Introduction
Atherosclerosis is considered to be an important
pathological manifestation of cardiovascular diseases and chronic
inflammatory disease (1).
Moreover, the disease is the major risk factor for cardiovascular
disease morbidity and mortality. The development of atherosclerosis
is associated with elements of the arterial wall, including T
cells, monocyte-derived macrophages, endothelial cells and vascular
smooth muscle cells (VSMCs) (2,3).
The accumulation of VSMCs contributes to occlusive cardiovascular
pathologies, including atherosclerosis (4). VSMCs reside within the media in a
quiescent state in the healthy blood vessel, whereas they migrate
into the intima after injury, and their growth can result in the
restriction of normal blood flow (5,6).
The proliferation of VSMCs plays an important role in the pathology
of atherosclerosis formation, coronary heart disease, hypertension
and restenosis following percutaneous transluminal coronary
angioplasty (PTCA) (7,8). As regards atherosclerosis, VSMC
proliferation is important for plaque formation. VSMC apoptosis
occurs in a number of arterial diseases, including angioplasty
restenosis, aneurysm formation and atherosclerosis (9). The apoptosis of VSMCs is involved in
atherosclerotic plaque rupture (10).
Osteopontin (OPN), a secreted protein, is expressed
by various cell types and has chemotactic, pro-adhesive and
cytokine-like properties. OPN is involved in a number of
physiological and pathological events, such as bone remodeling,
tumorigenesis, angiogenesis and apoptosis (11). Studies have demonstrated that OPN
is a multifunctional protein that is upregulated in a wide variety
of inflammatory conditions, such as autoimmune disease, fibrosis,
wound healing and atherosclerosis (12). The expression of OPN is
upregulated at sites with atherosclerotic plaques, particularly in
those associated with macrophages and foam cells (12). OPN, a critical mediator of
inflammation, plays an essential role in macrophage migration,
tissue infiltration (13) and
activation (14), as well as in
neutrophil migration and recruitment (15). Evidence suggests that OPN is also
expressed in smooth muscle-derived foam cells in human
atherosclerotic lesions of the aorta (16). Moreover, OPN can bind to several
types of integrin (such as αvβ3 integrin) to promote the attachment
of the cells to the extracellular matrix (ECM) and to regulate the
functions of these cells through certain signaling pathways
(17). It has been suggested that
the inhibition of the effects of αvβ3 integrin by disintegrin
agents, such as S247, can weaken cancer cell migration and invasion
(18). In the context of
atherosclerosis, OPN is generally regarded as a pro-atherogenic
molecule. An previous study indicated that OPN upregulates the
expression of matrix metalloproteinase (MMP)-9 through the αvβ3
integrin pathway in human chondrosarcoma cells (19).
A large number of studies have indicated that MMPs
play a role in regulating the behavior of VSMCs both in
vitro and in vivo (20). MMPs can regulate the migration,
proliferation and survival of VSMCs. MMP-9, a member of the MMP
family, can degrade basement membranes and promote neointima
formation in mouse models (21,22). Furthermore, there is evidence to
indicate that MMP-9 knockout (KO) mice show a reduced accumulation
of VSMCs in atherosclerotic plaques of brachiocephalic arteries
(23). These data highlight the
possibility that MMP-9 plays an essential role in the development
of atherosclerosis. To the best of our knowledge, in the present
study, we provide the first direct evidence that oxidized
low-density lipoprotein (oxLDL) induces the proliferation and
apoptosis of human VSMCs through the upregulation of OPN. We
demonstrate that the expression of OPN is associated with the
biological behavior of VSMCs by upregulating the levels of MMP-9
through the αvβ3 integrin pathway.
Materials and methods
Ethics statement
Ethical approval was obtained from the Ethics
Committee of Tangdu Hospital, the Fourth Military University,
Shaanxi, China. All patients participating in the study were of
Chinese origin and provided written informed consent for study
sample collection and permission for research use.
Antibodies
Mouse anti-OPN, mouse anti-MMP-9, mouse anti-αvβ3
integrin and mouse anti-β-actin monoclonal antibodies were
purchased from Abcam (Cambridge, MA, USA). HRP-conjugated goat
anti-mouse IgG was obtained from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA).
Low-density lipoprotein (LDL) preparation
and oxidation
Human LDL was purified from the fresh plasma of
healthy donors by sequential centrifugation, according to a
previously described method (24,25). The concentrations of LDL were
determined by the Lowry method (26). For the production of oxLDL, 200
μg/ml LDL were exposed to 20 μM CuSO4 in PBS for
oxidation and the oxidative reactions were terminated with 40 μM
butylhydroxytoluene in ethanol. Furthermore, oxLDL was dialyzed
against culture medium and sterile filtered.
VSMC culture
Human coronary artery smooth muscle cells (HCASMCs)
were obtained from PromoCell GmbH (Heidelberg, Germany). The cells
were cultured at 37°C under a humidified atmosphere of 5%
CO2 and 95% air and were grown in SMC growth medium
supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin
and 100 U/ml penicillin. HCASMCs between the third and sixth
passages were used throughout this study. Cells of these passages
were incubated for an additional 2 days to render them quiescent
prior to the initiation of each experiment, as previously described
(27). To establish the HCASMC
model of atherosclerosis, oxLDL was used as previously described
(7). The cells were divided into
4 groups: one group was treated with or without oxLDL (10–100
μg/ml); the second group was treated with or without small
interfering RNA (siRNA) against OPN; the third group was treated
with or without siRNA against MMP-9; and the fourth group was
treated with or without αvβ3 integrin antibody.
MTT proliferation assay
The proliferation of the HCASMCs was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay according to a previously described method with minor
modifications (7). Briefly,
approximately 1×104 cells were seeded into 96-well
plates and cultured for 24 h. SMC growth medium was used in the
normal control group, while 100 mg/l oxLDL was added to the cells
in the experimental groups. The cells were then cultured at 37°C
for 24 h. MTT (20 μl, 5 mg/ml) was then added to the HCASMCs for 4
h at 37°C and 200 μl DMSO were added to solubilize the crystals for
25 min at room temperature. The optical density (OD) value was
determined at 490 nm by a spectrophotometer (Multiskan MK3; Thermo
Fisher Scientific, Waltham, MA, USA). Each experimental group had
at least 5 wells and all experiments were performed at least 3
times and the average results were calculated.
Migration assay
HCASMC migration was detected by Transwell assay
using Transwell Costar inserts (pore size, 8 μm; diameter, 6.4 mm;
Corning Inc., Corning, NY, USA) according to a previously described
method with minor modifications (28). Briefly, the cells were treated
with siRNAs or αvβ3 integrin antibody prior to the migration
assays. The Transwells were coated with collagen (0.01%; Sigma, St.
Louis, MO, USA) overnight at 4°C. The HCASMCs were grown to 80%
confluence, and then detached with non-enzymatic cell dissociation
buffer. Cells were resuspended in serum-free SMC medium, followed
by transfer into the Transwells at a density of 1×104
cells/ml. The cells were placed in the upper chamber, while
chemoattractants were placed in the bottom of the Transwell, and
the cells were allowed to migrate for 4 h. Non-migrated cells were
removed from the upper chamber using a cotton bud, whereas the
migrated cells were stained with the Reastain Quick-Diff kit
(Reagena Ltd., Toivala, Finland) and then counted under a
brightfield microscope. All experiments were performed at least 3
times and the average results were calculated.
RNA extraction and RT-PCR
Total RNA was extracted from the cultured HCASMCs
using TRIzol reagent according to the manufacturer’s instructions
(Invitrogen, Carlsbad, CA, USA). Approximately 2 μg of total RNA
for each sample was reverse transcribed into first-strand cDNA for
RT-PCR analysis using a High-Capacity RNA-to-cDNA kit (Applied
Biosystems, Foster City, CA, USA) according to the instructions of
the manufacturer. The primer sequences used for RT-PCR were as
follows: OPN sense, 5′-CCAGCACAAGCAGACGTT-3′ and antisense,
5′-TCAGTCCATAAGCCAAGCTATAAC-3′; MMP-9 sense,
5′-CACTGTCCACCCCTCAGAGC-3′ and antisense,
5′-GCCACTTGTCGGCGATAAGC-3′; β-actin sense, 5′-CAT
CCGTAAAGACCTCTATGCCAAC-3′ and antisense, 5′-ATGGAGCCACCGATCCACA-3′.
The primers were all synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). The cycling conditions were as follows:
polymerase activation for 15 sec at 95°C; 40 cycles of
amplification at 95°C for 15 sec and 60°C for 60 sec. A cDNA
fragment of β-actin was amplified as the control. Relative gene
expression was calculated using the 2−ΔΔct method, as
previously described (29).
Western blot analysis
The total protein of HCASMCs was extracted using
RIPA buffer (Beyotime, Nantong, China) according to the
manufacturer’s instructions. Protein concentration was measured
using a BCA protein assay reagent kit (Pierce Chemical Co.,
Rockford, IL, USA). The proteins (40 μg/lane) were subjected to 15%
SDS-PAGE followed by western blot analysis. Mouse monoclonal
antibodies were used as the primary antibody and HRP-conjugated
goat anti-mouse IgG was used as the secondary antibody. Finally,
the blots were visualized using enhanced chemiluminescence
reagents. All experiments were repeated at least 3 times.
siRNA
siRNAs targeting human OPN and MMP-9 were designed
with a siRNA selection program available online at http://jura.wi.mit.edu/siRNAext/ and were
chemically synthesized by Genetimes Technology (Shanghai, China).
The nucleotide sequence for OPN was 5′-AAGCAGCUUUACAACAAAUACCC-3′
and 5′-AACATCACCTATTGGATCCAAACTAC-3′ for MMP-9. A non-specific
siRNA duplex containing the same nucleotides, but in an irregular
sequence (scrambled) served as the control. The cells were
transfected with siRNA (siOPN or siMMP-9) or control siRNA (siMock)
using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. The cells were analyzed 2 days after
transfection.
Statistical analysis
All data are expressed as the means ± standard error
of the mean (SEM). The differences between groups were compared by
Dunnett’s test subsequent to ANOVA. Differences were considered
statistically significant with a value of P<0.05. All
experiments were repeated at least 3 times.
Results
oxLDL accelerates the proliferation and
migration of HCASMCs
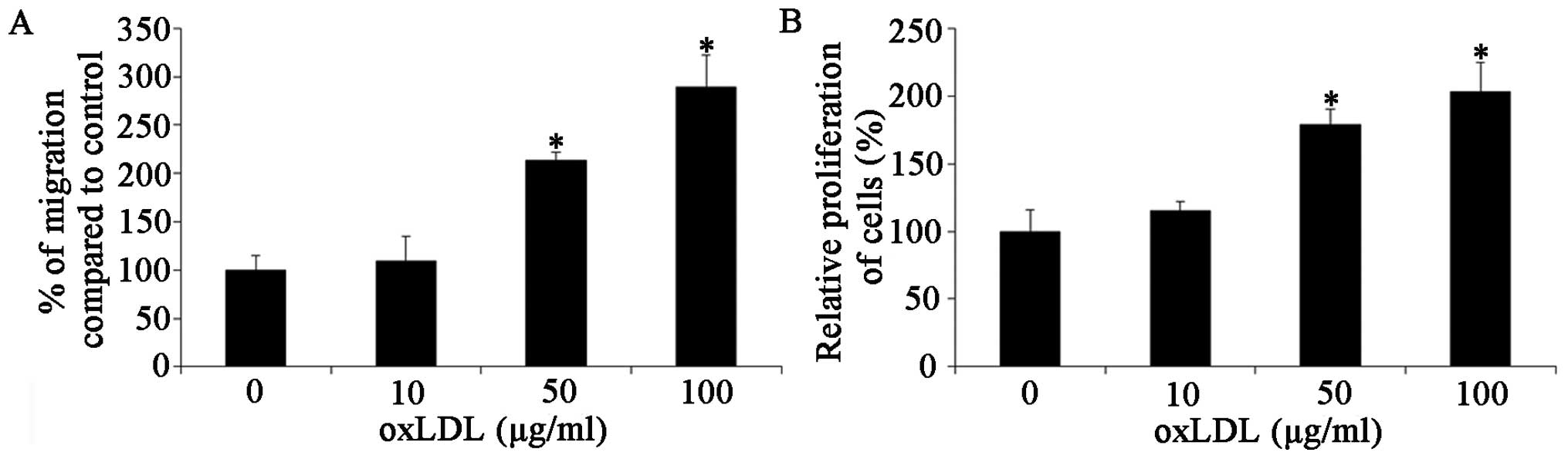
oxLDL-stimulated HCASMC responses were investigated.
The effects of oxLDL on cell proliferation were determined by MTT
assay. The results, shown in Fig.
1A, indicated that oxLDL at concentrations of 10, 50 and 100
μg/ml markedly accelerated HCASMC proliferation (P<0.05). oxLDL
promoted the proliferation of the HCASMCs in a dose-dependent
manner. We then determined the effects of oxLDL on cell migration
by cell migration assay, as described in Materials and methods.
Treatment of the HCASMCs with 10, 50 and 100 μg/ml oxLDL
significantly increased the migration ability of the cells to
109±26.0% at 10 μg/ml, 213±8.9% at 50 μg/ml (P<0.05) and
289±33.1% at 100 μg/ml (P<0.05) (Fig. 1B). These results demonstrate that
oxLDL accelerates the proliferation and migration of HCASMCs;
however, the exact mechanisms involved remain unelucidated. Thus,
further studies are required to determine the mechanisms of action
of oxLDL.
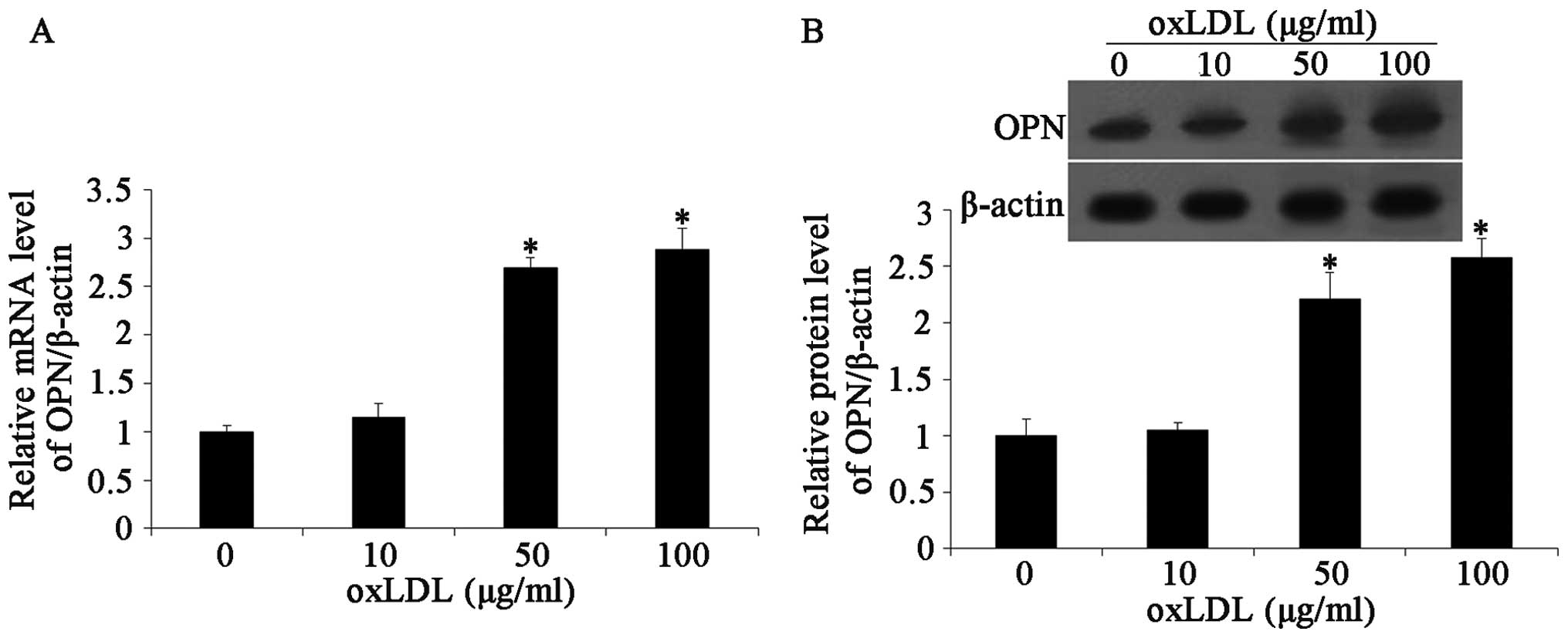
Effects of oxLDL on OPN expression
There is evidence to suggest that OPN plays an
important role in the development of atherosclerotic lesions.
Moreover, oxLDL increases the expression levels of OPN (11). Thus, in this study, we first
detected the expression of OPN in HCASMCs following treatment with
oxLDL. We used western blot analysis and RT-PCR to determine the
expression of OPN in the HCASMCs. OPN mRNA expression was
determined by RT-PCR following 24 h of incubation with oxLDL. The
results revealed that oxLDL (10 to 100 μg/ml) significantly
increased OPN mRNA expression in the HCASMCs treated with oxLDL
compared with the untreated cells (Fig. 2A). Following exposure to various
doses of oxLDL, OPN expression in the HCASMCs was measured by
western blot analysis. The results demonstrated that oxLDL
increased the expression level of OPN following treatment with
oxLDL at doses of 10 to 100 μg/ml compared with the controls
(untreated cells; Fig. 2B).
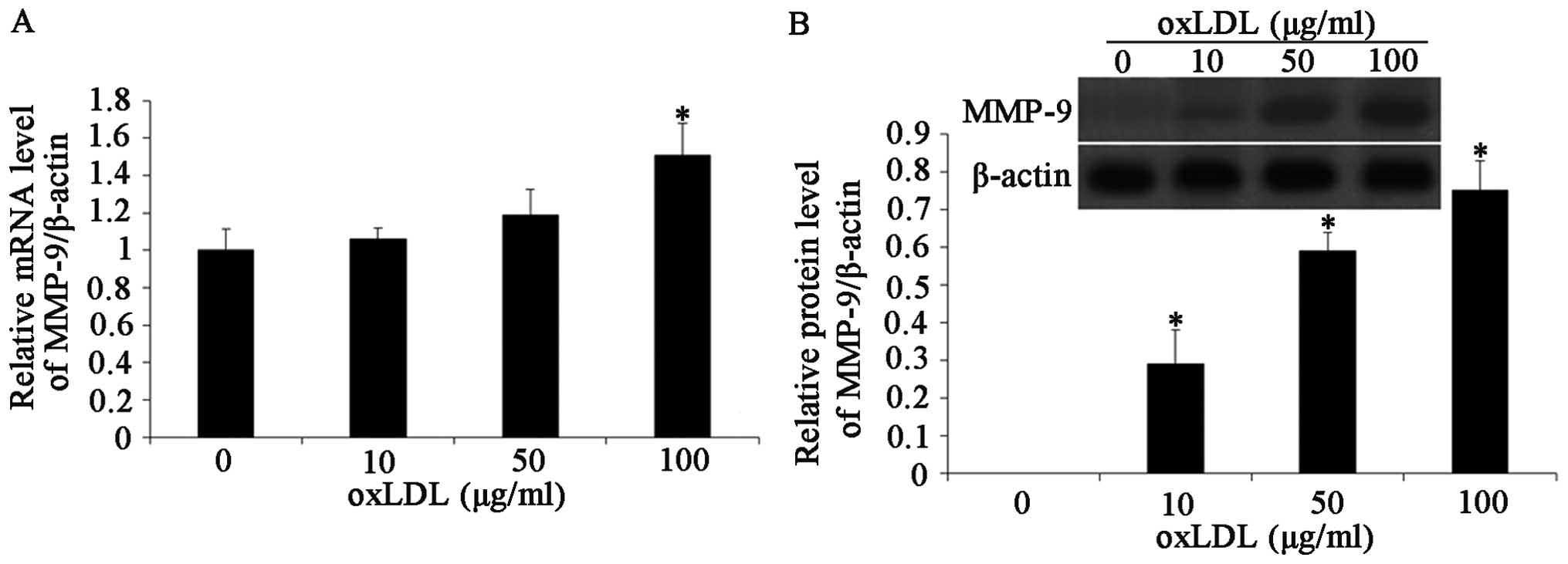
Effects of oxLDL on MMP-9 expression
A large number of studies have demonstrated that
MMPs play an important role in regulating the migration and
proliferation of VSMCs both in vitro and in vivo
(20). There is evidence to
suggest that the expression level of MMP-9 is increased during
atherosclerosis in rabbits (30).
The effects of oxLDL on MMP-9 expression were investigated in the
HCASMCs by western blot analysis and RT-PCR. The HCASMCs were
treated with oxLDL at concentrations of 10, 50 and 100 μg/ml. MMP-9
mRNA expression was significantly increased following exposure to
oxLDL in a dose-dependent manner (Fig. 3A). MMP-9 protein expression was
then determined by western blot analysis. As shown in Fig. 3B, the MMP-9 protein level was
increased in response to oxLDL at a concentration of 10 to 100
μg/ml (P<0.05).
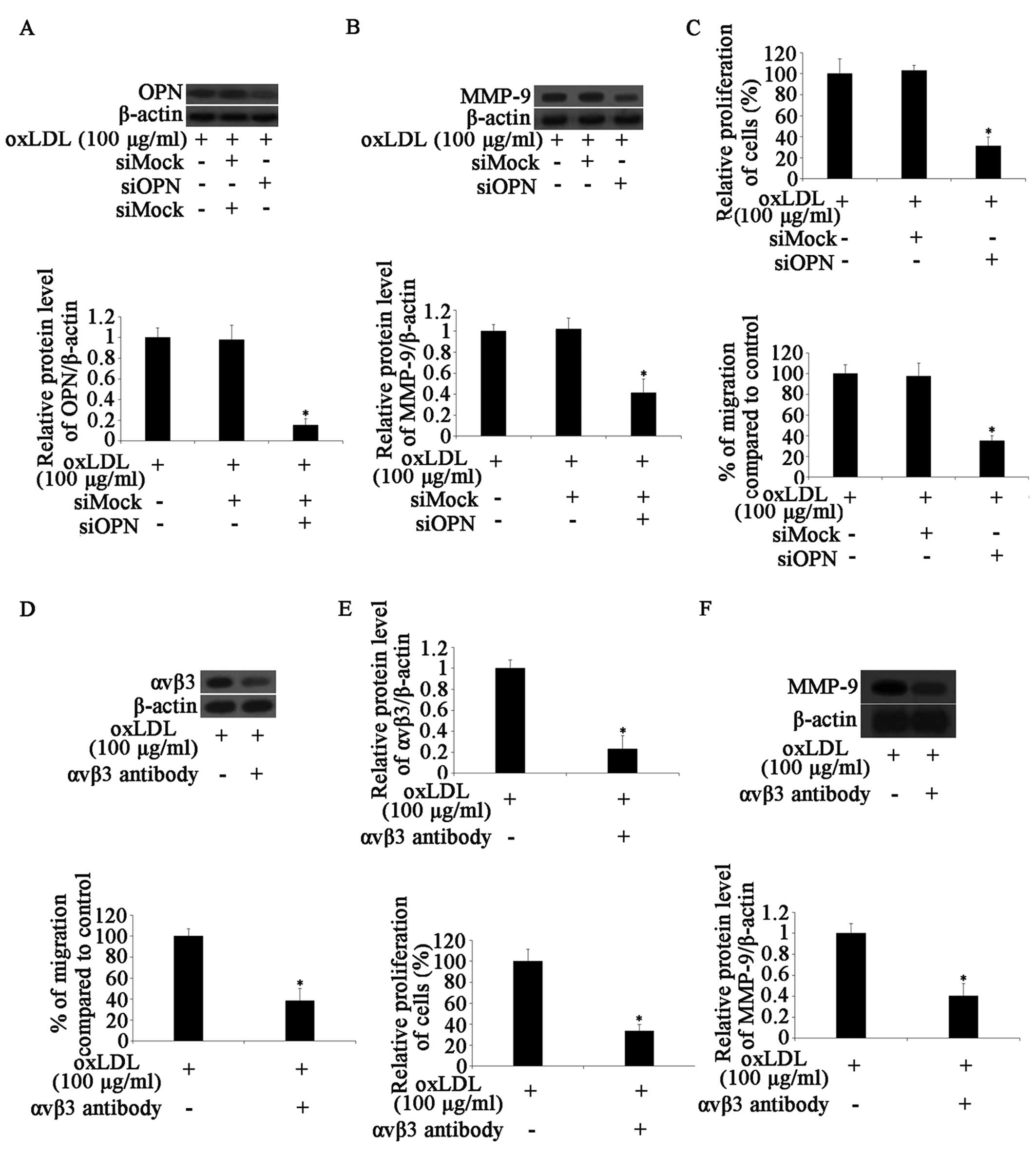
OxLDL-upregulated MMP-9 expression is
dependent on OPN expression through the αvβ3 integrin pathway
There is evidence to suggest that the treatment of
chondrosarcoma or melanoma cells with OPN induces the expression of
MMP-9 (19,31). In this study, we therefore
hypothesized that OPN may be involved in the oxLDL-induced
upregulation of MMP-9. Hence, a siRNA experiment was performed on
the HCASMCs. The OPN mRNA and protein expression levels in the
siOPN-transfected group were markedly lower than those in the
siMock-transfected group. The mRNA and protein expression of MMP-9
was not altered in the siOPN-transfected cells following treatment
with oxLDL (Fig. 4A and B). These
results indicated that the oxLDL-induced increased expression of
MMP-9 was partially dependent on OPN protein expression.
We then determined the proliferation and migration
ability of the HCASMCs. The results revealed that cell
proliferation and migration were markedly reduced following
treatment with OPN siRNA. The silencing of OPN partially reduced
the increased proliferation and migration resulting from treatment
with oxLDL (Fig. 4C). A previous
study suggested that OPN affects cell migration through αvβ3
integrin signaling (32). Thus,
we hypothesized that the αvβ3 integrin signaling pathway may be
involved in the biological actions of HCASMCs. Pre-treatment of the
cells for 30 min with 20 μg/ml anti-αvβ3 integrin monoclonal
antibody markedly reduced the expression of αvβ3 integrin (Fig. 4D). We then determined the effects
of anti-αvβ3 integrin monoclonal antibody on the proliferation and
migration ability of the HCASMCs. The results revealed that the
antibody inhibited oxLDL-induced cell proliferation and migration
(Fig. 4E). At the same time, the
expression of MMP-9 was detected. The results revealed that
pre-treatment of the cells with anti-αvβ3 antibody decreased
oxLDL-induced MMP-9 expression (Fig.
4F). These results indicated that oxLDL increased the
proliferation and migration of the HCASMCs through the upregulation
of OPN and MMP-9, and that MMP-9 expression is partly dependent on
OPN expression through the αvβ3 integrin pathway.
Effects of siMMP-9 on HCASMCs
To determine the effects of MMP-9 on the behavior of
HCASMCs, a siRNA experiment was performed. MMP-9 mRNA and protein
expression levels in the siMMP-9-transfected group were markedly
lower than those in the siMock-transfected group (Fig. 5A). Cell proliferation and
migration were markedly reduced after the cells were treated with
MMP-9 siRNA. The silencing of MMP-9 markedly reduced the effects of
oxLDL on HCASMC proliferation and migration (Fig. 5B). These results indicate that
MMP-9 plays an important role in the oxLDL-induced proliferation
and migration of HCASMCs.
Discussion
Elucidating the mechanisms that result in the
initiation and development of atherosclerosis is crucial for
identifying strategies to inhibit disease progression before it
leads to clinical consequences (33). A large number of studies have
indicated that many cell types, such as endothelial cells,
lymphocytes, macrophages and SMCs are associated with the formation
of atherosclerotic lesions (34).
There is evidence to suggest that SMC migration and proliferation
play a major role in the formation of fibroproliferative plaques
(35). A predominant feature of
atherogenesis is SMC migration into the intima of the arterial wall
where they synthesize the ECM, together with the accumulation of
cholesterol and proliferation by macrophages, leading to the
progression of atherosclerotic plaque (36). SMC proliferation is a critical
mechanism for the formation of plaque and fibrous caps; these cells
are also involved in endothelial cell injury, necrotic core
formation and plaque rupture (34,37).
A number of factors affect SMC proliferation and
migration, including hypertension, increased plasma cholesterol
levels and oxidative stress. oxLDL, present in atherosclerotic
lesions, is a well-established risk factor for atherosclerosis that
induces changes in gene expression (growth factors, cytokines and
adhesion molecules), lipoprotein metabolism, cell migration,
proliferation and apoptosis (38). An important finding of this study
was that OPN contributes to the oxLDL-induced migration and
proliferation of HCASMCs. In this study, we determined whether the
expression of OPN is upregulated when the HCASMCs were exposed to
oxLDL. The results demonstrated that oxLDL stimulated the
expression of OPN; there was a positive correlation between the OPN
expression level and HCASMC proliferation and migration.
There is evidence indicating that oxLDL upregulates
the expression of OPN (11).
Moreover, high levels of OPN are associated with the upregulation
of MMP-9 levels (19). We
therefore, hypothesized that OPN may be involved in the
oxLDL-induced upregulation of MMP-9. To further confirm the role of
OPN and to clarify the mechanisms underlying the oxLDL-induced
increase in cell proliferation and migration, MMP-9 expression was
detected in the HCASMCs. The results revealed that treatment with
oxLDL induced a significant increase in MMP-9 expression, which was
dependent on OPN expression levels. Previous studies have suggested
that OPN upregulates the expression of MMP-9 through the αvβ3
integrin pathway (19). Another
finding of this study was that oxLDL activates αvβ3 integrin in
HCASMCs. We found that the level of αvβ3 integrin was markedly
reduced following the treatment of the cells with antibody against
αvβ3. Moreover, MMP-9 expression, as well as the cell proliferation
and migration, were also reduced. To further confirm the role of
MMP-9 in HCASMC proliferation and migration, a siRNA experiment for
MMP-9 was performed. The results demonstrated that cell
proliferation and migration were markedly reduced following the
treatment of the cells with MMP-9 siRNA. These results indicate
that oxLDL increases the proliferation and migration of HCASMCs
through the upregulation of OPN and MMP-9, and that MMP-9
expression is partly dependent on OPN expression through the αvβ3
integrin pathway.
In conclusion, our data demonstrate that oxLDL
promotes the proliferation and migration of HCASMCs through the
upregulation of the expression of OPN and MMP-9. Furthermore, our
results suggest that the effects of oxLDL on the expression of
MMP-9 are dependent on the expression of OPN through the αvβ3
integrin pathway. The data presented in our study provide new
insight into the mechanisms through which oxLDL affects HCASMC
proliferation and migration.
Abbreviations:
|
SMCs
|
smooth muscle cells
|
|
HCASMCs
|
human coronary artery smooth muscle
cells
|
|
ox-LDL
|
oxidized low-density lipoprotein
|
|
OPN
|
osteopontin
|
|
ECM
|
extracellular matrix
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
OD
|
optical density
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Wang XH, Wang F, You SJ, et al:
Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide
pathway contributes to ox-LDL-induced inflammation in macrophage.
Cell Signal. 25:2255–2262. 2013.
|
|
2
|
Glass CK and Witztum JL: Atherosclerosis:
the road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González-Navarro H, Abu Nabah YN, Vinué Á,
et al: p19(ARF) deficiency reduces macrophage and vascular smooth
muscle cell apoptosis and aggravates atherosclerosis. J AM Coll
Cardiol. 55:2258–2268. 2010.PubMed/NCBI
|
|
4
|
Gerthoffer WT: Mechanisms of vascular
smooth muscle cell migration. Circ Res. 100:607–621. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murry CE, Gipaya CT, Bartosek T, Benditt
EP and Schwartz SM: Monoclonality of smooth muscle cells in human
atherosclerosis. Am J Pathol. 151:697–705. 1997.PubMed/NCBI
|
|
6
|
Johnson JL, Dwivedi A, Somerville M,
George SJ and Newby AC: Matrix metalloproteinase (MMP)-3 activates
MMP-9 mediated vascular smooth muscle cell migration and neointima
formation in mice. Arterioscl Thromb Vas. 31:e35–e44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li LX, Zhang XF, Bai X and Tong Q: SDF-1
promotes ox-LDL induced vascular smooth muscle cell proliferation.
Cell Biol Int. 37:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ross R: The pathogenesis of
atherosclerosis - an update. New Engl J Med. 314:488–500. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clarke MC, Figg N, Maguire JJ, et al:
Apoptosis of vascular smooth muscle cells induces features of
plaque vulnerability in atherosclerosis. Nat Med. 12:1075–1080.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennett MR and Boyle JJ: Apoptosis of
vascular smooth muscle cells in atherosclerosis. Atherosclerosis.
138:3–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazière C, Gomila C and Mazière JC:
Oxidized low-density lipoprotein increases osteopontin expression
by generation of oxidative stress. Free Radical Bio Med.
48:1382–1387. 2010.PubMed/NCBI
|
|
12
|
Cho HJ, Cho HJ and Kim HS: Osteopontin: a
multifunctional protein at the crossroads of inflammation,
atherosclerosis, and vascular calcification. Curr Atheroscler Rep.
11:206–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giachelli CM, Lombardi D, Johnson RJ,
Murry CE and Almeida M: Evidence for a role of osteopontin in
macrophage infiltration in response to pathological stimuli in
vivo. Am J Pathol. 152:353–358. 1998.PubMed/NCBI
|
|
14
|
Weber GF, Zawaideh S, Hikita S, Kumar VA,
Cantor H and Ashkar S: Phosphorylation-dependent interaction of
osteopontin with its receptors regulates macrophage migration and
activation. J Leukocyte Biol. 72:752–761. 2002.PubMed/NCBI
|
|
15
|
Koh A, Da Silva AP, Bansal AK, et al: Role
of osteopontin in neutrophil function. Immunology. 122:466–475.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeda T, Shirasawa T, Esaki Y, Yoshiki S
and Hirokawa K: Osteopontin mRNA is expressed by smooth
muscle-derived foam cells in human atherosclerotic lesions of the
aorta. J Clin Invest. 92:2814–2820. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zohar R, Suzuki N, Suzuki K, et al:
Intracellular osteopontin is an integral component of the CD44-ERM
complex involved in cell migration. J Cell Physiol. 184:118–130.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shannon KE, Keene JL, Settle SL, et al:
Anti-metastatic properties of RGD-peptidomimetic agents S137 and
S247. Clin Exp Metastasis. 21:129–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YJ, Wei YY, Chen HT, et al:
Osteopontin increases migration and MMP-9 up-regulation via αvβ3
integrin, FAK, ERK, and NF-κB-dependent pathway in human
chondrosarcoma cells. J Cell Physiol. 221:98–108. 2009.PubMed/NCBI
|
|
20
|
Newby AC: Matrix metalloproteinases
regulate migration, proliferation, and death of vascular smooth
muscle cells by degrading matrix and non-matrix substrates.
Cardiovasc Res. 69:614–624. 2006. View Article : Google Scholar
|
|
21
|
Johnson C and Galis ZS: Matrix
metalloproteinase-2 and -9 differentially regulate smooth muscle
cell migration and cell-mediated collagen organization. Arterioscl
Thromb Vasc Biol. 24:54–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuzuya M, Kanda S, Sasaki T, et al:
Deficiency of gelatinase a suppresses smooth muscle cell invasion
and development of experimental intimal hyperplasia. Circulation.
108:1375–1381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson JL, George SJ, Newby AC and
Jackson CL: Divergent effects of matrix metalloproteinases 3, 7, 9,
and 12 on atherosclerotic plaque stability in mouse brachiocephalic
arteries. Proc Natl Acad Sci USA. 102:15575–15580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belcher J, Egan JO, Bridgman G, Baker R
and Flack JM: A micro-enzymatic method to measure cholesterol and
triglyceride in lipoprotein subfractions separated by density
gradient ultracentrifugation from 200 microliters of plasma or
serum. J Lipid Res. 32:359–370. 1991.
|
|
25
|
Feng X, Zhang Y, Xu R, et al:
Lipopolysaccharide up-regulates the expression of Fcalpha/mu
receptor and promotes the binding of oxidized low-density
lipoprotein and its IgM antibody complex to activated human
macrophages. Atherosclerosis. 208:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
27
|
Shyu KG, Chen SC, Wang BW, Cheng WP and
Hung HF: Mechanism of the inhibitory effect of atorvastatin on
leptin expression induced by angiotensin II in cultured human
coronary artery smooth muscle cells. Clin Sci (Lond). 122:33–42.
2012. View Article : Google Scholar
|
|
28
|
Pellet-Many C, Frankel P, Evans IM, Herzog
B, Jünemann-Ramirez M and Zachary IC: Neuropilin-1 mediates PDGF
stimulation of vascular smooth muscle cell migration and signalling
via p130Cas. Biochem J. 435:609–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
30
|
Aikawa M, Rabkin E, Okada Y, et al: Lipid
lowering by diet reduces matrix metalloproteinase activity and
increases collagen content of rabbit atheroma: a potential
mechanism of lesion stabilization. Circulation. 97:2433–2444. 1998.
View Article : Google Scholar
|
|
31
|
Rangaswami H and Kundu GC: Osteopontin
stimulates melanoma growth and lung metastasis through
NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep.
18:909–915. 2007.PubMed/NCBI
|
|
32
|
Wai PY and Kuo PC: Osteopontin: regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doran AC, Meller N and McNamara CA: Role
of smooth muscle cells in the initiation and early progression of
atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Augé N, Maupas-Schwalm F, Elbaz M, et al:
Role for matrix metalloproteinase-2 in oxidized low-density
lipoprotein-induced activation of the sphingomyelin/ceramide
pathway and smooth muscle cell proliferation. Circulation.
110:571–578. 2004.
|
|
36
|
Ricciarelli R, Zingg JM and Azzi A:
Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36
scavenger receptor expression in cultured aortic smooth muscle
cells. Circulation. 102:82–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Auge N, Garcia V, Maupas-Schwalm F, Levade
T, Salvayre R and Negre-Salvayre A: Oxidized LDL-induced smooth
muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt
and the sphingolipid signaling pathways. Arterioscl Thromb Vasc
Biol. 22:1990–1995. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Witztum JL and Steinberg D: The oxidative
modification hypothesis of atherosclerosis: does it hold for
humans? Trends Cardiovas Med. 11:93–102. 2001. View Article : Google Scholar : PubMed/NCBI
|