Introduction
Primary osteosarcoma is the most common bone tumor
occurring in childhood and adolescence, comprising 2.4% of all
malignancies in pediatric patients (1,2).
Although neoadjuvant chemotherapy followed by surgical excision has
ameliorated the long-term survival of osteosarcoma patients,
patients that do not respond to commonly used drugs such as
cisplatin, doxorubicin and methotrexate have a poor prognosis
(3,4). The identification of the critical
molecules and/or signal transduction pathways responsible for
regulating development to drug resistance is therefore significant
for the development of novel treatment strategies for this type of
cancer.
Autophagy, a catabolic process for the
autophagosomic-lysosomal degradation of cytoplasmic contents, is
characterized by the formation of autophagosomes (double-membrane
vesicles) (5,6). Autophagy is associated with a number
of physiological processes including differentiation,
neurodegeneration, infection, and cancer (7). Findings of recent studies have
demonstrated that autophagy protects cancer cells from drug-induced
apoptosis and facilitates development to drug resistance (8,9).
Autophagy-related (Atg) genes are involved in the formation of
autophagosomes, which are delivered to lysosomes for degradation.
Atg14, also known as Beclin1-associated autophagy-related key
regulator (Barkor), localizes to autophagosomes, isolation
membranes, and endoplasmic reticulum (ER) and capable of enhancing
Vps34 activity. Knockdown of Barkor inhibits starvation-induced
autophagy (10,11). Furthermore, Barkor recruits a
series of class III PI3-kinase to the ER, where otherwise
phosphatidylinositol 3-phosphate (PI3P) is essentially absent. The
Barkor-dependent appearance of PI3P makes ER the platform for
autophagosome formation.
The ER-resident caspase-12 has been found to mediate
apoptosis signaling induced by ER stress (12). An initial study on caspase-12
knockout mice showed increased resistance to ER stress-induced
apoptosis (13). Another
protease, caspase-7, is also involved in the activation of
caspase-12 in response to ER stress and has been reported to
translocate from the cytosol to the ER to interact with caspase-12
leading to its activation (14–17).
The aim of the present study was to determine
whether knockdown of Barkor is crucial in osteosarcoma cell
chemosensitivity to cisplatin-induced apoptosis through the
activation of ER stress-associated apoptosis.
Materials and methods
Cell culture
The established human Saos-2 osteosarcoma cell line
(HTB-85™, ATCC) was supplied by the Cell Bank of the Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). The Saos-2 cell line was cultured for
<3 months in McCoy’s 5A medium supplemented with 1%
penicillin/streptomycin and 15% fetal bovine serum (Gibco, Grand
Island, NY, USA) at 37°C, 5% CO2.
Reagents and antibodies
Cisplatin (diluted in anhydrous DMF) (P4394) was
purchased from Sigma Chemical Company (St. Louis, MO, USA). The
enhanced chemiluminescence (ECL) kit was from Thermo Scientific
Pierce (no. 32109; Rockford, IL, USA). The Barkor and control siRNA
were purchased from Cell Signaling Technology (Danvers, MA, USA).
Barkor, cleaved PARP, cleaved caspase-9, Bcl-2, Bcl-xl, phospho-p38
MAPK and calpain antibodies were purchased from Cell Signaling
Technology, and the Annexin V-FITC/propidium iodine (PI) apoptosis
detection kit was purchased from Biouniquer Technology, Nanjing,
China.
CCK-8 viability assay
Saos-2 cells were seeded at 1×104
cells/well in a 96-well plate. The cells were treated for 48 h and
incubated for an additional 60 min at 37°C in 10% CCK-8 dye
(Dojindo, CK04). In the case of CCK-8 assay, water-soluble
tetrazolium salt (WST-8) was reduced by dehydrogenases in cells to
yield an orange-colored product (formosan), which is soluble in the
tissue culture medium. The amount of the formazan dye generated by
dehydrogenases in cells was directly proportional to the number of
viable cells. The absorbance was measured at 450 nm using a
microplate reader (Bio-Rad 550, Hercules, CA, USA).
siRNA knockdown of Barkor
The human small interference RNA was used to inhibit
mammalian Barkor/ATG14. Cells were transfected with a pre-designed
siRNA (100 nM) against Barkor (Cell Signaling Technology, 6286)
using the Lipofectamine™ 2000, according to the manufacturer’s
instructions. To assay the downregulation effect on Barkor, the
expression of protein was detected through western blot and
Quantitative real-time polymerase chain reaction (qRT-PCR)
following transfection with siRNA for 48 h. During the period of
maximal protein knockdown, the cells were treated with cisplatin
following siRNA-Barkor transfection.
Flow cytometry: Quantification of
apoptosis
To detect apoptosis, floating cells in the medium
and adherent cells were collected after 48 h of treatment. The
cells were stained using an Annexin V-FITC/PI Apoptosis Detection
Kit (Biouniquer), according to the manufacturer’s instructions.
Untreated cells were used as the control. The samples were analyzed
using a FACSCalibur flow cytometer (Becton-Dickinson, North Ryde,
New South Wales, Australia) within 45 min after the staining.
Analysis of apoptosis by DAPI staining
with laser confocal fluorescence microscopy
DNA damage characteristic of apoptosis was
identified by staining with 4′,6-diamidino-2-phenylindole (DAPI).
Osteosarcoma cells treated with GA for 48 h were washed three times
in phosphate-buffered saline (PBS), and fixed in 4.0%
paraformaldehyde at room temperature for 30 min. Samples were
stained with 1 μg/ml of DAPI (Sigma Chemical Company) for 15 min,
rinsed, and analyzed by laser confocal fluorescence microscopy.
Apoptotic cells were characterized by the decrease of nuclear
chromatin, fragmentation, or margination to the nuclear
membrane.
Western blot analysis
Total cellular protein extracts were prepared by
scraping the cells into Mammalian Protein Extraction Reagent
(Thermo Scientific Pierce, no. 78503). Protein concentration was
measured using BCA protein assay (Thermo Scientific Pierce). The
protein samples were separated on SDS-PAGE at 10–15%, and
electrotransferred onto nitrocellulose membranes (Amersham
Pharmacia Biotech, Zurich, Switzerland). The membranes were blocked
by 5% non-fat milk in Tris-buffered saline Tween-20 (TBST, pH 7.6)
for 60 min at room temperature. Primary antibodies (goat anti-mouse
IgG-HRP; Thermo Scientific Pierce) diluted in 5% BSA of TBST were
incubated overnight at 4°C. The membranes were incubated in
secondary antibodies (goat anti-rabbit IgG-HRP; Thermo Scientific
Pierce) for 90 min at room temperature. The membranes were washed
three times using the above-mentioned procedures. Proteins were
detected using electrochemiluminescence (ECL, Amersham Pharmacia
Biotech).
Gene expression detected by qRT-PCR
Total RNA was prepared using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Total RNA (2 μg) was used to synthesize the first
strand of cDNA. qRT-PCR was performed using Applied Biosystems 7500
Real-Time PCR System and SYBR® Premix Ex Taq™kit
(Perfect Real Time) (Takara Bio Inc., Shiga, Japan). β-actin was
applied as the input reference. Results are presented as CT values,
defined as the threshold PCR cycle number at which an amplified
product is first detected. The CT was determined as the mean of the
triplicate CT values for target gene minus the mean of the
triplicate CT values for β-actin. The primers used were: Barkor,
forward 5′-CACGCCTGTAATCCCAGCTACTC-3′, and reverse
5′-GCAATGGCACAATCTCGGCTCACT-3′; 18S rRNA, forward
5′-GACTCAACACGGGAAACCTCAC-3′, and reverse
5′-CCAGACAAATCGCTCCACCAAC-3′.
Statistical analysis
Data are expressed as mean ± standard deviation. The
mean values were calculated from data obtained in triplicate from
each experiment. The statistical significance of differences was
determined by Student’s two-tailed t-test in two groups and one-way
ANOVA in multiple groups. The data were analyzed using SPSS 17.0.
P-values were two-tailed and P<0.05 was considered statistically
significant. Asterisks indicate the level of significance.
Results
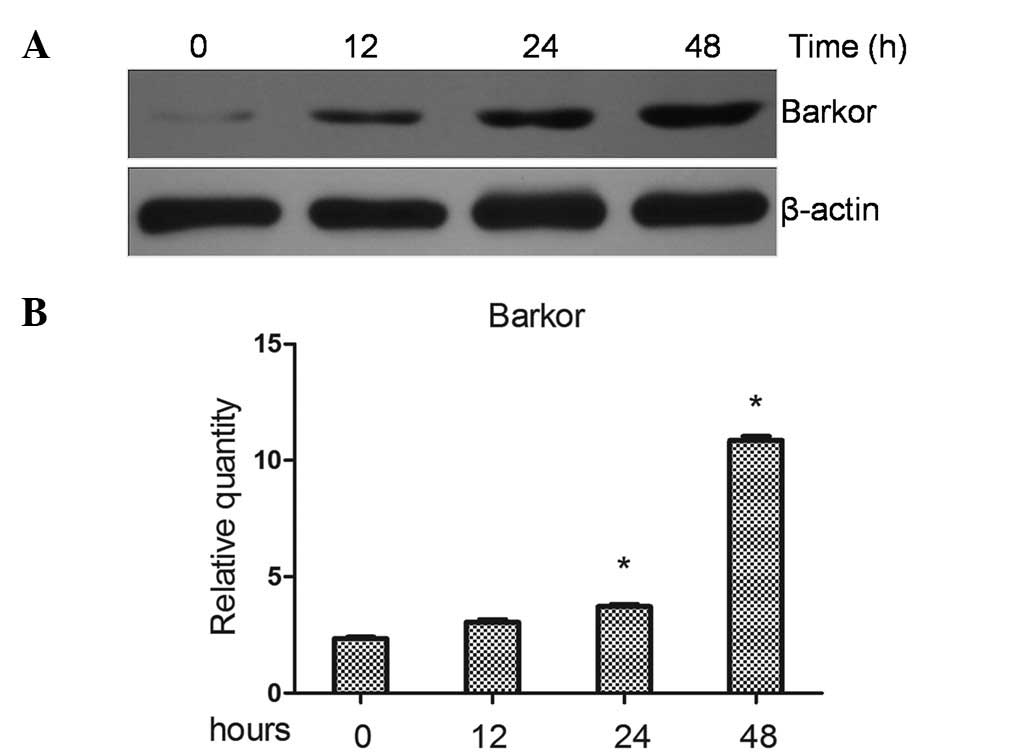
Upregulated expression of Barkor in
cisplatin-treated Saos-2 cells
Following exposure to apoptotic stimuli, Saos-2
cells may activate survival mechanisms to evade the induction of
cell death. It has been reported (8) that autophagy protects cancer cell
from chemotherapeutic drug-induced apoptosis, and Barkor is a key
regulator of autophagy. In this study, the Saos-2 cells were
treated with cisplatin and Barkor protein levels were observed by
western blotting. Results of the western blotting revealed that
cisplatin increased Barkor protein levels of the Saos-2 cell line
(Fig. 1A). By contrast, qRT-PCR
was used to investigate the effects of cisplatin on Barkor at the
transcriptional levels. Cisplatin significantly increased the mRNA
transcripts of Barkor in Saos-2 cell lines (P>0.05) (Fig. 1B).
Barkor siRNA transfection did not inhibit
Saos-2 cell growth
To elucidate the potential role of Barkor, Saos-2
cells were transfected with Barkor or control siRNA. The expression
of Barkor was observed by western blotting and qRT-PCR. The results
revealed that the downregulation of Barkor was significant
(transfection efficiency >70%) (Fig. 2A). A CCK-8 assay was performed to
determine the effects of the transfection of Barkor-siRNA on cell
growth and death. Control, control-siRNA-transfected and
Barkor-siRNA-transfected cells exhibited similar cell growth and
death (Fig. 2B). Silencing of
Barkor thus had no direct effect on host cell growth and
apoptosis.
Interference of Barkor-sensitized Saos-2
cells to cisplatin
Knockdown of Barkor in Saos-2 cells increased their
sensitivity to cisplatin. Fig. 4A
shows the decrease in cell proliferation as a function of drug
concentration following treatment with cisplatin for 48 h.
Barkor-silenced Saos-2 cells showed evident susceptibility to
cisplatin compared with the untransfected and
control-siRNA-transfected parent cells. No significant difference
in survival was observed between control-siRNA-transfected cells
and untransfected Saos-2 cells (Fig.
3A). Results of the CCK-8 assay examination showed that after
48 h treatment, IC50 values for cisplatin were 37.13 μM
in untransfected cells, 41.56 μM in control-siRNA-transfected cells
and 7.86 μM in Barkor-silenced cells (Fig. 3B).
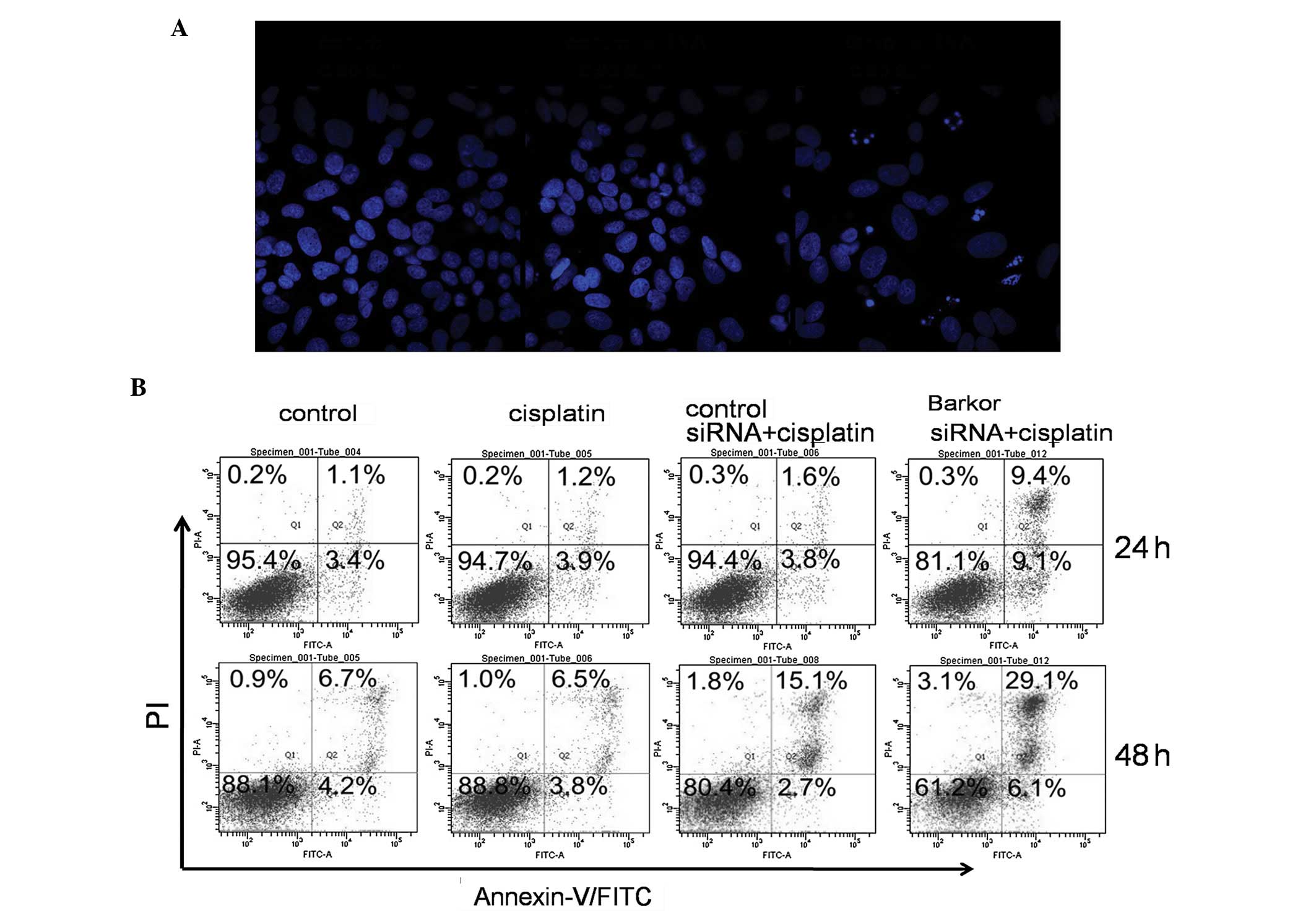
Apoptosis induced by cisplatin in
Barkor-silenced Saos-2 cells
We quantified cisplatin-induced apoptosis of
control- and siRNA-transfected Saos-2 cells. DAPI staining was used
to visualize nucleosomal DNA damage. Pyknosis, crescent-shaped edge
set, nuclear fragmentation and apoptotic bodies were evident in
Barkor-silenced Saos-2 cells treated with cisplatin (2 μM), while
these morphological features were not apparent in the
control-siRNA-transfected or untransfected Saos-2 cells treated
with cisplatin (2 μM) (Fig. 4A).
As shown in Fig. 4B,
Barkor-siRNA-transfected Saos-2 cells treated with cisplatin had
much higher rates of apoptosis than the control and
control-siRNA-transfected cells.
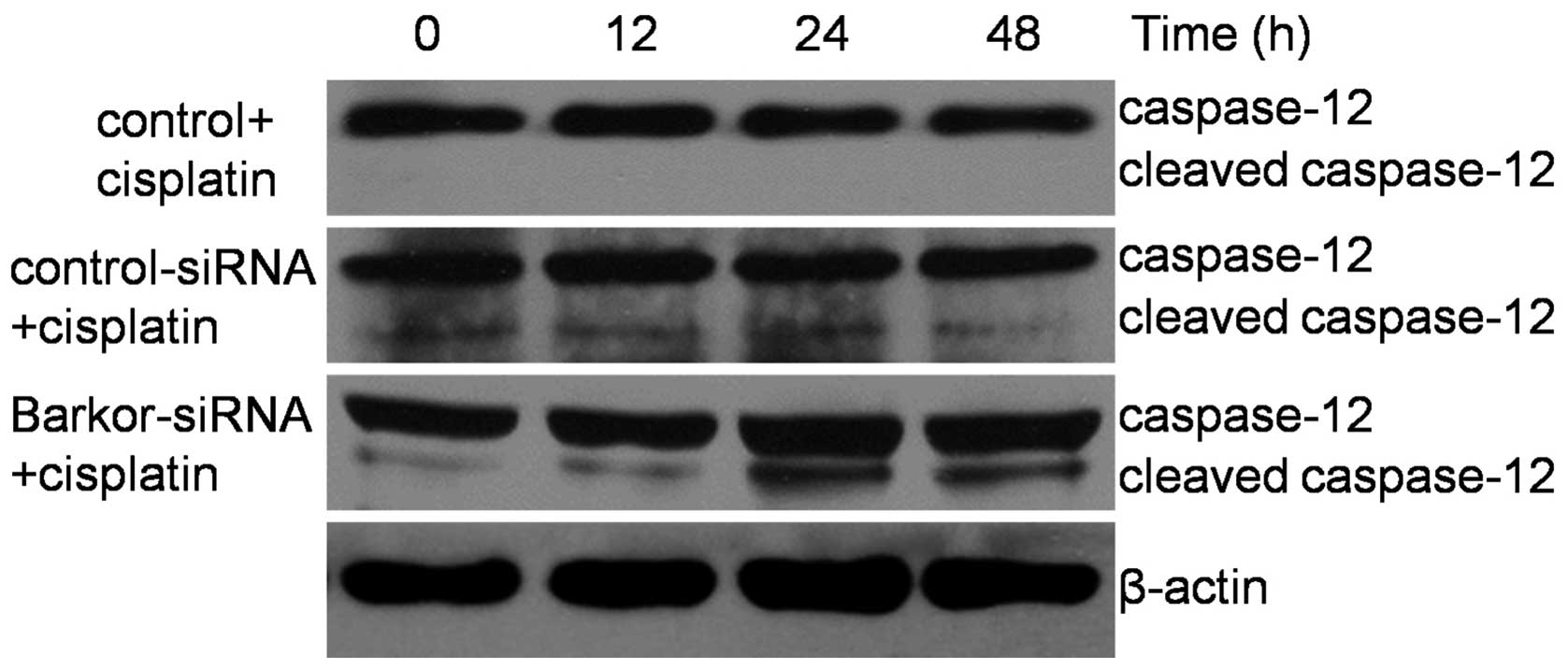
Knockdown of Barkor and upregulation of
caspase-12 combined with cisplatin treatment
Chemotherapeutic agents have previously been found
to activate autophagy in osteosarcoma (18). In the present study, treatment of
Saos-2 cells with cisplatin (10 μM) for 12–48 h induced an increase
in the Barkor level (Fig. 1).
Moreover, we investigated the effect of cisplatin on the caspase-12
protein expression, which is key in ER-mediated apoptosis. Of note,
caspase-12 was not cleaved in control-siRNA-transfected Saos-2
cells but in Barkor-siRNA-transfected Saos-2 cells undergoing ER
stress-induced apoptosis (Fig.
5). These findings suggest that the sensitization of cells by
silencing of Barkor occurs through activation of ER-mediated
apoptosis.
Endoplasmic reticulum- and
mitochondrial-mediated apoptosis induced by cisplatin in
Barkor-silenced Saos-2
To determine whether apoptosis induction by Barkor
knockdown and cisplatin treatment is ER- or mitochondrial-related,
western blotting was used to detect phospho-p38 MAPK, calpain,
PARP, Bcl-2 and Bcl-xl levels in Saos-2 cells treated with Barkor
knockdown and/or cisplatin treatment (1, 2 and 5 μM). As shown in
Fig. 6A, significantly detectable
activation of phospho-p38 MAPK, caspase-12 and cleaved calpain
suggested ER-mediated apoptosis was initiated. Fig. 6B shows that the upregulation of
cleaved caspase-9 and cleaved PARP expression was observed in
Barkor-transfected-Saos-2 cells treated with cisplatin, whereas
Bcl-2 was markedly downregulated in dose-dependent
cisplatin-treated Barkor-transfected-Saos-2 cells. However,
cisplatin had little effect on the expression of another
mitochondrial-mediated apoptosis marker, Bcl-xl. These results
indicated that Silencing of Barkor/ATG14 sensitizes
cisplatin-resistant osteosarcoma cells to ER stress- and
mitochondrial-associated apoptosis.
 | Figure 6Western blot analysis of phospho-p38
MAPK, calpain, cleaved caspase-9, cleaved PARP and antiapoptotic
proteins, Bcl-xL, Bcl-2 (A and B), in whole cell lysates of
Barkor-siRNA-transfected Saos-2 after treatment with 0, 1, 2, 5 μM
of cisplatin for 48 h. Downregulation of antiapoptotic marker Bcl-2
was evident in Barkor silenced cell lines treated with 5 μM of
cisplatin. Cisplatin apparently activated the expression of
phospho-p38 MAPK, calpain, cleaved caspase-9, cleaved PARP.
Barkor/ATG14, Beclin1-associated autophagy-related key
regulator. |
Discussion
Osteosarcoma is an aggressive neoplasm representing
the most common primary malignant bone tumor (19). The survival rate of patients with
osteosarcoma has increased as a result of rapid advancements of
comprehensive therapy, particularly adjuvant chemotherapy.
Nevertheless, the effect of cytotoxic drugs on osteosarcoma becomes
less useful due to acquired chemoresistance. The specific
drug-resistant mechanism and molecular target should be explored to
overcome resistance to chemotherapeutic drugs.
Autophagy, an intracellular catabolic process, has
been shown to play a critical role in multiple biological
functions, including mitochondrial turnover, neuronal function,
protein degradation, cell survival, and energy metabolism (20). The molecular machinery of
autophagy has been identified in yeast and is referred to as
autophagy-related (ATG) genes, one of which ATG14 (also known as
Barkor for Beclin 1-associated autophagy-related key regulator)
(21,22). Barkor is part of a protein complex
comprising Beclin 1, Vps15, and Vps34, and this Atg14-containing
complex is crucial in the initiation process of autophagy (21,22). Results of recent studies have
shown that autophagy may help cancer cells to survive in response
to growth-limiting conditions such as the presence of anticancer
drugs (8,9,18).
In the present study, the hypothesis that silencing of Barkor/ATG14
sensitizes cisplatin-resistant osteosarcoma cells to ER
stress-associated apoptosis was tested and verified.
In the present study, the results of the experiments
of molecular biology were based on the assumption of a cell
survival rate of >80%. As a consequence, the effect of Barkor
knockdown on osteosarcoma cell-enhanced apoptosis is probably not
due to toxicity of transfection reagent and plasmid. Barkor is an
evolutionarily conserved protein that exists in a variety of
organisms of yeast and mammals. The Atg14 protein has two
indispensable domain structures: the coiled-coil domain (CCD) in
the N-terminal region and the Barkor autophagosome targeting
sequence (BATS) domain in the C terminus (10,21,23). The coiled-coil domain is
responsible for the interaction with Beclin 1, and the BATS domain
is required for Atg14 targeting to an autophagosome. The critical
roles of Barkor in autophagy have been firmly established in
cell-based systems or lower organisms (10,11,21,23). However, its gene regulation and
physiological functions in cancer are unclear. Findings of the
present study showed that Atg14 is significantly upregulated in
cisplatin-treated the Saos-2 osteosarcoma cell line. Of note,
activation of caspase-12 and calpain was detected in
Barkor-inhibited Saos-2 cells, but not in untransfected and
control-siRNA-transfected Saos-2 cells. These results suggest that
Barkor regulates the ER stress-induced apoptosis-related genes
calpain and caspase-12.
To determine whether apoptosis induction by Barkor
knockdown and cisplatin treatment is ER stress-mediated, western
blotting, Annexin V-PI apoptosis assay and DAPI staining were used
to detect apoptosis in Saos-2 cells treated with Barkor knockdown
and/or cisplatin treatment. The results have shown that the protein
level of caspase-12 and calpain in cisplatin-induced
Barkor-silenced Saos-2 cells was significantly increased.
Additionally, apoptosis induction by Barkor knockdown and cisplatin
treatment is associated with ER stress and mitochondria.
In conclusion, findings of the present study have
demonstrated that Barkor as a critical regulator of autophagy and
protective molecule induced the cisplatin resistance of human
osteosarcoma cells in vitro. Inhibition of this survival
response is an effective method for chemosensitization of this
malignant osteosarcoma. The results of this study suggest that
knockdown of the Barkor expression may have clinical therapeutic
applications in enhancing the efficacy of cisplatin in
osteosarcoma.
Acknowledgements
This study was supported by the Technological
Project of Traditional Chinese Medicine of Zhejiang Province
(2012ZB092).
Abbreviations:
|
Barkor/ATG14
|
Beclin1-associated autophagy-related
key regulator
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
ATG
|
autophagy-related genes
|
|
ER
|
endoplasmic reticulum
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
BATS
|
Barkor autophagosome targeting
sequence
|
|
CCD
|
coiled-coil domain
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Wittig JC, Bickels J, Priebat D, et al:
Osteosarcoma: a multidisciplinary approach to diagnosis and
treatment. Am Fam Physician. 65:1123–1132. 2002.PubMed/NCBI
|
|
4
|
Meyers PA, Schwartz CL, Krailo M, et al:
Osteosarcoma: a randomized, prospective trial of the addition of
ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and
high-dose methotrexate. J Clin Oncol. 23:2004–2011. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reggiori F and Klionsky DJ: Autophagy in
the eukaryotic cell. Eukaryot Cell. 1:11–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Codogno P and Meijer AJ: Autophagy and
signaling: their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun WL, Chen J, Wang YP and Zheng H:
Autophagy protects breast cancer cells from epirubicin-induced
apoptosis and facilitates epirubicin-resistance development.
Autophagy. 7:1035–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Donovan TR, O’Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011.PubMed/NCBI
|
|
10
|
Zhong Y, Wang QJ, Li X, et al: Distinct
regulation of autophagic activity by Atg14L and Rubicon associated
with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol.
11:468–476. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsunaga K, Saitoh T, Tabata K, et al:
Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally
regulate autophagy at different stages. Nat Cell Biol. 11:385–396.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Momoi T: Caspases involved in ER
stress-mediated cell death. J Chem Neuroanat. 28:101–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Y, Dourdin N, Wu C, De Veyra T, Elce
JS and Greer PA: Ubiquitous calpains promote caspase-12 and JNK
activation during endoplasmic reticulum stress-induced apoptosis. J
Biol Chem. 281:16016–16024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masud A, Mohapatra A, Lakhani SA,
Ferrandino A, Hakem R and Flavell RA: Endoplasmic reticulum
stress-induced death of mouse embryonic fibroblasts requires the
intrinsic pathway of apoptosis. J Biol Chem. 282:14132–14139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao RV, Hermel E, Castro-Obregon S, et al:
Coupling endoplasmic reticulum stress to the cell death program.
Mechanism of caspase activation. J Biol Chem. 276:33869–33874.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinez JA, Zhang Z, Svetlov SI, Hayes
RL, Wang KK and Larner SF: Calpain and caspase processing of
caspase-12 contribute to the ER stress-induced cell death pathway
in differentiated PC12 cells. Apoptosis. 15:1480–1493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang J, Ni J, Liu K, et al: HMGB1
promotes drug resistance in osteosarcoma. Cancer Res. 72:230–238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar
|
|
20
|
Yang Z and Klionsky DJ: Eaten alive: a
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Itakura E, Kishi C, Inoue K and Mizushima
N: Beclin 1 forms two distinct phosphatidylinositol 3-kinase
complexes with mammalian Atg14 and UVRAG. Mol Biol Cell.
19:5360–5372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Q, Fan W, Chen K, Ding X, Chen S and
Zhong Q: Identification of Barkor as a mammalian autophagy-specific
factor for Beclin 1 and class III phosphatidylinositol 3-kinase.
Proc Natl Acad Sci USA. 105:19211–19216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan W, Nassiri A and Zhong Q:
Autophagosome targeting and membrane curvature sensing by
Barkor/Atg14(L). Proc Natl Acad Sci USA. 108:7769–7774. 2011.
View Article : Google Scholar : PubMed/NCBI
|