, Gu OW and Wan DC: Effects of cathelicidin
and its fragments on three key enzymes of HIV-1. Peptides.
6:1117–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Michaelis M, Cinatl J, Cinatl J, Pouckova
P, Langer K, Kreuter J and Matousek J: Coupling of the antitumoral
enzyme bovine seminal ribonuclease to polyethylene glycol chains
increases its systemic efficacy in mice. Anticancer Drugs.
13:149–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nasu M, Carbone M, Gaudino G, Ly BH,
Bertino P, Shimizu D, Morris P, Pass HI and Yang H: Ranpirnase
interferes with NF-κB pathway and MMP9 activity, inhibiting
malignant mesothelioma cell invasiveness and xenograft growth.
Genes Cancer. 2:576–584. 2011.PubMed/NCBI
|
|
59
|
Xia L, Chu KT and Ng TB: A low-molecular
mass ribonuclease from the brown oyster mushroom. J Pept Res.
66:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu Y, Wang H and Ng T: Purification and
characterization of a novel RNase with antiproliferative activity
from the mushroom Lactarius flavidulus. J Antibiot (Tokyo).
65:67–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lam SK and Ng TB: Isolation of a novel
thermolabile heterodimeric ribonuclease with antifungal and
antiproliferative activities from roots of the sanchi ginseng
Panax notoginseng. Biochem Biophys Res Commun. 285:419–423.
2001. View Article : Google Scholar : PubMed/NCBI
|