Introduction
Bone formation and remodeling occur throughout
development and adult life. The formation of new bone is a complex
cascade, involving cell proliferation, osteogenic cell
differentiation, extracellular matrix (ECM) maturation and matrix
mineralization. Bone remodeling depends on osteoblasts (OBs),
osteocytes and osteoclasts (1).
Mesenchymal stem cells (MSCs) differentiate into OBs and synthesize
and secrete bone matrix, which subsequently becomes mineralized
tissue. Once embedded into the bone matrix, OBs further
differentiate into osteocytes.
MSCs were first derived from bone marrow and are
characterized by their self-renewal ability and their capacity to
develop into a variety of mesenchymal tissues (2–4).
The expansion of human bone marrow-derived MSCs (BM-MSCs) in
vitro and their subsequent autoimplantation may be used for
stem cell therapy without the risk of rejection by the immune
system. BM-MSCs differentiate into OBs, chondrocytes and adipocytes
(5) and are therefore considered
the main source of bone regeneration and remodeling during
homeostasis (6–9). Much of this process depends on the
ability of MSCs to proliferate and differentiate under the
influence of biologically active molecules (i.e., growth factors)
(10–13). The role of growth factors in bone
repair is widely recognized, particularly for platelet-derived
growth factor (PDGF), insulin-like growth factor-I (IGF-I),
vascular endothelial growth factor (VEGF) and transforming growth
factor-β (TGF-β), all of which are inducers, particularly in
osteoprogenitor cells (14).
These growth factors are usually stored in the ECM; however,
following injury, they are actively released by the ECM, cells and
platelets.
TGF-β is one of the most abundant growth factors in
the bone matrix (15) and
regulates osteoblastic differentiation in a variety of ways, such
as by stimulating the proliferation and development of early OBs,
although it inhibits their maturation and mineralization (16). TGF-β is released from the bone
surface and recruits MSCs to bone-resorptive sites, where they
undergo differentiation into mature OBs, thus coupling bone
resorption with bone formation (17). TGF-β activates intracellular
effectors, such as mitogen-activated protein kinases (MAPKs) and
Sma- and Mad-related proteins (Smads) (18–20). There are at least three distinctly
regulated groups of MAPKs: extracellular signal-related kinases
(ERKs), Jun N-terminal kinases (JNKs) and p38 MAPKs (p38). The
activation of the ERK pathway mediates the differentiation of
BM-MSCs and that of the pre-adipocyte cell line, 3T3 L1, into
mature adipocytes. It also regulates the proliferation and
differentiation of bone cells and BM-MSCs during osteogenic
differentiation (21). JNK and
p38 are activated in human and mouse OBs to regulate bone
resorption (22,23).
PDGF is a polypeptide growth factor secreted from
cytokine-laden granules of aggregated platelets early after tissue
injury (24,25). PDGF is mainly produced by
platelets and has been implicated in the repair of tissue damage,
such as fractures (26). PDGF
consists of A, B, C and D isoforms, and forms homo or hetero
dimers, such as PDGF-AA or PDGF-AB (26). PDGF-BB exhibits the strongest
activity of these isoforms (26)
and has been approved by the US Food and Drug Administration (FDA)
for the treatment of patients with bone defects in oral and
maxillofacial regions (27–30). However, the specific molecular
mechanisms by which PDGF regulates the activity of multiple cell
types to control tissue development are not yet fully understood.
Much of the research in this area has focused on the role of PDGF
in controlling the vascularization of nascent tissue, forming
within the wound site (31). PDGF
indirectly regulates bone regeneration by increasing the expression
of angiogenic molecules, such as VEGF (32), hepatocyte growth factor (33) and that of the proinflammatory
cytokine, interleukin-6 (34);
VEGF is a particularly important molecule in bone regeneration
(35). In general, PDGF binding
leads to autophosphorylation on multiple tyrosine residues, thereby
activating several downstream cascades, such as ERK belonging to
MAPKs, phosphoinositide-3-kinase (PI3K)/Akt, Janus kinase (JAK) and
signal transducer and activator of transcription (STAT) pathways
(36,37). Osteogenic progenitor cells respond
to PDGF ligand-binding by the activation of Src tyrosine kinases
(38–40) and of the Akt protein kinase and
Grb2-mediated ERK-signaling (40). Consequently, PDGF increases the
pool of osteogenic cells at the injury site, acting as a
chemotactic agent and mitogen (41).
Even though the effects of TGF-β or PDGF alone on
the osteogenic differentiation of undifferentiated mesenchymal
cells have been reported in detail (17,40,42), their combined effects still remain
unknown to date. In this study, we investigated the osteogenic
differentiation of human MSCs (hMSCs) following stimulation with
exogenous TGF-β and PDGF. We also investigated the mechanisms
through which intracellular signals induced by TGF-β and/or PDGF
control the osteogenic differentiation of hMSCs.
Materials and methods
Reagents
Recombinant human TGF-β and PDGF, as well as the
MAPK/ERK kinase (MEK) inhibitor, U0126, and the PI3K inhibitor,
LY294002, were purchased from Calbiochem (La Jolla, CA, USA).
Cell culture and osteogenic
differentiation
The human BM-MSC line, UE7T-13, the lifespan of
which was prolonged by infection with a retrovirus encoding human
papillomavirus E7 and human telomerase reverse transcriptase
(hTERT) (43,44), was purchased from the Health
Science Research Resources Bank (JCRB no. 1154, Japan Health
Sciences Foundation, Tokyo, Japan). The UE7T-13 cells were cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St. Louis, MO,
USA) supplemented with 10% fetal bovine serum (FBS; PAA
Laboratories, Piscataway, NJ, USA) at 37°C in a humidified
incubator with an atmosphere of 5% CO2. To induce
osteogenic differentiation, the UE7T-13 cells were cultured in
24-well culture plates (Nunc, Roskilde, Denmark) containing basal
osteogenic differentiation medium (BODM) [α-MEM (Sigma)
supplemented with 100 nM dexamethasone (Sigma), 50 μg/ml ascorbic
acid (Nacalai Tesque, Kyoto, Japan), 10 mM β-glycerophosphate
(Sigma) and 10% FBS (PAA Laboratories)] containing TGF-β and/or
PDGF. Half of the medium in each dish was changed every 2–3
days.
Alkaline phosphatase (ALP) staining
The UE7T-13 cells were cultured in 24-well plastic
culture plates or Osteologic™ discs (BD Biosciences, Franklin
Lakes, NJ, USA) (a proprietary hydroxyapatite substitute for bone
mineral) containing BODM supplemented with TGF-β and/or PDGF for 1
week. The surface of the Osteologic cell culture disc is coated
with calcium phosphate. The cells were then stained with ALP using
the TRAP/ALP staining kit (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) according to the manufacturer’s instructions.
Alizarin red staining
Confluent UE7T-13 cells were cultured in 24-well
plastic culture plates containing BODM supplemented with TGF-β
and/or PDGF. After 2 weeks, bone matrix mineralization was
evaluated by Alizarin red S (Sigma) staining. Alizarin red was
extracted by the addition of 10% cetylpyridinium chloride (Sigma)
in 8 mM Na2HPO4 (Wako Pure Chemical) and 1.5
mM KH2PO4 (Wako Pure Chemical Industries,
Ltd.) while the absorbance was measured on an MPR-A4i microplate
reader (Tosoh Co., Tokyo, Japan) at 540 nm.
RNA isolation and quantitative RT-PCR
(qRT-PCR)
Confluent UE7T-13 cells in 24-well plastic culture
plates or Osteologic discs were cultured in BODM containing TGF-β
and/or PDGF. After 1 week of culture, total RNA was isolated using
Isogen reagent (Nippon Gene Co., Ltd., Tokyo, Japan) according to
the manufacturer’s instructions. cDNA was synthesized from total
RNA with the PrimeScript RT reagent kit (Takara Bio, Inc., Shiga,
Japan). qRT-PCR was performed on a Thermal Cycler Dice Real Time
System (Takara Bio, Inc.) with SYBR Premix Ex Taq II (Takara Bio,
Inc.) and specific oligonucleotide primers (presented in Table I). The mRNA expression levels of
runt-related transcription factor 2 (RUNX2), ALP, liver/bone/kidney
(ALPL), collagen, type I, alpha 1 (COL1A), secreted phosphoprotein
1 (osteopontin, SPP1), integrin-binding sialoprotein (bone
sialoprotein, IBSP), and bone gamma-carboxyglutamate (gla) protein
(osteocalcin, BGLAP) were normalized to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), and the relative expression levels were
expressed as the fold change relative to the corresponding
control.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Full name | Symbol | Primer sequence
(5′→3′) |
|---|
| Runt-related
transcription factor 2 | RUNX2 | Forward |
CACTGGCTGCAACAAGA |
| | Reverse |
CATTCCGGAGCTCAGCAGAATAA |
| Alkaline
phosphatase, liver/bone/kidney | ALPL | Forward |
GGACCATTCCCACGTCTTCAC |
| | Reverse |
CCTTGTAGCCAGGCCCATTG |
| Collagen, type I,
alpha 1 | COL1A | Forward |
TCTAGACATGTTCAGCTTTGTGGAC |
| | Reverse |
TCTGTACGCAGGTGATTGGTG |
| Secreted
phosphoprotein 1 | SPP1 | Forward |
ACACATATGATGGCCGAGGTGA |
| | Reverse |
TGTGAGGTGATGTCCTCGTCTGTAG |
| Integrin-binding
sialoprotein | IBSP | Forward |
GGCCACGATATTATCTTTACAAGCA |
| | Reverse |
TCAGCCTCAGAGTCTTCATCTTCA |
| Bone
gamma-carboxyglutamate (gla) protein | BGLAP | Forward |
AGGTGCAGCCTTTGTGTCCA |
| | Reverse |
GGCTCCCAGCCATTGATACAG |
|
Glyceraldehyde-3-phosphate
dehydrogenase | GAPDH | Forward |
GCACCGTCAAGGCTGAGAAC |
| | Reverse |
ATGGTGGTGAAGACGCCAGT |
Western blot analysis
The UE7T-13 cells were washed twice with PBS and
then lysed in RIPA buffer (50 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease
and phosphatase inhibitor cocktails (Sigma). The protein content
was measured with BCA reagent (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Equivalent protein samples were separated by
10–20% SDS-polyacrylamide gradient gel electrophoresis (SDS-PAGE)
and transferred onto a polyvinylidene difluoride (PVDF) membrane
(Millipore, Billerica, MA, USA). After blocking with 5% non-fat dry
milk in TTBS (50 mM Tris-HCl, pH 7.2, 150 mM NaCl, and 0.1%
Tween-20), the membrane was incubated with a primary anti-Akt (Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-phospho-Akt
(Ser473) (p-Akt, Cell Signaling Technology, Inc.), anti-p44/42 MAPK
(ERK1/2, Cell Signaling Technology, Inc.), anti-phospho-p44/42 MAPK
(Thr202/Tyr204) (p-ERK, Cell Signaling Technology, Inc.)
antibodies, and anti-β-actin (clone C4, Santa Cruz Biotechnology,
Santa Cruz, CA, USA) antibody as the loading control for
normalization. The blots were then incubated with ALP-conjugated
secondary antibody and developed using the BCIP/NBT membrane
phosphatase substrate system (KPL). Densitometry was performed
using ImageJ software (version 1.44). Data are expressed as the
ratio of phosphorylated to total molecular bands.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Statistical analysis was performed by using the Student’s
t-test, and values of p<0.05 were considered to indicate
statistically significant differences.
Results
PDGF markedly enhances the TGF-β-induced
ECM mineralization in hMSCs
In general, mesenchymal cells that differentiate
into OBs induce the mineralization of the the ECM (45–47). We investigated the TGF-β-mediated
induction of the osteogenic differentiation of the BM-MSC cell
line, UE7T-13, by using Alizarin red staining to assess ECM
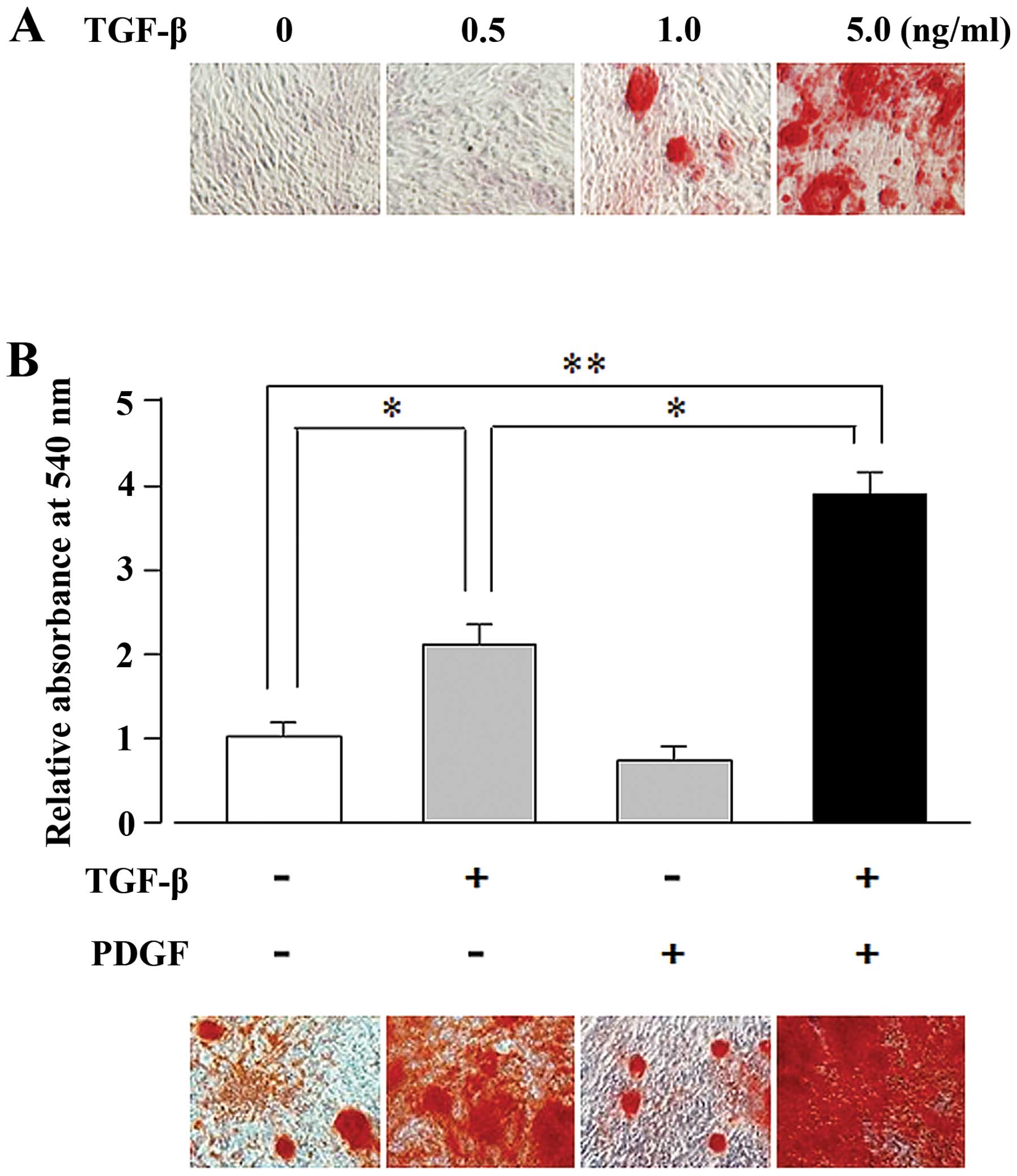
mineralization. As illustrated in Fig. 1A, TGF-β induced matrix
mineralization in the UE7T-13 cells in a dose-dependent manner
(1.0–5.0 ng/ml). We then examined the synergistic effects of PDGF
and TGF-β on osteogenic differentiation. Alizarin red staining of
TGF-β- and PDGF-stimulated cells revealed that the TGF-β (5
ng/ml)-induced matrix mineralization was enhanced by PDGF (10
ng/ml), whereas PDGF alone did not induce mineralization (Fig. 1B).
TGF-β and PDGF synergistically upregulate
the transcript levels of ALPL and IBSP in hMSCs
To elucidate the molecular mechanisms underlying the
synergistic effects of TGF-β and PDGF on osteogenic
differentiation, we investigated the transcript expression of
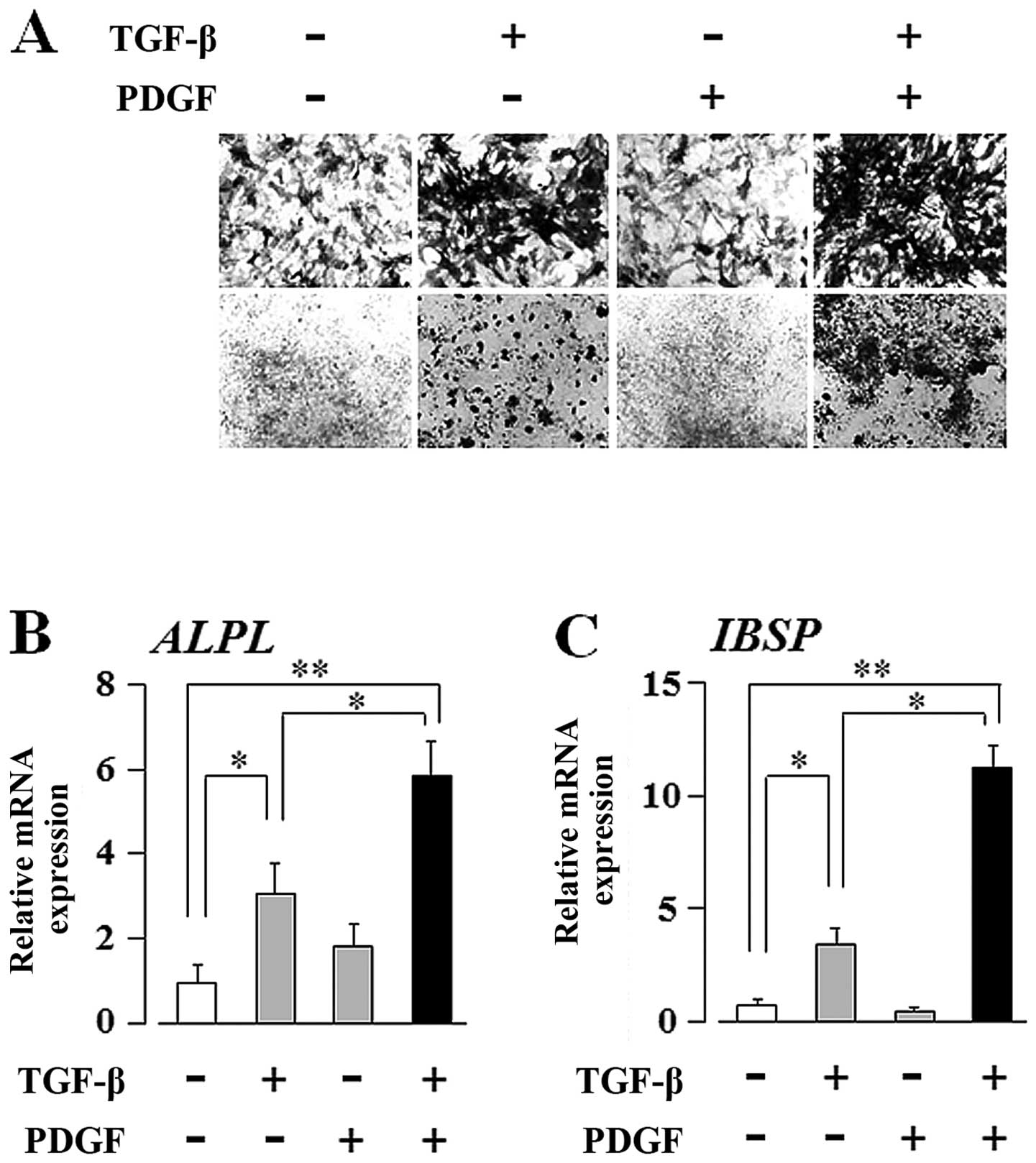
osteogenic differentiation markers in UE7T-13 cells by qRT-PCR. As
shown in Fig. 2B, TGF-β alone
markedly induced ALPL mRNA expression; PDGF alone had no effect on
ALPL expression. Intriguingly, PDGF markedly enhanced the
TGF-β-induced upregulation of ALP mRNA expression. In addition,
TGF-β and PDGF greatly induced IBSP expression, although neither
regulator alone was sufficient to influence IBSP expression
(Fig. 2E). These regulators,
either alone or synergistically, had no effect on the transcript
expression of other osteogenic differentiation marker genes, such
as RUNX2, COL1A1, SPP1 and BGLAP (Fig. 2A, C, D and F, respectively).
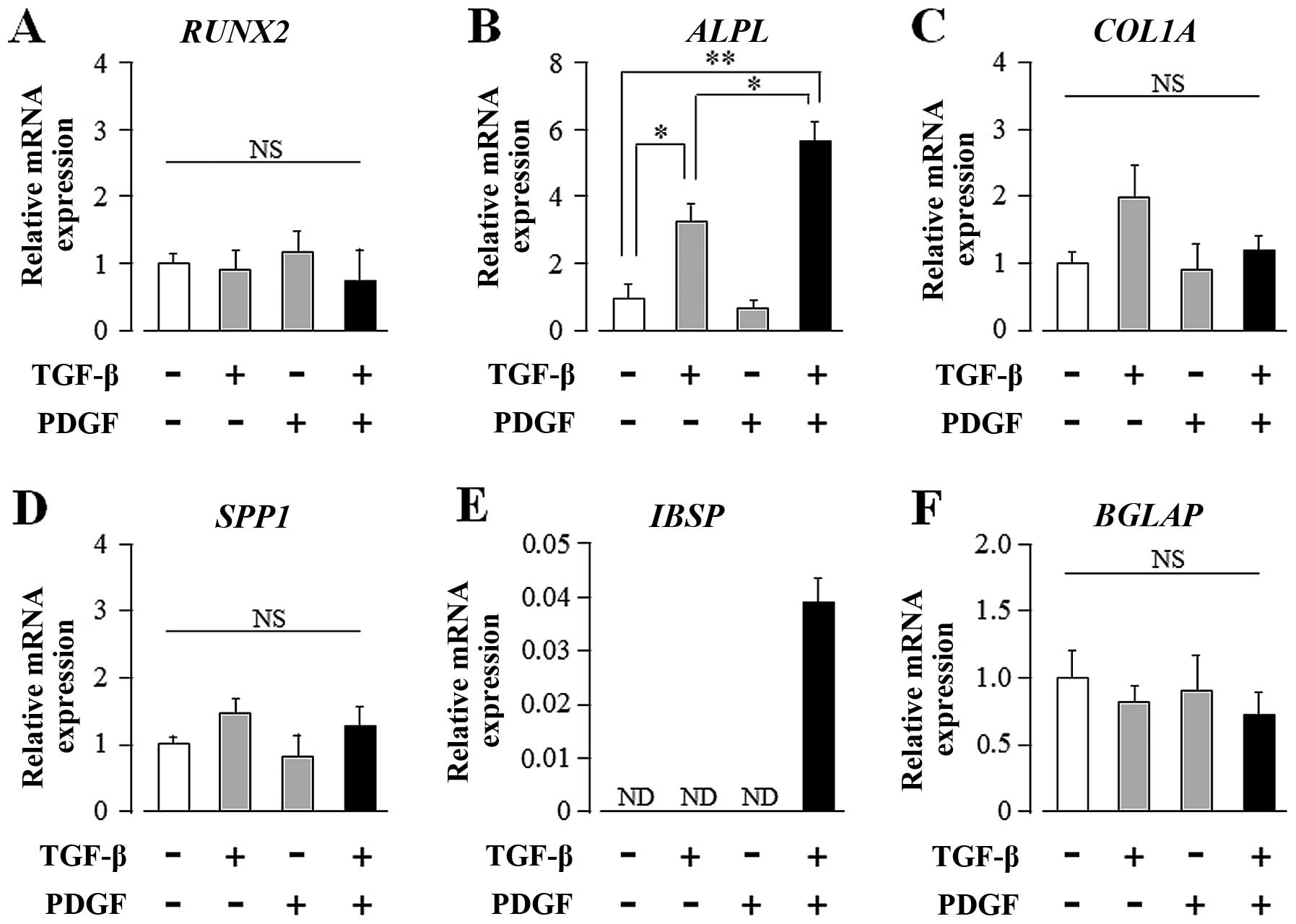 | Figure 2Transforming growth factor-β (TGF-β)
and platelet-derived growth factor (PDGF) synergistically
upregulate the transcript expression of alkaline phosphatase (ALP),
liver/bone/kidney (ALPL) and integrin-binding sialoprotein (bone
sialoprotein, IBSP) in human mesenchymal stem cells (hMSCs)
cultured on plastic culture plates. Confluent UE7T-13 cells were
cultured on 24-well plastic culture plates with basal osteogenic
differentiation medium (BODM) containing 5.0 ng/ml TGF-β and/or
10.0 ng/ml PDGF. After 1 week culture, qRT-PCR was performed with
specific oligonucleotide primers (presented in Table I). mRNA expression levels of (A)
runt-related transcription factor 2 (RUNX2), (B) ALPL, (C)
collagen, type I, alpha 1 (COL1A), (D) secreted phosphoprotein 1
(osteopontin, SPP1), (E) IBSP, and (F) bone gamma-carboxyglutamate
(gla) protein (osteocalcin, BGLAP) were normalized to GAPDH, and
the results are expressed as the fold change relative to the
respective control. Data are presented as the means ± SD.
*p<0.05, **p<0.02 indicate statistical
significance. |
PDGF enhances the TGF-β-induced
osteogenic differentiation of hMSCs on a proprietary hydroxyapatite
substitute for bone mineral (Osteologic discs)
As shown in Figs.
1 and 2B, PDGF enhanced the
TGF-β-induced osteogenic differentiation of hMSCs, whereas PDGF
alone had no effect on differentiation. These results suggest TGF-β
is superior to PDGF in the hierarchy that mediates the osteogenic
differentiation of hMSCs. However, as shown in Fig. 2E, it was unclear which growth
factor is the main regulator of IBSP mRNA expression. In order to
clearly rank these growth factors in the context of promoting OB
differentiation, we examined the mechanisms through which TGF-β
and/or PDGF affect the differentiation of hMSCs on a proprietary
hydroxyapatite substitute for bone mineral (Osteologic discs),
instead of the plastic culture plates utilized in Figs. 1 and 2. The surface of the Osteologic cell
culture disc is coated with calcium phosphate as described in
Materials and methods. As shown in Fig. 3A, ALP staining of the UE7T-13
cells cultured on Osteologic discs revealed that PDGF clearly
enhanced the TGF-β-induced upregulation of ALP expression, whereas
PDGF alone did not. In addition, as shown in Fig. 3B and C, qRT-PCR revealed that PDGF
clearly enhanced the TGF-β-induced upregulation of ALPL and IBSP
transcript expression, whereas PDGF alone did not. Thus, the
Osteologic culture system clearly demonstrated that TGF-β is
superior to PDGF in the regulation of the osteogenic
differentiation of hMSCs.
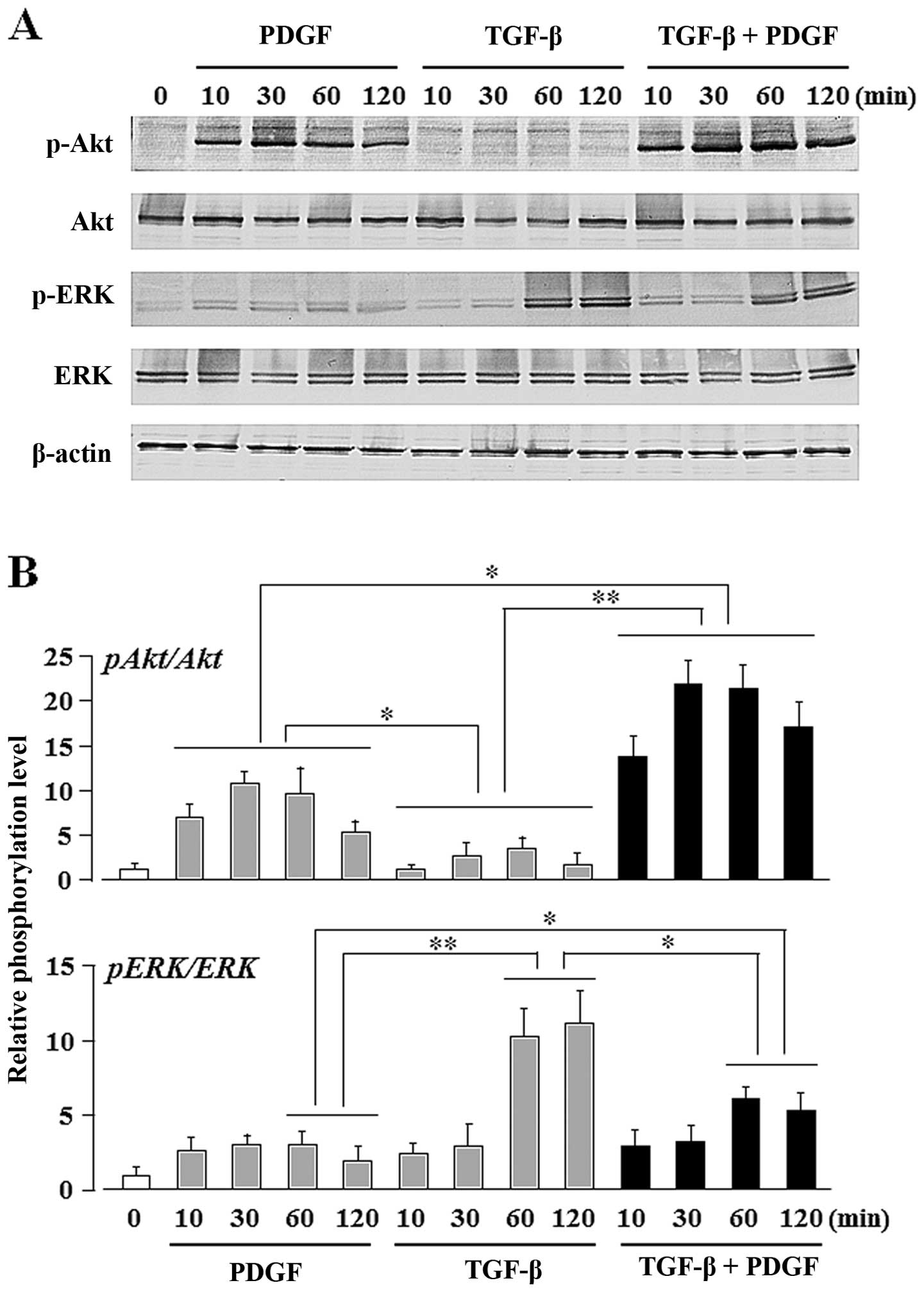
TGF-β upregulates the PDGF-induced Akt
activity, whereas PDGF downregulates the TGF-β-induced ERK
activity
In order to identify the signaling pathways
activated by TGF-β and/or PDGF during the osteogenic
differentiation of UE7T-13 cells, we evaluated the phosphorylation
status of the PI3K/Akt- and ERK-mediated pathways. Gharibi et
al previously reported that the PDGF BB-induced crosstalk
between these pathways affects the proliferation and adipogenic
commitment of hMSCs: PDGF-BB-induced PI3K/Akt signaling enhanced
the proliferative activity of the hMSCs, and PDGF-BB-induced
ERK-mediated signaling suppressed the adipogenic differentiation of
hMSCs (48). In general, cell
proliferation is poorly compatible with differentiation and
proliferation/differentiation switches have been demonstrated in
different cell types (49–51).
In addition, a reciprocal correlation exists between the
adipogenetic and osteogenetic differentiation of undifferentiated
mesenchymal cells (52–54). Thus, the crosstalk between the
PI3K/Akt- and ERK-mediated pathways appears to affect the
osteogenic commitment of hMSCs. In our study, ERK phosphorylation
was upregulated by stimulation with TGF-β alone, but Akt
phosphorylation was unaffected (Fig.
4). By contrast, Akt phosphorylation was upregulated by
stimulation with PDGF alone, but ERK phosphorylation was not. The
phosphorylation of both ERK and Akt was detected after
co-stimulation of PDGF and TGF-β. Notably, the combined stimulation
of TGF-β and PDGF strongly induced Akt phosphorylation (Fig. 4B; bar graph on upper panel, lanes
10–13), whereas PDGF alone only moderate induced Akt
phosphorylation (Fig. 4B; bar
graph on upper panel, lanes 2–5). By contrast, TGF-β alone markedly
induced ERK phosphorylation (Fig
4B; bar graph on lower panel, lanes 8 and 9), whereas the
combination of TGF-β and PDGF moderately induced ERK
phosphorylation (Fig 4B; bar
graph on lower panel, lanes 12 and 13). No phosphorylation of
Smad2/3 was detected, although it is one of the major pathways of
TGF-β stimulation in these cells (data not shown).
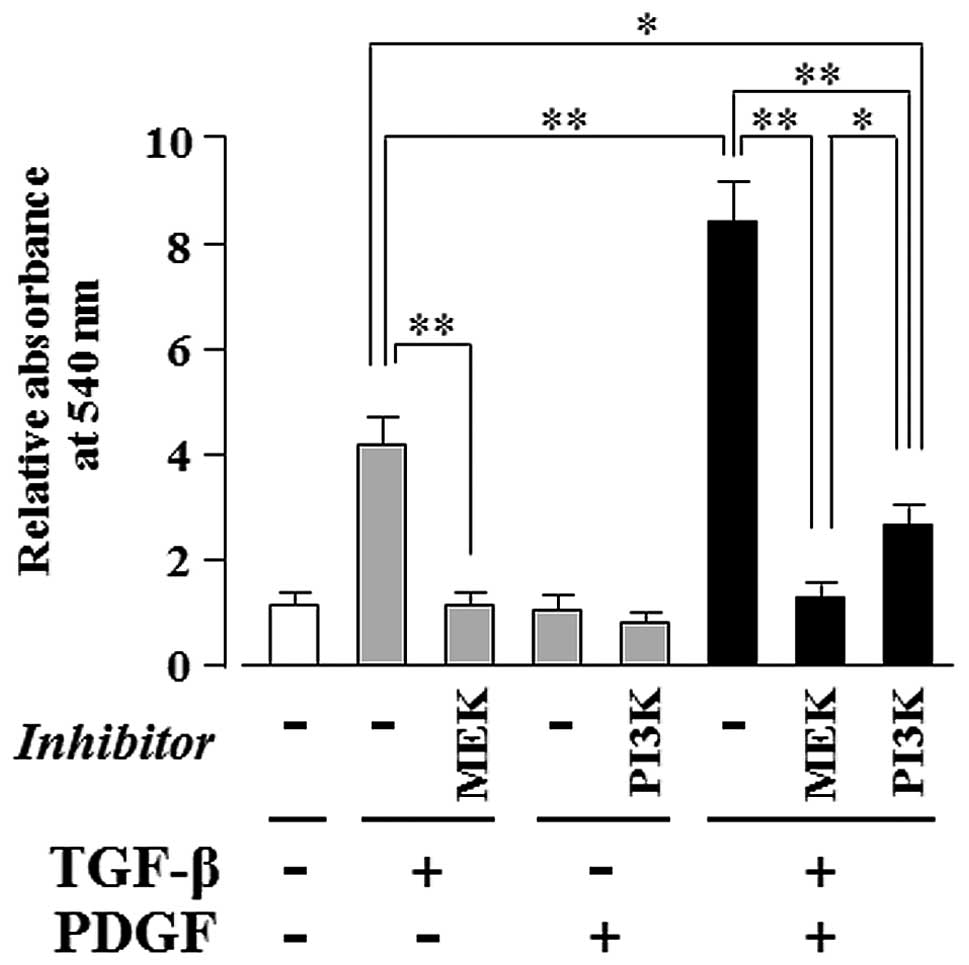
PDGF-induced PI3K-mediated signaling
enhances the TGF-β-induced, MEK-dependent osteogenic
differentiation of hMSCs
As shown in Fig.
5, TGF-β alone markedly induced ECM mineralization in a
MEK-dependent manner (Fig. 5;
lanes 2 and 3); moreover, PDGF clearly enhanced the TGF-β-induced
ECM mineralization (Fig. 5; lane
6), whereas PDGF alone had no effect (Fig. 5; lane 4). The synergistically
induced ECM mineralization was completely suppressed by U0126 (a
MEK inhibitor) (Fig. 5; lane 7)
and only partially suppressed by LY294002 (a PI3K inhibitor)
(Fig. 5; lane 8). The level of
ECM mineralization was lower in the culture supplemented with
TGF-β, PDGF and LY294002 (Fig. 5;
lane 8) than in the culture supplemented with TGF-β alone (Fig. 5; lane 2).
Discussion
TGF-β is crucial for connective tissue regeneration
and bone remodeling, as demonstrated by several in vivo and
in vitro studies. It affects osteogenic differentiation and
bone formation (55–59) and increases the mRNA levels of
osteogenic differentiation markers and ALP activity in murine bone
marrow MSCs (57). In this study,
we investigated whether TGF-β promotes the osteogenic
differentiation of the human bone marrow-derived MSC line, UE7T-13.
Our results demonstrated that TGF-β induced the osteogenic
differentiation of UE7T-13 in a dose-dependent manner (1.0–5.0
ng/ml) (Fig. 1A). Thus, we
focused on the synergistic effects of multiple growth factors on
the osteogenic differentiation of MSCs.
PDGF is mainly produced by platelets and has tissue
repair functions, such as fracture repair (26). In addition, PDGF is a
chemoattractant or mitogen of osteogenic mesenchymal cells
(41), and does not seem to
directly affect the osteogenic differentiation of MSCs. As shown in
Fig. 1B, PDGF alone did not
induce ECM mineralization in the UE7T-13 cells; however, PDGF
markedly enhanced the TGF-β-induced ECM mineralization.
We also investigated the synergistic effects of
TGF-β and PDGF on the mRNA expression of osteogenic differentiation
marker genes. qRT-PCR revealed that the transcript expression of
ALPL, a mineralization-associated enzyme, was increased by
stimulation with TGF-β alone (Fig.
2B). PDGF markedly enhanced the TGF-β induction of ALPL,
whereas PDGF alone had no effect. The TGF-β-induced ALPL expression
was similarly and greatly increased by PDGF stimulation, whereas
PDGF alone had no effect (data not shown). It is now well
established that osteogenic differentiation is marked by sequential
stages of cellular proliferation and ECM maturation (60). ALPL expression is a transient
early marker of osteogenic differentiation in MSCs, peaking at the
end of the proliferative stage before ECM maturation (61). Therefore, our findings suggest
that the PDGF support of TGF-β-induced osteogenic differentiation
may be important during the early stages of the osteogenesis of
MSCs.
IBSP expression is restricted to mineralized
connective tissues (62). IBSP is
a phosphorylated, sulfated glycoprotein that represents one of the
major non-collagenous ECM proteins associated with mineralized
tissues (63–65). A high expression of IBSP coincides
with de novo bone formation (62). IBSP is primarily expressed by
mature OBs and osteoclasts, as well as by hypertrophic chondrocytes
(66). We previously reported
that OB-like SaOS-2 cells have an increased expression of IBSP on
titanium surfaces coated with hydroxyapatite (67). Thus, the expression of IBSP is a
useful indicator of osteogenic differentiation. IBSP expression
occurs at the middle-to-late-stages of osteogenic differentiation
of undifferentiated mesenchymal cells (68). In our study, IBSP mRNA expression
was detected only in cultures containing both TGF-β and PDGF, but
was not detected in the cultures containing TGF-β or PDGF alone
(Fig. 2E). The bone surface is
comprised of hydroxyapatite, a calcium phosphate mineral, on which
MSCs differentiate into OBs; this of course differs significantly
from a polystyrene culture dish surface. Therefore, we examined the
osteogenic response of hMSCs to TGF-β and/or PDGF in cultures grown
on Osteologic discs, a proprietary hydroxyapatite substitute. As
shown in Fig. 3C, TGF-β alone
markedly induced IBSP mRNA expression in the UE7T-13 cells cultured
on Osteologic culture discs. In addition, PDGF clearly enhanced the
TGF-β-induced IBSP mRNA expression, whereas PDGF alone had no
effect on IBSP in this culture system. ALP staining and qRT-PCR
revealed that PDGF clearly enhanced the TGF-β-induced upregulation
of ALP protein and ALPL mRNA expression; PDGF alone had no effect
(Fig. 3A and B). Thus, the
Osteologic culture system demonstrated that TGF-β is superior to
PDGF in the osteogenic differentiation of hMSCs. The supportive
effect of PDGF seems to occur during the early stage (for ALPL
induction) to late stage (for IBSP induction) of osteogenic
differentiation.
In order to clarify the intracellular signaling
pathways that mediate the interaction between PDGF and TGF-β in the
induction of the osteogenic differentiation of MSCs, we evaluated
the phosphorylation status of the PI3K/Akt and ERK pathways. The
MEK inhibitor, U0126, completely suppressed the TGF-β-induced ECM
mineralization in the UE7T-13 cell culture (Fig. 5; lane 3); PDGF alone did not
promote osteogenic activity (Fig.
5; lane 4), but enhanced the TGF-β-induced ECM mineralization
(Fig. 5; lane 6). This
synergistic promotion of ECM mineralization was completely
suppressed by U0126 (Fig. 5; lane
7), strongly suggesting that PDGF enhances the TGF-β-induced
osteogenic differentiation of hMSCs in a TGF-β-activated
MEK-dependent manner. In addition, the synergistic differentiation
of hMSCs by both factors was partially suppressed by the PI3K
inhibitor, LY294002 (Fig. 5; lane
8). Taken together, our results indicate that PDGF-induced
PI3K-mediated signaling enhances the TGF-β-induced osteogenic
differentiation of hMSCs in a TGF-β-activated MEK-dependent manner.
Notably, the level of ECM mineralization in the presence of TGF-β,
PDGF and LY294002 (Fig. 5; lane
8) was markedly lower than that in the cultures with TGF-β alone
(Fig. 5; lane 2). As described
above, PDGF inhibits the TGF-β-induced MEK activity (Fig. 4B: bar graph on lower panel, lanes
12 and 13), whereas TGF-β enhances PDGF-induced Akt activity
(Fig. 4B: bar graph on upper
panel, lanes 10–13). These results suggest that ECM mineralization
may be predominantly induced by PI3K/Akt-mediated signaling than by
MEK/ERK-mediated signaling in the presence of both factors.
Thus, it can be concluded that PDGF-stimulated
PI3K/Akt-mediated signaling enhances the TGF-β-induced osteogenic
differentiation of hMSCs in a MEK/ERK-dependent manner. The
combination of PDGF-activated PI3K/Akt and TGF-β-activated MEK
mediates osteogenic differentiation which is important for
optimizing the potential therapeutic use of hMSCs for bone
formation. Our findings provide insight into the establishment of
novel therapeutic methods for bone formation by hMSCs.
Acknowledgements
This study was supported in part, by JSPS KAKENHI
(Grant nos. 25463053 to N.C., 24792149 to N.O., 25463224 to N.T.,
235928640 to H.K. and 23592896 to A.I.); the Open Research Project
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan, 2008–2012; and a Grant-in-Aid for Strategic
Medical Science Research Centre from the Ministry of Education,
Culture, Sports, Science, and Technology of Japan, 2010–2014.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Docheva D, Popov C, Mutschler W and
Schieker M: Human mesenchymal stem cells in contact with their
environment: surface characteristics and the integrin system. J
Cell Mol Med. 11:21–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baksh D, Song L and Tuan RS: Adult
mesenchymal stem cells: characterization, differentiation, and
application in cell and gene therapy. J Cell Mol Med. 8:301–316.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kassem M, Abdallah BM and Saeed H:
Osteoblastic cells: differentiation and trans-differentiation. Arch
Biochem Biophys. 473:183–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones E and Yang X: Mesenchymal stem cells
and bone regeneration: current status. Injury. 42:562–568. 2011.
View Article : Google Scholar
|
|
8
|
Proff P and Römer P: The molecular
mechanism behind bone remodelling: a review. Clin Oral Investig.
13:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazar-Karsten P, Dorn I, Meyer G, et al:
The influence of extracellular matrix proteins and mesenchymal stem
cells on erythropoietic cell maturation. Vox Sang. 101:65–76. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vidane AS, Zomer HD, Oliveira BM, et al:
Reproductive stem cell differentiation: extracellular matrix,
tissue microenvironment, and growth factors direct the mesenchymal
stem cell lineage commitment. Reprod Sci. 20:1137–1143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen BY, Wang X, Chen LW and Luo ZJ:
Molecular targeting regulation of proliferation and differentiation
of the bone marrow-derived mesenchymal stem cells or mesenchymal
stromal cells. Curr Drug Targets. 13:561–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soleymaninejadian E, Pramanik K and
Samadian E: Immunomodulatory properties of mesenchymal stem cells:
cytokines and factors. Am J Reprod Immunol. 67:1–8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chau JF, Leong WF and Li B: Signaling
pathways governing osteoblast proliferation, differentiation and
function. Histol Histopathol. 24:1593–1606. 2009.PubMed/NCBI
|
|
14
|
Pountos I, Georgouli T, Henshaw K, et al:
The effect of bone morphogenetic protein-2, bone morphogenetic
protein-7, parathyroid hormone, and platelet-derived growth factor
on the proliferation and osteogenic differentiation of mesenchymal
stem cells derived from osteoporotic bone. J Orthop Trauma.
24:552–556. 2010. View Article : Google Scholar
|
|
15
|
Bonewald LF and Mundy GR: Role of
transforming growth factor-beta in bone remodeling. Clin Orthop
Relat Res. 250:261–276. 1990.PubMed/NCBI
|
|
16
|
Bonewald LF and Dallas SL: Role of active
and latent transforming growth factor beta in bone formation. J
Cell Biochem. 55:350–357. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Y, Wu X, Lei W, et al:
TGF-beta1-induced migration of bone mesenchymal stem cells couples
bone resorption with formation. Nat Med. 15:757–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He S, Liu X, Yang Y, et al: Mechanisms of
transforming growth factor beta (1)/Smad signalling mediated by
mitogen-activated protein kinase pathways in keloid fibroblasts. Br
J Dermatol. 162:538–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012.
|
|
21
|
Mbalaviele G, Abu-Amer Y, Meng A, et al:
Activation of peroxisome proliferator-activated receptor-gamma
pathway inhibits osteoclast differentiation. J Biol Chem.
275:14388–14393. 2000. View Article : Google Scholar
|
|
22
|
Chaudhary LR and Avioli LV: Identification
and activation of mitogen-activated protein (MAP) kinase in normal
human osteoblastic and bone marrow stromal cells: attenuation of
MAP kinase activation by cAMP, parathyroid hormone and forskolin.
Mol Cell Biochem. 178:59–68. 1998. View Article : Google Scholar
|
|
23
|
Hu Y, Chan E, Wang SX and Li B: Activation
of p38 mitogen-activated protein kinase is required for osteoblast
differentiation. Endocrinology. 144:2068–2074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Einhorn TA and Trippel SB: Growth factor
treatment of fractures. Instr Course Lect. 46:483–486.
1997.PubMed/NCBI
|
|
25
|
Hart CE, Bailey M, Curtis DA, et al:
Purification of PDGF-AB and PDGF-BB from human platelet extracts
and identification of all three PDGF dimers in human platelets.
Biochemistry. 29:166–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hollinger JO, Hart CE, Hirsch SN, et al:
Recombinant human platelet-derived growth factor: biology and
clinical applications. J Bone Joint Surg Am. 90:48–54. 2008.
View Article : Google Scholar
|
|
27
|
Jayakumar A, Rajababu P, Rohini S, et al:
Multi-centre, randomized clinical trial on the efficacy and safety
of recombinant human platelet-derived growth factor with
β-tricalcium phosphate in human intra-osseous periodontal defects.
J Clin Periodontol. 38:163–172. 2011.PubMed/NCBI
|
|
28
|
Ridgway HK, Mellonig JT and Cochran DL:
Human histologic and clinical evaluation of recombinant human
platelet-derived growth factor and beta-tricalcium phosphate for
the treatment of periodontal intraosseous defects. Int J
Periodontics Restorative Dent. 28:171–179. 2008.
|
|
29
|
Nevins M, Giannobile WV, McGuire MK, et
al: Platelet-derived growth factor stimulates bone fill and rate of
attachment level gain: results of a large multicenter randomized
controlled trial. J Periodontol. 76:2205–2215. 2005. View Article : Google Scholar
|
|
30
|
McGuire MK, Kao RT, Nevins M and Lynch SE:
rhPDGF-BB promotes healing of periodontal defects: 24-month
clinical and radiographic observations. Int J Periodontics
Restorative Dent. 26:223–231. 2006.PubMed/NCBI
|
|
31
|
Distler JH, Hirth A, Kurowska-Stolarska M,
et al: Angiogenic and angiostatic factors in the molecular control
of angiogenesis. Q J Nucl Med. 47:149–161. 2003.PubMed/NCBI
|
|
32
|
Bouletreau PJ, Warren SM, Spector JA, et
al: Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression
in microvascular endothelial cells: implications for fracture
healing. Plast Reconstr Surg. 109:2384–2397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blanquaert F, Pereira RC and Canalis E:
Cortisol inhibits hepatocyte growth factor/scatter factor
expression and induces c-met transcripts in osteoblasts. Am J
Physiol Endocrinol Metab. 278:E509–E515. 2000.PubMed/NCBI
|
|
34
|
Franchimont N, Durant D, Rydziel S and
Canalis E: Platelet-derived growth factor induces interleukin-6
transcription in osteoblasts through the activator protein-1
complex and activating transcription factor-2. J Biol Chem.
274:6783–6789. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carano RA and Filvaroff EH: Angiogenesis
and bone repair. Drug Discov Today. 8:980–989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu E, Palmer N, Tian Z, et al:
Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR
genetically defined cells. PLoS One. 3:e37942008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Missbach M, Jeschke M, Feyen J, et al: A
novel inhibitor of the tyrosine kinase Src suppresses
phosphorylation of its major cellular substrates and reduces bone
resorption in vitro and in rodent models in vivo. Bone. 24:437–449.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martelli AM, Borgatti P, Bortul R, et al:
Phosphatidylinositol 3-kinase translocates to the nucleus of
osteoblast-like MC3T3-E1 cells in response to insulin-like growth
factor I and platelet-derived growth factor but not to the
proapoptotic cytokine tumor necrosis factor alpha. J Bone Miner
Res. 15:1716–1730. 2000. View Article : Google Scholar
|
|
40
|
Chaudhary LR and Hruska KA: The cell
survival signal Akt is differentially activated by PDGF-BB, EGF,
and FGF-2 in osteoblastic cells. J Cell Biochem. 81:304–311. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Antoniades HN: PDGF: a multifunctional
growth factor. Baillieres Clin Endocrinol Metab. 5:595–613. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou B, Hao Y, Wang C, et al: Conversion
of natively unstructured α-synuclein to its α-helical conformation
significantly attenuates production of reactive oxygen species. J
Inorg Biochem. 118:68–73. 2013.
|
|
43
|
Mori T, Kiyono T, Imabayashi H, et al:
Combination of hTERT and bmi-1, E6, or E7 induces prolongation of
the life span of bone marrow stromal cells from an elderly donor
without affecting their neurogenic potential. Mol Cell Biol.
25:5183–5195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shimomura T, Yoshida Y, Sakabe T, et al:
Hepatic differentiation of human bone marrow-derived UE7T-13 cells:
effects of cytokines and CCN family gene expression. Hepatol Res.
37:1068–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang H, Hu ZZ, Arighi CN and Wu CH:
Integration of bioinformatics resources for functional analysis of
gene expression and proteomic data. Front Biosci. 12:5071–5088.
2007.PubMed/NCBI
|
|
46
|
Dalle Carbonare L, Innamorati G and
Valenti MT: Transcription factor Runx2 and its application to bone
tissue engineering. Stem Cell Rev. 8:891–897. 2012.PubMed/NCBI
|
|
47
|
Neve A, Corrado A and Cantatore FP:
Osteoblast physiology in normal and pathological conditions. Cell
Tissue Res. 343:289–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gharibi B, Ghuman MS and Hughes FJ: Akt-
and Erk-mediated regulation of proliferation and differentiation
during PDGFRβ-induced MSC self-renewal. J Cell Mol Med.
16:2789–2801. 2012.PubMed/NCBI
|
|
49
|
Chen JF, Mandel EM, Thomson JM, et al: The
role of microRNA-1 and microRNA-133 in skeletal muscle
proliferation and differentiation. Nat Genet. 38:228–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Conti L, Sipione S, Magrassi L, et al: Shc
signaling in differentiating neural progenitor cells. Nat Neurosci.
4:579–586. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dugan LL, Kim JS, Zhang Y, et al:
Differential effects of cAMP in neurons and astrocytes. Role of
B-raf. J Biol Chem. 274:25842–25848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sottile V, Halleux C, Bassilana F, et al:
Stem cell characteristics of human trabecular bone-derived cells.
Bone. 30:699–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pereira RC, Delany AM and Canalis E:
Effects of cortisol and bone morphogenetic protein-2 on stromal
cell differentiation: correlation with CCAAT-enhancer binding
protein expression. Bone. 30:685–691. 2002. View Article : Google Scholar
|
|
54
|
Lecka-Czernik B, Moerman EJ, Grant DF, et
al: Divergent effects of selective peroxisome
proliferator-activated receptor-gamma 2 ligands on adipocyte versus
osteoblast differentiation. Endocrinology. 143:2376–2384. 2002.
|
|
55
|
Janssens K, ten Dijke P, Janssens S and
Van Hul W: Transforming growth factor-beta1 to the bone. Endocr
Rev. 26:743–774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee JY, Kim KH, Shin, et al: Enhanced bone
formation by transforming growth factor-beta1-releasing
collagen/chitosan microgranules. J Biomed Mater Res A. 76:530–539.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao L, Jiang S and Hantash BM:
Transforming growth factor beta1 induces osteogenic differentiation
of murine bone marrow stromal cells. Tissue Eng Part A. 16:725–733.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ripamonti U, Ferretti C, Teare J and Blann
L: Transforming growth factor-beta isoforms and the induction of
bone formation: implications for reconstructive craniofacial
surgery. J Craniofac Surg. 20:1544–1555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Quarles LD, Yohay DA, Lever LW, et al:
Distinct proliferative and differentiated stages of murine MC3T3-E1
cells in culture: an in vitro model of osteoblast development. J
Bone Miner Res. 7:683–692. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo X, Chen J, Song WX, et al: Osteogenic
BMPs promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen J, Shapiro HS and Sodek J:
Development expression of bone sialoprotein mRNA in rat mineralized
connective tissues. J Bone Miner Res. 7:987–997. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fisher LW, McBride OW, Termine JD and
Young MF: Human bone sialoprotein: deduced protein sequence and
chromosomal localization. J Biol Chem. 265:2347–2351.
1990.PubMed/NCBI
|
|
64
|
Ganss B, Kim RH and Sodek J: Bone
sialoprotein. Crit Rev Oral Biol Med. 10:79–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ogata Y: Bone sialoprotein and its
transcriptional regulatory mechanism. J Periodontal Res.
43:127–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bianco P, Fisher LW, Young MF, et al:
Expression of bone sialoprotein (BSP) in developing human tissues.
Calcif Tissue Int. 49:421–426. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chosa N, Taira M, Saitoh S, et al:
Characterization of apatite formed on alkaline-heat-treated Ti. J
Dent Res. 83:465–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Id Boufker H, Lagneaux L, Fayyad-Kazan H,
et al: Role of farnesoid X receptor (FXR) in the process of
differentiation of bone marrow stromal cells into osteoblasts.
Bone. 49:1219–1231. 2011.PubMed/NCBI
|