Introduction
Iron salts are used in the treatment of iron
deficiency anemia, as a supplemental intake of iron during
pregnancy and in multivitamin preparations. In the majority of
cases it is safe to use but toxic effects begin to appear at doses
>10–20 mg/kg of elemental iron, and ingestions of >50 mg/kg
are associated with severe toxicity or lethality (1,2).
Traditionally ferric chloride was used in an arterial thrombosis
model in rats to induce vessel damage resulting in blood clotting
(3). In the 19th century, ferrous
salts and ferrous chloride in particular were considered the most
effective agents in stanching the flow of blood from wounds
(4). Moreover, it was
demonstrated that ferric chloride treatment of mouse aorta ex
vivo caused endothelial denudation, collagen exposure and when
injected intravenously formed occlusive thrombi (5,6).
Diabetic patients are frequently anemic and treatment may include
oral or intravenous iron administration (7). Undas et al observed that
anemic patients on hemodialysis due to chronic kidney disease (CKD)
are at an increased risk of thromboembolic coronary events
associated with the formation of dense fibrin clots resistant to
fibrinolysis (8). Moreover, in
CKD patients a high labile plasma iron level (LPI) associated with
iron supplementation is involved in complications in dialyzed
patients such as myocardial infarction and bacterial infection
(9).
The role of iron treatment in the formation of
fibrin clots should therefore be investigated. Of note is that in
humans divalent iron, Fe+2, is rapidly oxidized to
trivalent iron, Fe3+, by ferroxidase (10). Additionally, ferric chloride and
in general Fe3+ ions show a markedly complex chemistry
producing a multiplicity of compounds over 29 days as shown in the
examples (11): FeCl3
+ 3H2O ⇌ Fe(OH)Cl2 + HCl + 2H2O ⇌
Fe(OH)2Cl + 2HCl + H2O ⇌ Fe(OH)3 +
3HCl. Feng and Nansheng provide additional species distribution of
three simple low-molecular-weight Fe3+ hydroxy complexes
(12): Fe3+ +
H2O → Fe(OH)2+ + H+;
Fe3+ + 2H2O → Fe(OH)2+
+ 2H+; Fe3+ + 2H2O →
Fe2(OH)24+ + 2H+. Moreover,
reactive free radicals are produced in the presence of ferric ions
alone by the Fenton reaction (10): Fe3+ + HO− →
Fe2+ + HO. These changes can be analyzed by UV
spectroscopy since different iron chemicals have distinct
λmax, for example:
Fe(H2O)63+ absorbs λmax
at 240 nm, Fe(OH)2+ shows λmax at 205 and 297
nm, and Fe2(OH)24+ comes into view
at λmax at 335 nm (12).
In the present study, we investigated clotting of
citrated plasma supplemented with Fe3+ (and calcium
Ca2+ to initiate clotting) by thromboelastometry and
electron microscopy. The results showed that iron changes plasma
clotting characteristics, kinetics and the dynamics of clot
formation in plasma. More changes were observed as the time of
storing stock solution of FeCl3 increased, possibly due
to different derivatives of Fe3+ being formatted over 29
days. Additionally, the morphology of clotted fibrin in the
Ca2+- and Fe3+-treated plasma was different
than the untreated, normal, control-clotted fibrin.
Materials and methods
Chemicals, plasticware and proteins
Kaolin, CaCl2 solution, pins and cups
were purchased from Haemoscope Co. (Neils, IL, USA). Fully active
human tissue plasminogen activator (tPA), product number HTPA-TC
was purchased from Molecular Innovations, Inc. (Novi, MI, USA).
Ferric chloride, fibrin and thrombin were purchased from
Sigma-Aldrich Co. LLC (St. Louis, MO, USA).
Preparation of plasma
Lyophilized specialty assayed reference plasma, cat.
no. 5185 (S.A.R.P., 10×1 ml) purchased from Helena Laboratories
(Beaumont, TX, USA) was prepared from a frozen pool of citrated
plasma obtained from healthy donors. S.A.R.P. has normal PT and
aPTT clotting times and may be used as reference data based on the
following parameters: fibrinogen**, factor
II*, factorV**, factor VII*,
factor VIII*, factor IX*, factor
X*, factor XI**, ristocetin
cofactor*, vWF:Ag*, factor XII, protein
C*, protein S - total, free) where (*) denotes samples
standardized according to World Health Organization (WHO)
regulations, and (**) denotes samples calibrated against ISTH
reference material. Plasma was stored at 4°C and reconstituted by
adding 1 ml of deionized water, followed by a 3-min rest. Plasma
for electron microscopy experiments was obtained from healthy
subjects aged between 20 and 25 years, both males and females.
Ethical approval was obtained from the University of Pretoria Human
Ethics Committee, and this study conforms to the principles of the
Declaration of Helsinki.
Analysis of plasma clot formation with
thromboelastography
Thromboelastography allows measurement of a total
coagulation profile and yields data on the kinetics and dynamics of
clot formation in plasma (13).
The essential part of the TEG® 5000
Thrombelastograph® Hemostasis Analyzer System
(Haemonetics Corporation, Braintree, MA, USA) is a pin hanging on a
torsion wire and inserted in a cup holding a sample (360 μl)
(13,14). This pin oscillates at 6 rpm at a
4°45′ angle at 37°C. When plasma viscosity changes during clot
formation, the pin motion is progressively restrained by the clot
and the cup. Sodium-citrated, reconstituted plasma was used for TEG
assays by mixing 1 ml of plasma with 20 μl of kaolin and in some
samples a constant amount of tPA was added [10 μl of tPA (2.1 mg/ml
in 0.4 M HEPES, 0.1 M NaCl, pH 7.4)] as a fibrinolytic agent
(15) to measure proteolysis
under controlled conditions (16,17). Subsequently, 320 μl of the mixture
was transferred to each cup and 20 μl of CaCl2 (0.2 M)
and/or FeCl3 (0.2 M) was added. In a separate experiment
1% of DMSO was added to the stock solution and plasma clotting was
analyzed as described above. The critical parameters of clotting
measured by TEG were: R was the time from initiation of the
reaction until a measurable clot was detected, K was the time from
the R point until a certain clot firmness ws achieved, (α) was the
maximum angle representing kinetics of clotting and LY30
(percentage) represented clot lysis 30 min after MA (maximum
amplitude) (13,18,19).
Electron microscopy
Purified fibrinogen (cat. no. F3879-250MG;
Sigma-Aldrich), human albumin (cat. no. A9511, Sigma-Aldrich)
samples were treated with 5 μl 0.2 M CaCl2, followed by
the addition of 5 μl of freshly prepared 0.2 M FeCl3.
After mixing, thrombin was added, to create an extensive fibrin
network. Human platelet rich plasma (PRP) samples were treated
(addition of CaCl2 and FeCl3) in the same
manner, but without thrombin. The samples were fixed immediately in
2.5% glutaraldehyde/formaldehyde in PBS solution, pH 7.4, for 30
min. The samples were then left for 16 min and 3 h, followed by
fixing in order to obtain a time-dependent analysis of the effect
of FeCl3 and CaCl2 on PRP. Smears were then
fixed followed by rinsing three times with PBS for 5 min prior to
being fixed for 30 min with 1% osmium tetraoxide (OsO4).
The samples were again rinsed three times with PBS for 5 min and
were dehydrated serially with 30, 50, 70 and 90% ethanol, and three
times with 100% ethanol. The material was mounted and coated with
carbon. A Zeiss ULTRA plus FEG-SEM with InLens capabilities
(Microscopy and Microanalysis Unit of the University of Pretoria,
Pretoria, South Africa) was used to study the surface morphology of
fibrin and micrographs were taken at 1 kV.
UV/VIS spectrometry
FeCl3 water solution was diluted at
1:1,000 from 0.2 M stock solution with or without DMSO and analyzed
on a UV/VIS spectrometer at a range of 230–800 nm. Samples were
analyzed at day 0 and periodically up to day 29 after
FeCl3 preparation.
Results
UV/VIS spectrometry
UV/VIS spectra of FeCl3 were altered over
the 29 days (Fig. 1). In general
an increase in the absorption of ~260 nm, and a slight increase of
~290 nm was observed. On day 14 and 29 the opposite changes were
detected. An increase of absorption when approaching 330 nm was
observed over the 29 days.
Analysis of plasma clot formation with
thromboelastography
There are no normal ranges of TEG parameters for
control plasma. However, the results yielded in this study were
very consistent: R (sec): 408, K (sec): 84, An (°): 70.6, MA (mm):
27.2, LY30 (%): 0 (all parameters ±10%) (20). Observed parameters for all the
controls were within these values. The addition of freshly prepared
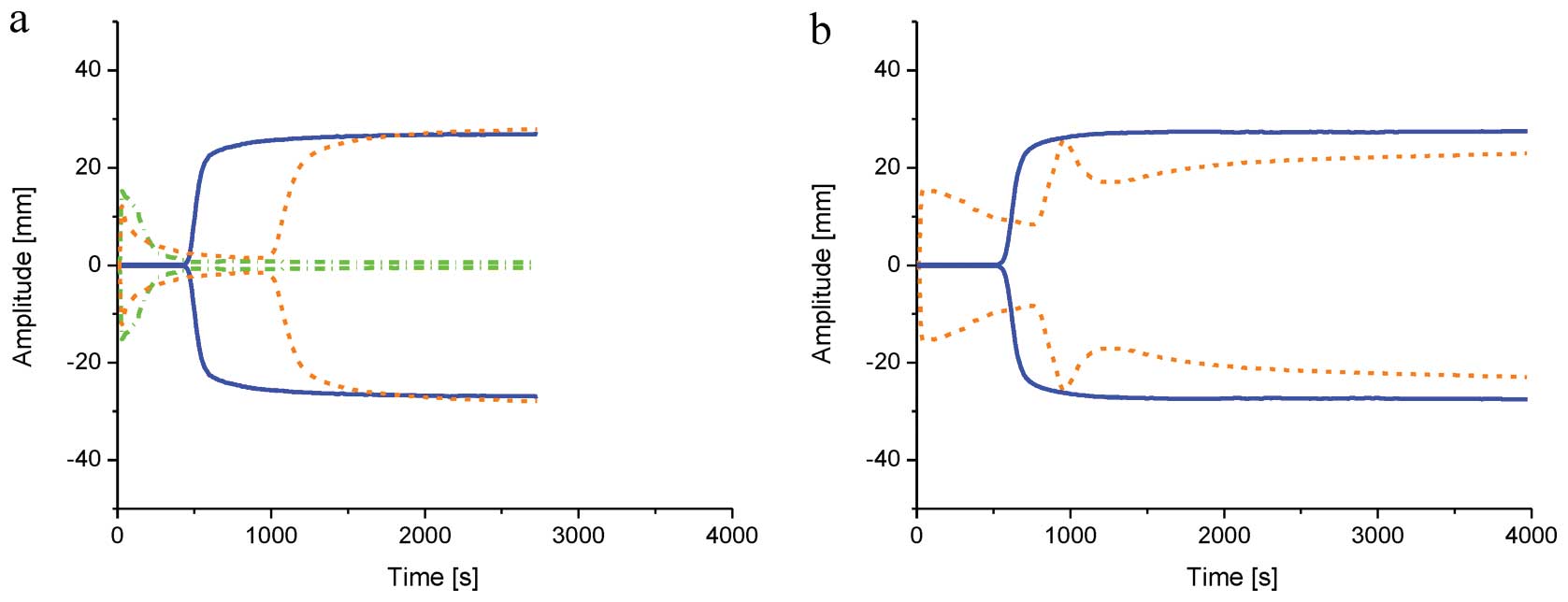
FeCl3 was manifested by an immediate increase of
viscosity/precipitation of plasma proteins [R (sec) ~10, K (sec)
N/A, MA (mm) ~15], followed by lysing as per classical
thromboelastography (Fig. 2).
Classic enzymatic coagulation appeared to be normal, with the
exception of extended R time (~1,050 sec). The remaining parameters
were normal (K, An, MA, LY30) although they were not recorded by
the TEG instrument as it is not designed to register parameters for
the second peak. The addition of tPA to plasma treated with
FeCl3 resulted in coagulation of proteins immediately
after measurement but no enzymatic coagulation (Fig. 2). FeCl3 was stored and
it was observed that over time initial MA increased, while a
secondary peak following initiation of enzymatic coagulation was
observed. By contrast, the strength of the clot as measured by MA
decreased. These changes were gradually more evident over time
(Fig. 2).
While FeCl3 stock solution with DMSO was
used all described effects of Fe3+ were less evident
confirming our previous suggestion that the free radicals were
playing role in coagulation (10).
Electron microscopy
Electron microscopy images revealed that
iron-treated plasma forms structures different from those of the
control fibrin where fibrin strands formed a solid and thick mesh
(Fig. 3). Proteins precipitated
immediately after the addition of Fe3+ did not contain
any typical fibrin fibers (Fig.
3a) but rather large aggregates with circular surface
depressions, as is evident in the morphology of clotted plasma at
~960 sec (time when the secondary peak on the thromboelastogram was
detected). Fig. 3b shows some
scattered fiber strands typical for fibrin in addition to flat,
irregular protein bodies. Images captured at the end of clotting
show numerous granules covering the fiber strands (Fig. 3c).
Discussion
General
The aim of this study was to investigate the effects
of Fe3+ on coagulation. However, during initial
experiments we observed that the stock solution of FeCl3
changed color and some changes were evident in the
thromboelastogram. Therefore, we prepared FeCl3 stock
solution and analyzed the samples obtained by spectroscopy from day
0 to 29. Spectrometric data strongly indicated that
FeCl3 undergoes gradual chemical modification. Our
results suggest that, for example, the concentration of
Fe2(OH)24+ (λmax at 335
nm) increases while that of Fe(OH)2+ (λmax at
297 nm) decreases, which is in agreement with observations from
previous studies (12). Clearly,
the UV data have shown that iron hydrates or other chemicals are
formed; however, they remain to be elucidated.
Fibrinogen coagulation
Simultaneously, we detected gradual changes in the
plasma clotting parameters. Divalent and trivalent metals can
change the clotting characteristics of plasma and blood (21). In the coagulation pathway, ions of
calcium activate prothrombin to thrombin which converts fibrinogen
to fibrin. Calcium is required for two distinct processes in
prothrombin activation: binding factor X and prothrombin to the
phospholipid surface. The first step is activated by numerous
cations such Mg2+, Ca2+, Sr2+,
Ba2+, Mn2+, Be2+, Fe2+,
Fe3+, Zn2+ (22). Replacement of calcium in this step
can slow coagulation depending on the metal (22–26). However, the calcium binding sites
involved in the protein-phospholipid structure, show exceptional
selectivity for cations required for the protein transition, with
the exception of strontium and barium, which can replace calcium in
this role. The other metals form a protein-phospholipid complex
with a different structure resulting in inhibition of the
coagulation reactions (22,23). It is plausible that
Fe3+ interferes in the mechanism of factor X-initiated
prothrombin transformation resulting in slowing clot formation.
Findings of previous studies have shown that iron prevents/slows
the coagulation of normal plasma or blood while Mg2+
increases the clotting time of human plasma (21,27,28). However, we have found that iron
modifies coagulation in a more complex manner than the simple
extension of clot formation.
In the present study, we have established that
coagulation parameters change as FeCl3 storing time
increases. Thus, Fe3+ as well as the hydrolytic
Fe3+ species interact with proteins of the coagulation
cascade. We also observed clot lysis following its initial
formation. This happened a few days after the preparation of
FeCl3 stock solution and was more evident (Fig. 2b) over time. When fibrin is formed
it is relatively unstable. The fibrin clot is stabilized
catalytically by factor XIII and is, not only mechanically stronger
than the non-cross-linked, but also less vulnerable to premature
fibrinolysis degradation (10).
Therefore it is possible that hydrolytic Fe3+ species
inactivates factor XIII making possible a premature partial lysing
of fibrin. Literature on iron and factor XIII is rather sparse, but
it was reported that a severe iron intoxication in a 15-year-old
girl resulted in numerous proteins of the fibrinolytic cascade,
especially factor VIII and XIII, being affected (29). Additionally, Fe3+ or
its hydrolytic species interacts with fibrin or fibrinogen-changing
morphology (Fig. 3c). Tightly
bound fibrin fibers and spherical structures are clearly visible
and this image differs significantly from that of the normal clot
(Fig. 3d). Similar dense matted
deposits and some spherical structures were observed even with
lower concentrations of Fe3+ (30). Furthermore, it has been found that
human fibrinogen directly recognizes iron ions and changes in the
morphology of fibrin may be a result of this modification (31). The experiments conducted on the
animals revealed that iron induces coagulopathy in a dose-dependent
manner. It prolonged the prothrombin, thrombin, and partial
thromboplastin time in animals as well as and in the human plasma.
It was found that thrombin was markedly inhibited by iron in its
clotting effect on fibrinogen. The inhibitory effect was reversible
subsequent to iron removal by EDTA chelation and gel filtration.
Additionally, amidolytic activity of thrombin, factor Xa,
kallikrein, and trypsin were reversibly inhibited by
Fe3+. The coagulopathy was likely induced by
Fe3+ as serine proteases are capable of binding
Fe3+ ion(s) (28).
Free radicals are known to affect coagulation and
fibrinolysis, and free radical scavengers normalize these processes
(32) as was evident from results
of our experiments with DMSO. It was reported that hydroxyl
radical-induced modification of fibrin(ogen) molecules makes them
resistant to fibrinolytic degradation (33). Subsequently, we treated plasma
with Ca2+, Fe3+ and tPA. Non-fibrinogen
coagulation was identified when Fe3+ was added as
expected. However, a fibrin clot was not formed, which may be
attributed to delayed fibrin formation in the presence of
Fe3+, and degradation of fibrinogen and fibrin by
plasmin activated by tPA (34–36). However, in that experiment we
identified some residual but not lysed clots, which may be
explained by the presence of fibrinogen molecules resistant to
fibrinolytic degradation, as described by Lipinski et al
(33).
Non-enzymatic
coagulation/precipitation
In the Fe3+-treated samples instantaneous
formation of insoluble coagulums was observed. This effect was more
prominent over time and was the effect of Fe3+ and its
hydrolytic species (Fig. 2a and
b). The thromboelastograms show that after protein(s)
precipitation these coagulates were lysed, which may be an
artifact. It seems that initially formed large aggregates with
circular surface depressions (Fig.
3a) were self-aggregated to form some scattered fiber strands
typical for fibrin in addition to spherical and flat, irregular
protein bodies. Additionally, after removal of the pin from the TEG
cup a reddish-colored clump was present in all
Fe3+-treated samples (Fig.
4), which may be due to initially formed, loosely connected,
precipitated viscous proteins being clumped by oscillation of the
pin inside of cup. This clump of proteins were rotated inside the
cup with less resistance resulting in instrument interpretation of
this as proteolysis.
We also attempted to identify the proteins that were
precipated following iron addition. Albumin is the most abundant
protein in the circulation and represents 52–60% of the total
plasma protein. It plays an important role in the transportation
and storage of hormones, fatty acids and drugs, and in the
transportation of essential metal ions. Both Fe2+ and
Fe3+ ions bind to heme serum albumin through the heme
iron complex but only Fe3+ binds to heme-free albumin.
Fe3+ ions are transported in plasma mainly by a non-heme
iron-binding glycoprotein transferrin, which composes ~7–10% of
plasma protein (37). Iron can
denature proteins in general and albumin in particular (38,39). The two proteins constitute up to
70% of total plasma proteins. In a separate experiment we therefore
show that Fe3+ precipitates albumin (Fig. 3f). The reddish color observed
sugggests that transferrin possibly co-precipitates among the other
proteins incorporated into these particles (40,41).
FeCl3 is used in animal models to study
early arterial thrombus formation as a result of rapid endothelial
injury, and the associated thrombotic formation. FeCl3
application is a valuable model for investigation into thrombosis
and atherosclerosis. However, caution should be applied since iron
interacts with various proteins from the coagulation cascade and
its effects depend on storage of the stock solution (42).
In conclusion, trivalent iron is involved in
coagulation in a complex manner. It extends the clotting of plasma
by interacting with proteins of the coagulation cascade.
Fe3+ and/or its hydrolytic species interact with
fibrinogen and/or fibrin, changing their morphology and properties.
Moreover, when stored, FeCl3 produces derivatives that
potentiate changes in plasma clotting, some of which can be
attributed to free radicals formed during FeCl3 storage.
In general FeCl3 is able to weaken the fibrin clot while
precipitating plasma proteins immediately after application. This
property can be exploited therapeutically in stanching the flow of
blood from wounds when optimum concentrations of FeCl3
are found.
Acknowledgements
This study was supported in part by grants from the
Frank Stranahan Endowed Chair and Children Miracle Network.
References
|
1
|
Chang TP and Rangan C: Iron poisoning: a
literature-based review of epidemiology, diagnosis, and management.
Pediatr Emerg Care. 27:978–985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mills KC and Curry SC: Acute iron
poisoning. Emerg Med Clin North Am. 12:397–413. 1994.
|
|
3
|
Elg M, Gustafsson D and Carlsson S:
Antithrombotic effects and bleeding time of thrombin inhibitors and
warfarin in the rat. Thromb Res. 94:187–197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Augustus F, Barnard P and Guyot A: New
Universal Cyclopedia: A Scientific and Popular Treasury of Useful
Knowledge. 2. 1st edition. A. J. Johnson & Co; New York: pp.
821881
|
|
5
|
Eckly A, Hechler B, Freund M, et al:
Mechanisms underlying FeCl3-induced arterial thrombosis.
J Thromb Haemost. 779–789. 2011.
|
|
6
|
Woollard KJ, Sturgeon S, Chin-Dusting JP,
Salem HH and Jackson SP: Erythrocyte hemolysis and hemoglobin
oxidation promote ferric chloride-induced vascular injury. J Biol
Chem. 284:13110–13118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’Mara NB: Anemia in patients with chronic
kidney disease. Diab Spectr. 21:12–19. 2008.
|
|
8
|
Undas A, Kolarz M, Kopec G and Tracz W:
Altered fibrin clot properties in patients on long-term
haemodialysis: relation to cardiovascular mortality. Nephrol Dial
Transplant. 23:2010–2015. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esposito BP, Breuer W, Slotki I and
Cabantchik ZI: Labile iron in parenteral iron formulations and its
potential for generating plasma nontransferrin-bound iron in
dialysis patients. Eur J Clin Invest. 32(Suppl 1): 42–49. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipinski B and Pretorius E: Novel pathway
of ironinduced blood coagulation: implications for diabetes
mellitus and its complications. Pol Arch Med Wewn. 122:115–122.
2012.PubMed/NCBI
|
|
11
|
Chlorek żelaza(III). Wikipedia, 2013.
http://pl.wikipedia.org/wiki/Chlorek_%C5%BCelaza%28III%29.
Wikipedia; 2013, (In Polish).
|
|
12
|
Feng W and Nansheng D: Photochemistry of
hydrolytic iron (III) species and photoinduced degradation of
organic compounds. A minireview. Chemosphere. 41:1137–1147. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Evans PA, Hawkins K, Lawrence M, Barrow
MS, Williams PR and Williams RL: Studies of whole blood coagulation
by oscillatory shear, thromboelastography and free oscillation
rheometry. Clin Hemorheol Microcirc. 38:267–277. 2008.PubMed/NCBI
|
|
14
|
Gallimore MJ, Harris SL, Tappenden KA,
Winter M and Jones DW: Urokinase induced fibrinolysis in
thromboelastography: a model for studying fibrinolysis and
coagulation in whole blood. J Thromb Haemost. 3:2506–2513. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carr ME Jr, Krishnamurti C and Alving BM:
Effect of plasminogen activator inhibitor-1 on tissue-type
plasminogen activator-induced fibrinolysis. Thromb Haemost.
67:106–110. 1992.PubMed/NCBI
|
|
16
|
Jankun J, Aleem AM, Selman SH, et al:
Highly stable plasminogen activator inhibitor type one (VLHL PAI-1)
protects fibrin clots from tissue plasminogen activator-mediated
fibrinolysis. Int J Mol Med. 20:683–687. 2007.
|
|
17
|
Jankun J, Aleem AM, Selman SH, Basrur V
and Skrzypczak-Jankun E: VLHL plasminogen activator inhibitor
spontaneously reactivates from the latent to active form. Int J Mol
Med. 23:57–63. 2009.PubMed/NCBI
|
|
18
|
Jankun J, Keck R, Selman SH and
Skrzypczak-Jankun E: Systemic or topical application of plasminogen
activator inhibitor with extended half-life (VLHL PAI-1) reduces
bleeding time and total blood loss. Int J Mol Med. 26:501–504.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jankun J, Selman SH, Keck RW,
Lysiak-Szydlowska W and Skrzypczak-Jankun E: Very long half-life
plasminogen activator inhibitor type 1 reduces bleeding in a mouse
model. BJU Int. 105:1469–1476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jankun J, Skotnicka M, Lysiak-Szydlowska
W, Al-Senaidy A and Skrzypczak-Jankun E: Diverse inhibition of
plasminogen activator inhibitor type 1 by theaflavins of black tea.
Int J Mol Med. 27:525–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jankun J, Skrzypczak-Jankun E and Lipinski
B: Complex function of magnesium in blood clot formation and lysis.
Cent Eur J Immunol. 38:149–153. 2013. View Article : Google Scholar
|
|
22
|
Nelsestuen GL, Broderius M and Martin G:
Role of gamma-carboxyglutamic acid. Cation specificity of
prothrombin and factor X-phospholipid binding. J Biol Chem.
251:6886–6893. 1976.PubMed/NCBI
|
|
23
|
Soriano-Garcia M, Padmanabhan K, de Vos AM
and Tulinsky A: The Ca2+ ion and membrane binding
structure of the Gla domain of Ca-prothrombin fragment 1.
Biochemistry. 31:2554–2566. 1992.
|
|
24
|
Urano T, Ihara H, Suzuki Y, Takada Y and
Takada A: Coagulation-associated enhancement of fibrinolytic
activity via a neutralization of PAI-1 activity. Semin Thromb
Hemost. 26:39–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Urano T, Ihara H, Takada Y, Nagai N and
Takada A: The inhibition of human factor Xa by plasminogen
activator inhibitor type 1 in the presence of calcium ion, and its
enhancement by heparin and vitronectin. Biochim Biophys Acta.
1298:199–208. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Urano T, Nagai N, Matsuura M, Ihara H,
Takada Y and Takada A: Human thrombin and calcium bound factor Xa
significantly shorten tPA-induced fibrin clot lysis time via
neutralization of plasminogen activator inhibitor type 1 activity.
Thromb Haemost. 80:161–166. 1998.PubMed/NCBI
|
|
27
|
Abou-Shady EA, Farrag HE, el-Damarawy NA,
Mohamed FA, Kamel AM and Massoud AA: In vitro effects of trace
elements on blood clotting and platelet function. A--Iron, copper,
and gold. J Egypt Public Health Assoc. 66:21–48. 1991.PubMed/NCBI
|
|
28
|
Rosenmund A, Haeberli A and Straub PW:
Blood coagulation and acute iron toxicity. Reversible iron-induced
inactivation of serine proteases in vitro. J Lab Clin Med.
103:524–533. 1984.PubMed/NCBI
|
|
29
|
Henriksson P, Nilsson L, Nilsson IM and
Stenberg P: Fatal iron intoxication with multiple coagulation
defects and degradation of factor VIII and factor XIII. Scand J
Haematol. 22:235–240. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pretorius E and Lipinski B: Differences in
morphology of fibrin clots induced with thrombin and ferric ions
and its pathophysiological consequences. Heart Lung Circ.
22:447–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orino K: Functional binding analysis of
human fibrinogen as an iron- and heme-binding protein. Biometals.
26:789–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishina M, Komaba Y, Kobayashi S, et al:
Administration of free radical scavenger edaravone associated with
higher frequency of hemorrhagic transformation in patients with
cardiogenic embolism. Neurol Med Chir (Tokyo). 48:292–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lipinski B, Pretorius E, Oberholzer HM and
Van Der Spuy WJ: Iron enhances generation of fibrin fibers in human
blood: implications for pathogenesis of stroke. Microsc Res Tech.
75:1185–1190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dempfle CE, Argiriou S, Alesci S, et al:
Fibrin formation and proteolysis during ancrod treatment. Evidence
for des-A-profibrin formation and thrombin independent factor XIII
activity. Ann NY Acad Sci. 936:210–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drinane MC, Sherman JA, Hall AE, Simons M
and Mulligan-Kehoe MJ: Plasminogen and plasmin activity in patients
with coronary artery disease. J Thromb Haemost. 4:1288–1295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Talbot K, Meixner SC and Pryzdial EL:
Enhanced fibrinolysis by proteolysed coagulation factor Xa. Biochim
Biophys Acta. 1804:723–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu X, Zhang L, Shen D, Wu H and Liu Q:
Oxygen-dependent oxidation of Fe(II) to Fe(III) and interaction of
Fe(III) with bovine serum albumin, leading to a hysteretic effect
on the fluorescence of bovine serum albumin. J Fluoresc.
18:193–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davies MJ, Gilbert BC and Haywood RM:
Radical-induced damage to bovine serum albumin: role of the
cysteine residue. Free Radic Res Commun. 18:353–367. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zardeneta G, Milam SB and Schmitz JP:
Iron-dependent generation of free radicals: plausible mechanisms in
the progressive deterioration of the temporomandibular joint. J
Oral Maxillofac Surg. 58:302–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crichton RR: Proteins of iron storage and
transport. Adv Protein Chem. 40:281–363. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crichton RR and Charloteaux-Wauters M:
Iron transport and storage. Eur J Biochem. 164:485–506. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tseng MT, Dozier A, Haribabu B and Graham
UM: Transendothelial migration of ferric ion in FeCl3
injured murine common carotid artery. Thromb Res. 118:275–280.
2006. View Article : Google Scholar : PubMed/NCBI
|