Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic
stromal cells, which retain the ability to self-renew and
differentiate into mesenchymal cells, such as osteoblasts,
adipocytes, chondrocytes and skeletal muscle cells (1). Various surface markers, including
CD49a, CD73, CD105, vascular cell adhesion molecule-1
(VCAM-1)/CD106, CD140b, CD146, CD271, mesenchymal stromal cell
antigen-1 (MSCA-1) and STRO-1, have been used alone or in
combination to identify and isolate human MSCs (hMSCs) (2–8).
STRO-1 is a popular MSC marker and is often used in combination
with VCAM-1 for MSC isolation. A number of studies have analyzed
the changes in surface marker expression caused by prolonged
cultivation, and have reported the attenuated expression of several
markers, including VCAM-1 (9,10).
Cell adhesion molecules, such as VCAM-1 mediate the interaction of
MSCs with endothelial cells (ECs), which is essential for MSC
homing. Therefore, the reduction in VCAM-1 expression during
expansion in culture may be related to the decreased homing ability
of senescent MSCs. Mabuchi et al recently demonstrated that
clones of hMSCs retaining high rates of colony-forming unit
fibroblasts (CFU-Fs) expressing the surface markers, CD271/low
affinity nerve growth factor receptor (LNGFR), thymocyte antigen-1
(Thy-1) and VCAM-1, exhibited robust multilineage differentiation
and self-renewal ability (11).
An in-depth investigation of MSC markers has made it possible to
identify and purify MSCs; for instance, anti-CD49a antibody is
useful for identifying hMSCs (12,13). Notably, rapidly expanding clones
of hMSCs express high levels of CD49a and VCAM-1 and are highly
migratory (11). These results
suggest that VCAM-1 can be used as a marker for enriching
migratory, multipotent and proliferative cells from
culture-expanded MSCs.
Cadherins are members of a family of transmembrane
proteins involved in mediating homophilic adhesion in a
Ca2+-dependent manner. These proteins are major
components of adherence junctions (AJs) in cells. E-cadherin is the
main cadherin in the AJs of epithelial cells, whereas other
cadherins, including N-cadherin, P-cadherin, R-cadherin and
VE-cadherin form AJs in other cell types. Although fibroblasts
express a number of different cadherins, including P-cadherin,
R-cadherin, OB-cadherin and fat-like cadherins (14,15), N-cadherin is the predominant
cadherin expressed by these cells (14,16). N-cadherin-mediated AJs are of
great importance in connective tissue physiology and are critical
for the regulation of cell attachment and migration (17), wound healing (18), metastatic potential (19) and embryonic development (20,21), as well as the differentiation and
formation of numerous specialized tissues, including fibrous
connective tissues (22–26). N-cadherin is considered to be the
key factor in directing cell-cell interactions during mesenchymal
condensation, a process mediated by surface contact that results in
the aggregation of progenitor cells (27–30). Studies have indicated that the
expression of deletion mutant forms of N-cadherin, which lack
either the extracellular homotypic interaction domains or the
intracellular β-catenin binding site, results in decreased cellular
condensation and impaired chondrogenesis (31,32). These findings suggest that both
extracellular homotypic interaction and intracellular interaction
with the catenin complex are essential for proper N-cadherin
signaling (33).
In a recent study of ours, we found that the
expression level of VCAM-1/CD106 was markedly upregulated in human
bone marrow-derived MSCs, UE7T-13 cells, under conditions of high
cell density (34). The high cell
density-induced expression of VCAM-1 was markedly suppressed by
nuclear factor-κB (NF-κB) signaling-related protein kinase
inhibitors, such as the IκB kinase-2 (IKK-2) inhibitor VI, a
phosphoinositide 3-kinase (PI3K) inhibitor, an Src inhibitor and a
protein kinase C (PKC) inhibitor. Therefore, the high cell
density-induced VCAM-1 expression was regulated by the NF-κB
pathway in human bone marrow-derived MSCs. However, the identities
of the inducing factor(s) that activate the NF-κB pathway in MSC
cell-cell adhesion are not yet clear. Herein, we demonstrate that
cell-cell adhesion by N-cadherin activates the NF-κB pathway via
platelet-derived growth factor (PDGF) receptor (PDGFR)-β in a
ligand-independent manner.
Materials and methods
Reagents
The SCADS inhibitor kit, including various protein
kinase inhibitors was generously supplied by the Screening
Committee of Anticancer Drugs supported by a Grant-in-Aid for
Scientific Research on Priority Area ‘Cancer’ from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Cell culture
Human bone marrow-derived MSCs, UE7T-13 cells, the
life span of which was prolonged by infection with a retrovirus
encoding human papillomavirus E7 and human telomerase reverse
transcriptase (hTERT) (35,36), were purchased from the Health
Science Research Resources Bank (JCRB no. 1154, Japan Health
Sciences Foundation, Japan). UE7T-13 cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) supplemented with 10% fetal bovine serum (FBS; HyClone,
Logan, UT, USA) at 37ºC in a humidified incubator with an
atmosphere of 5% CO2 (3.0×103
cells/cm2, ‘low’ cell density; 1.0×105
cells/cm2, ‘high’ cell density).
RNA isolation and quantitative RT-PCR
(qRT-PCR)
Total RNA from low, medium and high cell density
cultured UE7T-13 cells was isolated using Isogen reagent (Nippon
Gene, Tokyo, Japan) according to the manufacturer’s instructions.
First-strand cDNA was synthesized from total RNA by using the
PrimeScript RT reagent kit (Takara, Kyoto, Japan). qRT-PCR was
performed on a Thermal Cycler Dice Real-time System (Takara) using
SYBR Premix Ex Taq II (Takara) with specific oligonucleotide
primers (presented in Table I).
The mRNA expression levels for VCAM-1, PDGFRα and PDGFRβ were
normalized to those obtained for glyceraldehyde adenosine-phosphate
dehydrogenase (GAPDH), and the relative expression levels were
shown as fold-increase or decrease relative to the control.
 | Table ISequences of the qRT-PCR primers used
in this study. |
Table I
Sequences of the qRT-PCR primers used
in this study.
| Gene | Primer
sequences | Location (bp) | Product size
(bp) |
|---|
| VCAM-1 | Foward:
5′-CGAAAGGCCCAGTTGAAGGA-3′ | 2086–2226 | 141 |
| Reverse:
5′-GAGCACGAGAAGCTCAGGAGAAA-3′ | | |
| PDGFRα | Forward:
5′-GTGCGAAGACTGAGCCAGATTG-3′ | 5708–5828 | 121 |
| Reverse:
5′-CGATAAACAGAATGCTTGAGCTGTG-3′ | | |
| PDGFRβ | Forward:
5′-TGCCTTGCCAGCACTAACATTC-3′ | 4769–4908 | 140 |
| Reverse:
5′-CCAGAGTGTGATGTGTGATCTGGA-3′ | | |
| GAPDH | Forward:
5′-GCACCGTCAAGGCTGAGAAC-3′ | 248–389 | 142 |
| Reverse:
5′-ATGGTGGTGAAGACGCCAGT-3′ | | |
Western blot analysis
The UE7T-13 cells were washed twice with ice-cold
PBS and then lysed in RIPA buffer (50 mM Tris-HCl, pH 7.2, 150 mM
NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) containing
protease and phosphatase inhibitor cocktails (Sigma-Aldrich). The
protein content of the samples was measured using the BCA reagent
(Pierce Biotechnology, Rockford, IL, USA). Samples containing equal
amounts of protein were separated by 12.5% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene
difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After
being blocked with 5% non-fat dry milk in T-TBS (50 mM Tris-HCl, pH
7.2, 150 mM NaCl and 0.1% Tween-20), the membrane was incubated
with primary anti-Src (Cell Signaling, Technologies, Beverly, MA,
USA) and anti-phospho-Src (p-Src, Cell Signaling) antibodies, using
an anti-β-actin (clone C4, Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) antibody as a loading control for normalization. The
blots were subsequently incubated with horseradish
peroxidase-conjugated secondary antibody and developed using
chemiluminescence with the Amersham ECL western blot analysis
system (GE Healthcare Biosciences, Pittsburgh, PA, USA). The
detected blots were photographed using the photo image detection
system CL-Cube L (Tohoku Electronic Industrial Co., Ltd., Sendai,
Japan) and densitometrically measured using ImageJ software
(version 1.44). Data are expressed as the ratio of phosphorylated
to total molecular bands.
siRNA transfection for PDGFRs
Three sets of Stealth siRNA oligonucleotide duplexes
targeting PDGFRα and PDGFRβ were designed using the online BLOCK-iT
RNAi Designer software (Invitrogen, Carlsbad, CA, USA). Sequences
of the siRNA oligonucleotide duplexes are listed in Table II. UE7T-13 cells were seeded in
24-well culture plates without antibiotic selection at a density of
2.0×104 cells/well, 24 h before siRNA transfection.
Subsequently, transcriptional knockdown was performed by
transfection of the cells with siRNA oligonucleotide duplexes at a
final concentration of 20 nM in DMEM using Lipofectamine RNAiMAX
(Invitrogen) for 24 h according to the manufacturer’s instructions.
The effects of RNAi knockdown of target genes were assayed by
qRT-PCR. Stealth siRNA Negative Control medium GC Duplex
(Invitrogen) was also included as a control for sequence
independent RNAi knockdown.
 | Table IIOligonucleotide sequences of the
Stealth siRNA duplex. |
Table II
Oligonucleotide sequences of the
Stealth siRNA duplex.
| Gene | siRNA no. | RNA duplex
sequence |
|---|
| PDGFRα | −387 | Sense: |
5′-AAUGAAAGCUGGCAGAGGAUUAGGC-3′ |
| | Antisense: |
5′-GCCUAAUCCUCUGCCAGCUUUCAUU-3′ |
| −751 | Sense: |
5′-UAUAAUGGCAGAAUCAUCAUCCUCC-3′ |
| | Antisense: |
5′-GGAGGAUGAUGAUUCUGCCAUUAUA-3′ |
| −843 | Sense: |
5′-UUAAAGCCCUGUCUGCUGUCGUAGG-3′ |
| | Antisense: |
5′-CCUACGACAGCAGACAGGGCUUUAA-3′ |
| PDGFRβ | −803 | Sense: |
5′-CAAAGAUGUAGAGCCGUUUCCGCUC-3′ |
| | Antisense: |
5′-CCUACGACAGCAGACAGGGCUUUAA-3′ |
| −1061 | Sense: |
5′-CAUAGUAGGCAUCAGAAUCCACCUC-3′ |
| | Antisense: |
5′-GAGGUGGAUUCUGAUGCCUACUAUG-3′ |
| −1506 | Sense: |
5′-UUGUCUUUGAACCACAGGACAGUGG-3′ |
| | Antisense: |
5′-CCACUGUCCUGUGGUUCAAAGACAA-3′ |
Overexpression of N-cadherin
cDNA encoding full-length N-cadherin
(Met1-Asp906) or an N-cadherin deletion
mutant lacking the C-terminal intercellular domain
(Met1-Trp745) was amplified by PCR. The cDNA
was then subcloned into the pcDNA/V5/GW/D-TOPO vector (Invitrogen)
using the pcDNA Gateway Directional TOPO Expression kit
(Invitrogen) according to the manufacturer’s instructions. The
overexpression vectors, containing full-length N-cadherin
(pCDH2-full) or the deletion mutant (pCDH2-Δ), were transfected
into the UE7T-13 cells using Lipofectamine LTX (Invitrogen) for 48
h according to the manufacturer’s instructions. The effects of
N-cadherin overexpression were assayed by flow cytometric analysis.
The transfected UE7T-13 cells were stripped with cell dissociation
buffer (Invitrogen) and washed with PBS containing 0.5% FBS and 2
mM EDTA. The cells (1.0×105) were then incubated with an
anti-N-cadherin antibody (clone GC-4; Abcam, Cambridge, MA, USA)
for 1 h at room temperature. The cells were then incubated with
phycoerythrin-conjugated secondary antibody for 1 h. Image
acquisition was performed using the EPICS XL ADC System (Beckman
Coulter, Brea, CA, USA).
Cell proliferation assay
Cell proliferation was analyzed using a colorimetric
assay for the quantification of the cleavage of the tetrazolium
salt WST-1 (Roche Applied Science, Basel, Switzerland) by
mitochondrial dehydrogenases in viable cells. The dye formed can be
quantified using a spectrophotometer and directly correlates with
the number of metabolically active cells in the culture. The cells
were grown in 96-well plates (Nunclon®; Sigma-Aldrich)
for 1, 3 and 5 days treated with or without 10 ng/ml of recombinant
human PDGF-BB (Acris Antibodies, San Diego, CA, USA). After each
incubation period, the cells were incubated for a further 1 h at
37ºC with 100 μl medium containing 10 μl WST-1 reagent. The samples
were shaken for 1 min, and absorbance was measured at 450 nm using
a MPR-A4i microplate reader (Tosoh Corp., Tokyo, Japan).
Statistical analysis
All experiments were repeated at least 3 times.
Representative images or data are shown. Data are presented as the
means ± standard deviation (SD). Differences between averages and
percentages between the control and test samples were statistically
analyzed using paired two-tailed Student’s t-tests. Values of
p<0.05 were considered to indicate statistically significant
differences.
Results
Effect of receptor tyrosine kinase (RTK)
inhibitors on high cell density-induced VCAM-1 expression
In a recent study, we demonstrated that high cell
density induces VCAM-1 expression through the NF-κB pathway in
UE7T-13 cells (34) and that the
expression of VCAM-1 in high cell density culture was significantly
inhibited by treatment with IKK-2 inhibitor VI. In the present
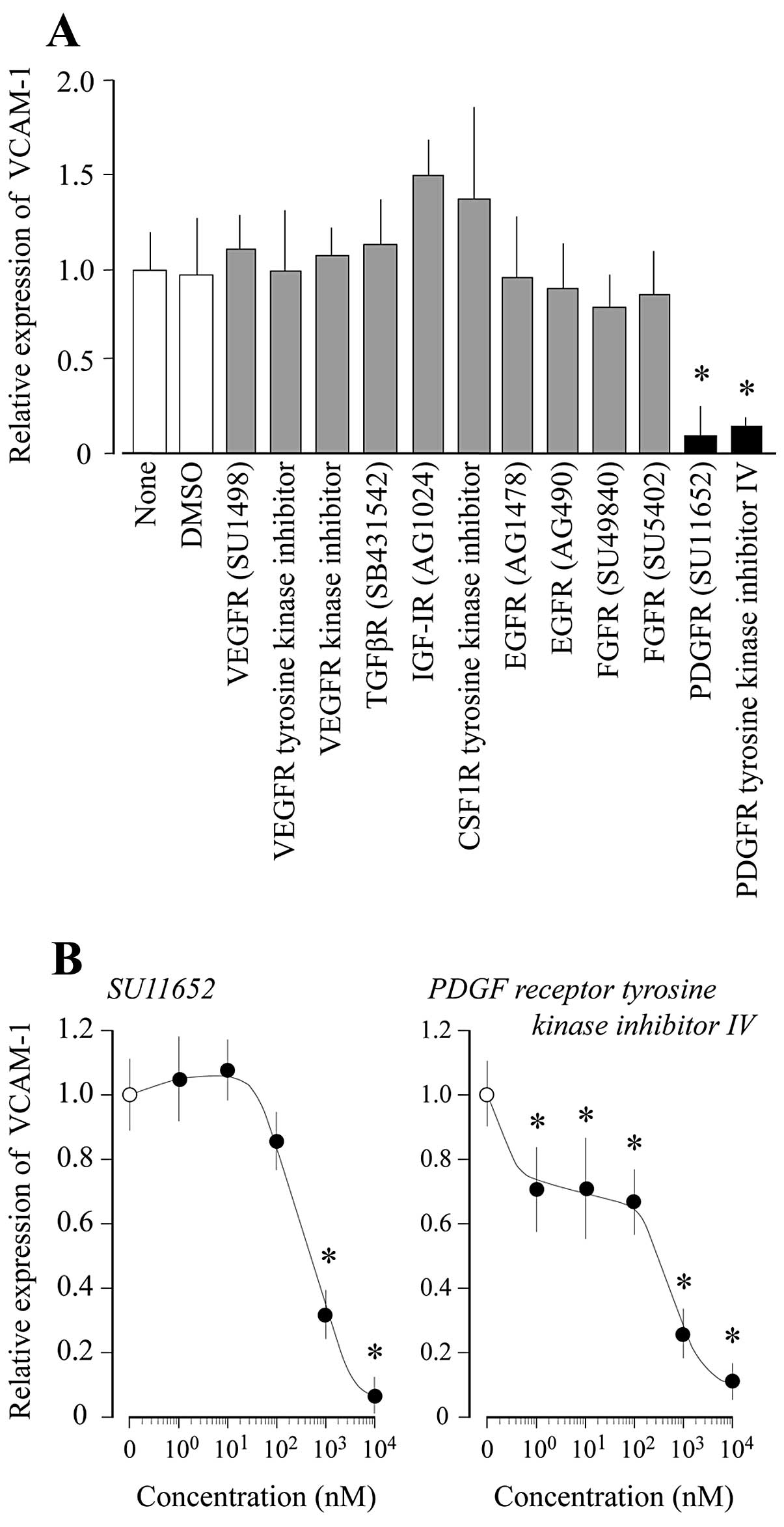
study, we investigated the effects of RTK inhibitors on VCAM-1
expression. As illustrated in Fig.
1, the high cell density-induced VCAM-1 expression decreased in
a dose-dependent manner upon the addition of PDGFR inhibitors
(SU11652 and PDGFR tyrosine kinase inhibitor IV) to the cell
culture. However, the addition of the following RTK inhibitors did
not decrease VCAM-1 expression: vascular endothelial growth factor
receptor (VEGFR) inhibitors (SU1498 and VEGFR kinase inhibitor I),
an insulin-like growth factor-1 receptor (IGF-1R) inhibitor
(AG1024), an epidermal growth factor receptor (EGFR) inhibitor
(AG1478), fibroblast growth factor receptor (FGFR) inhibitors
(SU4984 and SU5402), and a colony stimulating factor-1 receptor
(CSF1R) tyrosine kinase inhibitor (Fig. 1A). Similarly, the addition of a
receptor type serine/threonine kinase inhibitor, such as a
transforming growth factor-β receptor (TGFβR) inhibitor (SB431542)
did not decrease VCAM-1 expression (Fig. 1A).
Role of PDGFRβ on high cell
density-induced VCAM-1 expression
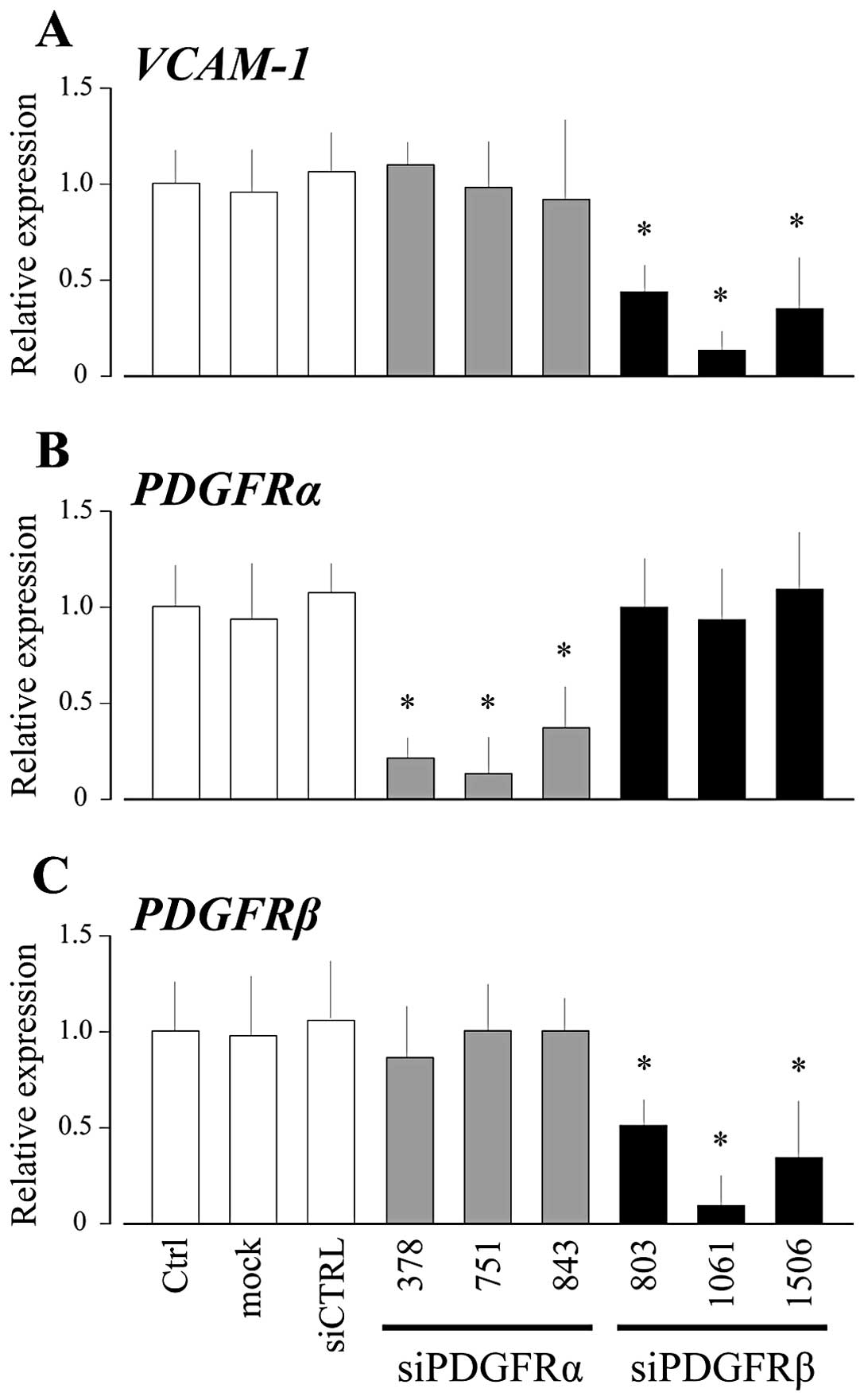
In order to determine the role of PDGFRs in the high
cell density-induced intracellular signal transduction culminating
into VCAM-1 expression in UE7T-13 cells, we investigated the
effects of knocking down PDGFRα and PDGFRβ expression on VCAM-1
expression. As shown in Fig. 2,
VCAM-1 mRNA expression in high cell density culture was reduced
effectively by transfection with siRNA targeting PDGFRβ, but not
siRNA targeting PDGFRα. In order to investigate whether PDGFRβ
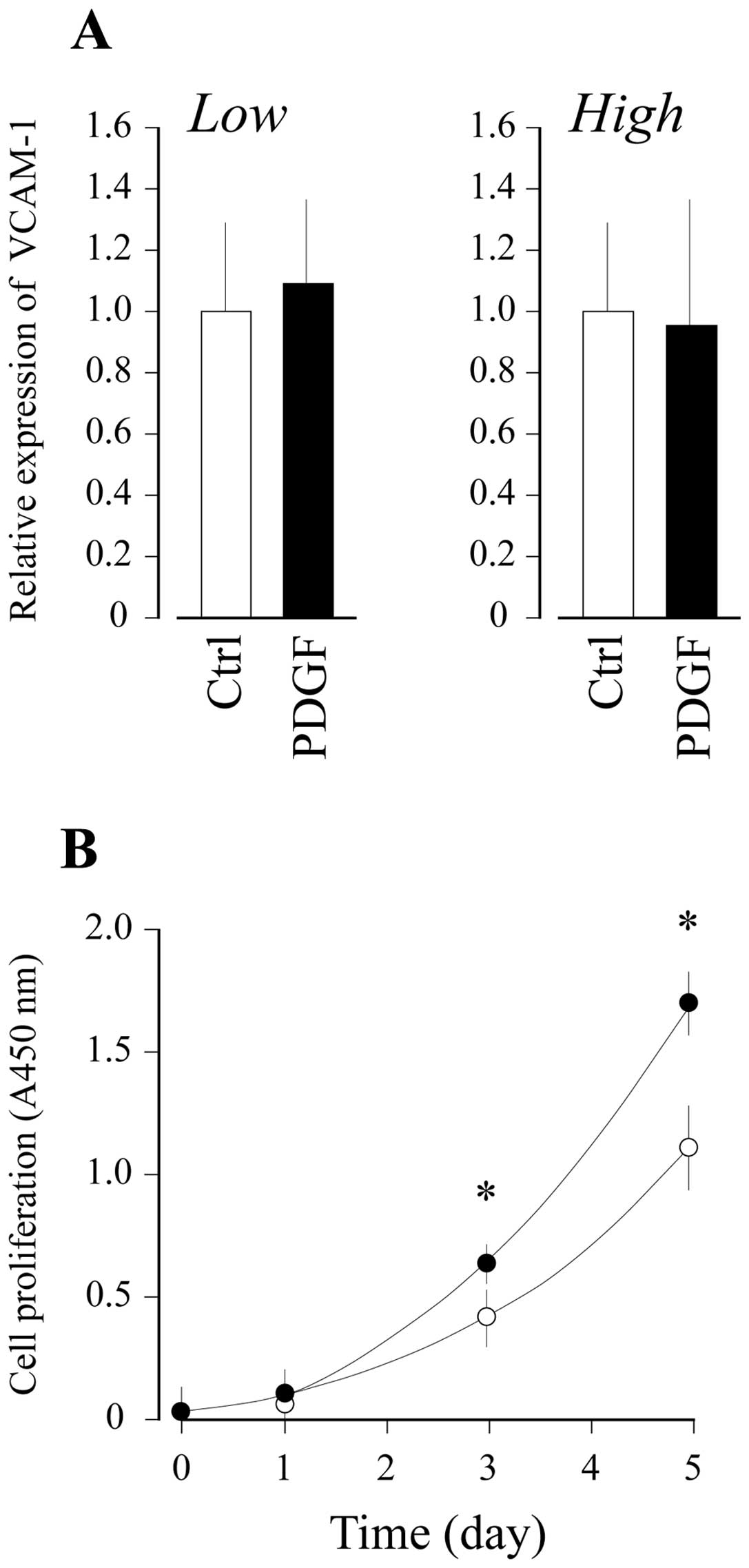
mediates the intracellular signal for VCAM-1 expression in a
ligand-dependent manner, PDGF-BB was added to the UE7T-13 cell
culture at high and low cell densities. As shown in Fig. 3A, PDGF-BB did not affect the level
of VCAM-1 mRNA expression in the MSCs at low or high cell
densities. By contrast, exogenously added recombinant human PDGF-BB
markedly accelerated the proliferation of UE7T-13 cells, suggesting
that functional PDGFR is present at the cell surface in UE7T-13
cells (Fig. 3B).
Role of N-cadherin in high cell
density-induced VCAM-1 expression
A previous study revealed that a
mechanostress-induced intracellular signal is mediated by an RTK in
ECs, even if the receptor was not stimulated with any ligand:
vascular endothelial (VE)-cadherin forms a mechanosensory complex
with VEGFR2 in ECs in a ligand-independent manner (37). Therefore, in this study, we
investigated the role of N-cadherin as a major structural molecule
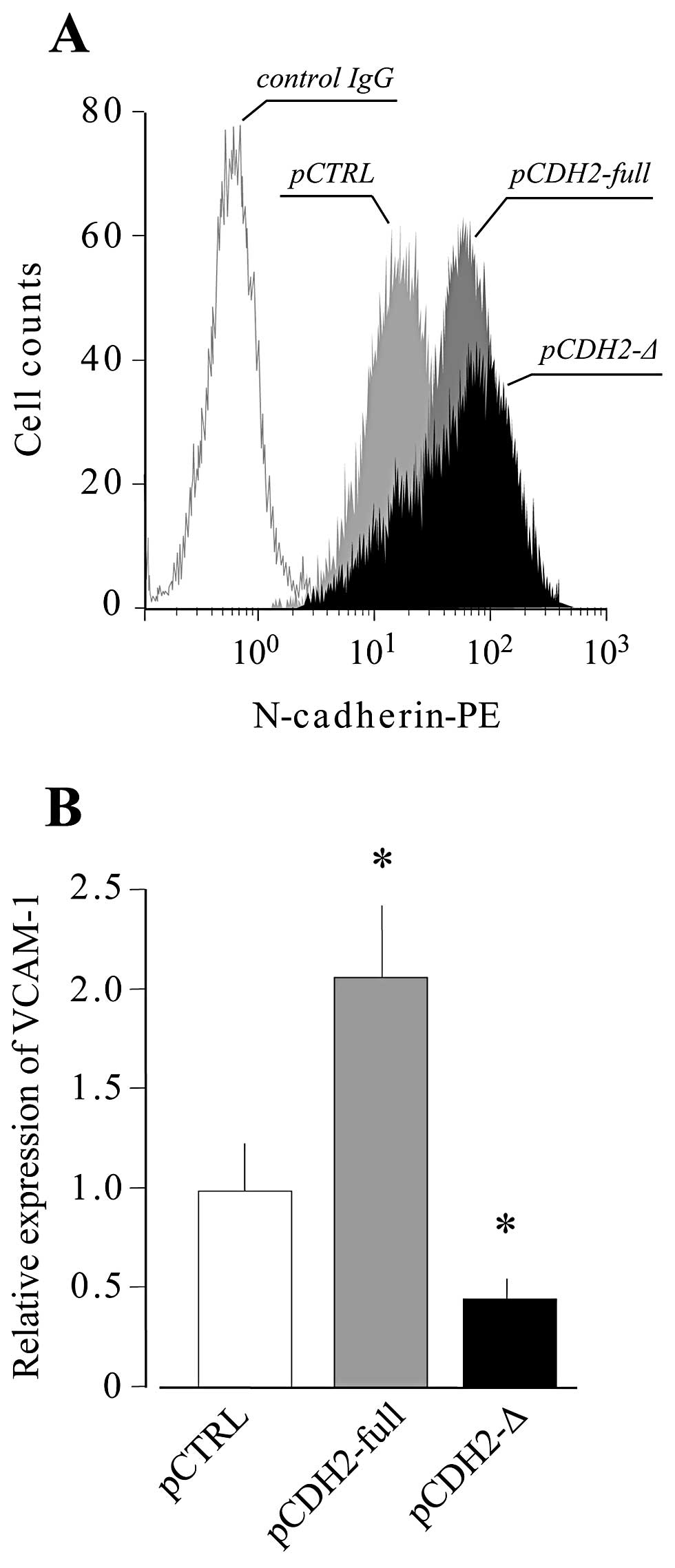
in AJs in MSCs on high cell density-induced VCAM-1 expression.
UE7T-13 cells were transfected with vectors expressing either
full-length N-cadherin or a truncated version lacking the
intracellular domain. The expression of N-cadherin, which localizes
on the cell surface, was confirmed by flow cytometry (Fig. 4A), and then the cells were seeded
at a high density. Under conditions of high density, the
overexpression of full-length N-cadherin markedly increased the
level of VCAM-1 mRNA expression. By contrast, the overexpression of
the truncated N-cadherin markedly reduced the high cell
density-induced VCAM-1 expression (Fig. 4B). These results indicate that, in
MSCs, the high cell density induction of VCAM-1 expression is
mediated by N-cadherin.
Signaling pathway for high cell
density-induced VCAM-1 expression
In ECs, even if VEGFR2 is not stimulated with VEGF,
the VE-cadherin-VEGFR2 complex responds to a subset of endothelial
shear stresses, resulting in the activation of NF-κB-mediated Src
signaling that upregulates VCAM-1 expression (37). Therefore, in this study, we
investigated the phosphorylation status of Src by western blot
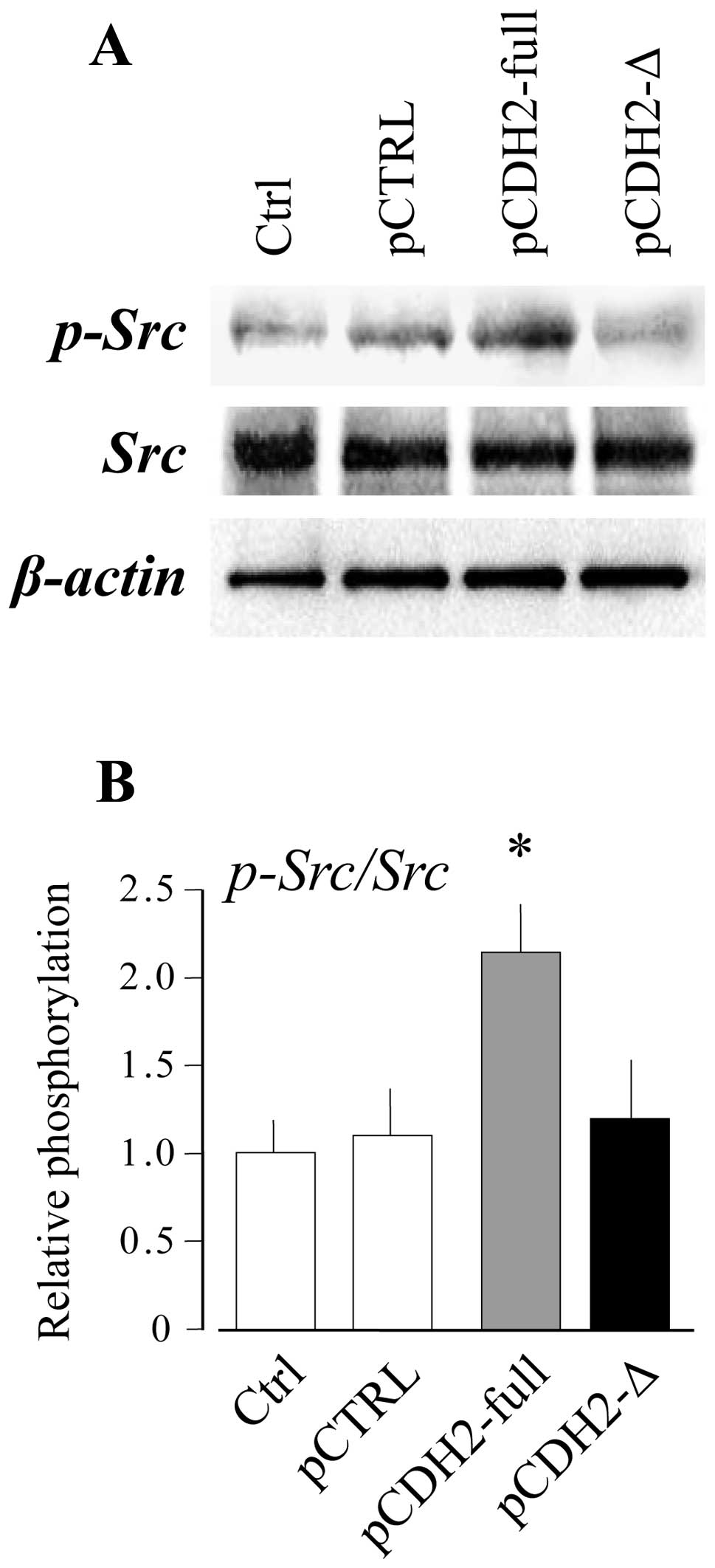
analysis. As shown in Fig. 5, the
phosphorylation levels of Src in UE7T-13 cells were markedly
upregulated by the overexpression of full-length N-cadherin
(pCDH2-full). On the other hand, the overexpression of the
truncated mutant (pCDH2-Δ) had no effect compared with the control
vector-transfected cells (pCTRL). This result strongly suggests
that the phosphorylation of Src through the intracellular domain of
N-cadherin plays an important role in the regulation of VCAM-1
expression by cell-cell adhesion.
Discussion
VCAM-1 is an important marker for enriching
migratory, multipotent and proliferative cells from
culture-expanded MSCs (11). In
addition, it has been shown that VCAM-1 is upregulated in bone
marrow-derived MSCs cultured to overconfluency (38). However, a comprehensive analysis
of the types of adhesion molecules that play important roles in
cell-cell contact between MSCs has not been performed. In a recent
study, we demonstrated that VCAM-1 expression was markedly
upregulated in human bone marrow-derived MSCs at high cell density
through the activation of the NF-κB pathway (34).
Notably, a previous study revealed that a
mechanostress-induced intracellular signal is mediated by an RTK in
ECs in a ligand-independent manner: VE-cadherin forms a
mechanosensory complex with VEGFR2 in ECs that responds to a subset
of endothelial shear stresses, resulting in the activation of
NF-κB-mediated Src signaling, upregulating VCAM-1 expression, even
when VEGFR2 is not stimulated with VEGF (37). Therefore, we hypothesized that an
RTK may similarly mediate the cell-cell contact-induced signal
responsible for upregulating VCAM-1 expression in MSCs. When we
evaluated the effects of various RTK inhibitors on high cell
density-induced VCAM-1 expression, we found that VCAM-1 expression
was decreased by PDGFR inhibitors (SU11652 and PDGFR tyrosine
kinase inhibitor IV) in a dose-dependent manner (Fig. 1). However, other RTK inhibitors,
e.g., inhibitors of VEGFR, IGF1R, EGFR and FGFR, as well as a
receptor type serine/threonine kinase TGFβR inhibitor, did not
decrease VCAM-1 expression (Fig.
1A). In general, PDGFR is activated by PDGF as its ligand. PDGF
has four isoforms (A–D) that form homo- or heterodimers, such as
PDGF-AA, PDGF-AB and PDGF-BB (39). Of these, PDGF-BB exhibits the
strongest activity (39) and has
been approved by the FDA for the treatment of patients with bone
defects in the oral and maxillofacial regions (40–43). PDGF-BB is mainly produced by
platelets and has been implicated in tissue repair (fracture
repair) (39). PDGFR has two
isoforms (α and β) that also form homo- or heterodimers, such as
PDGFRα/α, α/β and β/β (39).
PDGFRα is reportedly expressed in MSCs and osteoblast progenitor
cells, and PDGFRα-positive cells exhibit a high osteoblastic
differentiation capacity (44).
In MSCs, a recent study provided evidence that PDGF-BB promotes
PDGFα-positive cell migration into artificial bones without
inhibiting osteoblastogenesis (45). In this study, high cell
density-induced VCAM-1 expression was significantly suppressed by
the knockdown of PDGFRβ (Fig. 2).
Of note, the PDGF-BB-induced PDGFR activation did not affect the
level of VCAM-1 mRNA expression in MSCs at both low and high cell
densities (Fig. 3A), although
PDGF-BB clearly upregulated MSC proliferation (Fig. 3B). These results strongly suggest
that the high cell density-induced intracellular signal to
upregulate VCAM-1 expression in MSCs was mediated by PDGFRβ in a
PDGF-BB-independent manner.
In MSCs, VE-cadherin does not exhibit dominant
expression, although N-cadherin does (46). Therefore, it may be possible that
the formation of a mechanosensory interaction occurs between
N-cadherin and PDGFRβ in high cell density cultures and that this
interaction may then activate the NF-κB-mediated signaling pathway,
thus upregulating VCAM-1 expression. In support of this hypothesis,
in the present study, the overexpression of full-length N-cadherin
in UE7T-13 cells markedly increased high cell density-induced
VCAM-1 expression, whereas the overexpression of truncated
N-cadherin lacking its intracellular domain suppressed the high
cell density-induced VCAM-1 expression (Fig. 4). As, in ECs, the
VE-cadherin-VEGFR2 complex responds to a subset of endothelial
shear stresses by activating NF-κB-mediated Src signaling (37). Therefore, we examined the
phosphorylation status of Src by western blot analysis and found
that the phosphorylation levels of Src in UE7T-13 cells were
enhanced at high cell density (Fig.
5A). Src phosphorylation levels in the UE7T-13 cells were also
upregulated by the overexpression of full-length N-cadherin
(Fig. 5B). By contrast, the
overexpression of the truncated mutant did not affect Src
phosphorylation. In addition, in a previous study, we demonstrated
that high cell density-induced VCAM-1 expression was decreased by
the Src inhibitor, PP2 analogue (34). These results strongly suggest that
the phosphorylation of Src through the intracellular domain of
N-cadherin plays an important role in the upregulation of VCAM-1 by
cell-cell adhesion.
In conclusion, the data presented in our study
demonstrate that cell-cell adhesion induced by high cell density
through N-cadherin enhances the expression of VCAM-1 via PDGFRβ in
a ligand-independent manner in human bone marrow-derived MSCs.
These findings may eventually lead to the development of new,
MSC-based clinical therapies in regenerative medicine.
Acknowledgements
This study was supported in part by JSPS KAKENHI
Grant nos. 25463053 to N.C., 23592896 to A.I.; the Open Research
Project from the Ministry of Education, Culture, Sports, Science
and Technology of Japan, 2008–2012; and Grant-in-Aid for Strategic
Medical Science Research Centre from the Ministry of Education,
Culture, Sports, Science and Technology of Japan, 2010–2014.
References
|
1
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aslan H, Zilberman Y, Kandel L, et al:
Osteogenic differentiation of noncultured immunoisolated bone
marrow-derived CD105+ cells. Stem Cells. 7:1728–1737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Battula VL, Treml S, Bareiss PM, et al:
Isolation of functionally distinct mesenchymal stem cell subsets
using antibodies against CD56, CD271, and mesenchymal stem cell
antigen-1. Haematologica. 94:173–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boiret N, Rapatel C, Veyrat-Masson R, et
al: Characterization of nonexpanded mesenchymal progenitor cells
from normal adult human bone marrow. Exp Hematol. 33:219–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bühring HJ, Battula VL, Treml S, et al:
Novel markers for the prospective isolation of human MSC. Ann NY
Acad Sci. 1106:262–271. 2007.PubMed/NCBI
|
|
6
|
Gronthos S, Zannettino AC, Hay SJ, et al:
Molecular and cellular characterisation of highly purified stromal
stem cells derived from human bone marrow. J Cell Sci.
116:1827–1835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quirici N, Soligo D, Bossolasco P, et al:
Isolation of bone marrow mesenchymal stem cells by anti-nerve
growth factor receptor antibodies. Exp Hematol. 30:783–791. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sacchetti B, Funari A, Michienzi S, et al:
Self-renewing osteoprogenitors in bone marrow sinusoids can
organize a hematopoietic microenvironment. Cell. 131:324–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honczarenko M, Le Y, Swierkowski M, et al:
Human bone marrow stromal cells express a distinct set of
biologically functional chemokine receptors. Stem Cells.
24:1030–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wagner W, Horn P, Castoldi M, et al:
Replicative senescence of mesenchymal stem cells: a continuous and
organized process. PLoS One. 3:e22132008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mabuchi Y, Morikawa S, Harada S, et al:
LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct
subpopulations in mesenchymal stem cells. Stem Cell Reports.
1:152–165. 2013.
|
|
12
|
Deschaseaux F, Gindraux F, Saadi R, et al:
Direct selection of human bone marrow mesenchymal stem cells using
an anti-CD49a antibody reveals their CD45med, low phenotype. Br J
Haematol. 122:506–517. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones EA, English A, Kinsey SE, et al:
Optimization of a flow cytometry-based protocol for detection and
phenotypic characterization of multipotent mesenchymal stromal
cells from human bone marrow. Cytometry B Clin Cytom. 70:391–399.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuyoshi N and Imamura S: Multiple
cadherins are expressed in human fibroblasts. Biochem Biophys Res
Commun. 235:355–358. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simonneau L, Kitagawa M, Suzuki S and
Thiery JP: Cadherin 11 expression marks the mesenchymal phenotype:
towards new functions for cadherins? Cell Adhes Commun. 3:115–130.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hatta K and Takeichi M: Expression of
N-cadherin adhesion molecules associated with early morphogenetic
events in chick development. Nature. 320:447–449. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akitaya T and Bronner-Fraser M: Expression
of cell adhesion molecules during initiation and cessation of
neural crest cell migration. Dev Dyn. 194:12–20. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Wever O, Westbroek W, Verloes A, et al:
Critical role of N-cadherin in myofibroblast invasion and migration
in vitro stimulated by colon-cancer-cell-derived TGF-beta or
wounding. J Cell Sci. 117:4691–4703. 2004.
|
|
19
|
Kashima T, Nakamura K, Kawaguchi J, et al:
Overexpression of cadherins suppresses pulmonary metastasis of
osteosarcoma in vivo. Int J Cancer. 104:147–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radice GL, Rayburn H, Matsunami H, et al:
Developmental defects in mouse embryos lacking N-cadherin. Dev
Biol. 181:64–78. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
García-Castro MI, Vielmetter E and
Bronner-Fraser M: N-Cadherin, a cell adhesion molecule involved in
establishment of embryonic left-right asymmetry. Science.
288:1047–1051. 2000.PubMed/NCBI
|
|
22
|
Hinz B, Pittet P, Smith-Clerc J, et al:
Myofibroblast development is characterized by specific cell-cell
adherens junctions. Mol Biol Cell. 15:4310–4320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomasek JJ, Gabbiani G, Hinz B, et al:
Myofibroblasts and mechano-regulation of connective tissue
remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marthiens V, Gavard J, Lambert M and Mège
RM: Cadherin-based cell adhesion in neuromuscular development. Biol
Cell. 94:315–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krauss RS, Cole F, Gaio U, et al: Close
encounters: regulation of vertebrate skeletal myogenesis by
cell-cell contact. J Cell Sci. 118:2355–2362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marie PJ: Role of N-cadherin in bone
formation. J Cell Physiol. 190:297–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahrens PB, Solursh M and Reiter RS:
Stage-related capacity for limb chondrogenesis in cell culture. Dev
Biol. 60:69–82. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
San Antonio JD and Tuan RS: Chondrogenesis
of limb bud mesenchyme in vitro: stimulation by cations. Dev Biol.
115:313–324. 1986.PubMed/NCBI
|
|
29
|
Oberlender SA and Tuan RS: Spatiotemporal
profile of N-cadherin expression in the developing limb mesenchyme.
Cell Adhes Commun. 2:521–537. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tavella S, Raffo P, Tacchetti C, et al:
N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp
Cell Res. 215:354–362. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DeLise AM and Tuan RS: Alterations in the
spatiotemporal expression pattern and function of N-cadherin
inhibit cellular condensation and chondrogenesis of limb
mesenchymal cells in vitro. J Cell Biochem. 87:342–359. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delise AM and Tuan RS: Analysis of
N-cadherin function in limb mesenchymal chondrogenesis in vitro.
Dev Dyn. 225:195–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuan RS: Cellular signaling in
developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J Bone
Joint Surg Am. 85:137–141. 2003.PubMed/NCBI
|
|
34
|
Nishihira S, Okubo N, Takahashi N, et al:
High-cell density-induced VCAM-1 expression inhibits the migratory
ability of mesenchymal stem cells. Cell Biol Int. 35:475–481. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mori T, Kiyono T, Imabayashi H, et al:
Combination of hTERT and bmi-1, E6, or E7 induces prolongation of
the life span of bone marrow stromal cells from an elderly donor
without affecting their neurogenic potential. Mol Cell Biol.
25:5183–5195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimomura T, Yoshida Y, Sakabe T, et al:
Hepatic differentiation of human bone marrow-derived UE7T-13 cells:
Effects of cytokines and CCN family gene expression. Hepatol Res.
37:1068–1079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Sweet DT, Irani-Tehrani M, et al:
Shc coordinates signals from intercellular junctions and integrins
to regulate flow-induced inflammation. J Cell Biol. 182:185–196.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee RH, Seo MJ, Pulin AA, et al: The
CD34-like protein PODXL and alpha6-integrin (CD49f) identify early
progenitor MSCs with increased clonogenicity and migration to
infarcted heart in mice. Blood. 113:816–826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hollinger JO, Hart CE, Hirsch SN, et al:
Recombinant human platelet-derived growth factor: biology and
clinical applications. J Bone Joint Surg Am. 90:48–54. 2008.
View Article : Google Scholar
|
|
40
|
Jayakumar A, Rajababu P, Rohini S, et al:
Multi-centre, randomized clinical trial on the efficacy and safety
of recombinant human platelet-derived growth factor with
β-tricalcium phosphate in human intra-osseous periodontal defects.
J Clin Periodontol. 38:163–172. 2011.PubMed/NCBI
|
|
41
|
Ridgway HK, Mellonig JT and Cochran DL:
Human histologic and clinical evaluation of recombinant human
platelet-derived growth factor and beta-tricalcium phosphate for
the treatment of periodontal intraosseous defects. Int J
Periodontics Restorative Dent. 28:171–179. 2008.
|
|
42
|
Nevins M, Giannobile WV, McGuire MK, et
al: Platelet-derived growth factor stimulates bone fill and rate of
attachment level gain: results of a large multicenter randomized
controlled trial. J Periodontol. 76:2205–2215. 2005. View Article : Google Scholar
|
|
43
|
McGuire MK, Kao RT, Nevins M and Lynch SE:
rhPDGF-BB promotes healing of periodontal defects: 24-month
clinical and radiographic observations. Int J Periodontics
Restorative Dent. 26:223–231. 2006.PubMed/NCBI
|
|
44
|
Morikawa S, Mabuchi Y, Kubota Y, et al:
Prospective identification, isolation, and systemic transplantation
of multipotent mesenchymal stem cells in murine bone marrow. J Exp
Med. 206:2483–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshida S, Iwasaki R, Kawana H, et al:
PDGFBB promotes PDGFRα-positive cell migration into artificial bone
in vivo. Biochem Biophys Res Commun. 421:785–789. 2012.
|
|
46
|
Wuchter P, Boda-Heggemann J, Straub BK, et
al: Processus and recessus adhaerentes: giant adherens cell
junction systems connect and attract human mesenchymal stem cells.
Cell Tissue Res. 328:499–514. 2007. View Article : Google Scholar : PubMed/NCBI
|