Introduction
Hearing impairment is one of the most common
disabling sensory defects in humans and varies according to age of
onset and severity. Approximately 1 in 500 newborns has congenital
hearing loss (1,2). Hearing impairment is a genetically
heterogeneous disease; over 300 genes are thought to be necessary
for hearing and over 50 deafness-causing genes have been identified
(3,4). These genes encode a number of
important proteins, such as myosin motors, gap junction proteins,
ion channels, transcription factors, F-actin bundle proteins,
F-actin crosslinking proteins and other gene expression products
that have unknown functions (5–10).
Studies on the localization and function of these genes have
provided a great deal of insight into the mechanisms of
hearing.
Hearing in mammals is characterized by high
sensitivity, a wide dynamic range and sharp frequency selectivity.
In the mammalian auditory pathway, the sensory receptor cells from
the organ of Corti have differentiated into 1 row of inner hair
cells (IHCs) on the modiolar side of the tunnel and 3 rows of outer
hair cells (OHCs) on the lateral side. OHCs play a considerable
role in frequency selectivity and in signal amplification through
many cytoskeleton proteins, such as myosin, actin and prestin. The
slow and fast motility or the electromotility of OHCs is involved
in this process (11–14). The slow motile shortening of OHCs
is likely performed by the phosphorylation of proteins in the
actin-spectrin network of the cortical cytoskeleton in the lateral
cell wall. The plasma membrane of the OHC lateral wall and
stereocilia contain a number of proteins, such as myosin, actin,
prestin, ankyrin, spectrin and calmodulin (15). It has been previously suggested
that a number of conventional myosins play an important role in the
development of the inner ear (16); however, the underlying mechanisms
remain unknown. Myosin may be regulated by myosin light chain
kinase (MLCK) through the phosphorylation of the regulatory light
chain (RLC) in smooth muscle cells. Therefore, we hypothesized that
MLCK may play an important role in hearing.
MLCK is the principal regulator of various forms of
eukaryotic motility. MLCK plays an important role in many functions
of non-muscle cells, including cell spreading and migration,
neurite growth cone advancement, cytokinesis, cytoskeletal
clustering, stress fiber formation, platelet shape changes,
secretion, transepithelial permeability and cytoskeletal
arrangements, which affect ion currents or ion exchange at the
plasma membrane (17–20). The smooth muscle gene Mylk
expresses 3 transcripts using alternative promoters, including
short MLCK, long MLCK and telokin. Short MLCK is expressed in hair
cells and is necessary for maintaining membrane stability in IHCs
(21). Short MLCK has a catalytic
core, a regulatory segment, 3 immunoglobulin-like modules, a
fibronectin module, a PEVK repeat-rich region and a 3DFRXXL F-actin
binding motif in the N terminus (22–24). MLCK has been shown to be expressed
in IHCs (21). In a recent study,
mice carrying a specific deletion of MLCK in the IHCs
(MLCKIKO mice) presented with impaired hearing, whose
mutant IHCs produced ball-like structures around their hair bundles
in vivo, displayed less resistance to hypoosmotic solution,
manifested less membrane F-actin and reduced the phosphorylation of
myosin light chain in vitro. The authors demonstrated that
MLCK is necessary for maintaining the membrane stability of IHCs
(21). The exact role of MLCK in
OHCs remains unclear. In the present study, using an animal model
of mice with a specific deletion of MLCK in OHCs, we investigated
the function and regulatory mechanisms of MLCK in OHCs, and found
that MLCK has important functions in OHCs.
Materials and methods
Generation of MLCK OHC-specific knockout
mice
The Institutional Animal Care and Use Committee
(IACUC) of the Model Animal Research Center of Nanjing University
approved all animal procedures. All experiments were conducted in
accordance with the IACUC guidelines (Nanjing, China) (permit no.
AP#MZ3).
Floxed Mylk mice (Mylkflox/flox)
with a congenic background (B6:129) which were crossed with OHC-Cre
transgenic mice (25), were
kindly provided by the Department of Development Neurobiology, St.
Jude Children’s Research Hospital (Chicago, IL, USA). The OHC-Cre
transgenic mice were generated with a BAC containing the prestin
gene to specifically enforce Cre expression in OHCs. The resultant
mice (Mylkflox/flox: OHC-Cre; designated as
MLCKOKO) were OHC-specific knockout mice for MLCK. The
littermates of MLCKOKO (Mylkflox/+:
OHC-Cre) were used as controls (CTR). These mice were maintained on
a 12-h light/dark cycle under specific pathogen-free (SPF)
conditions in standard animal rooms of the National Resource Center
for Mutant Mice (NRCMM) of China.
Hearing tests
Acoustic brainstem responses (ABRs) reflect the
response of the auditory system to acoustic stimuli. Distortion
product otoacoustic emissions (DPOAEs) measure acoustic energy,
which is generated in the form of otoacoustic emissions (OAEs),
that are produced by OHCs in the cochlea. We measured ABRs and
DPOAEs to perform a robust assessment of hearing impairment in
mice.
MLCKOKO and CTR mice, ranging in age from
2 to 9 months, were used in the experiments. All tests were
performed in a single-walled, soundproof booth and were repeated 3
times for each mouse. The mice were anesthetized by an
intraperitoneal injection of avertin at an initial dose of 500
mg/kg body weight and maintained with a half-dose every 20 min.
After testing was completed, all mice were kept warm on a heating
pad, at 37°C, until they fully recovered from the anesthesia.
ABR recordings
ABR waveforms were recorded with subcutaneous needle
electrodes at the vertex (active), posterior bulla region of the
right ear (reference) and tip of the nose (ground). An outlay
trumpet was placed 10 cm in front of the nasal tip. Click and tone
pips of 8, 16 and 32 kHz were generated using the evoked generation
workstation system 3 (Tucker Davis Technologies Inc.; Gainesville,
FL, USA) with computerized SigGen32 software. The response was
averaged (n=1,024) and displayed from 110 to 0 dB with decreasing
steps of 5 dB. The threshold of hearing was determined by observing
the lowest intensity of sound required to elicit a characteristic
waveform.
DPOAE recordings
DPOAEs were recorded and analyzed with workstation
system 3 (Tucker Davis Technologies Inc.) using computerized
SigGen32 software. The acoustic probe was lengthened with a tapered
plastic tube to ensure a tight fit in the external ear canal and
the formation of a closed acoustic system. The f2/f1 frequency
ratio was maintained at 1.20, and the value of f2 varied from 1 to
12 kHz (8 points). The sound intensities at each frequency (f1 and
f2) were 65 dB. The signal-to-noise ratios (SNRs) of the DPOAE
(2f1-f2) data at the various f2 values were obtained.
Histopathological analysis
Mice were euthanized with an overdose of avertin and
were then perfused with phosphate-buffered solution (PBS) followed
by 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA). The
acoustic capsule was removed. The cochleae were removed through an
open window over the apical turn of the cochlea and stored
overnight in 4% paraformaldehyde in PBS at 4°C. For
decalcification, the cochleae were incubated in 10%
ethylenediaminetetraacetic acid (EDTA) (pH 7.4) for 5 days at 4°C
prior to standard histological examination. Briefly, the specimens
were dehydrated in a graded series of ethanol solutions before
being embedded in paraffin and the serial sections (7 μm) of the
cochleae were stained with hematoxylin and eosin (H&E).
Scanning electron microscopy (SEM)
assay
Mice were euthanized with an overdose of avertin and
perfused with PBS followed by glutaraldehyde fixative (2.5%
glutaraldehyde in PBS). Cochleae were selected and post-fixed again
in glutaraldehyde fixative at 4°C for 4–6 h followed by
decalcification. For SEM, the bone and stria vascularis surrounding
the cochleae were dissected, and the tectorial membrane was removed
to expose the organ of Corti. The organ of Corti was fixed in 1%
osmium tetroxide, dehydrated and critical point-dried. The organ of
Corti was sputter-coated with gold and the images were collected
using an S-3000 N scanning electron microscope (Hitachi, Tokyo,
Japan) at 15 kV.
Immunofluorescence assay for inner ear
sensory epithelia
The cochleae were removed through the round window,
infused with 4% paraformaldehyde in PBS and post-fixed at 4°C for 3
h. Following decalcification in 10% EDTA overnight at room
temperature, the Corti sensory epithelium was dissected from the
soft cochlea. The sensory epithelia were permeabilized with 0.5%
Triton X-100 in PBS for 20 min and washed 3 times with PBS.
Non-specific binding was blocked in PBS buffer containing 5% goat
serum, 1% bovine serum albumin (BSA) and 0.1% Triton X-100 for 1 h
at room temperature. The tissues were incubated with primary
antibody overnight at 4°C and washed 3 times with 0.1% Tween in PBS
followed by incubation with the secondary antibody for 1.5 h at
room temperature. In the experiments, anti-phospho myosin light
chain 2 (Ser 19) antibody (1:150 dilution; Cell Signaling; Cell
Signaling Technology, BSN, USA) was used as the primary antibody,
and Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor
555-conjugated goat anti-mouse or anti-rabbit IgG were used as the
secondary antibodies (Molecular Probes; Carlsbad, CA, USA). F-actin
was labeled by Alexa Fluor 488-conjugated Phalloidin (Invitrogen;
Carlsbad, CA, USA), while DAPI (Sigma; Carlsbad, CA, USA) was used
to stain the nucleus. Immunofluorescent signals were examined under
an Olympus confocal microscope (Olympus, Tokyo, Japan).
Isolation of OHCs and measurement of the
OHC diameter
Three-month-old mice of either gender were
anesthetized by an intraperitoneal injection of avertin. The bullae
were excised, and the lateral wall of the cochleae and stria
vascularis were removed with a fine needle under a dissection
microscope. The organ of Corti was dissected, and OHCs were
enzymatically isolated at room temperature with collagenase (type
IV Sigma-Aldrich, 1 mg/ml). The isolated OHCs were transferred with
a glass pipette to a chamber filled with D-Hank’s solution, and
their diameter was measured using an Olympus confocal microscope.
The D-Hank’s solution contained 136.9 mM/l NaCl, 5.4 mM/l KCl, 0.3
mM/l Na2HPO4, 4.2 mM/l NaHCO3 and
0.4 mM/l KH2PO4 (300 mOsm/l, pH 7.2). ImageJ
software was used to analyze the diameter of the OHCs.
Statistical analysis
The data are expressed as the means ± SEM.
Differences concerning the hearing and the diameter of the OHCs
between the 2 groups were compared with one-way analysis of
variance followed by a Student-Newman-Keul’s t-test with a value of
P<0.05 considered to indicate a statistically significant
difference. We used the χ2 test to analyze the data of
the missing and disordered OHC stereocilia in the apex turn with
P<0.01 as the level of significance. SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA) software was used for statistical analysis.
Results
MLCKOKO mice display an
increased ABR threshold and decreased DPOAE amplitudes
We recorded and characterized the ABRs of
MLCKOKO mice that were between 2 and 9 months of age and
compared them with those of the control mice. An example of ABR
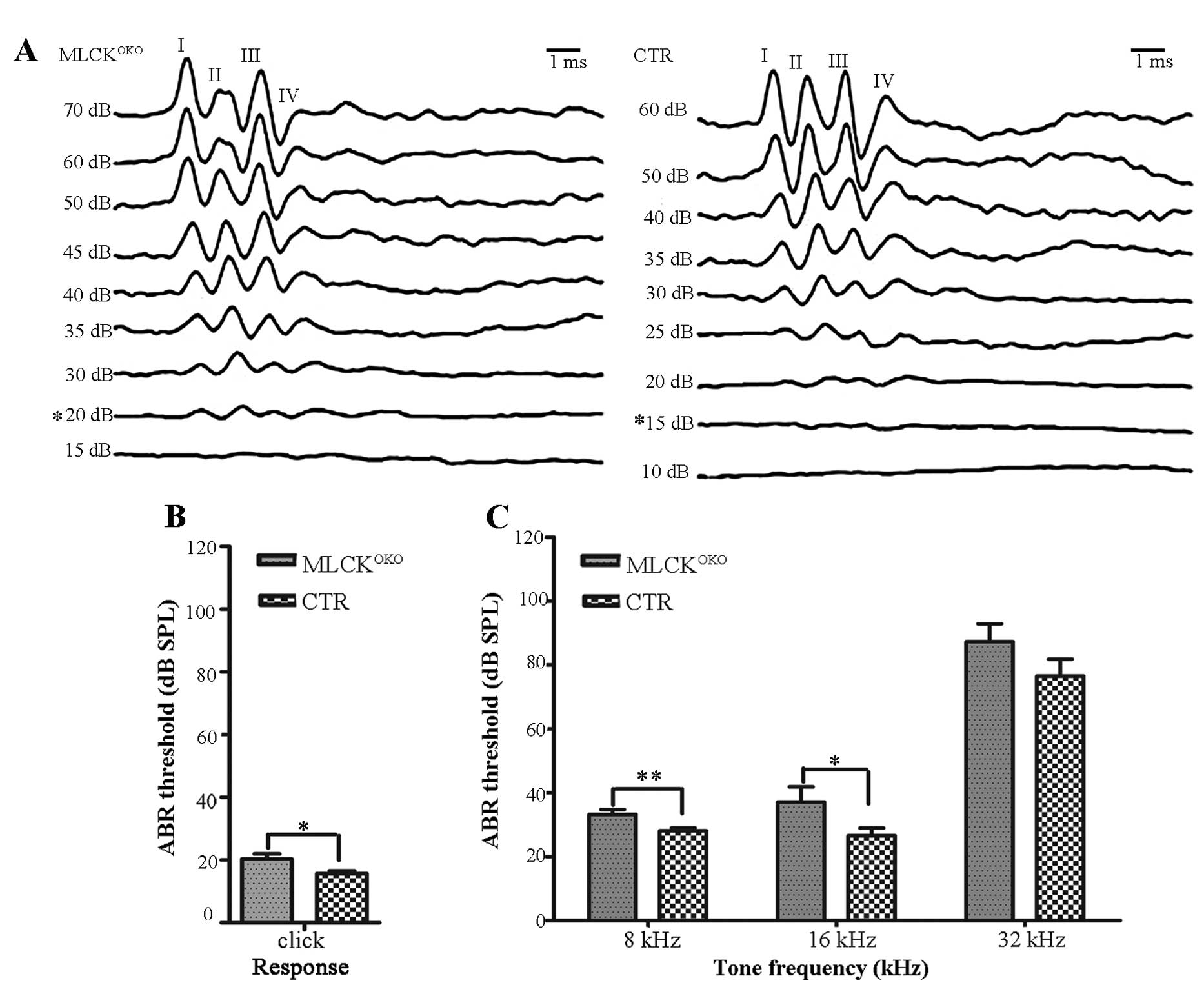
waves is presented in Fig. 1A.
The recordings were 10-msec-long and included a 2-msec pre-stimulus
period. There were typically 4–5 waves in each 10-msec trace, as
reported previously by Song et al (26). Table
I shows the ABR thresholds of the mice of different ages and at
different frequencies.
 | Table IThe mean acoustic brainstem response
(ABR) thresholds and their variance estimates (standard deviations)
for each test, age group of mice and auditory stimulus, and the
numbers of mice tested in each group. |
Table I
The mean acoustic brainstem response
(ABR) thresholds and their variance estimates (standard deviations)
for each test, age group of mice and auditory stimulus, and the
numbers of mice tested in each group.
| | | Tone frequency
(kHz) | |
|---|
| | |
| |
|---|
| Mice | Age | Click | 8 | 16 | 32 | N |
|---|
|
MLCKOKO | 2–3 M | 15.29±4.13 | 25.29±5.72 | 21.17±4.51 | 48.23±7.69 | 17 |
| CTR | | 15.00±4.33 | 26.76±3.03 | 22.05±3.09 | 45.88±4.75 | 17 |
|
MLCKOKO | 4–5 M | 15.47±3.84 | 26.19±5.45 | 23.09±8.58 | 55.0±16.12 | 21 |
| CTR | | 14.76±3.34 | 26.66±4.28 | 20.95±5.38 | 51.9±16.46 | 21 |
|
MLCKOKO | 6–7 M | 20.35±8.49a | 33.21±8.18b | 37.1±25.14a | 87.3±29.64 | 28 |
| CTR | | 15.68±4.76a | 28.10±4.89b | 26.5±13.03a | 76.5±28.78 | 29 |
|
MLCKOKO | 8–9 M | 24.5±11.76 | 38.7±20.68 | 42.5±29.88 | 85.0±30.96 | 12 |
| CTR | | 25.4±16.19 | 35.9±17.00 | 38.6±21.10 | 88.0±23.85 | 11 |
The ABR thresholds did not differ significantly
between the MLCKOKO mice and CTR mice at 2–5 and 8–9
months of age (P>0.05 for all frequencies). Compared with the
CTR mice, the MLCKOKO mice (6–7 months old) displayed a
significantly higher threshold in response to clicks (20.35±8.49 dB
vs. 15.68±4.76 dB of CTR, P<0.05) and tones (8 kHz: 33.21±8.18
dB vs. 28.10±4.89 dB of CTR, P<0.01; 16 kHz: 37.14±25.14 dB vs.
26.55±13.03 of CTR, P<0.05). The mean ABR thresholds between the
2 experimental groups (CTR and MLCKOKO mice) are
graphically illustrated in Fig. 1B
and C for the 6- to 7-month-old age groups.
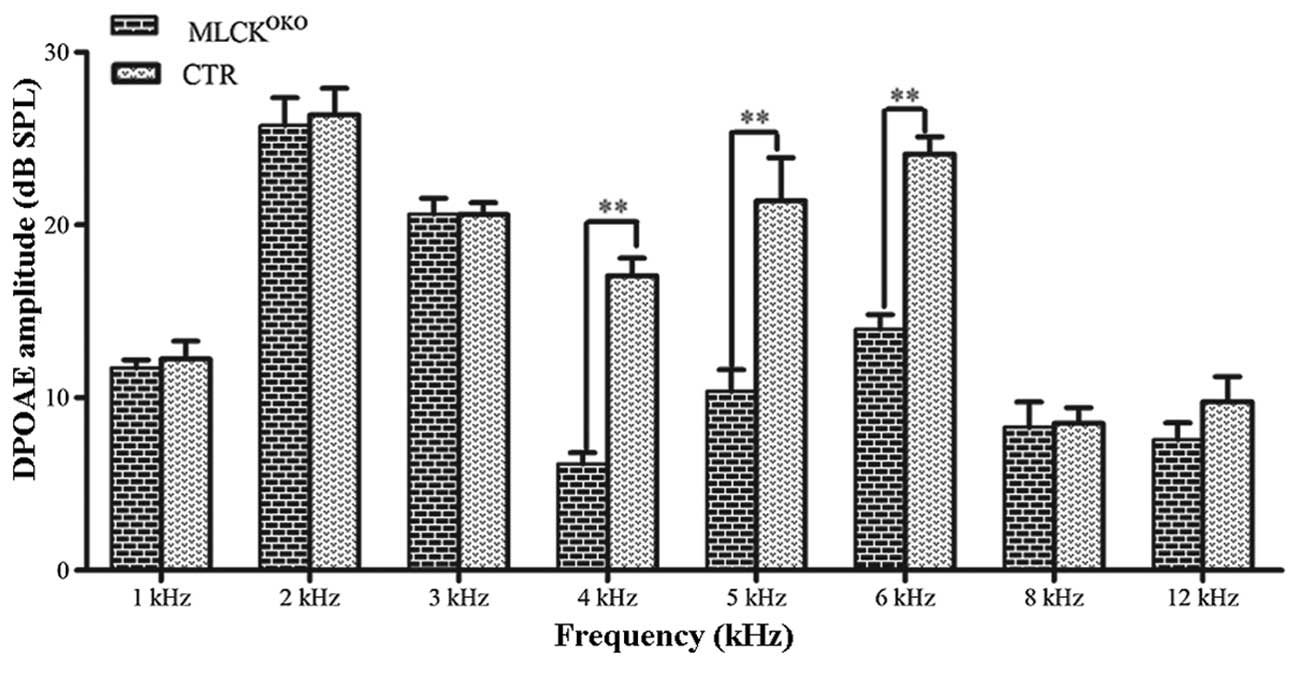
ABR was used to assess the function of the entire
auditory pathway objectively, whereas DPOAE evaluated cochlear
function. We measured the DPOAE thresholds of the
MLCKOKO mice and CTR mice at the age of 3 months. In
DPOAE testing, the distortion product 2f1-f2 was significantly
decreased in the MLCKOKO mice compared with the control
mice. Fig. 2 illustrates the
comparison of the mean and standard error of DPOAE thresholds
between the CTR and MLCKOKO mice. Significant
differences were observed between the 2 groups of mice (t-test,
P<0.01 at 4, 5 and 6 kHz frequencies). The threshold shifts
occurred at 4, 5 and 6 kHz frequencies, and in the
MLCKOKO mice, the average amplitudes of the DPOAEs
decreased by >10 dB.
Histological analysis of the cochlea
structure
Light microscopy analysis of the cochleae sections
displayed the normal architecture of the organ of Corti with IHCs,
OHCs, Hensen’s cells, stria vascularis and spiral ganglion cells in
3-month-old MLCKOKO mice, although these mice displayed
impaired hearing function (data not shown).
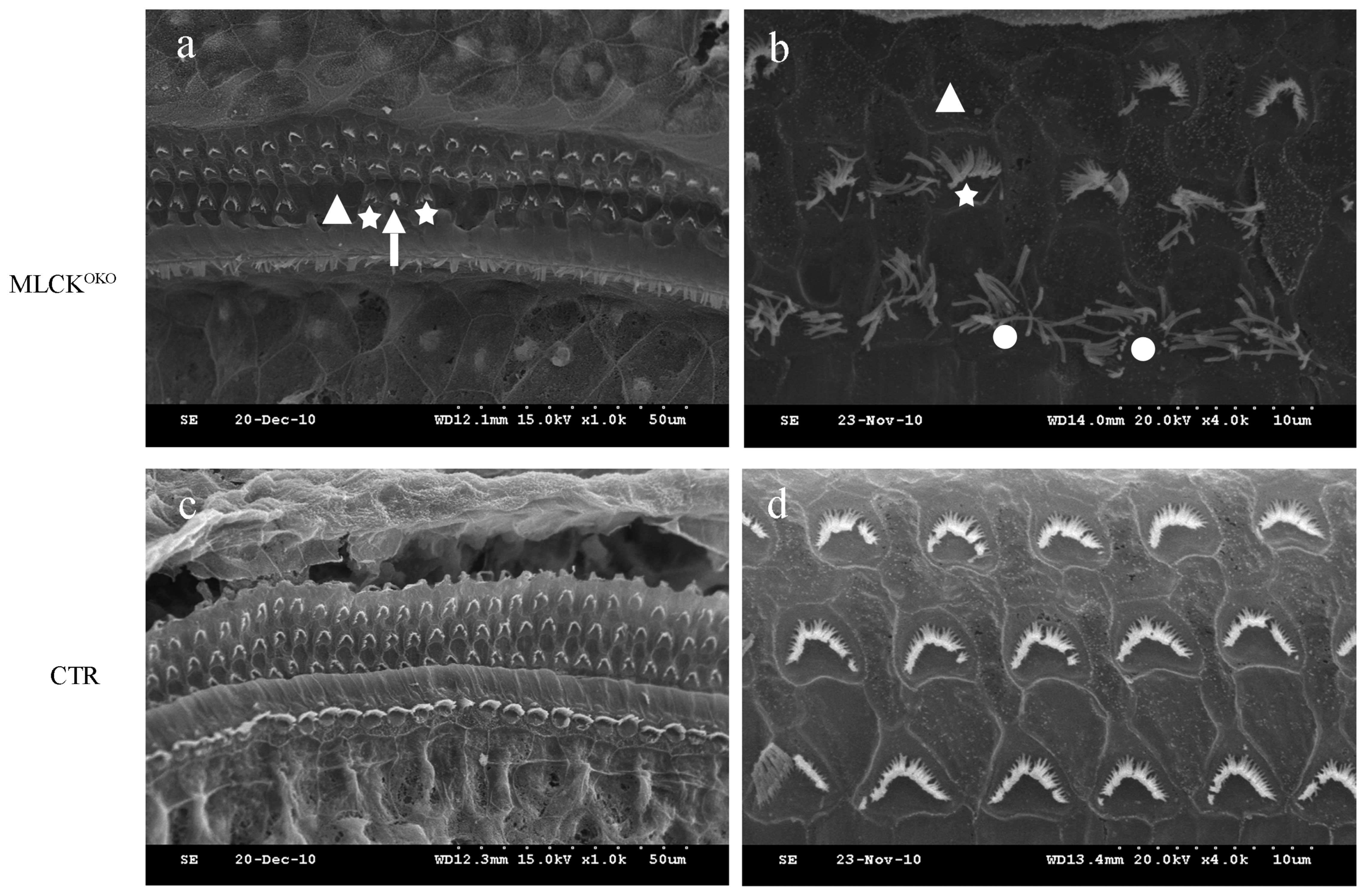
By contrast, SEM assays of the MLCKOKO
mice at 3, 5, 6, 7 and 9 months of age revealed the degeneration of
the OHC stereocilia. On the surface preparation of each cochlea,
the missing hair cell stereocilia were observed primarily in the
OHCs towards the apex turn of the cochlea, which indicated that
MLCK may have some influence on the aggregation of the stereocilia.
However, we found fewer OHCs missing stereocilia in the cochlear
basilar membrane of the CTR mice. The disarrangement of the OHC
stereociliary bundles was another morphological finding. There was
no observable loss of IHC stereocilia through the entire basilar
membrane of the cochlea (Fig.
3).
We separately counted the number of missing and
disordered OHC stereocilia in the apex turn, and we conducted a
χ2 test to analyze these data using SPSS 17.0 software
with P<0.05 considered to indicate a statistically significant
difference. The results are presented in Table II.
 | Table IIComparative analysis of the missing
and disordered outer hair cell (OHC) stereocilia in the apex turn
between the CTR and MLCK OHC-specific knockout (MLCKOKO)
mice. |
Table II
Comparative analysis of the missing
and disordered outer hair cell (OHC) stereocilia in the apex turn
between the CTR and MLCK OHC-specific knockout (MLCKOKO)
mice.
| Age (months) | Mice | Missing | Remaining | P-value | Abnormal | Normal | P-value |
|---|
| 3 |
MLCKOKO | 59 | 498 | <0.001 | 395 | 164 | <0.001 |
| CTR | 2 | 402 | | 48 | 228 | |
| 5 |
MLCKOKO | 30 | 308 | <0.001 | 269 | 68 | <0.001 |
| CTR | 6 | 301 | | 19 | 213 | |
| 6 |
MLCKOKO | 30 | 310 | <0.001 | 302 | 38 | <0.001 |
| CTR | 6 | 297 | | 147 | 113 | |
| 7 |
MLCKOKO | 103 | 456 | <0.001 | 421 | 139 | <0.001 |
| CTR | 18 | 751 | | 263 | 509 | |
| 9 |
MLCKOKO | 136 | 633 | <0.001 | 611 | 158 | <0.001 |
| CTR | 19 | 402 | | 307 | 321 | |
MLCK-deficient OHCs present reduced
F-actin and RLC phosphorylation
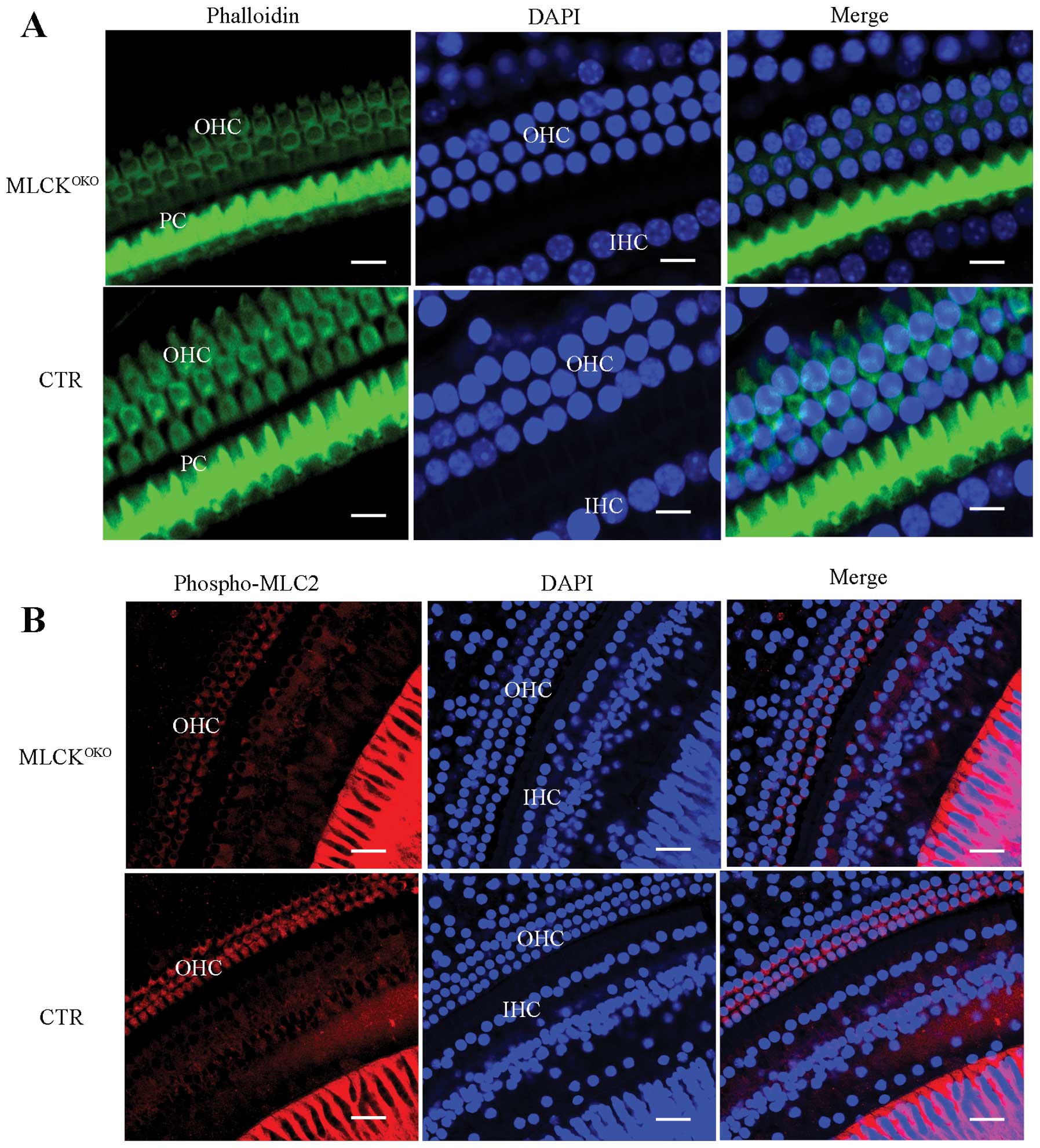
In order to determine whether the density of F-actin
was altered, we stained the inner ear cells with phalloidin. Most
of the phalloidin signal was distributed around the OHC cell
membrane and the button area. The CTR IHCs had strong and
continuous F-actin staining in addition to membrane structure.
However, F-actin staining in the MLCK-deficient OHCs was weak and
discontinuous (Fig. 4A).
MLCK is a dedicated kinase for myosin light chain
phosphorylation. RLC phosphorylation is also involved in various
cellular processes, in addition to its important role in smooth
muscle contraction. It has been demonstrated that RLC
phosphorylation enhances the formation of polymerized F-actin
(27). Thus, we measured RLC
phosphorylation in OHCs by staining the phosphorylated RLCs with a
specific antibody. In the MLCKOKO cochleae, many OHCs
showed obvious weak staining of the phosphorylated RLCs in contrast
to the cochleae of the CTRs (Fig.
4B).
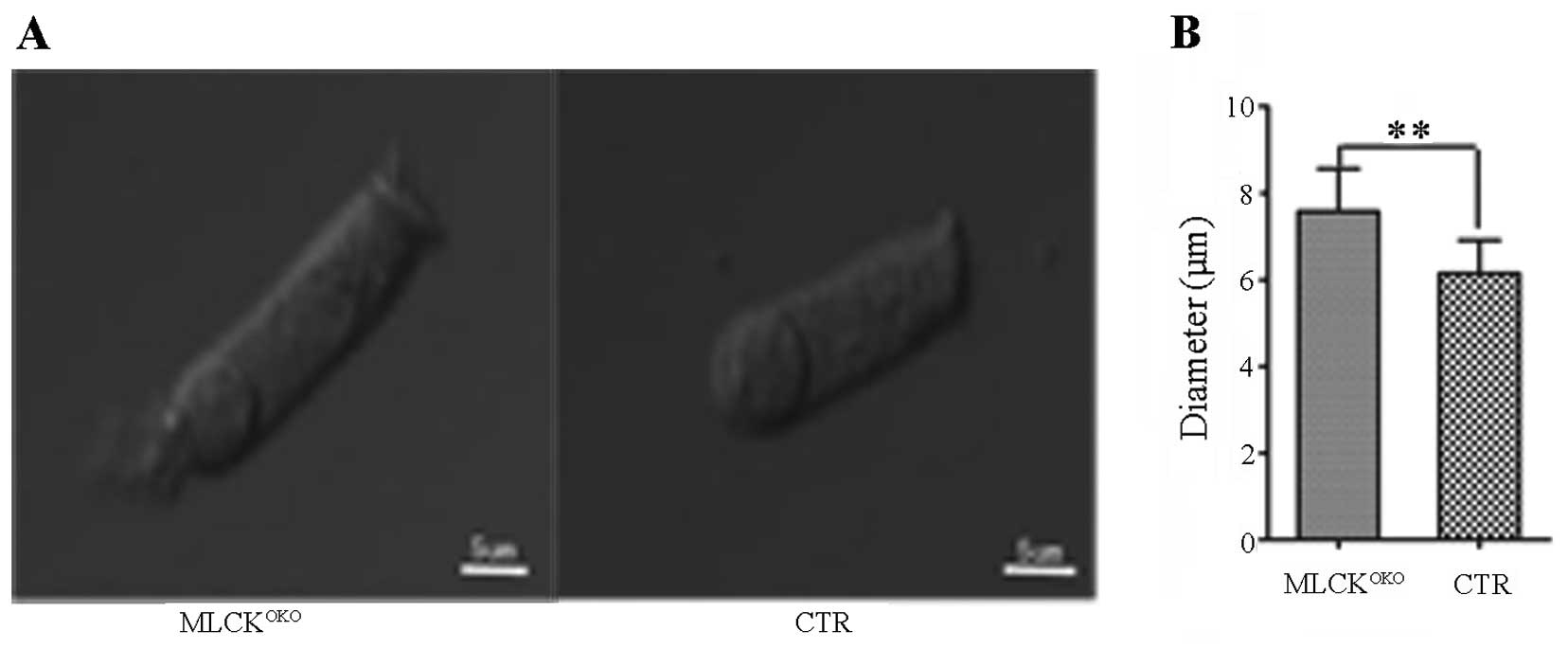
Diameter of MLCK-deficient OHCs is
increased
Changes in the volume of hair cells are important
for hearing sensitivity, and cell volume is primarily regulated by
membrane tethering and cytoskeleton organization (28,29). A comparison of the OHC diameters
between 3-month-old MLCKOKO and control mice revealed
that the diameters differed (Fig.
5). Compared with the CTR mice, the OHCs of the
MLCKOKO mice were longer and narrower (7.48±1.29 μm vs.
6.12±0.88 μm in diameter of CTR, P<0.01).
Discussion
Genetic and environmental factors are the two main
causes of hearing loss. Genetic factors constitute 60% or more of
the main reason of hearing loss (30). Clearly identifying causative genes
is the key to the study of hereditary hearing loss. The completion
of the Human Genome Project has provided us with an important
platform for hereditary hearing loss research. Notably, mice
provide a good model system for studying human hearing loss due to
the anatomical, functional, physiological and pathological
similarities between humans and mice. When a candidate gene related
to hearing loss in humans is proposed, we can verify the hypothesis
by engineering similar mutations in mice.
It has been documented that there are hereditary
deafness-related genes, including cytoskeletal proteins,
extracellular matrix proteins, channel and gap junction proteins,
transcription factors, mitochondrial genes and numerous other
structures and signaling molecules. However, the mechanisms of
action of these genes and their role in hearing remains
unclear.
The cytoskeleton is a fibrous protein filament that
maintains cell shape, cell movement, information transmission,
energy conversion and other functions. The auditory system is
complex, and the hair cells in the inner ear play a significant
role in hearing. The protein components of the cytoskeleton of hair
cells, such as actin, actin-binding protein, myosin, cadherin and
Rho GTPases, are closely related to the mechano-chemical and
-electrical transduction processes in hearing. Mutations of these
proteins may lead to abnormalities in the structure and function of
the hair cell bodies and stereocilia. Actin and myosin are the
basic components of the hair cell structure (31–35). There are a variety of regulatory
pathways involved in the interaction of actin and myosin in the
smooth muscle. The phosphorylation of the Ca2+-dependent
RLC is important in regulating MLCK enzyme activity (36–38). MLCK may play an important role in
active OHC regulation, as the cell body and stereocilia of the OHCs
have a large amount of actin and myosin. We specifically deleted
the Mylk gene in OHCs by crossing floxed Mylk mice
with transgenic mice that expressed Cre in their OHCs. This type of
mouse model is useful for clarifying the function of MLCK in
OHCs.
OHCs can regulate cochlear sensitivity to sound
stimulation and adjust the gain of the cochlear amplifier to
moderate sound intensities. This process involves the stereociliary
and somatic motility of OHCs. Thus, we specifically knocked out the
Mylk gene in OHCs to elucidate its role in the hearing
process.
We evaluated hearing impairment by testing ABRs and
DPOAEs in mice. Compared with the controls, 6- to 7-month-old
MLCK-deficient mice showed impaired hearing with a 5- to 10-dB SPL
increase in ABR thresholds in response to clicks and tones, and
3-month-old MLCK-deficient mice had significantly reduced DPOAE
amplitudes at low frequencies. The SEM results revealed that the
stereocilia of the OHCs were missing, and stereocilia bundles were
scattered on the apex turn. It has been documented that
low-frequency hearing corresponds to OHCs in the apex turn. These
results demonstrate that the deletion of MLCK has some influence on
the active regulation of OHCs.
Our results aslo demonstrated that F-actin and RLC
phosphorylation staining in MLCK-deficient OHCs was weak, and that
their diameter was increased. MLCK may strengthen the cell membrane
through non-kinase activity. MLCK can bundle F-actin and other
motor proteins through the non-catalytic N-terminal extension to
enhance the cytoskeletal structure and cell membrane. However,
there was no significant difference in histology. Perhaps the role
of MLCK in the regulation of OHC somatic motility is small, and is
more dependent on prestin.
Transducer channels located at the tips of the
stereocilia are mechanically gated by mechanoelectrical
transduction. The channels open when the cilia are bent toward the
tallest one, and close when they bend towards the opposite
direction (39). Stereocilia are
rich in actin and myosin, and stereociliary motility is associated
with Ca2+. The receptor potential of stereocilia is
formed by K+ influx and cell depolarization. It opens
L-type voltage-dependent calcium channels and allows
Ca2+ influx, causing a series of reactions (40). This model predicts that MLCK
deletion can alter the architecture of the stereocilia and thus
affect the transduction channels at the tips of the
stereocilia.
Acknowledgements
We thank Professor Minsheng Zhu, Dr Weiqi He, Dr
Yajing Peng, Dr Chen Chen, Dr Chenghai Zhang, Dr Yanning Qiao, Dr
Caiping Chen and Dr Tao Tao of the Model Animal Research Center of
Nanjing University in China. This study was supported by grants
from the National Natural Science Funding of China (nos. 30973302
and 81371090), and the Medical Youth Priming Project of Nanjing
(QYK1162).
References
|
1
|
Thomas PC: Of specialty interest:
publications of the National Institute on Deafness and Other
Communication Disorders. ORL Head Neck Nurs. 20:26–30.
2002.PubMed/NCBI
|
|
2
|
Battey JF Jr: News from the National
Institute on Deafness and Other Communication Disorders. Am J Otol.
19:263–265. 1998.PubMed/NCBI
|
|
3
|
Steel KP and Kros CJ: A genetic approach
to understanding auditory function. Nat Genet. 27:143–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnos KS: The implications of genetic
testing for deafness. Ear Hear. 24:324–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krendel M and Mooseker MS: Myosins: tails
(and heads) of functional diversity. Physiology. 20:239–251. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Etournay R, Zwaenepoel I, Perfettini I,
Legrain P, Petit C and El-Amraoui A: Shroom2, a myosin-VIIa- and
actin-binding protein, directly interacts with ZO-1 at tight
junctions. J Cell Sci. 120:2838–2850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman TB, Sellers JR and Avraham KB:
Unconventional myosins and the genetics of hearing loss. Am J Med
Genet. 89:147–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mermall V, Post PL and Mooseker MS:
Unconventional myosins in cell movement, membrane traffic, and
signal transduction. Science. 279:527–533. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Libby RT and Steel KP: The roles of
unconventional myosins in hearing and deafness. Essays Biochem.
35:159–174. 2000.PubMed/NCBI
|
|
10
|
Redowicz MJ: Myosins and deafness. J
Muscle Res Cell Motil. 20:241–248. 1999. View Article : Google Scholar
|
|
11
|
Ashmore JF: A fast motile response in
guinea-pig outer hair cells: the cellular basis of the cochlear
amplifier. J Physiol. 388:323–347. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dallos P and Fakler B: Prestin, a new type
of motor protein. Nat Rev Mol Cell Biol. 3:104–111. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dulon D and Schacht J: Motility of
cochlear outer hair cells. Am J Otol. 13:108–112. 1992.PubMed/NCBI
|
|
14
|
Santos-Sacchi J: New tunes from Corti’s
organ: the outer hair cell boogie rules. Curr Opin Neurobiol.
13:459–468. 2003.
|
|
15
|
Knipper M, Zimmermann U, Köpschall I,
Rohbock K, Jüngling S and Zenner HP: Immunological identification
of candidate proteins involved in regulating active shape changes
of outer hair cells. Hear Res. 86:100–110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto N, Okano T, Ma XF, Adelstein RS
and Kelley MW: Myosin II regulates extension, growth and patterning
in the mammalian cochlear duct. Development. 136:1977–1986. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoenwaelder SM and Burridge K:
Bidirectional signaling between the cytoskeleton and integrins.
Curr Opin Cell Biol. 11:274–286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bresnick AR: Molecular mechanisms of
nonmuscle myosin-II regulation. Curr Opin Cell Biol. 11:26–33.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szaszi K, Kurashima K, Kapus A, Paulsen A,
Kaibuchi K, Grinstein S and Orlowski J: RhoA and rho kinase
regulate the epithelial Na+/H+ exchanger
NHE3. Role of myosin light chain phosphorylation. J Biol Chem.
275:28599–28606. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aromolaran AS, Albert AP and Large WA:
Evidence for myosin light chain kinase mediating
noradrenaline-evoked cation current in rabbit portal vein myocytes.
J Physiol. 524:853–863. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu GJ, Wang F, Chen C, Xu L, Zhang WC,
Fan C, Peng YJ, Chen J, He WQ, Guo SY, Zuo J, Gao X and Zhu MS:
Myosin light-chain kinase is necessary for membrane homeostasis in
cochlear inner hair cells. Plos One. 7:e348942012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Birukov KG, Schavocky JP, Shirinsky VP,
Chibalina MV, Van Eldik LJ and Watterson DM: Organization of the
genetic locus for chicken myosin light chain kinase is complex:
multiple proteins are encoded and exhibit differential expression
and localization. J Cell Biochem. 70:402–413. 1998. View Article : Google Scholar
|
|
23
|
Smith AF, Bigsby RM, Word RA and Herring
BP: A 310-bp minimal promoter mediates smooth muscle cell-specific
expression of telokin. Am J Physiol. 274:C1187–C1195.
1998.PubMed/NCBI
|
|
24
|
Watterson DM, Schavocky JP, Guo L, Weiss
C, Chlenski A, Shirinsky VP, Van Eldik LJ and Haiech J: Analysis of
the kinase-related protein gene found at human chromosome 3q21 in a
multi-gene cluster: organization, expression, alternative splicing,
and polymorphic marker. J Cell Biochem. 75:481–491. 1999.
View Article : Google Scholar
|
|
25
|
Li MY, Tian Y, Fritzsch B, Gao JG, Wu XD
and Zuo J: Inner hair cell Cre-expressing transgenic mouse.
Genesis. 39:173–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song L, McGee JA and Walsh EJ:
Consequences of combined maternal, fetal and persistent postnatal
hypothyroidism on the development of auditory function in Tshrhyt
mutant mice. Brain Res. 1101:59–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goeckeler ZM: Myosin light chain
kinase-regulated endothelial cell contraction: the relationship
between isometric tension, actin polymerization, and myosin
phosphorylation. J Cell Biol. 130:613–627. 1995. View Article : Google Scholar
|
|
28
|
Li J and Verkman AS: Impaired hearing in
mice lacking aquaporin-4 water channels. J Biol Chem.
276:31233–31237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frolenkov GI, Belyantseva IA, Friedman TB
and Griffith AJ: Genetic insights into the morphogenesis of inner
ear hair cells. Nat Rev Genet. 5:489–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marazita ML, Ploughman LM, Rawlings B,
Remington E, Arnos KS and Nance WE: Genetic epidemiological studies
of early-onset deafness in the U.S. school-age population. Am J Med
Genet. 46:486–491. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lynch ED, Lee MK, Morrow JE, Welcsh PL,
Leon PE and King MC: Nonsyndromic deafness DFNA1 associated with
mutation of a human homolog of the Drosophila gene
diaphanous. Science. 278:1315–1318. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu XZ, Walsh J, Mburu P, Kendrick-Jones
J, Cope MJ, Steel KP and Brown SD: Mutations in the myosin VIIA
gene cause non-syndromic recessive deafness. Nat Genet. 16:188–190.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson SM, Householder DB, Coppola V,
Tessarollo L, Fritzsch B, Lee EC, Goss D, Carlson GA, Copeland NG
and Jenkins NA: Mutations in Cdh23 cause nonsyndromic hearing loss
in waltzer mice. Genomics. 74:228–233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lalwani AK, Goldstein JA, Kelley MJ,
Luxford W, Castelein CM and Mhatre AN: Human nonsyndromic
hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin
MYH9. Am J Hum Genet. 67:1121–1128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grimsley-Myers CM, Sipe CW, Geleoc GS and
Lu X: The small GTPase Rac1 regulates auditory hair cell
morphogenesis. J Neurosci. 29:15859–15869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Walker JW, Gilbert SH, Drummond RM, Yamada
M, Sreekumar R, Carraway RE, Ikebe M and Fay FS: Signaling pathways
underlying eosinophil cell motility revealed by using caged
peptides. Proc Natl Acad Sci USA. 95:1568–1573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding HL, Ryder JW, Stull JT and Kamm KE:
Signaling processes for initiating smooth muscle contraction upon
neural stimulation. J Biol Chem. 284:15541–15548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Breckenridge MT, Dulyaninova NG and
Egelhoff TT: Multiple regulatory steps control mammalian nonmuscle
myosin II assembly in live cells. Mol Biol Cell. 20:338–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheatham MA, Huynh KH, Gao J, Zuo J and
Dallos P: Cochlear function in Prestin knockout mice. J Physiol.
560:821–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mills DM and Schmiedt RA: Metabolic
presbycusis: differential changes in auditory brainstem and
otoacoustic emission responses with chronic furosemide application
in the gerbil. J Assoc Res Otolaryngol. 5:1–10. 2004. View Article : Google Scholar
|