Introduction
In 1984, Torres and Hart found that serine and
threonine residues in nuclear and cytoplasmic proteins can undergo
O-linked N-acetylglucosamine (O-GlcNAc) modification (1). The difference with various other
modifications, is that O-GlcNAc modification is regulated by a
single pair of enzymes: the glycosyltransferase, O-GlcNAc
transferase (OGT) (EC 2.4.1.94; GI: 6006036), and the glycosidase,
O-GlcNAcase (OGA) (EC .3.2.1.52; GI: 13646137). There are >1,000
types of proteins that can be O-GlcNAcylated. O-GlcNAcylation
affects protein interactions, cellular localization, protein
degradation and participates in the regulation of various
biological functions (2). The
role of O-GlcNAc modification in type 2 diabetes, neurological
degenerative diseases, as well as its role in cardiovascular and
cerebrovascular diseases has been investigated in depth and is
becoming a key factor in regulating cellular processes (3).
Certain studies have revealed that O-GlcNAc
modification mediates diabetic vascular dysfunction (4). Increased O-GlcNAcylation has been
shown to enhance reactivity to constrictor stimuli (5); O-GlcNAcylation has also been shown
to enhance vascular contraction contribute through the activation
of the endothelin-1 (ET-1) or RhoA kinase pathway (6).
Although O-GlcNAcylation and diabetes mellitus (DM)
have been extensively studied in the cardiovascular and
neuroendocrine fields, reports on the alterations that occur in
retinal O-GlcNAcylation levels in diabetic retinopathy are limited.
More precisely, the location of the retinal cells which are
affected by protein O-GlcNAcylation under hypoxic conditions, as
well as the effects of O-GlcNAcylation on the blood retinal
barrier, have not been reported. In the current study, we examined
whether hypoxia increases O-GlcNAcylation in retinal vascular cells
under high glucose conditions and whether hypoxia-inducible factor
1α (HIF1α) activation is consistent with the response to and
activation of O-GlcNAcylation in retinal lesions in diabetic
retinopathy. In addition, the effects of O-GlcNAcylation on the
blood-retinal barrier were verified in vitro by the
inhibition of O-GlcNAcylation.
An adequate supply of oxygen and nutrients is
critical to retinal function. Prolonged hypoxia induced by high
glucose can induced changes in the retina. Any changes that occur
in oxygen or glucose levels can lead to cellular damage at the
molecular level. Elucidating these mechanisms will not only enhance
our understaning of the pathogenesis of diabetic retinopathy, but
may also lead to the development of novel therapeutic strategies
(7).
Materials and methods
Reagents
The reagents purchased were as follows: HIF1α
antibody (Bethyl Laboratories, Inc., Montgomery, TX, USA); CTD110.6
antibody (Cell Signaling Technology, Inc., Beverly, MA, USA); OGT
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA);
anti-von Willebrand Factor antibody (Abcam, Cambridge, MA, USA;
VWF, ab6994); Alexa Fluor 488-labeled goat anti-Rabbit IgG and
occludin (Abcam); 4′,6′-diamidino-2-phenylindole hydrochloride
(DAPI) and heparin (Sigma, St. Louis, MO, USA); Dulbecco’s modified
Eagle’s medium (DMEM; high glucose medium, 4.5 g/l glucose and low
glucose medium, 1 g/l glucose) (Gibco, Carlsbad, CA, USA); MEM,
HEPES, collagenase type IV, DNase, Pronase, thiamet G, alloxan,
AG490 and bovine serum albumin (BSA) (all obtained from Sigma);
fetal calf serum (FBS) Trypsine-EDTA (Invitrogen, Carlsbad, CA,
USA); extracellular matrix (ECM), ECGS (100X), penicillin,
streptomycin, ascorbic acid, and amphotericin-B (ScienCell,
Carlsbad, CA, USA); plastic tissue culture flask (Costar,
Cambridge, MA, USA); and Immobilon-NC Transfer Membrane (Millipore,
Billerica, MA, USA). Leica confocal laser scanning microscope
(Leica Microsystems, Mannheim, Germany) was used for scanning. The
db/db mice and db/m mice were purchased from SLRC Laboratory Animal
Centre of Shanghai Institutes for Biological Sciences.
Isolation of bovine retinal vascular
endothelial cells (BRVECs) and primary cell culture
The isolation of BRVECs was performed according to a
modified method originally developed by Banumathi et al
(8). Briefly, the freshly
isolated retinas from bovine eyes were washed with ice-cold
CO2-independent medium, 100 U/ml penicillin and 100
μg/ml streptomycin. In a laminar flow hood, the retinas were washed
with the same solution, homogenized on ice 6 times and centrifuged
at 400 × g for 10 min at 4°C. The pelleted retinal tissue was
resuspended in 10 ml of serum-free MEM. The retinal sections were
transferred to a tube containing 4 ml of an enzyme cocktail which
consisted of 500 μg/ml collagenase type IV, 200 μg/ml DNase and 200
μg/ml pronase in 10 mM phosphate-buffered saline (PBS) containing
0.5% BSA. The retinal sections along with the enzyme cocktail were
incubated at 37°C for 30 min. The pellet was passed through a 53-μm
steel mesh. The trapped blood vessels were removed with the use of
sterile forceps and then washed 3 times with cold MEM by
centrifugation at 400 × g for 5 min. The pellet containing
microvessel fragments was suspended in DMEM supplemented with 10%
FBS, 90 μg/ml heparin, 5 μg/ml ascorbic acid, ECGS 100 U/ml
penicillin-G, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin-B.
The cells were then incubated at 37°C in a 5% CO2
incubator. The culture medium was replaced every 3 days and after
reaching confluence, the cells were passaged with a 0.25% solution
of trypsin-EDTA. von Willebrand factor (vWF) and CD31 antibodies
were used for the identification of BRVECs. The BRVECs were then
cultured under high and low glucose conditions for 72 h and were
starved in serum-free DMEM for a further 24 h. The cells were then
treated with chemicals (thiamet G or OGT siRNA) for 24 h. In the
hypoxia group, the cells were cultured in low and high glucose
medium, and were then exposed to a culture chamber containing 1–3%
O2 for 2, 6, 12, 24 and 48 h.
Immunofluorescence staining of BRVECs for
vWF
The BRVECs were fixed overnight in 4%
paraformaldehyde, then washed 3 times with PBS for 5 min. They were
pre-treated with 0.5% H2O2-methanol and then
blocked and permeabilized by incubation in 5% goat serum containing
0.1% Triton X-100 and 3% BSA. After having been washed with PBS,
the cells were incubated overnight with the primary vWF antibody,
followed by incubation with the Alexa Fluor 488-conjugated
secondary antibody in PBS solution for 1 h at room temperature. The
cells were then washed several times with PBS and incubated for 10
min with DAPI. After having been washed extensively with PBS, the
stained cells were examined under a Leica immunofluorescence
microscope.
siRNA tansfection and treatment with
thiamet G
Total protein O-GlcNAcylation was inhibited with the
use of OGT siRNA or alloxan and was enhanced with the use of
thiamet G. siRNA targeting OGT and the negative control siRNA were
purchased from GenePharma (Shanghai, China). siRNA (ON-TARGETplus
SMARTpool; 100 nM) was incubated with Lipofectamine 2000
(Invitrogen) in 1.0 ml of serum-free medium for 30 min. The
siRNA-Lipofectamine 2000 complex was then added to the cells in 4.0
ml of serum-free medium and maintained for days. On the 3rd day,
the cells were incubated with high glucose for 48 h. The other cell
groups were incubated with thiamet G 48 h followed by high glucose
medium.
Treatment with AG490
The BRVECs were treated with 80 μM AG490 for 24 h,
then cell lysis was prepared for western blot analysis. AG490, a
Janus kinase 2 (JAK2) inhibitor, can reduce the phosphorylation of
JAK2, as a protective factor of the retinal barrier. Cells that
were treated with AG490 were used as the positive control. AG490
can not only reduce the expression of VEGF, but can also increase
the expression of occludin.
Western blot analysis
Cell lysates and the retinal tissues from db/db and
db/m mice were treated with lysis buffer [10 mM Tris (pH 7.5), 150
mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM DTT, 0.1 mM PMSF, 10%
glycerol and a protease inhibitor cocktail tablet] for 40 min on
ice. The retinal tissues were homogenized in lysis buffer followed
by centrifugation at 13,500 rpm for 10 min. The total protein
concentration was calculated using the Enhanced BCA protein assay
kit (Beyotime Institute of Biotechnology, Haimen, China). Lysate
protein (30 μg) was subjected to 4–10% SDS-PAGE and
electrophoretically transferred onto a nitrocellulose (NC)
membrane. After blotting, the membrane was blocked in 5% fat-free
dry milk for 1 h and then incubated with the specific primary
antibody overnight at 4°C. Protein bands were detected with the use
of an enhanced chemiluminescence (ECL) detection kit (Amersham)
following hybridization with the HRP-conjugated secondary antibody
or with the use of LI-COR Odyssey infrared laser imaging system in
order to detect the scanned image. Densitometric analysis was
performed using Image J software (version 1.43, Broken Symmetry
Software, Bethesda, MD, USA). For each experiment, the measurements
were repeated 3 times.
Experimental animals and treatment
We used C57BLKS/J db/db mice as an animal model of
type 2 diabetes, while C57BLKS/J db/m mice were selected as the
control group. Male C57BLKS/J db/db (n=20, 10 weeks old) and db/m
mice (n=10, 10 weeks old) were purchased from the SLRC Laboratory
Animal Centre of Shanghai Institutes for Biological Sciences. They
were housed in cages in a constant environment (room temperature,
20–22°C; room humidity, 40–60%) with a 12 h/12 h light/dark cycle.
Blood glucose levels remained at 19.8–29.8 mM. All procedures were
approved by the Animal Ethics Committee of Tongji University,
Shanghai, China. The db/db mice were divided into 2 groups: an
early-stage diabetic retinopathy group (DM, 12–16 weeks, n=10) and
a late-stage diabetic retinopathy group (DM, 24–32 weeks, n=10).
The eyes were immediately enucleated, and the retinas were then
dissected. Retinal tissues were kept at −80°C until further
analysis.
Immunofluorescence staining of mouse
retinas
The eyes from the db/db mice with varying stages of
diabetic retinopathy (12, 24 and 32 weeks) and from the control
db/m mice were enucleated and embedded with optimal cutting
temperature (OCT) compound and then frozen at −80°C. The
10-mm-thick frozen sections were fixed in 4% paraformaldehyde
overnight. Following pre-treatment with 0.5%
H2O2-methanol for 30 min and PBS containing
5% normal goat serum, 0.5% BSA and 0.1% Triton X-100 for 30 min at
room temperature, the sections were incubated overnight at 4°C with
anti-O-GlcNAc [anti-mouse CTD 110.6 (1:100), anti-OGT antibody
(1:100)] and anti-HIF1α (1:100) antibodies. The sections were then
rinsed with PBS and incubated at room temperature for 1 h with
Alexa Fluor 488-conjugated secondary antibody. All antibodies were
diluted in PBS containing 0.5% goat serum, 0.5% BSA, and 0.1%
Triton X-100. Instead of a primary antibody, goat serum was used as
the negative control. The sections were examined with a Leica
confocal laser scanning microscope. Images were captured and
processed.
In situ hybridization of HIF1α of mouse
retinas
The antisense and sense oligonucleotide probes
(Boster Biological Technology Co., Wuhan, China) were designed
according to the mouse HIF-1a transcript sequences: i)
5′-TTATGAGCTTGCTCATCAGTTGCCACTTCC-3′; ii)
5′-CTCAGTTTGAACTAACTGGACACAGTGTGT-3′; iii)
5′-GGCCGCTCAATTTATGAATATTATCATGCT-3′.
The tissue sections were fixed at room temperature
for 20–30 min in 4% paraformaldehyde in 0.1 M PBS (PH 7.4),
containing 1/1,000 diethylpyrocarbonate DEPC. Endogenous peroxidase
and biotin were blocked with 30%
H2O2-methanol (1:50) for 30 min. The sections
were then permeabilized with proteinase K (Catalog no. S3020; Dako
Denmark A/S, Glostrup, Denmark) and washed 3 times with
Tris-buffered saline. The sections were fixed again with the
aforementioned solution. Biotinylated probes designed for HIF1α
were mixed in hybridization buffer (50% formamide) at a
concentration of 200 ng/ml. Probes were added to the sections,
which were then covered with coverslips and heated at 92°C for 5
min. Hybridization was carried out in a humidified chamber at 37°C
overnight. The sections were washed in 2X saline-sodium citrate
(SSC) for 10 min followed by 3 washes in Tris-buffered saline and
then blocked at 37°C for 30 min with blocking solution. The
sections were incubated with biotinylated mouse anti-digoxin
solution at 37°C for 60 min, then washed 4 times with PBS. For
hybridization signal detection, SABC and DAB kits were used
according to the manufacturer’s instructions (Boster Biological
Technology Co.).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 13.0; SPSS, Chicago, IL, USA). The unpaired
Student’s t-test was used to assess the significance between 2
groups. One-way ANOVA was used to compare 3 or more groups, while
the Mann-Whitney U test or Rank Cases method were used to examine
the heterogeneity of variance. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
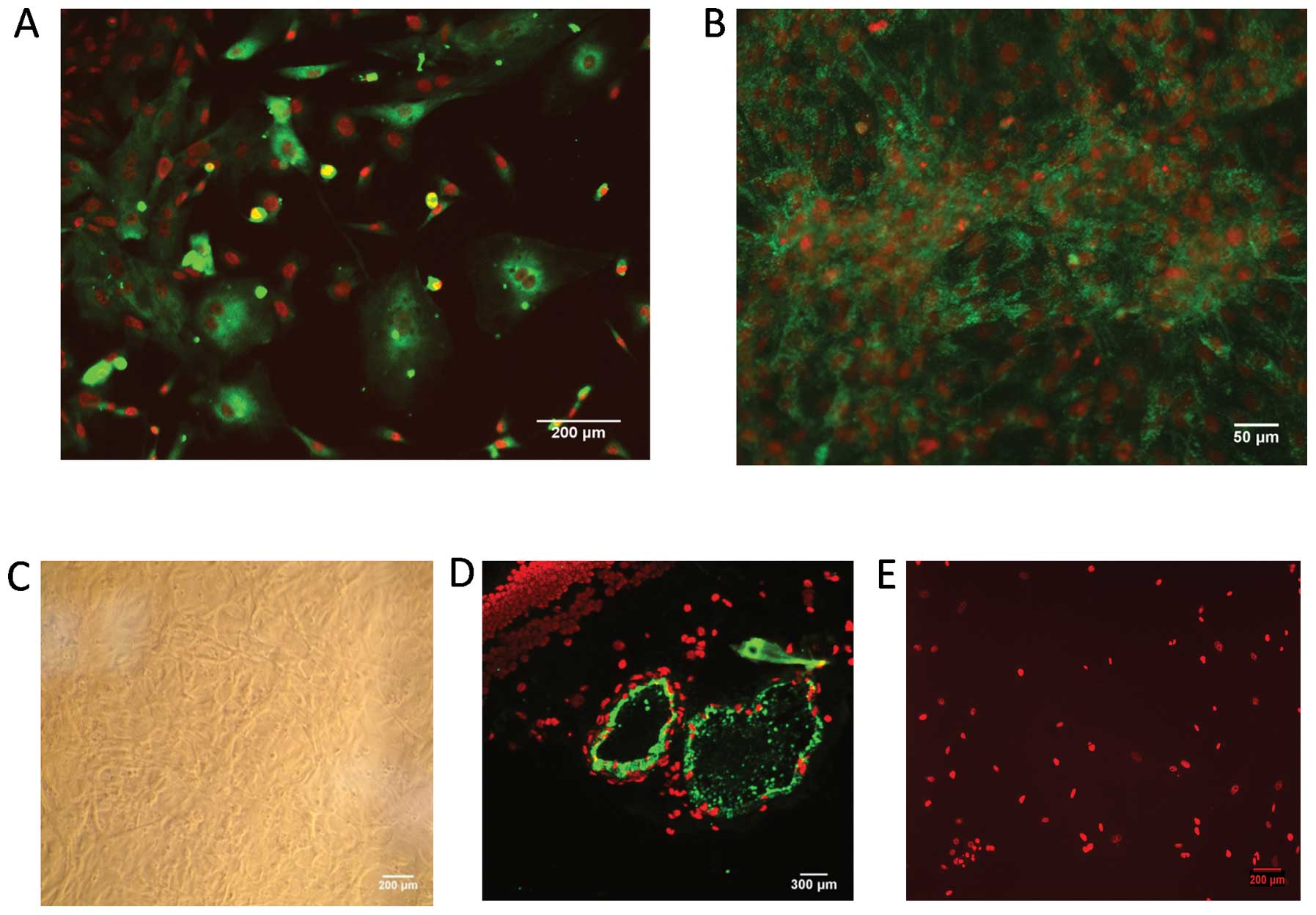
Characteristics of BRVECs
The cells initially grew as capillary-like
structures and demonstrated the typical cobblestone morphology of
endothelial cells at confluence (Fig.
1B and C). These cells were characterized as vascular
endothelial cells by VWF antigen expression. Using
immunofluorescence, the positive expression of vWF antigen in the
BRVECs was demonstrated by green particles in the cytoplasm
(Fig. 1A and B). BSA staining was
used as a negative control (Fig.
1E) while bovine retinal vascular tissue immunostaining was
used as a positive control (Fig.
1D).
Total protein O-GlcNAcylation and HIF1α
retinal distribution in db/db mice with varying stages of diabetic
retinopathy
Protein O-GlcNAcylation in the retinas of db/db mice
was detected by CTD110.6 immunofluorescence and presented a
distribution pattern consistent with HIF1α distribution as found by
immunofluorescence and in situ hybridization (Fig. 2). O-GlcNAcylation appeared in the
retinal ganglion cell, inner nuclear and retina pigment epithelium
(RPE) layers which are first affected by diabetic retinopathy
(Fig. 2B and D), and consequently
in the inner plexiform layer (Fig. 2F
and H). HIF1α (Fig. 2J and G)
and CTD110.6 expression levels (Fig.
2L) were observed at almost undetectable levels in the db/m
mouse retinas (negative controls).
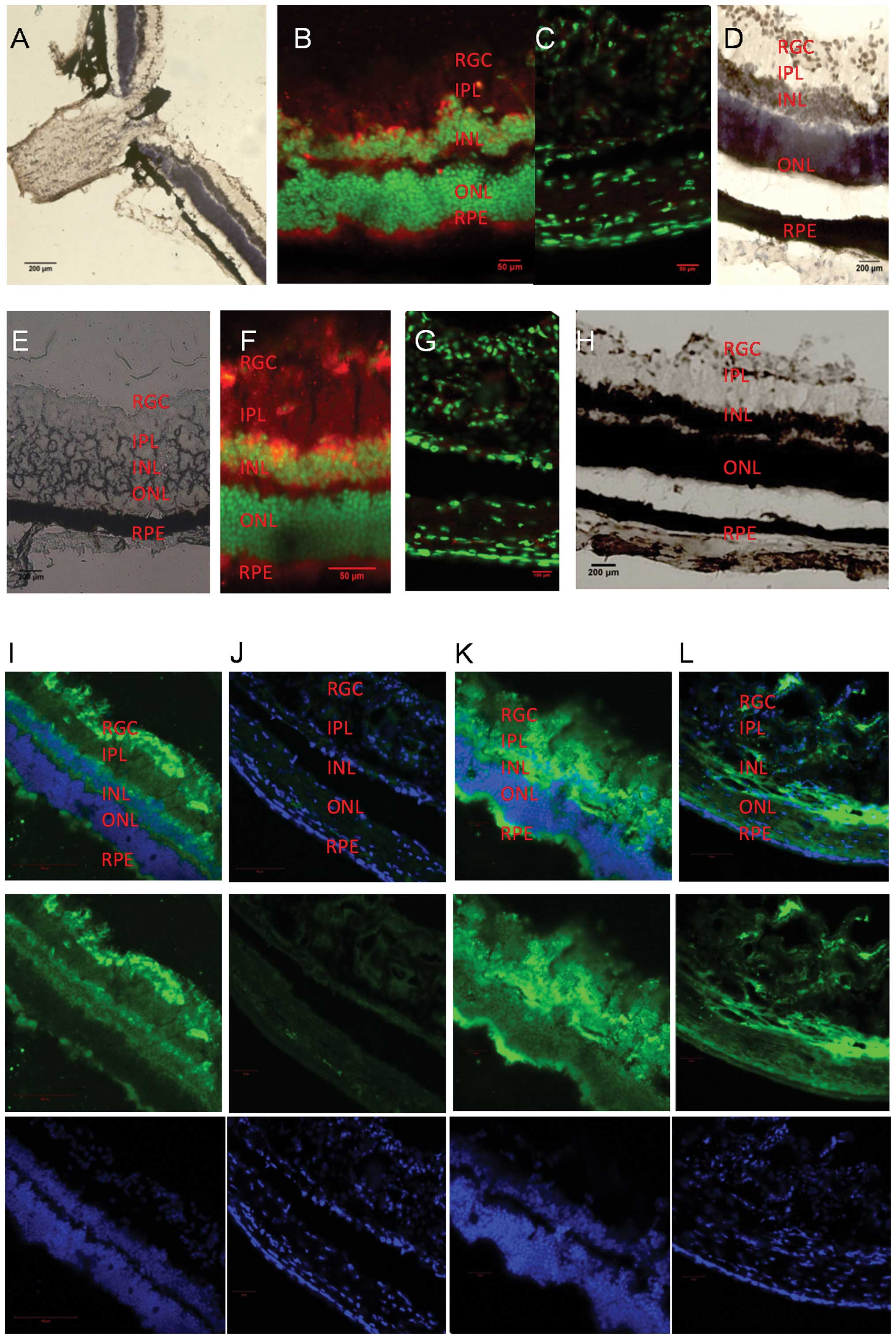 | Figure 2(A) HIF1α in situ
hybridization of db/db mouse retinas (magnification, ×100). (B)
HIF1α immunofluorescence of retinas of 12-week-old db/db mice
(magnification, ×200). HIF1α is stained red and is expressed mainly
in the retinal ganglion cell (RGC) layer, the inner nuclear layer
(INL) and the retinal pigment epithelium (RPE) layer. The outer
nuclear layer (ONL) shows negative staining. (C) HIF1α
immunofluorescence of 12-week-old db/m mouse retinas of the control
group, (magnification, ×400). (D) HIF1α in situ
hybridization of the retinas of 12-week-old db/db mice
(magnification, ×400) present the same distribution of
immunostaining. (E) H&E staining of db/db mouse retinas. (F)
HIF1α immunofluorescence of 32-week-old db/db mouse retinas
(magnification, ×400). HIF1α is stained red and appears mainly in
the RGC layer, the INL, the inner plexiform layer (IPL) and the RPE
layer. The ONL shows negative staining (G) HIF1α immunofluorescence
of 32-week-old db/m mouse retinas of the control group
(magnification, ×400). (H) HIF1α in situ hybridization of
the retinas of 32-week-old db/db mice (magnification, ×200); brown
shows the positive staining. (I) HIF1α immunofluorescence of
24-week-old db/db mouse retinas (magnification, ×200). HIF1α is
expressed mainly in the RGC, the INL, the IPL and the RPE layer.
(K) Retinal CTD110.6 immunofluorescence of 24-week-old db/db mice
(magnification, ×200), presented a positive staining pattern
similar to that of HIF1α. Mainly the RGC, the INL, the RPE layer
and the IPL were stained. (J and L) Retinal HIF1α (magnification,
×200, J) and CTD110.6 (magnification, ×200, L) immunofluorescence
of the control group db/m mice. Low HIF1α and CTD110.6 expression
levels were observed. |
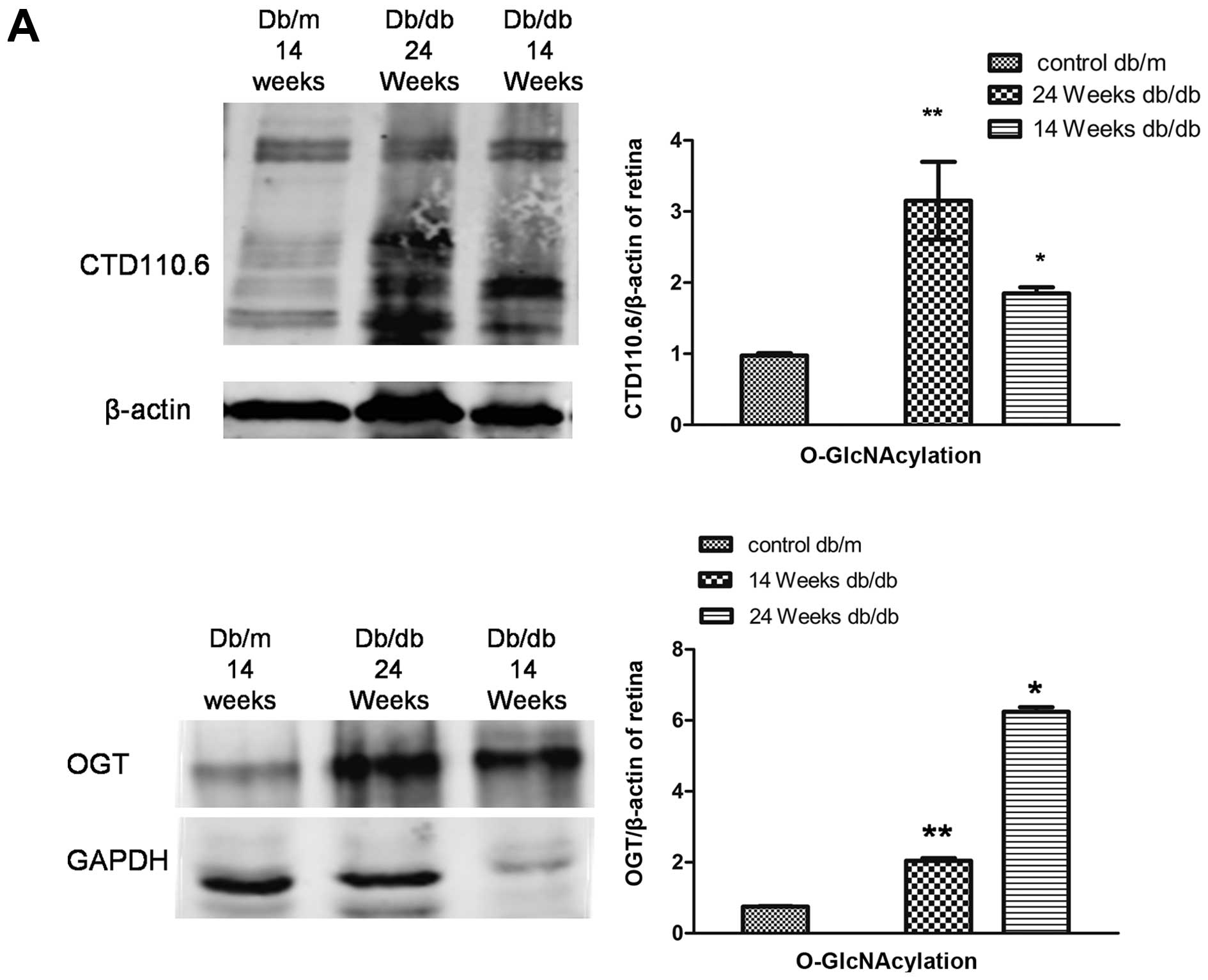
O-GlcNAcylation levels in vivo and in
vitro
The protein expression of O-GlcNAc was determined by
western blot analysis using CTD110.6 and OGT antibodies as
described in Materials and methods. O-GlcNAcylation was increased
in the retinas of 14- and 24-week-old db/db mice compared to those
of the age-matched control mice (Fig.
3A). A similar increase was observed in O-GlcNAcylation in
BRVECs cultured under high glucose conditions (Fig. 3B). In addition, culture under
hypoxic conditions induced a time-dependent increase in
O-GlcNAcylation (Fig. 3C).
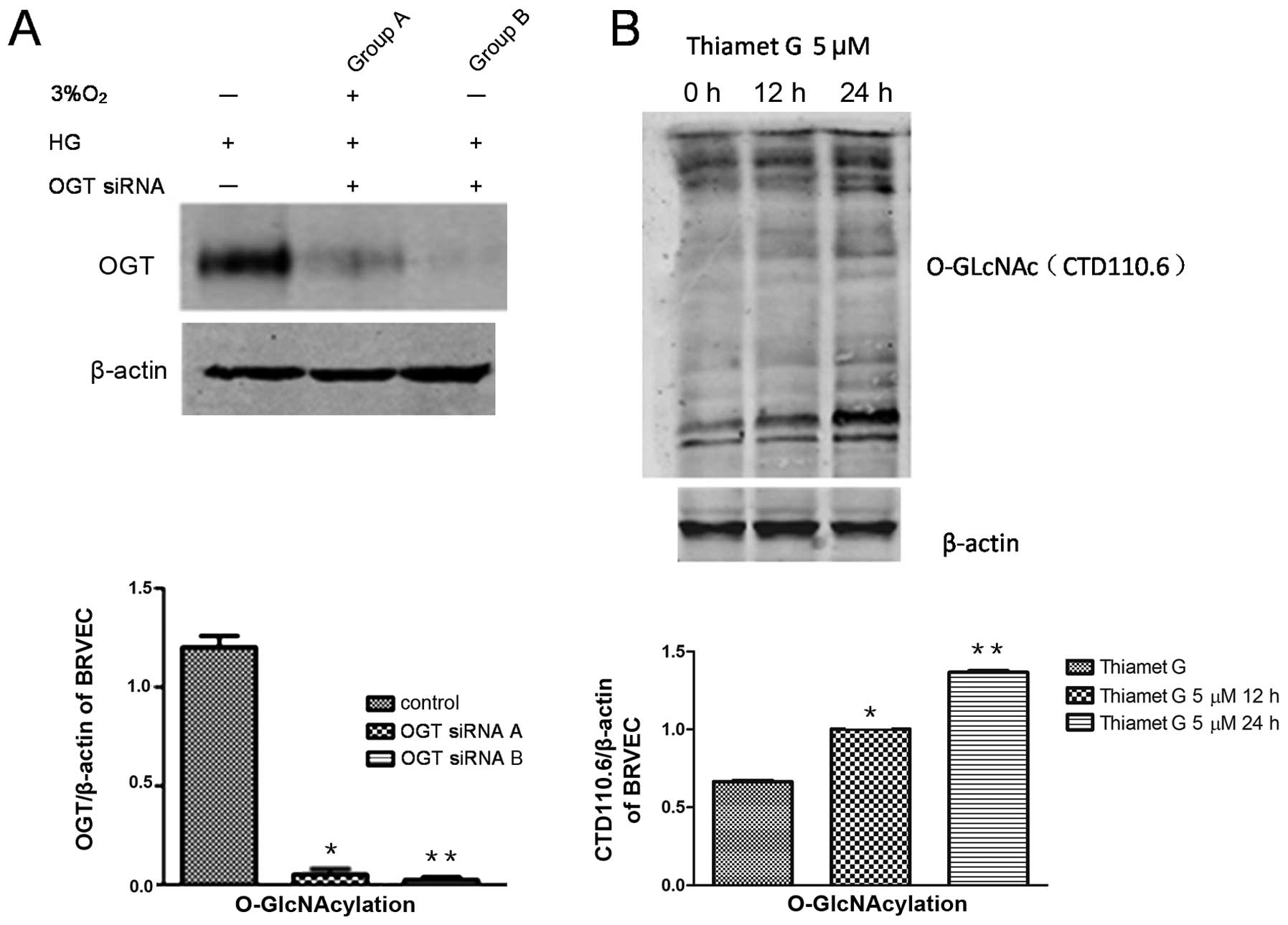
Changes in O-GlcNAcylation levels in
BRVECs and the blood-retinal barrier
OGT siRNA inhibition of O-GlcNAcylation in BRVECs in
under a high glucose state (Fig.
4A) led to an increased expression of occludin and to reduced
VEGF expression levels (Fig. 4C),
while increased O-GlcNAcylation was observed following treatment
with thiamet G (Fig. 4B and C).
These results indicate that inhibiting O-GlcNAcylation in BRVECs
may protect the blood-retinal barrier and reduce VEGF
expression.
Discussion
Diabetic retinopathy is a serious complication of
diabetes, leading to blindness in individuals with diabetes
(9). O-GlcNAc signaling
participates in the pathogenesis of diabetes (10–13). Recent studies have suggested that
it also participates in the pathogenesis of diabetic retinopathy
(14). The elevated expression of
O-GlcNAc has emerged as a regulator of the cellular stress response
(15). One of the major causes of
cellular stress is hypoxia (16,17) and it is associated with the
development of diabetic retinopathy (18,19). In other studies, when multiple
cell lines were subjected to diverse types of stress (including
hypoxia), there was a rapid and global increase in O-GlcNAc
expression levels (20). Hypoxia
upregulates the expression of the epitope H containing an O-GlcNAc
residue in human ependymal cells (21). Increased OGT levels enhance
O-GlcNAcylation and reduce cardiomyocyte death following hypoxia
(22). O-GlcNAcylation levels
have been shown to increase in cardiac cells folloiwng exposure to
hypoxia for 4 h and early reoxygenation; this was confirmed in a
follow-up study by the same group (23).
In the current study, it was demonstrated that the
O-GlcNAc expression levels increased in vitro and in
vivo not only in a high glucose state but also under hypoxic
conditions. BRVEC O-GlcNAcylation in a high glucose state was
exacerbated by hypoxia. In diabetic retinopathy, the reduced
expression levels of occludin represent a decline in blood-retinal
barrier function. OGT siRNA, which inhibits O-GlcNAcylation can
protect the blood-retinal barrier by increasing the expression
levels of occludin in BRVECs in vitro under high glucose
conditions. Thus, it is reported herein that O-GlcNAc signaling,
which is associated with HIF1α, is one of the regulatory factors of
blood-retinal barrier function in the pathogenesis of diabetic
retinopathy.
The possible mechanisms involved in triggering the
increase in O-GlcNAcylation under hypoxic conditions are the
following: Firstly, O-GlcNAc-mediated cytoprotection, as well as
hypoxia which causes endoplasmic reticulum (ER) stress, have been
shown to be involved in the pathogenesis of diabetes (24–26). The enhancement of O-GlcNAcylation
has been shown to exert cytoprotective effects during hypoxia,
ischemia and oxidative stress (27–29). The mitochondria are critical
targets of O-GlcNAc-mediated cytoprotection (22,27,29,30). Moreover, O-GlcNAc signaling can
directly attenuate oxidative stress-induced cellular dysfunction
(27) and calcium overload
(29,31). Further studies are required in
order to gain a better understanding of diabetic retinopathy.
Secondly, it has been demonstrated that the
hexosamine signaling pathway (HSP) is linked to O-GlcNAc cycling
and plays an important role in nutrient sensing (32). HSP expression represents an
ubiquitous molecular mechanism which prevents the deleterious
effects caused by stress (33),
while O-GlcNAcylation plays an important role in regulating DNA
damage or repair through signaling pathways following stress
(15).
It is still unclear as to the exact changes that
occur in O-GlcNAcylation levels in the diabetic retina. In the
present study, we aimed to determine which retinal layer is
affected first and to a greater extent by O-GlcNAcylation during
diabetic retinopathy in db/db mice.
The results of immunofluorescence and in situ
hybridization (Fig. 2) revealed
that the distribution of the O-GlcNAcylated factors is consistent
with HIF1α distribution in the db/db mouse retina. During the early
stages of diabetic retinopathy (12 weeks), protein O-GlcNAcylation
is distributed in the retinal ganglion cell layer, inner nuclear
layer and RPE layer. During the later stages of the disease (24 and
32 weeks), it is not only distributed in the aforementioned layers
but also in the inner plexiform layer. During the early stages of
diabetic retinopathy, high glucose levels lead to retina hypoxia
(34). According to a recent
study, HIF1α expression levels have been shown to increase
significantly in the vitreous fluid of surgically-treated eyes with
proliferative diabetic retinopathy (PDR) (35), which also verifies the existence
of hypoxia throughout the entire duration of diabetes. The data
reported herein demostrate that hypoxia is accompanied by protein
O-GlcNAcylation, a finding that reveals that oxygen is necessary
for O-GlcNAcylation of the retinal protein.
The photoreceptors of the inner layer, the outer
plexiform layer and the deeper regions of the inner plexiform layer
are considered dominant oxygen consumers of the rat retina
(36). The higher oxygen demand
of these layers including the ‘OFF’ pathway ganglion cells which
are normally located in the deeper inner plexiform layer, may
render them more sensitive to hypoxia (36).
In the hyperoxia state, such as respired 100%
oxygen, oxygen consumption of the inner retinal layer exceeds that
of the photoreceptor layer (37–39). Studies have also shown that VEGF
mRNA expression levels in the retina of diabetic rats are
significantly increased, mainly in the retinal ganglion cell layer
and the inner nuclear layer (40). These data are iin accordance with
our observation that HIF1α is distributed first in the inner
nuclear layer, retinal ganglion cell layer and RPE layer, and then
in the inner plexiform layer in db/db mouse retinas. HIF1α is a
molecular level oxygen sensor and represents the degree of hypoxia
(41). Hyperglycemia-induced
O2 consumption activates HIF1α and other
hypoxia-associated genes (42).
Hypoxia induces a decrease in adenosine triphosphate (ATP) levels,
ion imbalance and free radicals which cause ganglion cell, inner
nuclear layer cell and RPE cell apoptosis (43). The distribution of O-GlcNAc is
consistent with the distribution of HIF1α in the db/db mouse retina
and reveals that the change in retinal O-GlcNAcylation levels is
mainly influenced by the location where hypoxia occurs. This may be
a compensatory response which leads to tissue damage.
In this study, we also investigated the decreased
expression levels of occludin in cultured BRVECs under high glucose
conditions; these levels increased following transfection with OGT
siRNA. Further studies are required to confirm whether this change
occurs due to the direct regulation of occludin phosphorylation, or
to other kinase-mediated signal transduction pathways.
Occludin is known as a transmembrane component of
tight junctions (TJs) and together with ZO-1, constitute the TJ
between cells (44). It has also
been confirmed that occludin plays a role in the blood-retinal
barrier breakdown during diabetic retinopathy (45, 46). Under hypoxic conditions, the
transient increase in intracellular Ca2+ activates
extracellular signal-related kinases, such as calmodulin-dependent
kinase, triggering the cascade reaction, causing changes in the
mRNA levels and protein expression of occludin and other
TJ-associated proteins, eventually leading to cell barrier function
damage (47).
It is more evident when occludin is phosphorylated.
Occludin serine/threonine phosphorylation aggregates in the TJ
location, while fewer occludin phosphorylated residues are
distributed in the cytoplasm of the basement membrane side. The
increased serine/threonine phosphorylation of occludin can enhance
the barrier function (48).
Factors, such as lack of calcium, phorbol (phorbol ester)
stimulation (44) and
pathological conditions (45),
that destroy TJs, can change the state of occludin phosphorylation,
which influences TJ barrier functions (46). It is well known that O-GlcNAc
modification due to glycosylation and phosphorylation, is a
complementary adjustment, which functions in a regulatory mode, as
suppressing glycosylated occludin expression may enhance its
phosphorylation (1), leading to
the increase of TJ barrier functions. There exists a novel
interplay between occludin and O-β-glycosylation (49). Due to the complexity of the
measurement methods, further studies arerequired to confirm whether
occludin itself exerts O-GlcNAcylation.
The present study demonstrates that during the
course of diabetic retinopathy, the degree of retinal hypoxia
becomes more severe and O-GlcNAcylation is closely associated with
hypoxia in the retinas of db/db mice with type 2 diabetes. Protein
O-GlcNAcylation appears mainly first in the ganglion cell layer,
the inner nuclear layer and the RPE layer and at a later stage, in
the inner plexiform layer. In addition, we found that the
inhibition of BRVEC O-GlcNAcylation under high glucose conditions
can prevent the increase in occludin and the decrease in VEGF
expression levels, and may thus contribute to the protection of
blood-retinal barrier in diabetic retinopathy. Further studies are
required to gain a better understanding of the molecular basis of
O-GlcNAcylation which plays a role in the pathogenesis of diabetic
retinopathy.
Acknowledgements
This study was supported in part by the project of
Shen Kang Hospital Development Center (SHDC12010207, Shanghai,
China). We are grateful for the assistance of Dr Bebee and Dr
Yingbo Shui of the Research Centre of Ophthalmology at Washington
University (USA).
References
|
1
|
Torres CR and Hart GW: Topography and
polypeptide distribution of terminal N-acetylglucosamine residues
on the surfaces of intact lymphocytes. Evidence for O-linked
GlcNAc. J Biol Chem. 259:3308–3317. 1984.
|
|
2
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lima VV, Rigsby CS, Hardy DM, Webb RC and
Tostes RC: O-GlcNAcylation: a novel post-translational mechanism to
alter vascular cellular signaling in health and disease: focus on
hypertension. J Am Soc Hypertens. 3:374–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lima VV, Spitler K, Choi H, Webb RC and
Tostes RC: O-GlcNAcylation and oxidation of proteins: is signalling
in the cardiovascular system becoming sweeter? Clin Sci (Lond).
123:473–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lima VV, Giachini FR, Carneiro FS, et al:
Increased vascular O-GlcNAcylation augments reactivity to
constrictor stimuli - VASOACTIVE PEPTIDE SYMPOSIUM. J Am Soc
Hypertens. 2:410–417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim do H, Seok YM, Kim IK, Lee IK, Jeong
SY and Jeoung NH: Glucosamine increases vascular contraction
through activation of RhoA/Rho kinase pathway in isolated rat
aorta. BMB Rep. 44:415–420. 2011.
|
|
7
|
Grimm C and Willmann G: Hypoxia in the
eye: a two-sided coin. High Alt Med Biol. 13:169–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banumathi E, Haribalaganesh R, Babu SS,
Kumar NS and Sangiliyandi G: High-yielding enzymatic method for
isolation and culture of microvascular endothelial cells from
bovine retinal blood vessels. Microvasc Res. 77:377–381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ali TK and El-Remessy AB: Diabetic
retinopathy: current management and experimental therapeutic
targets. Pharmacotherapy. 29:182–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan HB, Singh JP, Li MD, Wu J and Yang X:
Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab.
24:301–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jensen RV, Zachara NE, Nielsen PH, Kimose
HH, Kristiansen SB and Botker HE: Impact of O-GlcNAc on
cardioprotection by remote ischaemic preconditioning in
non-diabetic and diabetic patients. Cardiovasc Res. 97:369–378.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett CE, Johnsen VL, Shearer J and
Belke DD: Exercise training mitigates aberrant cardiac protein
O-GlcNAcylation in streptozotocin-induced diabetic mice. Life Sci.
92:657–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McLarty JL, Marsh SA and Chatham JC:
Post-translational protein modification by O-linked
N-acetyl-glucosamine: its role in mediating the adverse effects of
diabetes on the heart. Life Sci. 92:621–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gurel Z, Sieg KM, Shallow KD, Sorenson CM
and Sheibani N: Retinal O-linked N-acetylglucosamine protein
modifications: implications for postnatal retinal vascularization
and the pathogenesis of diabetic retinopathy. Mol Vis.
19:1047–1059. 2013.
|
|
15
|
Zachara NE, Molina H, Wong KY, Pandey A
and Hart GW: The dynamic stress-induced ‘O-GlcNAc-ome’ highlights
functions for O-GlcNAc in regulating DNA damage/repair and other
cellular pathways. Amino Acids. 40:793–808. 2011.
|
|
16
|
Tai TC, Wong-Faull DC, Claycomb R and Wong
DL: Hypoxic stress-induced changes in adrenergic function: role of
HIF1 alpha. J Neurochem. 109:513–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Witt KA, Mark KS, Huber J and Davis TP:
Hypoxia-inducible factor and nuclear factor kappa-B activation in
blood-brain barrier endothelium under hypoxic/reoxygenation stress.
J Neurochem. 92:203–214. 2005. View Article : Google Scholar
|
|
18
|
Arden GB and Sivaprasad S: Hypoxia and
oxidative stress in the causation of diabetic retinopathy. Curr
Diabetes Rev. 7:291–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Chen P, Zhang J, et al:
Enzyme-induced vitreolysis can alleviate the progression of
diabetic retinopathy through the HIF-1alpha pathway. Invest
Ophthalmol Vis Sci. 54:4964–4970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zachara NE, O’Donnell N, Cheung WD, Mercer
JJ, Marth JD and Hart GW: Dynamic O-GlcNAc modification of
nucleocytoplasmic proteins in response to stress. A survival
response of mammalian cells. J Biol Chem. 279:30133–30142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arvanitis LD, Vassiou K, Kotrotsios A and
Sgantzos MN: Hypoxia upregulates the expression of the O-linked
N-acetylglucosamine containing epitope H in human ependymal cells.
Pathol Res Pract. 207:91–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ngoh GA, Watson LJ, Facundo HT, Dillmann W
and Jones SP: Non-canonical glycosyltransferase modulates
post-hypoxic cardiac myocyte death and mitochondrial permeability
transition. J Mol Cell Cardiol. 45:313–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ngoh GA, Facundo HT, Zafir A and Jones SP:
O-GlcNAc signaling in the cardiovascular system. Circ Res.
107:171–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Araki E, Oyadomari S and Mori M:
Endoplasmic reticulum stress and diabetes mellitus. Intern Med.
42:7–14. 2003. View Article : Google Scholar
|
|
25
|
Harding HP, Zeng H, Zhang Y, et al:
Diabetes mellitus and exocrine pancreatic dysfunction in
perk−/− mice reveals a role for translational control in
secretory cell survival. Mol Cell. 7:1153–1163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozcan U, Cao Q, Yilmaz E, et al:
Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones SP, Zachara NE, Ngoh GA, et al:
Cardioprotection by N-acetylglucosamine linkage to cellular
proteins. Circulation. 117:1172–1182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Marchase RB and Chatham JC:
Increased O-GlcNAc levels during reperfusion lead to improved
functional recovery and reduced calpain proteolysis. Am J Physiol
Heart Circ Physiol. 293:H1391–H1399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ngoh GA, Facundo HT, Hamid T, Dillmann W,
Zachara NE and Jones SP: Unique hexosaminidase reduces metabolic
survival signal and sensitizes cardiac myocytes to
hypoxia/reoxygenation injury. Circ Res. 104:41–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Champattanachai V, Marchase RB and Chatham
JC: Glucosamine protects neonatal cardiomyocytes from
ischemia-reperfusion injury via increased protein O-GlcNAc and
increased mitochondrial Bcl-2. Am J Physiol Cell Physiol.
294:C1509–C1520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ngoh GA, Hamid T, Prabhu SD and Jones SP:
O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte
death. Am J Physiol Heart Circ Physiol. 297:H1711–H1719. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanover JA, Krause MW and Love DC: The
hexosamine signaling pathway: O-GlcNAc cycling in feast or famine.
Biochim Biophys Acta. 1800:80–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guinez C, Mir AM, Leroy Y, Cacan R,
Michalski JC and Lefebvre T: Hsp70-GlcNAc-binding activity is
released by stress, proteasome inhibition, and protein misfolding.
Biochem Biophys Res Commun. 361:414–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kennedy A and Frank RN: The influence of
glucose concentration and hypoxia on VEGF secretion by cultured
retinal cells. Curr Eye Res. 36:168–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loukovaara S, Koivunen P, Ingles M,
Escobar J, Vento M and Andersson S: Elevated protein carbonyl and
HIF-1alpha levels in eyes with proliferative diabetic retinopathy.
Acta Ophthalmol. May 29–2013.(Epub ahead of print).
|
|
36
|
Yu DY and Cringle SJ: Oxygen distribution
and consumption within the retina in vascularised and avascular
retinas and in animal models of retinal disease. Prog Retin Eye
Res. 20:175–208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu DY, Cringle SJ, Yu PK and Su EN:
Intraretinal oxygen distribution and consumption during retinal
artery occlusion and graded hyperoxic ventilation in the rat.
Invest Ophthalmol Vis Sci. 48:2290–2296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cringle SJ and Yu DY: A multi-layer model
of retinal oxygen supply and consumption helps explain the muted
rise in inner retinal PO(2) during systemic hyperoxia. Comp Biochem
Physiol A Mol Integr Physiol. 132:61–66. 2002. View Article : Google Scholar
|
|
39
|
Yu DY, Cringle SJ and Su EN: Intraretinal
oxygen distribution in the monkey retina and the response to
systemic hyperoxia. Invest Ophthalmol Vis Sci. 46:4728–4733. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sandercoe TM, Geller SF, Hendrickson AE,
Stone J and Provis JM: VEGF expression by ganglion cells in central
retina before formation of the foveal depression in monkey retina:
evidence of developmental hypoxia. J Comp Neurol. 462:42–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semenza GL: Hydroxylation of HIF-1: oxygen
sensing at the molecular level. Physiology (Bethesda). 19:176–182.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bensellam M, Duvillie B, Rybachuk G, et
al: Glucose-induced O(2) consumption activates hypoxia inducible
factors 1 and 2 in rat insulin-secreting pancreatic beta-cells.
PLoS One. 7:e298072012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang L, Tan P, Zhou W, et al:
N-acetylcysteine protects against hypoxia mimetic-induced autophagy
by targeting the HIF-1 alpha pathway in retinal ganglion cells.
Cell Mol Neurobiol. 32:1275–1285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feldman GJ, Mullin JM and Ryan MP:
Occludin: structure, function and regulation. Adv Drug Deliv Rev.
57:883–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cummins PM: Occludin: one protein, many
forms. Mol Cell Biol. 32:242–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dorfel MJ and Huber O: Modulation of tight
junction structure and function by kinases and phosphatases
targeting occludin. J Biomed Biotechnol. 2012:8073562012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clarke H, Soler AP and Mullin JM: Protein
kinase C activation leads to dephosphorylation of occludin and
tight junction permeability increase in LLC-PK1 epithelial cell
sheets. J Cell Sci. 113(Pt 18): 3187–3196. 2000.PubMed/NCBI
|
|
48
|
Simonovic I, Arpin M, Koutsouris A,
Falk-Krzesinski HJ and Hecht G: Enteropathogenic Escherichia
coli activates ezrin, which participates in disruption of tight
junction barrier function. Infect Immun. 69:5679–5688.
2001.PubMed/NCBI
|
|
49
|
Butt AM, Feng D, Nasrullah I, et al:
Computational identification of interplay between phosphorylation
and O-beta-glycosylation of human occludin as potential mechanism
to impair hepatitis C virus entry. Infect Genet Evol. 12:1235–1245.
2012. View Article : Google Scholar : PubMed/NCBI
|