Introduction
Skin aging occurs in an age-dependent (internal
aging) and environment-dependent (external aging) manner (1). Photoaging is a main component of
extrinsic aging and is an important etiology of several skin
diseases, such as photodermatoses, actinic keratosis and skin
cancer (2). Photoaged skin
exhibits severe alterations in the cellular component and
extracellular matrix with atrophy of elastin, its microfibillar
component fibrillin and interstitial collagens, major structural
proteins of the dermis connective tissue (3). Ultraviolet (UV) radiation is a
significant source of photoaging and although most UV radiation is
blocked by the ozone layer of the stratosphere, there is increased
awareness of the interactions between ozone depletion and climate
change (global warming) that may have an impact on human exposure
to terrestrial UV (4).
The effects of UV on human skin at the physiologic
and molecular biologic levels have been previously investigated. UV
radiation generates reactive oxygen species and DNA damage that
induce cell cycle arrest and apoptosis (2). In addition, UV radiation
transcriptionally induces the expression of matrix
metalloproteinases (MMPs) and elastases, which affect cell
migration and wrinkle formation through the degradation of collagen
and elastin in dermal fibroblast cells (5). Mitogen-activated protein kinases
(MAPKs) and nuclear factor-κB (NF-κB) are post-translationally
activated by UV through phosphorylation and their signaling
pathways constitute a major molecular defense against UV radiation
(6). Recently, gene expression
profiling by microarray-based analysis revealed that many genes are
regulated by UV-mediated signaling pathways (7,8).
Although there is extensive transcriptomic and proteomic analysis
of UV signaling in skin cells, post-transcriptional responses to UV
radiation, such as microRNAs, have not been widely studied.
MicroRNAs (miRNAs) are short RNA sequences ranging
from 16 to 35 nucleotides that can directly interact with target
mRNAs via complementary base pairing at a specific target site
(9). The interaction between
miRNA and target mRNA inhibits translation of the target, and
results of previous studies suggested that ~30% of all mRNAs in
humans may be post-transcriptionally regulated by miRNAs (9,10).
In human dermal fibroblast cells, microarray-based studies have
previously documented the molecular mechanisms underlying
photodamage and skin carcinogenesis by UVB and UVA, and several
miRNAs have been identified to be involved in this process
(11,12). In addition, miRNA-based studies
have demonstrated that miR-34c-5p and miR-22 are UVB-response
miRNAs and that the elevated expression of these miRNAs is capable
of regulating UVB-mediated senescence and apoptosis in dermal
fibroblasts (13,14). Previously, we demonstrated that a
titrated extract of Centella asiatica and epigallocatechin
gallate (EGCG) have UVB protective effects in dermal fibroblast
cells by altering miRNA expression profiles (15,16). The abovementioned studies
suggested that miRNA is likely a significant regulator of
UVB-mediated cellular mechanisms and may also have protective
functions in response to UVB.
Arctiin, a lignin compound derived from several
plants including Arctium lappa, a herb used widely in
traditional Chinese medicine for the treatment of the common cold
(17). Findings of previous
pharmacological studies revealed that arctiin possesses protective
potential against lipopolysaccharide (LPS)-induced inflammation and
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhlP)-induced
hepatocarcinogenesis and exerts an ameliorative effect on
glomerulonephritis in Sprague-Dawley rat models (18–20). However, its protective effect in
skin cells has not been investigated. To the best of our knowledge,
this is the first study to demonstrate that arctiin exerts a
protective effect against UVB radiation in normal human dermal
fibroblast (NHDF) cells by changing miRNA expression profiles.
Materials and methods
Cell culture and chemical treatment
NHDF cells (Lonza, Basel, Switzerland) were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL,
Invitrogen Life Technologies, Gaithersburg, MD, USA) containing 10%
fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA).
Arctiin was purchased from Sigma-Aldrich and dissolved in DMSO. To
evaluate the cytotoxicity and UVB protective effects of arctiin,
NHDF cells were seeded in 96-well and 60-mm culture plates at a
density of 4×104 cells/well and 7×105
cells/plate, respectively.
UVB irradiation
UVB irradiation of NHDF cells was performed as
described previously (15).
Briefly, NHDF cells were seeded in 96-well and 60-mm plates and
cultured in growth media overnight. When the cells reached ~70%
confluence they were pretreated with various doses of arctiin at
different time points and then exposed to UVB irradiation. An
irradiation dose of 100 mJ/cm2 was used throughout the
study. Prior to UVB irradiation the medium was replaced with
phosphate-buffered saline (PBS) and the cells were exposed to UVB
without the culture plate cover. Subsequent to UV exposure, the PBS
was immediately replaced with growth medium containing DMSO or
arctiin.
Cell viability assay
The cytotoxicity and UVB protective effects of
arctiin on NHDF cells were investigated using a water-soluble
tetrazolium salt (WST-1) assay (EZ-Cytox cell viability assay kit;
Itsbio, Seoul, Korea). At the end of the experiments, 1/10 volume
of WST-1 solution was added to the cells and incubated at 37°C for
0.5 h. Cell viability was determined by measuring the absorbance at
450 nm using an iMark microplate reader (Bio-Rad, Hercules, CA,
USA). Results are presented as the means ± standard deviation (SD)
of three independent experiments. P<0.05 as determined by
Student’s t-test was considered significant.
Analysis of cell cycle by flow
cytometry
To distinguish cells in different phases of the cell
cycle, UVB-irradiated NHDF cells pretreated with or without arctiin
were fixed by the addition of cold 70% ethanol and stained with a
fluorescent dye, propidium iodide (PI) (Sigma-Aldrich). The PI
fluorescence intensity was detected using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA). The mean PI
fluorescence intensity was obtained from 10,000 cells using the
FL2-H channel.
Wound healing assay
NHDF cells were seeded in 60-mm culture plates and
grown overnight. When the cells reached ~90% confluence they were
pretreated with 10 μM acrtiin for 6 h and a wound was formed by
scraping the cells with a 20-μl pipette tip and washing with PBS.
After wound formation the cells were exposed to UVB irradiation and
cultured in growth media containing DMSO or arctiin for 24 h.
Migration of the wounded cells was evaluated 0 and 24 h after
wounding by recording photographic images using a phase-contrast
Olympus CKX41 microscope (Olympus, Tokyo, Japan).
Luciferase-based DNA repair assay
To determine the effect of arctiin on DNA repair,
the pGL3 luciferase reporter vector (Promega, Madison, WI, USA) was
damaged with 2,000 J/m2 UVC radiation as described
previously (21). Control or
damaged pGL3 vector was co-transfected into NHDF cells with
pSV-β-galactosidase plasmid (as a transfection control)
using Lipofectamine 2000 reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). After 24 h the transfected cells were lysed
using Passive Lysis buffer (Promega), luciferin was added, and the
luciferase activity of each cell lysate was analyzed using a
Veritas Luminometer (Turner Designs, Sunnyvale, CA, USA). The
results were normalized to β-galactosidase activity and
presented as percentages of the control with SD. Results shown are
the averages of three independent experiments.
Total RNA purification
Total RNAs were purified using TRIzol reagent
(Invitrogen Life Tecnologies) according to the manufacturer’s
instructions. The RNA integrity, concentration and purity were
estimated using a Bioanalyzer 2100 (Agilent Technologies Inc.,
Santa Clara, CA, USA) and MaestroNano (Maestrogen, Las Vegas, NV,
USA), respectively (15). RNA
samples that showed A260/280 and A260/A230 values >1.8, and an
RNA integrity number (RIN) >8.0 were subjected to microRNA-based
microarray.
Microarray analysis of miRNA
expression
Microarray analysis was performed using SurePrint G3
Human V16 miRNA 8×60K (Agilent Technologies Inc.), according to a
previously described protocol (15). Briefly, 100 ng total RNA was
labeled with cyanine 3-pCp and hybridized to the probes on the
microarray. The microarray slide was scanned and data derived from
the image were analyzed using GeneSpring GX software version 11.5
(Agilent Technologies Inc.). The raw data were filtered using FLAG
and t-tests, and applied to the fold-change analysis. Significant
miRNAs were determined using the fluorescence ratio between two
samples, and miRNAs exhibiting a >2-fold increase or decrease in
expression were selected for subsequent bioinformatic analysis.
Bioinformatic analysis of deregulated
miRNAs
To investigate the biological significance of the
differentially expressed miRNAs, we first predicted putative target
genes of the miRNAs using the DIANA-microT bioinformatic tool
(http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index)
(22). The prediction of target
genes was limited by setting a 0.8-threshold in the program. The
putative target genes of each miRNA were then analyzed for
biological function using the Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathways and Database for Annotation, Visualization
and Interrogate Discovery (DAVID) (http://david.abcc.ncifcrf.gov/home.jsp) Bioinformatics
Resources version 6.7 according to the developer’s protocol
(23). For example, the predicted
593 target genes of hsa-miR-1290 were uploaded into the DAVID web
server and analyzed using the ‘functional annotation tool’ of
DAVID. The ‘KEGG pathway’ category was then processed by setting a
Ease score of 0.5. Involved KEGG pathways exhibiting a value of
>2% (percentage of involved target genes/total target genes)
were selected.
Results
Arctiin promotes UVB protection in NHDF
cells
To determine whether arctiin is involved in the
protection against UVB irradiation in NHDF cells, we first
investigated the cytotoxic concentration range of arctiin.
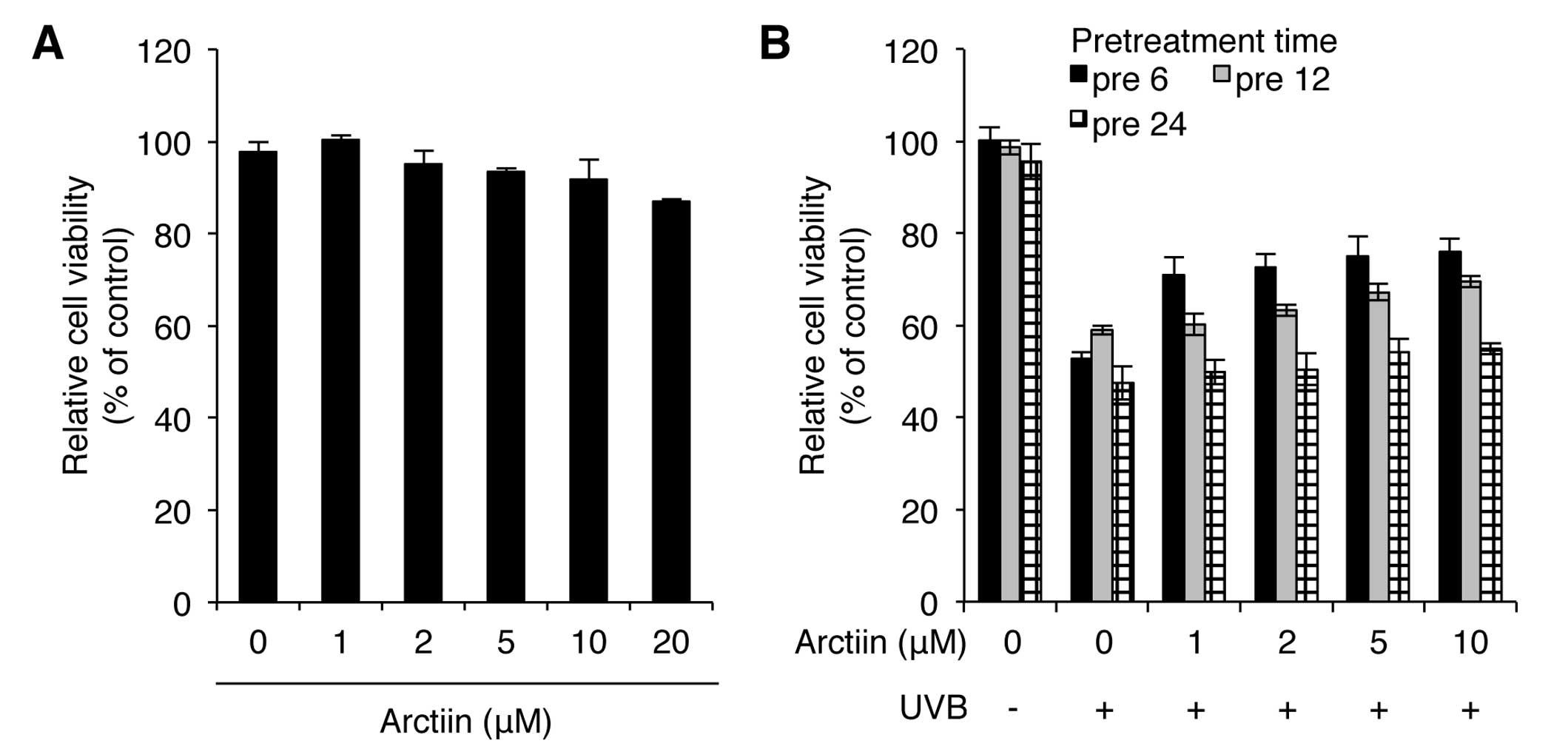
Treatment with 1–10 μM arctiin resulted in a <10% decrease in
cell viability whereas treatment with 20 μM arctiin exhibited a
higher cytotoxicity in NHDF cells (Fig. 1A). We also determined the UVB
protection effect of arctiin by pretreating cells with arctiin at
different concentrations (1, 2, 5 and 10 μM) and at different time
points (6, 12 and 24 h) prior to UVB (100 mJ/cm2)
irradiation. Pretreatment with 10 μM arctiin for 6 h showed the
lowest decrease in viability after UVB irradiation, suggesting that
this dose of arctiin has a photoprotective effect on UVB radiation
in NHDF cells (Fig. 1B). Based on
these results, pretreatment with 10 μM arctiin for 6 h was used
throughout the study.
Arctiin rescues UVB-induced apoptosis in
NHDF cells
Treatment of NHDF cells with high doses of UVB
irradiation leads to cell cycle arrest and apoptosis (24). To determine whether arctiin
pretreatment affected the UVB-mediated physiological defects its
effect on cell cycle distribution were analyzed using PI staining
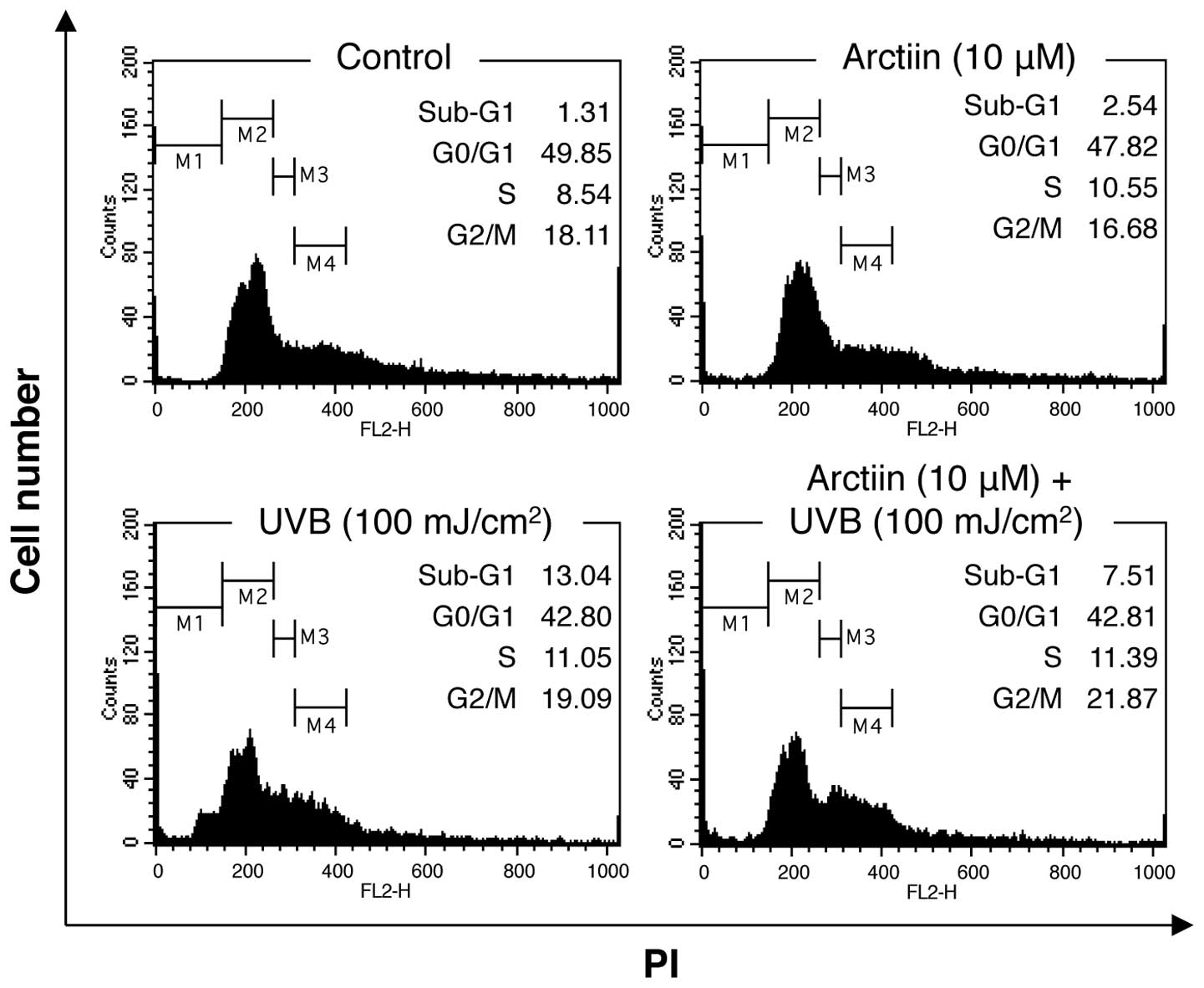
and flow cytometry. Treatment of NHDF cells with arctiin alone
induced few changes in cell cycle distribution compared with the
control cells (Fig. 2). UVB
irradiation (100 mJ/cm2) induced an increase in the
number of sub-G1 cells (13.4%), indicating that the dose of UVB
radiation used in this study induced apoptosis in NHDF cells.
Pre-treatment with arctiin prior to irradiation decreased the
sub-G1 fraction (7.51%), suggesting that arctiin protects against
UVB-induced apoptosis in NHDF cells.
Arctiin rescues UVB-mediated migration
defects in NHDF cells
Migration of NHDF cells is important for skin wound
healing (25). To explore the
possibility that arctiin rescues the UVB-induced migration defect
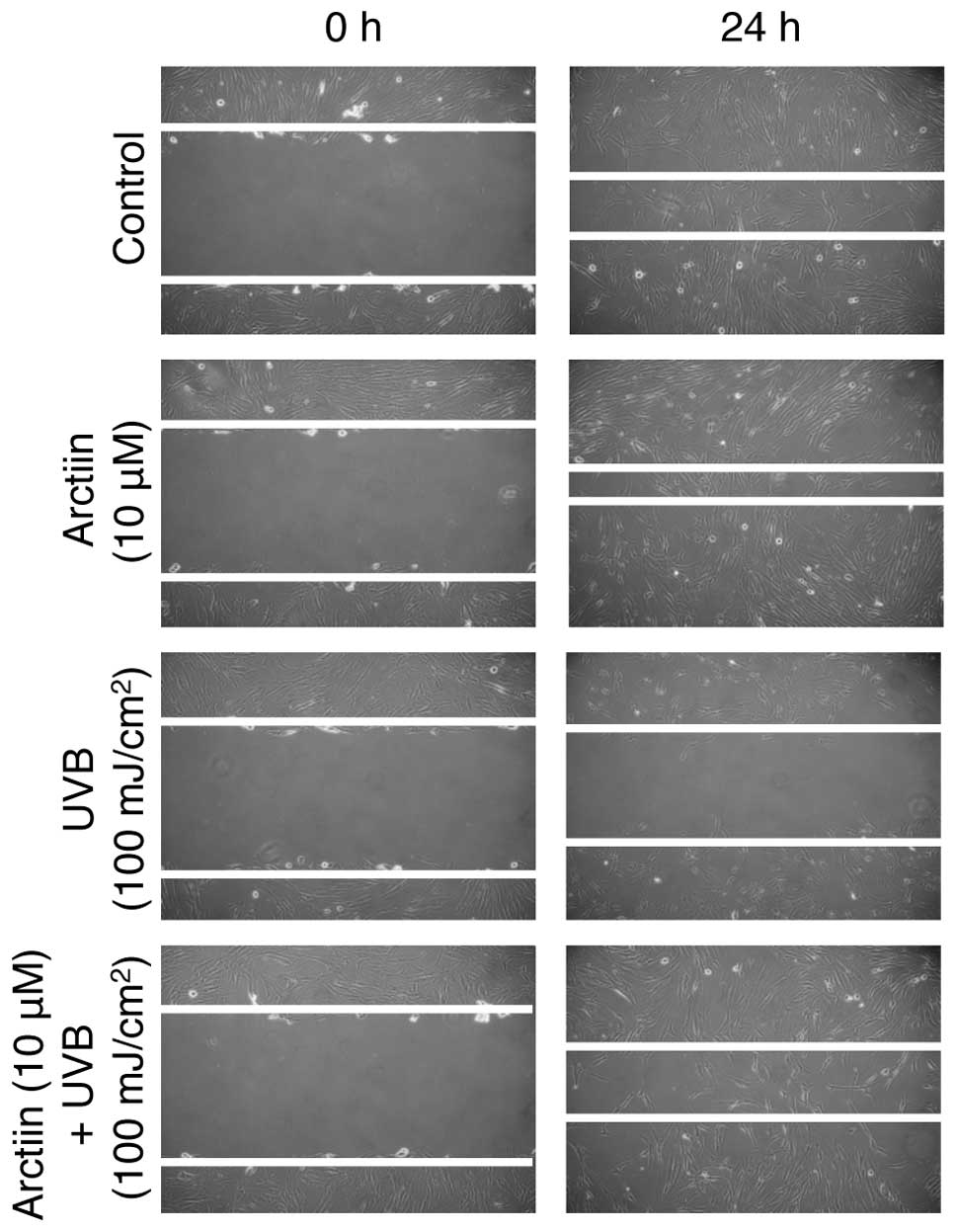
in NHDF cells, we first analyzed whether arctiin induces NHDF
migration. Arctiin-treated cells showed a higher rate of migration
than non-treated control cells after 24-h incubation, suggesting
that arctiin accelerates the migration of NHDF cells (Fig. 3, upper two panels). We also
confirmed that UVB irradiation markedly decreased the migration
rate (Fig. 3, third panel)
compared with the control. Arctiin pretreatment prior to UVB
irradiation reduced the wound size compared with UVB irradiation
alone. These results suggested that the UVB-mediated defect in
wound healing was rescued by arctiin in NHDF cells.
Arctiin promotes UVB-mediated DNA damage
repair
UV radiation induces cell death as a result of
accumulation of DNA damage (26),
thus we determined whether arctiin is involved in the repair of DNA
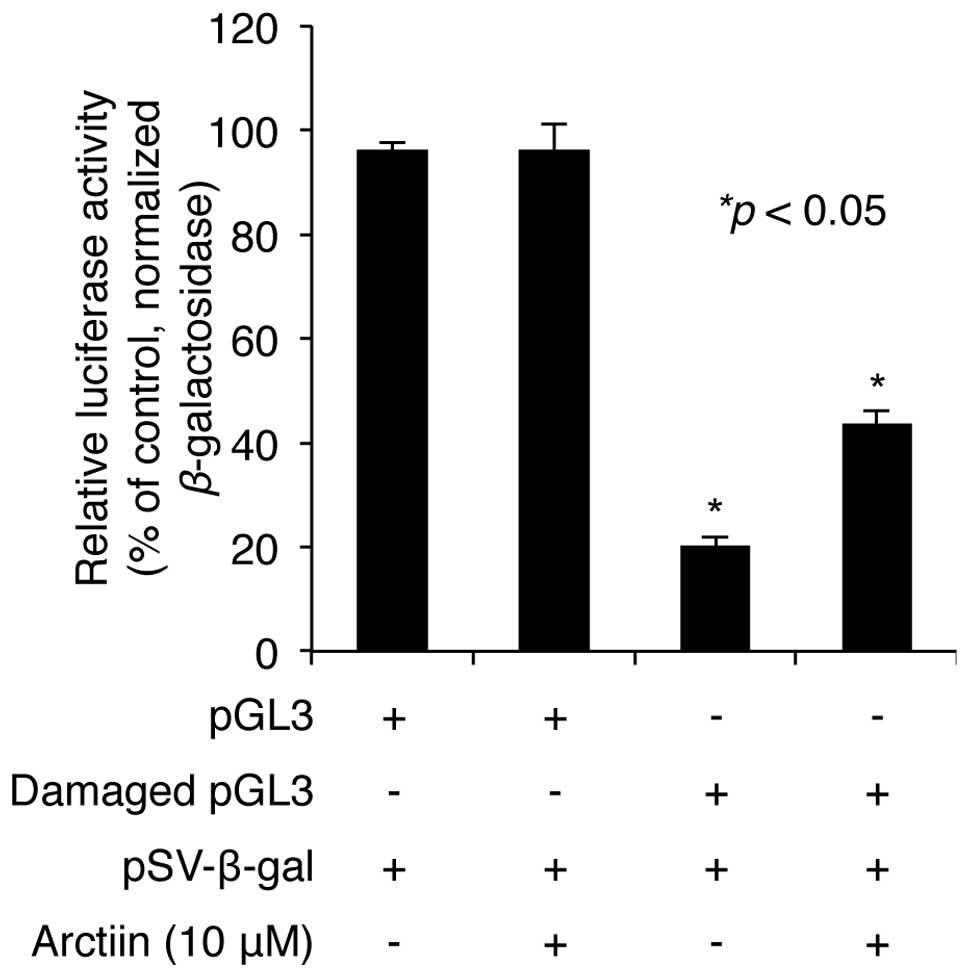
damage induced by UV radiation in NHDF cells. pGL3 plasmid
containing the luciferase gene was exposed to a 2,000
J/m2 dose of UVC in vitro. Untreated or
UVC-treated plasmids were cotransfected into NHDF cells with
pSV-β-galactosidase plasmid as a transfection control.
Following transfection, the cells were treated with 10 μM arctiin
for 24 h and the luciferase activities of cell lysates were
measured as described in Materials and methods. The results showed
that the luciferase activity decreased to 20.3% after UVC damage
compared with the undamaged control (100% luciferase activity).
Pretreatment with arctiin did not affect luciferase activity of
control pGL3 (Fig. 4), but
rescued the decrease in luciferase activity induced by UVC
irradiation to 43.8% (Fig. 4),
suggesting that arctiin plays a protective role through the
promotion of DNA damage repair in NHDF cells.
The UVB protection effect of arctiin is
associated with changes in the expression of specific miRNAs in
NHDF cells
UV radiation can alter the expression profiles of
mRNA and microRNA (miRNA) in skin cells including NHDF cells
(27). To explore the role of
miRNAs in the arctiin-mediated UVB protection effect, we performed
miRNA microarray analysis on NHDF cells. Total RNA from
UVB-irradiated and arctiin-pretreated/UVB-irradiated NHDF cells was
hybridized against the SurePrint G3 Human v16 miRNA 8×60K
microarray as described in Materials and methods. Numerous miRNAs
showed significant differential expression, suggesting that arctiin
induced expression changes in specific miRNAs to protect NHDF cells
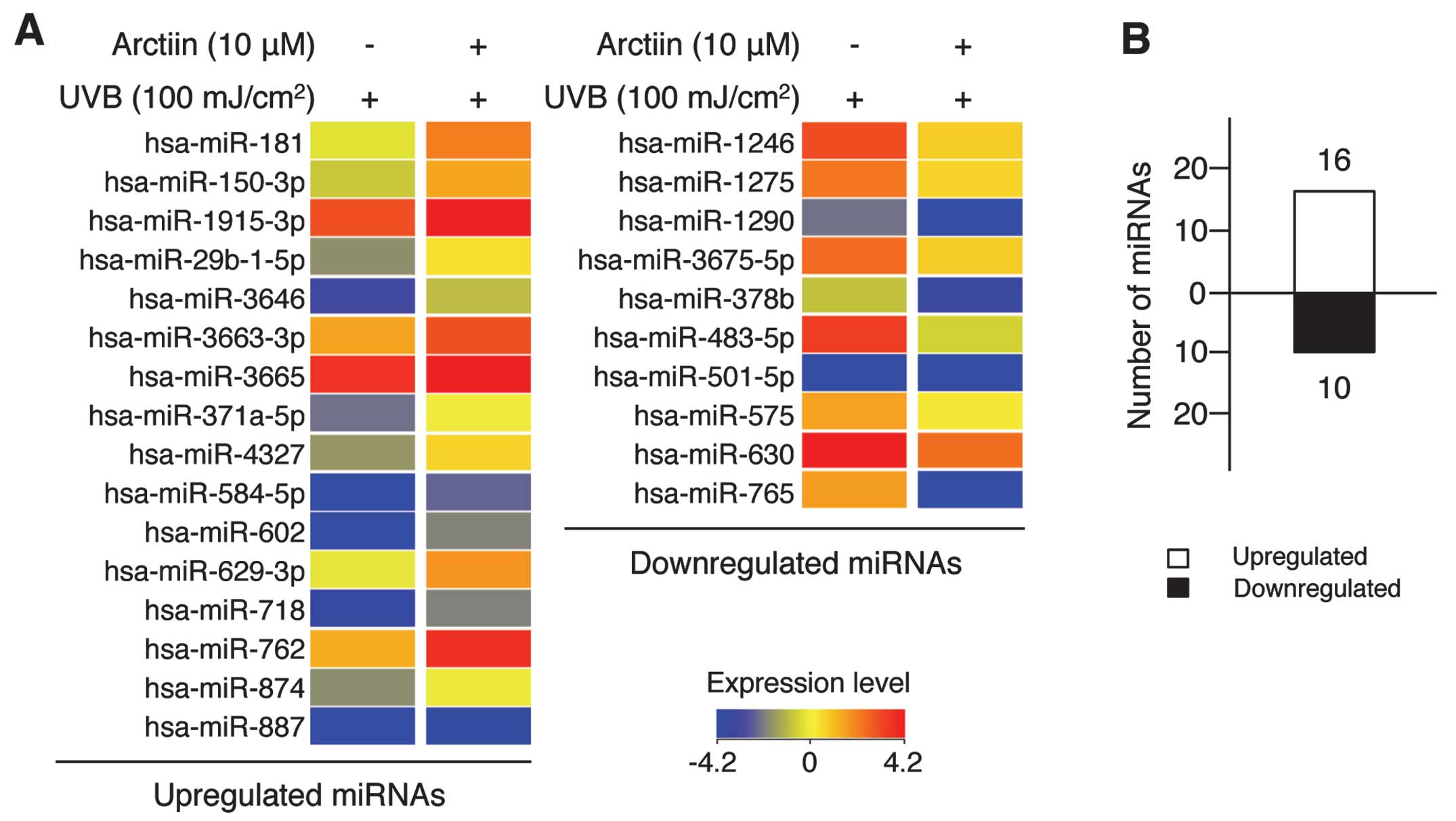
from UVB damage (Fig. 5A).
Specifically, the expression level of 16 miRNAs was upregulated
(Fig. 5A, left panel) and that of
10 miRNAs was downregulated (Fig.
5A, right panel) in the arctiin-mediated UVB protection system
(Fig. 5B). Of the 26 miRNAs,
hsa-miR-602 and -762 were upregulated 5.74- and 4.09-fold,
respectively, and hsa-miR-765 and -483-5p were downregulated 11.86-
and 8.45-fold, respectively. The complete list of differentially
expressed miRNAs is provided in Table
I.
 | Table ImiRNAs showing >2-fold expression
change in NHDF cells pretreated with arctiin prior to UVB
irradiation. |
Table I
miRNAs showing >2-fold expression
change in NHDF cells pretreated with arctiin prior to UVB
irradiation.
| miRNA | Change relative to
controls | Direction of
regulation | Chr. | miRNA | Change relative to
controls | Direction of
regulation | Chr. |
|---|
| hsa-miR-1181 | 3.88 | Up | 19 | hsa-miR-762 | 4.09 | Up | 16 |
| hsa-miR-150-3p | 3.17 | Up | 19 | hsa-miR-874 | 2.03 | Up | 5 |
|
hsa-miR-1915-3p | 2.12 | Up | 10 | hsa-miR-887 | 2.90 | Up | 5 |
|
hsa-miR-29b-1-5p | 2.72 | Up | 7 | hsa-miR-1246 | −3.81 | Down | 2 |
| hsa-miR-3646 | 2.53 | Up | 20 | hsa-miR-1275 | −2.63 | Down | 6 |
|
hsa-miR-3663-3p | 2.51 | Up | 10 | hsa-miR-1290 | −2.37 | Down | 1 |
| hsa-miR-3665 | 2.03 | Up | 13 |
hsa-miR-3679-5p | −2.73 | Down | 2 |
|
hsa-miR-371a-5p | 2.64 | Up | 19 | hsa-miR-378b | −2.42 | Down | 3 |
| hsa-miR-4327 | 2.95 | Up | 21 | hsa-miR-483-5p | −8.45 | Down | 11 |
| hsa-miR-584-5p | 2.31 | Up | 5 | hsa-miR-501-5p | −2.25 | Down | X |
| hsa-miR-602 | 5.74 | Up | 9 | hsa-miR-575 | −2.08 | Down | 4 |
| hsa-miR-629-3p | 2.95 | Up | 15 | hsa-miR-630 | −3.62 | Down | 15 |
| hsa-miR-718 | 2.09 | Up | X | hsa-miR-765 | −11.86 | Down | 1 |
Differentially regulated miRNAs may be
involved in the regulation of key pathways involved in the UVB
protection effect in NHDF cells
miRNA is an important regulator of cell
proliferation, senescence and apoptosis through the modulation of
target mRNA translation (28).
Therefore, we investigated the biological significance of the
deregulated miRNAs in the arctiin-mediated UVB protection effect.
First, the predicted target genes of each miRNA were identified
using the DIANA-microT-CDS (v5.0) bioinformatic tool as described
in Materials and methods. To improve the accuracy of the target
search, the threshold of the tool was fixed at 0.8. After the
target search, information on the Ensembl transcript ID of target
genes was collected and the ID lists of target genes were analyzed
to identify their biological functions using DAVID bioinformatic
resources. Biological significance was extracted from the large
gene lists using one of the analysis tools available in the DAVID
database or the KEGG pathway. To improve accuracy, the Ease score,
which is a modified Fisher’s exact P-value, was fixed at 0.5 and
meaningful KEGG pathways showing a value of >2% (percentage of
involved target genes/total target genes) were selected. The
results suggested these miRNAs were present in the signaling
pathways of cell cycle, cell proliferation, cancer,
ubiquitin-mediated proteolysis, insulin, focal adhesion, MAPK, Wnt
and ErbB (Tables II and III). In particular, almost all of the
upregulated miRNAs were involved in MAPK, Wnt and cancer signaling
pathways, whereas the downregulated miRNAs were mainly involved in
MAPK, ErbB, focal adhesion, cell cycle and cancer signaling
pathways. Of note, the MAPK signaling pathway, which involves
MAPKK, p38, JNK and ERK1/2, was one of the most significant
pathways identified for up- and downregulated miRNAs.
 | Table IIMain functions of upregulated miRNAs
predicted by bioinformatics analysis. |
Table II
Main functions of upregulated miRNAs
predicted by bioinformatics analysis.
| miRNA | Total targets | KEGG pathway | Target count in
pathway | %a | P-value |
|---|
| hsa-miR-602 | 302 | MAPK signaling
pathway | 7 | 2.3 | 2.20E-01 |
| | Insulin signaling
pathway | 6 | 2 | 5.30E-02 |
| | Alzheimer’s
disease | 6 | 2 | 1.00E-01 |
| | Calcium signaling
pathway | 6 | 2 | 1.30E-01 |
| hsa-miR-762 | 534 | Axon guidance | 16 | 3 | 6.60E-07 |
| | MAPK signaling
pathway | 16 | 3 | 2.90E-03 |
| | Pathways in
cancer | 15 | 2.8 | 3.70E-02 |
| | Wnt signaling
pathway | 13 | 2.4 | 4.00E-04 |
| | Regulation of actin
cytoskeleton | 11 | 2.1 | 4.50E-02 |
| hsa-miR-1181 | 2 | - | - | - | - |
| hsa-miR-150-3p | 184 | Wnt signaling
pathway | 5 | 2.7 | 6.00E-02 |
| | Neurotrophin
signaling pathway | 4 | 2.2 | 1.20E-01 |
| | Ubiquitin-mediated
proteolysis | 4 | 2.2 | 1.50E-01 |
| | MAPK signaling
pathway | 4 | 2.2 | 4.90E-01 |
| hsa-miR-629-3p | 445 | Pathways in
cancer | 10 | 2.3 | 2.10E-01 |
| hsa-miR-4327 | 112 | MAPK signaling
pathway | 4 | 3.6 | 1.20E-01 |
| | Pathways in
cancer | 4 | 3.6 | 1.80E-01 |
| | Melanoma | 3 | 2.7 | 4.00E-02 |
| | Calcium signaling
pathway | 3 | 2.7 | 1.90E-01 |
| hsa-miR-887 | 10 | - | - | - | - |
|
hsa-miR-29b-1-5p | 265 | - | - | - | - |
|
hsa-miR-371a-5p | 351 | Spliceosome | 8 | 2.3 | 4.20E-03 |
| | Wnt signaling
pathway | 7 | 2 | 3.60E-02 |
| hsa-miR-3646 | 569 | - | - | - | - |
|
hsa-miR-3663-3p | 305 | MAPK signaling
pathway | 12 | 3.9 | 5.90E-03 |
| | Pathways in
cancer | 11 | 3.6 | 5.50E-02 |
| | Neurotrophin
signaling pathway | 7 | 2.3 | 2.00E-02 |
| | Focal adhesion | 7 | 2.3 | 1.30E-01 |
| | Cytokine-cytokine
receptor interaction | 7 | 2.3 | 3.00E-01 |
| hsa-miR-584-5p | 288 | MAPK signaling
pathway | 8 | 2.8 | 9.70E-02 |
| | Pathways in
cancer | 8 | 2.8 | 2.10E-01 |
|
hsa-miR-1915-3p | 351 | Wnt signaling
pathway | 8 | 2.3 | 5.60E-03 |
| | Pathways in
cancer | 7 | 2 | 3.30E-01 |
| hsa-miR-718 | 18 | - | - | - | - |
| hsa-miR-874 | 176 | B- and T-cell
receptor signaling pathway | 4 | 2.3 | 2.20E-02 |
| | MAPK signaling
pathway | 4 | 2.3 | 3.70E-01 |
| hsa-miR-3665 | 195 | Neurotrophin
signaling pathway | 4 | 2.1 | 1.10E-01 |
| | Insulin signaling
pathway | 4 | 2.1 | 1.30E-01 |
| | MAPK signaling
pathway | 4 | 2.1 | 4.70E-01 |
 | Table IIIMain functions of downregulated
miRNAs predicted by bioinformatics analysis. |
Table III
Main functions of downregulated
miRNAs predicted by bioinformatics analysis.
| miRNA | Total targets | KEGG pathway | Target count in
pathway | %a | P-value |
|---|
| hsa-miR-765 | 548 | Cytokine-cytokine
receptor interaction | 11 | 2 | 2.00E-01 |
| hsa-miR-483-5p | 32 | Focal adhesion | 2 | 6.2 | 1.50E-01 |
| hsa-miR-1246 | 290 | Neurotrophin
signaling pathway | 7 | 2.4 | 3.20E-03 |
| hsa-miR-630 | 54 | Alanine and
glutamate metabolism | 2 | 3.7 | 4.80E-02 |
|
hsa-miR-3679-5p | 238 | Calcium signaling
pathway | 7 | 2.9 | 1.80E-02 |
| | ErbB signaling
pathway | 5 | 2.1 | 2.00E-02 |
| | Insulin signaling
pathway | 5 | 2.1 | 7.80E-02 |
| | MAPK signaling
pathway | 5 | 2.1 | 4.00E-01 |
| hsa-miR-1275 | 268 | Tight junction | 8 | 3 | 1.10E-03 |
| | Wnt signaling
pathway | 6 | 2.2 | 3.60E-02 |
| | Endocytosis | 6 | 2.2 | 7.20E-02 |
| hsa-miR-378b | 162 | Pathways in
cancer | 8 | 4.9 | 3.60E-02 |
| | Cell cycle | 4 | 2.5 | 1.20E-01 |
| hsa-miR-1290 | 593 | Pathways in
cancer | 17 | 2.9 | 4.00E-02 |
| | Focal adhesion | 14 | 2.4 | 7.90E-03 |
| | Insulin signaling
pathway | 13 | 2.2 | 7.60E-04 |
| | MAPK signaling
pathway | 12 | 2 | 1.90E-01 |
| hsa-miR-501-5p | 301 | Ubiquitin-mediated
proteolysis | 9 | 3 | 7.60E-04 |
| | MAPK signaling
pathway | 8 | 2.7 | 9.20E-02 |
| | Regulation of actin
cytoskeleton | 6 | 2 | 2.00E-01 |
| hsa-miR-575 | 241 | MAPK signaling
pathway | 8 | 3.3 | 7.70E-02 |
| | Non-small cell lung
cancer | 6 | 2.5 | 8.60E-04 |
| | Prostate
cancer | 6 | 2.5 | 7.70E-03 |
| | Melanoma | 5 | 2.1 | 1.70E-02 |
| | Cell cycle | 5 | 2.1 | 9.60E-02 |
Discussion
Although arctiin, a major lignin of Arctium
lappa, has been reported to have various biological functions
including anti-microbial, anti-inflammatory and anticancer
properties (19,29,30), the antiproliferative functions of
arctiin, which are documented in PC3 prostate cancer cells, HepG2
hepatocarcinoma cells and HaCaT keratinocytes (30–32), remain controversial. Matsuzaki
et al (30) showed that
relatively high doses of arctiin (25–250 μM) decreased the number
of viable cells and increased the fraction of cells in G1 phase to
10%, but did not induce apoptosis. In addition, Huang et al
(31) showed that arctiin
preferentially induced cell detachment, but did not exert
anti-proliferative or cytotoxic effects in PC3 cells. Furthermore,
our results show that relatively low doses (1, 2, 5 and 10 μM) of
arctiin exhibited little cytotoxicity but 20 μM arctiin induced a
14% decrease in cell viability. These results suggest that,
although arctiin induces cell detachment in specific cell types,
the antiproliferative effect may be evident only at high doses, and
low concentrations of arctiin may exert other biological activities
that have not yet been identified.
To the best of our knowledge we have demonstrated,
for the first time, that a low concentration of arctiin protects
NHDF cells against UVB damage. Results of the WST-1-based cell
viability assay revealed that pretreatment with 10 μM arctiin for 6
h conferred maximum UVB protection. We also confirmed that arctiin
inhibits UVB-induced apoptosis, cell migration defect and DNA
damage. Notably, longer pretreatment (12 and 24 h) with arctiin did
not enhance the protection effect over a 6-h treatment. Viability
of cells pretreated for 24 h with various doses of arctiin was
comparable to that of UVB-irradiated cells. It has been reported
(30,31) that arctiin-mediated inhibition of
cell growth is dependent on treatment time. Cells treated with or
without arctiin for <24 h showed a similar growth rate, but
cells treated with arctiin for >24 h showed decreased growth
rates. Similarly, treatment with 3 and 10 μM arctiin for short
periods of time exert no cytotoxic effects, whereas treatment for
>30 h resulted in a significantly decreased cell growth rate.
Therefore, the arctiin-mediated UVB protection effect is dependent
on treatment time.
A recent study focusing on the possible role and
function of miRNAs in UVB-mediated skin diseases such as skin aging
and cancer provided a novel viewpoint on the pathogenesis of UV
radiation-related diseases in human skin (27). miRNAs have been shown to be
important in a large number of specific skin physiological
processes, including keratinocyte differentiation, melanogenesis,
development of skin stem cells and dermal fibroblast senescence
(14,33–36). Microarray analyses have revealed
that the expression profiles of miRNAs are altered in photodamage
and skin carcinogenesis induced by UV radiation, and numerous
miRNAs are involved in this process (11,27,34). Moreover, changes in miRNA profiles
may be directly related to the pathogenesis of photoaging and skin
cancer. However, the UVB protective effect of miRNAs in NHDF cells
has not been extensively investigated and little is known regarding
the potential role of photodamage-regulated miRNAs in cell
function, which may be an important factor in the progression of
diseases related to UVB radiation. In the present study, the
miRNA-based microarray analysis revealed that the arctiin-mediated
UVB protection effect involved deregulation of 26 miRNAs in NHDF
cells, of which 16 were upregulated and 10 were downregulated. Of
the 26 miRNAs, the expression of 8 miRNAs altered >3-fold. In
their study, Zhou et al (14) showed that UVB radiation, not only
changed the expression profiles of miRNAs, but that specific miRNAs
are major inducers of UVB-mediated senescence. These deregulated
miRNAs potentially have an important effect on biological pathways
essential for protection against UVB in skin cells.
It has been reported that >30% of protein-coding
genes are post-transcriptionally regulated by miRNAs, where each
miRNA is able to target ~200 transcripts and a single mRNA may also
be targeted by >1 miRNA (9,10).
To determine the possible biological effects of the miRNAs that are
deregulated during arctiin-mediated UVB protection in NHDF cells,
we first predicted the target genes of the miRNAs using
bioinformatic tools with a high threshold, and then performed a
functional gene-annotation enrichment analysis using the KEGG
pathway database. The results showed that the deregulated miRNAs
were primarily associated with the cancer, MAPK, Wnt, insulin
signaling and neurotrophin signaling pathways. Of these, the MAPK
pathway was one of the most significantly involved pathways
affected by miRNAs that were up- or downregulated in
arctiin-treated UVB-irradiated cells. The upregulated miRNAs,
including miR-602, -762, -150-3p, -4327, -584-5p, -874 and -3665
were all strongly predicted to affect target genes involved in the
MAPK pathway. Several of the downregulated miRNAs, including
miR-3679-5p, -1290 and -575, were also predicted to be involved in
the MAPK pathway. This signaling cascade is involved in a number of
cell functions, including proliferation, differentiation and
apoptosis. In a recent study, UV was found to activate MAPKs such
as p38 MAPK, JNK and ERK1/2, which play important roles in the UV
response in skin cells (6).
Notably, activation of p38 MAPK and JNK is critically involved in
UV-mediated proapoptotic and antiapoptotic responses (6), whereas activated ERK1/2 has been
reported only in UV-mediated proapoptotic signal transduction
(6). Therefore, ERK1/2 activation
may be specifically regulated by the miRNAs that are upregulated by
arctiin in NHDF cells. The study also reported that UV-mediated
activation of those MAPKs is not post-transcriptional, but is
induced by post-translational modifications such as phosphorylation
(6). Therefore, our finding that
the target genes of up- and downregulated miRNAs are significantly
involved in the MAPK pathway raises the possibility that upstream
or downstream proteins of MAPKs are post-transcriptionally
regulated by the deregulated miRNAs and may contribute to the
arctiin-mediated UVB protection effect in NHDF cells.
The role of miRNA-602 in cell functions has
previously been studied in hepatitis B virus-mediated
hepatocellular carcinoma (37).
The data revealed that miR-602 regulated the tumor suppressor gene
RASS1FA, which affects several cell functions including
proliferation, migration, senescence and apoptosis (38). Loss of RASSF1 expression is
associated with the status of K-ras, which is an effector molecule
of MAPK signal transduction (39). Considering that miR-602 was the
most highly upregulated miRNA in our arctiin-mediated UVB
protection system, it is likely that the upregulated expression of
miR-602 contributes at least in part to UVB protection in NHDF
cells. miR-765 and -483-5p were the most highly downregulated
miRNAs identified in the present study. The cell function of those
miRNAs has not been investigated; however, the aberrant expression
of miR-765 and -483-5p may also be involved in UVB protection.
In summary, to the best of our knowledge, we have
demonstrated for the first time that a low dose of arctiin has UVB
protective activity in NHDF cells. We also confirmed that arctiin
inhibits the UVB-mediated cell growth defect, apoptosis, cell
migration defect and DNA damage in these cells. Furthermore,
arctiin treatment induces expression changes in specific miRNA
profiles, and some of the deregulated miRNAs have predicted roles
in the regulation of MAPK and cell growth signal pathways. Although
future studies should be performed to validate the deregulated
miRNAs, results of this study have provided novel information on
miRNA-mediated UVB protection in NHDF cells.
Acknowledgements
We would like to thank all other members of Coreana
Cosmetics Co., Ltd. for their support. This study was supported by
the KU Research Professor Program of Konkuk University and grant
from the Ministry of Science, ICT and Future Planning (grant no.
20110028646) of the Republic of Korea.
References
|
1
|
Sjerobabski-Masnec I and Situm M: Skin
aging. Acta Clin Croat. 49:515–518. 2010.
|
|
2
|
Wang B: Photoaging: a review of current
concepts of pathogenesis. J Cutan Med Surg. 15(Suppl 1): S374–S377.
2011.PubMed/NCBI
|
|
3
|
Wlaschek M, Tantcheva-Poór I, Naderi L, et
al: Solar UV irradiation and dermal photoaging. J Photochem
Photobiol B. 63:41–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diffey B: Climate change, ozone depletion
and the impact on ultraviolet exposure of human skin. Phys Med
Biol. 49:R1–R11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muthusamy V and Piva TJ: The UV response
of the skin: a review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Travers JB, Edenberg HJ, Zhang Q, et al:
Augmentation of UVB radiation-mediated early gene expression by the
epidermal platelet-activating factor receptor. J Invest Dermatol.
128:455–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casati P and Walbot V: Gene expression
profiling in response to ultraviolet radiation in maize genotypes
with varying flavonoid content. Plant Physiol. 132:1739–1754. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Zhou BR, Hua LJ, Guo Z and Luo D:
Differential miRNA profile on photoaged primary human fibroblasts
irradiated with ultraviolet A. Tumour Biol. Jul 7–2013.(Epub ahead
of print).
|
|
12
|
Lu C, Ding ZH and Zhou MJ: Mechanisms of
ultraviolet B irradiation-induced injuries in 16HBE cells. Nan Fang
Yi Ke Da Xue Xue Bao. 31:57–60. 2011.(In Chinese).
|
|
13
|
Tan G, Shi Y and Wu ZH: MicroRNA-22
promotes cell survival upon UV radiation by repressing PTEN.
Biochem Biophys Res Commun. 417:546–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou BR, Guo XF, Zhang JA, et al: Elevated
miR-34c-5p mediates dermal fibroblast senescence by ultraviolet
irradiation. Int J Biol Sci. 9:743–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An IS, An S, Kang SM, et al: Titrated
extract of Centella asiatica provides a UVB protective
effect by altering microRNA expression profiles in human dermal
fibroblasts. Int J Mol Med. 30:1194–1202. 2012.
|
|
16
|
An IS, An S, Park S, Lee SN and Bae S:
Involvement of microRNAs in epigallocatechin gallate-mediated UVB
protection in human dermal fibroblasts. Oncol Rep. 29:253–259.
2013.PubMed/NCBI
|
|
17
|
Sun WJ, Sha ZF and Gao H: Determination of
arctiin and arctigenin in Fructus Arctii by reverse-phase
HPLC. Yao Xue Xue Bao. 27:549–551. 1992.
|
|
18
|
Hirose M, Yamaguchi T, Lin C, et al:
Effects of arctiin on PhIP-induced mammary, colon and pancreatic
carcinogenesis in female Sprague-Dawley rats and MeIQx-induced
hepatocarcinogenesis in male F344 rats. Cancer Lett. 155:79–88.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee S, Shin S, Kim H, et al:
Anti-inflammatory function of arctiin by inhibiting COX-2
expression via NF-κB pathways. J Inflamm (Lond).
8:162011.PubMed/NCBI
|
|
20
|
Wu JG, Wu JZ, Sun LN, et al: Ameliorative
effects of arctiin from Arctium lappa on experimental
glomerulonephritis in rats. Phytomedicine. 16:1033–1041. 2009.
View Article : Google Scholar
|
|
21
|
Cui X, Zhang J, Du R, et al: HSF4 is
involved in DNA damage repair through regulation of Rad51. Biochim
Biophys Acta. 1822:1308–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maragkakis M, Reczko M, Simossis VA, et
al: DIANA-microT web server: elucidating microRNA functions through
target prediction. Nucleic Acids Res. 37:W273–W276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI
|
|
24
|
Lee CH, Wu SB, Hong CH, Yu HS and Wei YH:
Molecular mechanisms of UV-induced apoptosis and its effects on
skin residential cells: the implication in UV-based phototherapy.
Int J Mol Sci. 14:6414–6435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sinha RP and Häder DP: UV-induced DNA
damage and repair: a review. Photochem Photobiol Sci. 1:225–236.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Syed DN, Khan MI, Shabbir M and Mukhtar H:
MicroRNAs in skin response to UV radiation. Curr Drug Targets.
14:1128–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subramanyam D and Blelloch R: From
microRNAs to targets: pathway discovery in cell fate transitions.
Curr Opin Genet Dev. 21:498–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayashi K, Narutaki K, Nagaoka Y, Hayashi
T and Uesato S: Therapeutic effect of arctiin and arctigenin in
immunocompetent and immunocompromised mice infected with influenza
A virus. Biol Pharm Bull. 33:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuzaki Y, Koyama M, Hitomi T, et al:
Arctiin induces cell growth inhibition through the down-regulation
of cyclin D1 expression. Oncol Rep. 19:721–727. 2008.PubMed/NCBI
|
|
31
|
Huang DM, Guh JH, Chueh SC and Teng CM:
Modulation of anti-adhesion molecule MUC-1 is associated with
arctiin-induced growth inhibition in PC-3 cells. Prostate.
59:260–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moritani S, Nomura M, Takeda Y and
Miyamoto K: Cytotoxic components of bardanae fructus (goboshi).
Biol Pharm Bull. 19:1515–1517. 1996. View Article : Google Scholar
|
|
33
|
Yi R and Fuchs E: MicroRNA-mediated
control in the skin. Cell Death Differ. 17:229–235. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou BR, Xu Y, Permatasari F, et al:
Characterization of the miRNA profile in UVB-irradiated normal
human keratinocytes. Exp Dermatol. 21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bemis LT, Chen R, Amato CM, et al:
MicroRNA-137 targets microphthalmia-associated transcription factor
in melanoma cell lines. Cancer Res. 68:1362–1368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hildebrand J, Rütze M, Walz N, et al: A
comprehensive analysis of microRNA expression during human
keratinocyte differentiation in vitro and in vivo. J Invest
Dermatol. 131:20–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang L, Ma Z, Wang D, Zhao W, Chen L and
Wang G: MicroRNA-602 regulating tumor suppressive gene RASSF1A is
overexpressed in hepatitis B virus-infected liver and
hepatocellular carcinoma. Cancer Biol Ther. 9:803–808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fernandes MS, Carneiro F, Oliveira C and
Seruca R: Colorectal cancer and RASSF family--a special emphasis on
RASSF1A. Int J Cancer. 132:251–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao D, Chen Y, Tang Y, et al: Loss of
RASSF1A expression in colorectal cancer and its association with
K-ras status. Biomed Res Int. 2013:9767652013.PubMed/NCBI
|