Introduction
Hepatocellular carcinoma (HCC) is the most common
cause of cancer mortality worldwide (1). Potentially curative treatments
include hepatic resection, liver transplantation, microwave
coagulation and radiofrequency ablation. Palliative therapy
involves transarterial chemoembolization (TACE), systemic
chemotherapy and targeted therapy with sorafenib (2,3).
Liver transplantation and surgical resection improve survival in
patients with small, non-invasive and non-metastatic tumors.
However, there are still no effective treatment for advanced
disease (4,5).
Lignans are a group of complex polyphenolic
antioxidants found in plants. Clinical studies suggest that lignans
are one of the most promising classes of dietary agents for cancer
prevention (6,7). A number of lignans have been
investigated in preclinical tumor xenograft models, prospective and
case-control epidemiologic studies, and in some clinical trials
(6–10). In the absence of data from
randomized clinical trials, it is not known whether lignans can
reduce cancer growth. However, several biomarker-based neoadjuvant
trials have demonstrated that dietary intake of flaxseed lignan
inhibits tumor cell proliferation and induces apoptosis (7,11).
Results of a large prospective study demonstrated an association
between high dietary lignan intake and reduced risk of breast
cancer (10). Previously, it was
reported that isolation of a unique class of vitexins, neolignan
compounds from the seed of Vitex negundo, a Chinese herb
(12), and vitexin compound 1
(VB-1), the purified vitexin compound-1 (a neolignan compound), had
potent cytotoxic effects in various cancer cell lines (12). However, the molecular mechanisms
by which VB-1 induced growth inhibition in HCC cells remain to be
clarified.
Constitutive activation of Akt has been shown to be
associated with increased cell growth, proliferation and
angiogenesis (13–15). Overexpression of Akt has been
reported in a variety of human cancers, including HCC. Cells that
express elevated level of phosphorylated Akt protein, negatively
regulate forkhead transcription family members, in particular
forkhead box protein O3a (FOXO3a) (16–19). These FOXO proteins indirectly
inhibit cell growth and angiogenesis (20). Findings of recent studies have
demonstrated that the amplification or activation of Akt is
required for the growth and survival of HCC cell lines (21,22), supporting the participation of an
activated Akt pathway in the tumorigenesis of HCC.
FOXO3a is a forkhead/winged helix box class O (FOXO)
transcription factor that is involved in a variety of cell
processes such as cell cycle progression, stress detoxification,
DNA damage repair, glucose metabolism and differentiation (23). It is regulated by a number of
mechanisms, including phosphorylation. Phosphorylated FOXO3a
proteins have been shown to bind to 14-3-3 chaperone proteins
causing them to become sequestered in the cytoplasm, where they are
unable to regulate gene expression. When active, FOXO3a induces
cell cycle arrest and apoptosis, by negatively mediating
angiogenesis signaling and acting as an anti-proliferative
factor.
Studies in mammalian cells have identified FOXO3a as
a target gene that regulates other genes such as survivin,
p27Kip1, p21 and vascular endothelial growth factor
(VEGF) (24). FOXO3a
transcription factors are known to be cellular targets of antitumor
drugs used to treat HCC (25) and
chronic myeloid leukemia (26).
However, whether VB-1 induces cell cycle arrest and angiogenesis
inhibition in HCC cells by regulating FOXO3a transcription factor
has yet to be determined.
In this study, we investigated the anticancer
activity of VB-1 and analyzed its underlying molecular mechanisms.
The results demonstrated that VB-1 selectively induces cytotoxicity
in HCC cells. It inhibits phosphorylation of Akt and FOXO3a
transcription factor, leading to cell cycle arrest and inhibition
of angiogenesis.
Materials and methods
Cell lines and cell culture
Human HCC cell lines Hep3B, Huh-7 and HepG2 and
human embryo liver L-02 cells were purchased from the China Centre
for Type Culture Collection (CCTCC, Wuhan, China). The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen-Gibco, Grand Island, NY, USA), 100
U/ml penicillin and 100 U/ml streptomycin and were kept in a
humidified atmosphere with 5% CO2 at 37°C.
Reagents
VB-1
(6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydro-methyl-7-methoxy-3,4-dihydro-2-naphthaldehyde)
was purified from EVn-50, a mixture of lignan compounds from
Vitex negundo seed, as described previously (12).
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), fluorouracil (5-FU), crystal violet, low-gelling point
SeaPlaque agarose and propidium iodide (PI) were purchased from
Sigma Chemical Co. (St. Louis, MO, USA). Antibodies against
phospho-PI3K, PI3K, phospho-Akt, Akt, p21/CIP1, p27/KIP1, cyclin
D1, phospho-FOXO3a, FOXO3a and β-actin were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Enhanced
chemiluminescence (ECL) Western Blot Detection reagents were
obtained from Amersham Life Sciences, Inc. (Arlington Heights, IL,
USA).
MTT assay
Cells were seeded in a 96-well plate at a density of
0.5×104 cells/well and maintained in serum-free medium
for 24 h. Various concentrations of experimental agents were added
to each well and cultured for 48 h, followed by incubation with
media containing 0.5 mg/ml MTT for 4 h. The supernatant was removed
by centrifugation (1,000 rpm, 5 min) DMSO (100 μl) was added and
absorbance at 570 nm (A570) was measured using an
enzyme-labeling instrument (ELX-800 type; Bio-Tek, Shanghai,
China). The relative cell viability rate was calculated as:
(average A570 of the experimental
group/average A570 of the control
group) ×100%.
Clonogenic assay
Cells were plated in 24-well plates at a density of
300 cells/well for 24 h, prior to the addition of various
concentrations of experimental agents. After 48 h the
drug-containing medium was removed and replaced with complete
growth medium. The medium was then replaced every 3 days for 7–10
days until visible colonies formed. Colonies were simultaneously
fixed and stained with 0.5% crystal violet in methanol. Individual
stained colonies in each well were manually counted and the colony
formation fraction was calculated as: colony number/(number of
cells seeded x plating efficiency). Plating efficiency was
estimated as the colony number divided by the number of cells
seeded in the drug-free medium.
Soft agar cloning assay
The base layer consisted of 0.6% low-gelling point
SeaPlaque agarose in complete DMEM culture medium. Soft agar
consisting of 0.3% SeaPlaque agarose in complete DMEM culture
medium was mixed with 1×104 HCC cells and plated on top
of the base layer in a 60 mm diameter culture dish. Soft agar
cultures were maintained at 37°C and observed with a Leica DMI
4000B microscope imaging system to evaluate colony counts.
Cell cycle analysis by flow
cytometry
Cells were plated in 6-well plates at a density of
1×106 cells/well for 24 h, prior to the addition of
various concentrations of test agents. After 24 h the cells were
harvested, and DNA content was stained for 15 min at 37°C with a
solution containing 0.4% Triton X-100 (Sigma), 50 μg/ml of PI
(Sigma), and 2 μg/ml of DNase-free RNase (Roche, Indianapolis, IN,
USA). The plates were then analyzed for cell cycle perturbation
using a FACSCalibur system (FACS 420; Becton-Dickinson, Franklin
Lakes, NJ, USA). The CellQuest program was used to quantify the
distribution of cells in each cell cycle phase: G1, S and G2/M.
DNA transfection
The small interfering RNA (siRNA) duplexes targeting
the sequence 5′-UAAUGUGCCCGUCCUU GUCUU-3′ of the human Akt
gene, the sequence 5′-ACU CCGGGUCCAGCUCCAC-3′ of the FOXO3a
gene and control siRNA oligonucleotides were purchased from
Dharmacon Research, Inc. (Lafayette, CO, USA). For silencing of Akt
or FOXO3a, HepG2 cells were transfected with double stranded siRNA
of Akt or FOXO3a using a Signal Silence siRNA kit from Cell
Signaling Technology, Inc. Briefly, 1×106 cancer cells
were plated in 60 mm Petri dishes for 24 h and exposed to 3 ml of
transfection medium containing 20 μg Lipofectamine 2000 and 100 nM
siRNA for 24 h. Gene silencing in transfected cells was confirmed
by western blotting.
Western blot analysis
Western blot analyses were performed as described
previously (12). In brief, cells
were lysed in RIPA buffer containing 1X protease inhibitor
cocktail. Protein concentrations were determined using the Bradford
assay (Bio-Rad, Philadelphia, PA, USA). Proteins were separated
using 10–12.5% SDS/PAGE and transferred to membranes (Millipore,
Bedford, MA, USA) in a Tris (20 mM), glycine (150 mM) and methanol
(20%) buffer at 55 V for 4 h at 4°C. After blocking in 5% non-fat
dry milk in TBS, the membranes were incubated with the primary
antibodies at 1:1,000 dilution in TBS overnight at 4°C. The
membranes were then washed three times with TBS-Tween-20, and
incubated with secondary antibodies conjugated with horseradish
peroxidase at 1:5,000 dilutions in TBS for 1 h at room temperature.
The membranes were washed another three times in TBS-Tween-20 at
room temperature. Protein bands were visualized on X-ray film using
an enhanced chemiluminescence detection system.
Determination of VEGF
Cells were seeded in 6-well plates
(1.0×105 cells/well) and incubated at 37°C. After 24 h,
the cell culture supernatant was collected, and the cells were
counted after trypsinization. After collection, the medium was
centrifuged at 800 × g for 3 min at 4°C to remove cell debris. The
supernatant was frozen at −20°C for subsequent VEGF assay or
assayed immediately using commercially available ELISA kits
(R&D Systems, Minneapolis, MN, USA).
Matrigel in vitro endothelial tube
formation assay
Cells were cultured in serum-free DMEM for 24 h. The
conditioned media were collected, centrifuged, and transferred to
fresh tubes and stored at −20°C. Growth factor-reduced Matrigel
(125 μl), was thawed on ice, and plated in a 6-well plate. The
plate was then incubated at 37°C for 30 min to allow the Matrigel
to polymerize. HUVECs were trypsinized and seeded (5×104
cells/well) in each well with 250 μl of conditioned medium
from VB-1-treated or non-treated control HepG2 cells. The plate was
incubated for 6 h. Each well was photographed using an inverted
microscope with digital camera. The assessment of the vessel number
of the vessel perimeter in each field was calculated using the
Scion Image analysis program.
Statistical analysis
Statistical analysis was performed using Prism
statistical analysis software (GrafPad Software, Inc., San Diego,
CA, USA). The data are presented as means ± standard deviations of
experiments performed in triplicate for each experimental group.
Differences between groups were analyzed by one- or two-way
analysis of variance (ANOVA), followed by Bonferoni’s multiple
comparison tests. P<0.05 was considered to indicate statistical
significance.
Results
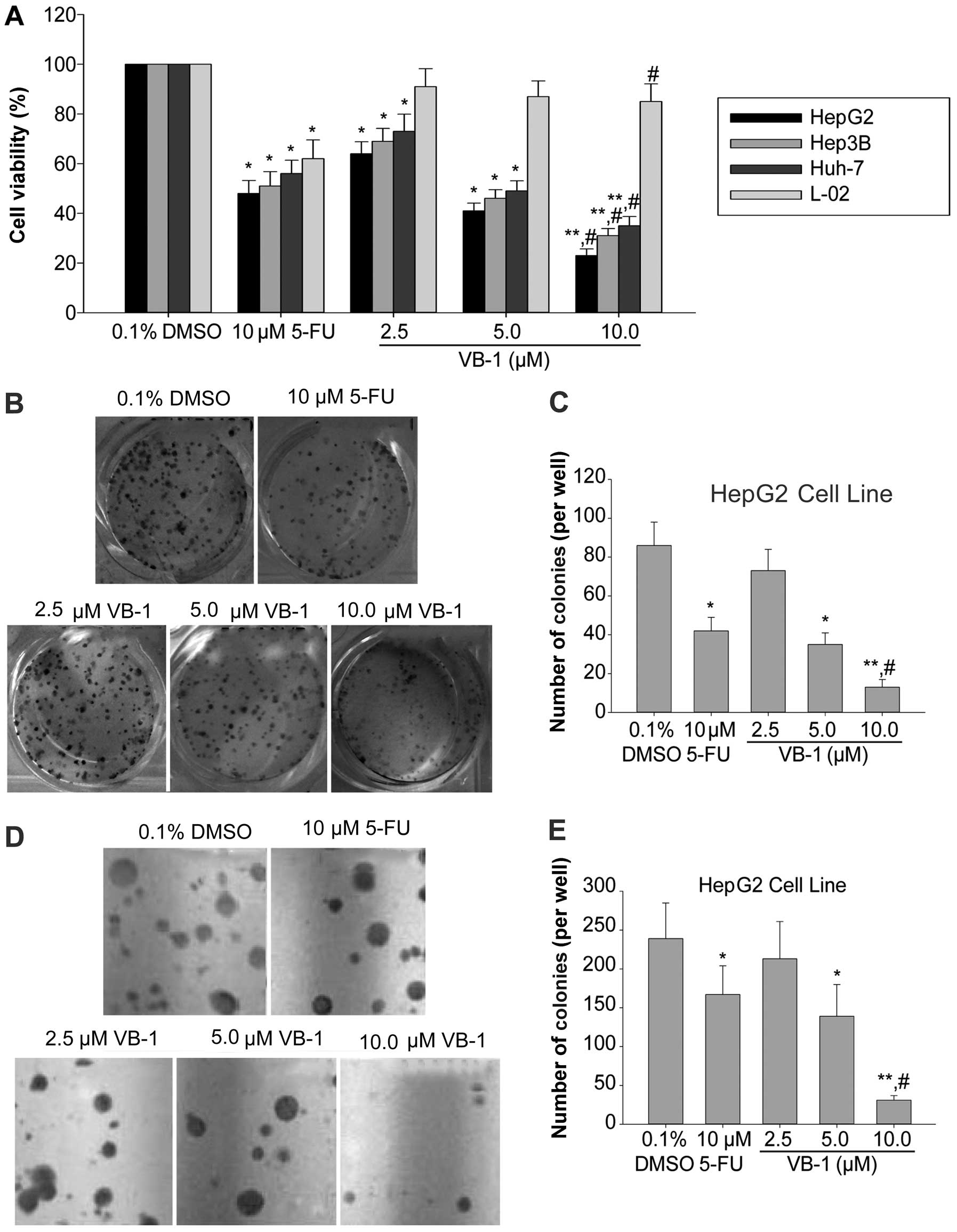
VB-1 inhibits the growth of human HCC
cells
VB-1 has been shown to inhibit the growth of several
cancer cell lines, including HCC cells (12). Therefore, its effects on cell
viability in the HCC cell lines HepG2, Hep3B and Huh-7 were
examined using an MTT assay. Fig.
1A shows that VB-1 inhibited cell viability in a
concentration-dependent manner. The HepG2 cell line was most
sensitive, the Hep3B cell line was moderately sensitive, and the
Huh-7 cell line was least sensitive. VB-1 had little effect on the
human embryo liver L-02 cell line. These data suggested that VB-1
is likely a selective agent that inhibits HCC cell
proliferation.
We examined the effects of VB-1 on colony formation
of the HCC cell line HepG2 on agar plates and showed that VB-1
inhibited anchorage-dependent growth in a dose-dependent manner
(Fig. 1B and C). Additionally
VB-1 inhibited colony formation in a dose-dependent manner in a
soft agar assay (Fig. 1D and E).
These data suggest that VB-1 acts as a potent chemopreventive agent
for HCC.
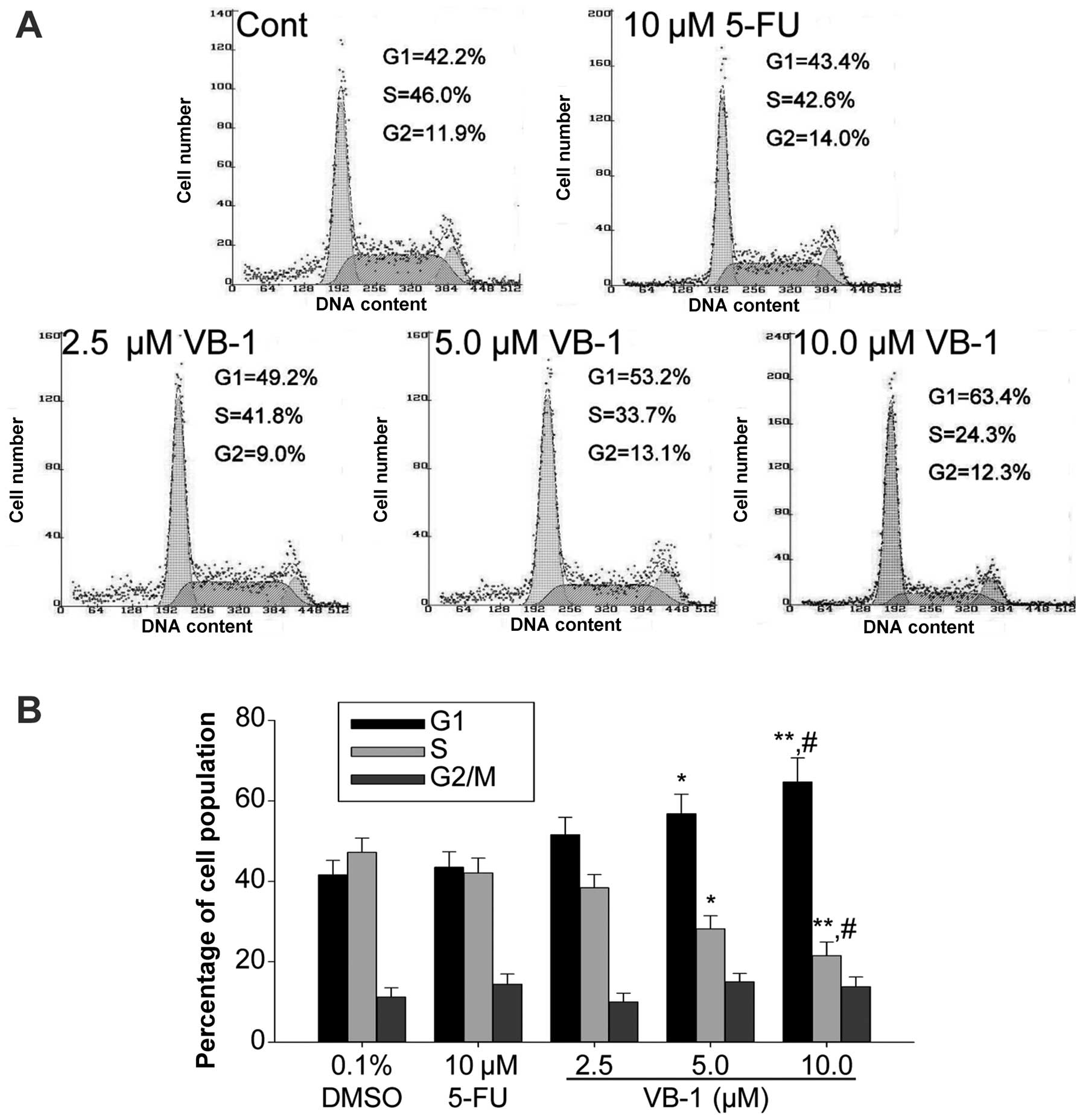
VB-1 arrests the cell cycle at the G1/G0
phase in HepG2 cells
It has been previously demonstrated that VB-1 is
capable of inducing G1/G0 phase arrest in breast cancer cells
(12). Therefore, we investigated
whether a similar effect occurs in HepG2 cells. The effect of VB-1
on the cell cycle was determined using FCM analysis. VB-1 resulted
in an accumulation of cells in the G1/G0 phase (Fig. 2). This concentration-dependent
effect suggests that VB-1 induces cell growth inhibition in HepG2
cells via G1/G0 phase arrest.
VB-1 decreases PI3K and Akt
phosphorylation in HepG2 cells
Akt is constitutively active in most cancer cells,
and it enhances cell proliferation (27). In order to understand the
correlation between PI3K/Akt and VB-1-induced growth inhibition, we
determined the expression of protein phosphorylation of PI3K and
Akt in cells exposed to VB-1. VB-1 resulted in slight inhibition of
PI3K phosphorylation in HepG2 cells (Fig. 3A) and relatively marked inhibition
of Akt phosphorylation (Fig. 3B).
VB-1 had no effect on total PI3K and Akt expression (Fig. 3A and B). These data suggest that
VB-1 inhibits cell proliferation by inhibiting PI3K and Akt
phosphorylation.
To investigate the role of Akt in VB-1-mediated
growth inhibition, we used a siRNA that specifically silences Akt.
Expression of siRNAs silence gene expression resulting in
functional inactivation (6,7).
Western blotting showed that Akt was downregulated after
transfection with a siRNA that targeted Akt in HepG2 cells
(Fig. 3C). In addition, siRNA Akt
enhanced the ability of VB-1 to inhibit cell viability in HepG2
cells (Fig. 3D). These results
suggested that VB-1-mediated Akt signaling inhibition may
contribute to growth inhibition of HepG2 cells.
VB-1 regulates the expression of
phosphorylated FOXO3a and downstream target genes associated with
cell cycle regulation
Akt kinase has been shown to regulate the
phosphorylation of FOXO3a protein (28,29). The phosphorylation of FOXO3a
proteins was determined using western blotting. VB-1 inhibited the
phosphorylation of FOXO3a protein. However, VB-1 had no effect on
the total protein of FOXO3a expression (Fig. 4A).
Downregulation of FOXO3a by siRNA transfection
reduced the expression of FOXO3a protein, as confirmed by western
blotting (Fig. 4B). Additionally,
VB-1 inhibited the viability of HepG2 cells, and inhibition of
FOXO3a expression by siRNA suppressed VB-1-induced growth
inhibition (Fig. 4C). These
results suggested that VB-1 inhibited growth by regulating
FOXO3a.
The effects of VB-1 on cell cycle regulatory genes
were also examined. VB-1 induced the expression of the cell cycle
inhibitors p21CIP1, p27Kip1 and inhibited
cyclin D1 expression in HepG2 cells (Fig. 4D). These results suggested that
VB-1 induced growth arrest by regulating the expression of cell
cycle genes.
Conditioned medium from cells exposed to
VB-1 inhibits VEGF activity and tube formation of HUVECs
induced
FOXO3a has been shown to regulate VEGF signaling in
various cell types (30). To
determine whether VB-1 reduces VEGF protein expression and activity
by activating FOXO3a, we examined the levels of VEGF protein
expression and activity in culture medium. The results showed that
VB-1 reduced VEGF protein expression and decreased the levels of
VEGF secreted in the culture medium (Fig. 5A and B). However, there was a
marked increase in the activity of VEGF in FOXO3a siRNA transfected
HepG2 cells (data not shown).
It has been suggested that the activation of FOXO3a
transcription factor is an important physiological process that
inhibits angiogenesis and ultimately controls tumor growth
(31). FOXO3a has been shown to
inhibit angiogenesis and metastasis in certain tumor models
(22). Since VB-1 inhibited VEGF
expression and secretion in HepG2 cells, we examined whether
conditioned media from VB-1-treated cells were able to reduce tube
formation, which is an indirect measure of angiogenesis. An in
vitro tube formation assay was performed in growth
factor-reduced Matrigel. Conditioned media from VB-1-treated cells
significantly reduced tube formation of HUVECs after 6 h incubation
compared with the medium from control cells (Fig. 5C and D). By contrast, conditioned
media from FOXO3a siRNA-transfected HepG2 cells increased tube
formation of HUVECs after 6 h incubation compared with the medium
from control siRNA-transfected cells (data not shown).
Discussion
Surgery, chemotherapy, and radiotherapy are
generally used to treat HCC, however, no effective therapy for
advanced HCC is currently available. Thus, there is a need to
identify other therapeutic agents against this disease. VB-1, the
purified vitexin compound 1 present in Vitex negundo seed,
has been shown to exert anticancer activity and to be associated
with low toxicity (12). In the
present study, we showed that VB-1 suppressed the proliferation of
HCC cell lines HepG2, Hep3B, Huh-7, but had little effect on L-02
cells. Additionally, VB-1 significantly inhibited the
anchorage-dependent and anchorage-independent growth of HepG2 cells
in a concentration-dependent manner by inducing cell cycle arrest
at G1/G0. VB-1 also reduced the secretion of VEGF, resulting
in inhibition of the endothelial tube formation. To the best of our
knowledge, we have shown for the first time that VB-1-induced
growth inhibition and cell cycle arrest are mediated by regulation
of the Akt/FOXO3a pathway.
The Akt pathway is crucial in sustaining survival
against the programmed death in cancer cells. It has been widely
shown that hyperactivation of Akt is a common event in many human
cancer types, and activation of the PI3K/Akt pathway has been
reported to contribute to chemotherapy resistance (32–34). This pathway, therefore, is an
important target for anticancer therapies. Identification of a safe
and effective therapeutic inhibitor of PI3K or Akt continues to be
a challenge. This study has demonstrated that VB-1 inactivates Akt,
which, in turn, reduces the phosphorylation of FOXO3a, leading to
an increased expression of downstream target genes, such as p21 and
p27, and a decreased expression of cyclin D1 exprotein.
Upregulation of p21 and p27, which bind to CDK2/CD4, have
previously been shown to sequester the procedure involved in cell
cycle arrest (35,36). This was also demonstrated in our
study using VB-1-treated HepG2 cells. In-depth studies are required
to clarify whether VB-1 is a direct or indirect inhibitor of Akt.
However, the pharmacological and toxicological profiles of VB-1
partly revealed in this study, as well as its in vivo
effectiveness in inhibiting xenografted tumor growth (12), suggest that the compound may
become an anti-Akt candidate for HCC therapy.
In conclusion, taken together our results
demonstrate that VB-1 induces cell cycle arrest and growth
inhibition by the inactivation of Akt which in turn regulates a
FOXO3a transcription factor. Genetic inhibition of Akt pathways are
known to have synergistic effects on the activation of FOXO3a
transcription factor through dephosphorylation. Thus, VB-1 appears
to be an attractive agent for the prevention and treatment of
HCC.
Acknowledgements
We express our thanks to Dr Jian-Guo Cao (Medical
College, Hunan Normal University, Changsha, Hunan, China) for
critical input into the scientific content of the manuscript. This
study was supported by the Major State Science and Technology
Special Purpose of China (2009ZX09102-109), the National Science
and Technology Major Projects for ‘Major New Drugs Innovation and
Development’ (2012ZX09303014001), the International Science and
Technology Cooperation Program of China (2011DFA30620) and the
Hunan Province Science and Technology Project (2009FG3142).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32:225–237. 2000. View Article : Google Scholar
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai EC and Lau WY: The continuing
challenge of hepatic cancer in Asia. Surgeon. 3:210–215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson LU, Chen JM, Li T,
Strasser-Weippl K and Goss PE: Dietary flaxseed alters tumor
biological markers in postmenopausal breast cancer. Clin Cancer
Res. 11:3828–3835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JB, Lee MS, Cha EY, et al:
Magnolol-induced apoptosis in HCT-116 colon cancer cells is
associated with the AMP-activated protein kinase signaling pathway.
Biol Pharm Bull. 35:1614–1620. 2012.PubMed/NCBI
|
|
8
|
Kuijsten A, Arts IC, Hollman PC, van’t
Veer P and Kampman E: Plasma enterolignans are associated with
lower colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev.
15:1132–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adlercreutz H: Lignans and human health.
Crit Rev Clin Lab Sci. 44:483–525. 2007. View Article : Google Scholar
|
|
10
|
Bergman Jungestrom M, Thompson LU and
Dabrosin C: Flaxseed and its lignans inhibit estradiol-induced
growth, angiogenesis, and secretion of vascular endothelial growth
factor in human breast cancer xenografts in vivo. Clin Cancer Res.
13:1061–1067. 2007.PubMed/NCBI
|
|
11
|
Cuca LE, Coy ED, Alarcón MA, Fernández A
and Aristizábal FA: Cytotoxic effect of some natural compounds
isolated from Lauraceae plants and synthetic derivatives.
Biomedica. 31:335–343. 2011.PubMed/NCBI
|
|
12
|
Zhou Y, Liu YE, Cao J, et al: Vitexins,
nature-derived lignan compounds, induce apoptosis and suppress
tumor growth. Clin Cancer Res. 15:5161–5169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Xiao Y, Yuan Y, et al: Effects of
oleic acid on cell proliferation through an integrin-linked kinase
signaling pathway in 786-O renal cell carcinoma cells. Oncol Lett.
5:1395–1399. 2013.PubMed/NCBI
|
|
14
|
Vene R, Benelli R, Minghelli S, Astigiano
S, Tosetti F and Ferrari N: Xanthohumol impairs human prostate
cancer cell growth and invasion and diminishes the incidence and
progression of advanced tumors in TRAMP mice. Mol Med.
18:1292–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubinfeld H, Cohen-Kaplan V, Nass D, et
al: Heparanase is highly expressed and regulates proliferation in
GH-secreting pituitary tumor cells. Endocrinology. 152:4562–4570.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Wu X, Zhong D, Zhai W, Ding Z and
Zhou Y: Short hairpin RNA (shRNA) Ether à go-go 1 (Eag1) inhibition
of human osteosarcoma angiogenesis via VEGF/PI3K/AKT signaling. Int
J Mol Sci. 13:12573–12583. 2012.
|
|
17
|
Khaidakov M and Mehta JL: Oxidized LDL
triggers pro-oncogenic signaling in human breast mammary epithelial
cells partly via stimulation of MiR-21. PLoS One. 7:e469732012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Dijk M, van Bezu J, van Abel D, et al:
The STOX1 genotype associated with pre-eclampsia leads to a
reduction of trophoblast invasion by alpha-T-catenin upregulation.
Hum Mol Genet. 19:2658–2667. 2010.PubMed/NCBI
|
|
19
|
Biggs WH III, Meisenhelder J, Hunter T,
Cavenee WK and Arden KC: Protein kinase B/Akt-mediated
phosphorylation promotes nuclear exclusion of the winged helix
transcription factor FKHR1. Proc Natl Acad Sci USA. 96:7421–7426.
1999. View Article : Google Scholar
|
|
20
|
Band AM, Björklund M and Laiho M: The
phosphatidylinositol 3-kinase/Akt pathway regulates transforming
growth factor-{beta} signaling by destabilizing ski and inducing
Smad7. J Biol Chem. 284:35441–35449. 2009.
|
|
21
|
Wang L, Jia D, Duan F, et al: Combined
anti-tumor effects of IFN-alpha and sorafenib on hepatocellular
carcinoma in vitro and in vivo. Biochem Biophys Res Commun.
422:687–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanbrocklin MW, Robinson JP, Lastwika KJ,
McKinney AJ, Gach HM and Holmen SL: Ink4a/Arf loss promotes tumor
recurrence following Ras inhibition. Neuro Oncol. 14:34–42. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim BC: FoxO3a mediates transforming
growth factor-beta1-induced apoptosis in FaO rat hepatoma cells.
BMB Rep. 41:728–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang S, Chong ZZ, Shang YC and Maiese K:
WISP1 neuroprotection requires FoxO3a post-translational modulation
with autoregulatory control of SIRT1. Curr Neurovasc Res. 10:54–69.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang C, Chen W, Zhi X, et al: Serotonin
promotes the proliferation of serum-deprived hepatocellular
carcinoma cells via upregulation of FOXO3a. Mol Cancer. 12:142013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naka K, Hoshii T, Muraguchi T, et al:
TGF-beta-FOXO signalling maintains leukaemia-initiating cells in
chronic myeloid leukaemia. Nature. 463:676–680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Jiang H, Gao H, et al: The
monoclonal antibody CH12 enhances the sorafenib-mediated growth
inhibition of hepatocellular carcinoma xenografts expressing
epidermal growth factor receptor variant III. Neoplasia.
14:509–518. 2012.
|
|
28
|
Chung YW, Kim HK, Kim IY, Yim MB and Chock
PB: Dual function of protein kinase C (PKC) in
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced manganese
superoxide dismutase (MnSOD) expression: activation of CREB and
FOXO3a by PKC-alpha phosphorylation and by PKC-mediated
inactivation of Akt, respectively. J Biol Chem. 286:29681–29690.
2011.
|
|
29
|
Kashiwagi A, Fein MJ and Shimada M:
Calpain modulates cyclin-dependent kinase inhibitor 1B (p27(Kip1))
in cells of the osteoblast lineage. Calcif Tissue Int. 89:36–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dormond O, Madsen JC and Briscoe DM: The
effects of mTOR-Akt interactions on anti-apoptotic signaling in
vascular endothelial cells. J Biol Chem. 282:23679–23686. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davis R, Singh KP, Kurzrock R and Shankar
S: Sulforaphane inhibits angiogenesis through activation of FOXO
transcription factors. Oncol Rep. 22:1473–1478. 2009.PubMed/NCBI
|
|
32
|
Wu HH, Wu JY, Cheng YW, et al: cIAP2
upregulated by E6 oncoprotein via epidermal growth factor
receptor/phosphatidylinositol 3-kinase/AKT pathway confers
resistance to cisplatin in human papillomavirus 16/18-infected lung
cancer. Clin Cancer Res. 16:5200–5210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kreutzer JN, Ruzzene M and Guerra B:
Enhancing chemosensitivity to gemcitabine via RNA interference
targeting the catalytic subunits of protein kinase CK2 in human
pancreatic cancer cells. BMC Cancer. 10:4402010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fleischer B, Schulze-Bergkamen H,
Schuchmann M, et al: Mcl-1 is an anti-apoptotic factor for human
hepatocellular carcinoma. Int J Oncol. 28:25–32. 2006.PubMed/NCBI
|
|
35
|
He L, Yang X, Cao X, Liu F, Quan M and Cao
J: Casticin induces growth suppression and cell cycle arrest
through activation of FOXO3a in hepatocellular carcinoma. Oncol
Rep. 29:103–108. 2013.PubMed/NCBI
|
|
36
|
Nayak G and Cooper GM: p53 is a major
component of the transcriptional and apoptotic program regulated by
PI 3-kinase/Akt/GSK3 signaling. Cell Death Dis. 3:e4002012.
View Article : Google Scholar : PubMed/NCBI
|