Introduction
Melanoma is an extremely aggressive cancer with a
high metastatic potential and is responsible for the majority of
skin cancer-related mortality (1). It is now the 5th and 6th leading
cause of cancer in men and women, respectively (2). For several years, melanoma has been
considered to be a highly radioresistant tumor due to its efficient
DNA repair mechanisms (3).
However, a number of studies have suggested that melanoma cells are
heterogeneous and hence should not be generally considered
radioresistant (4–7). Finding agents that sensitize
malignant cells to radiation would augment tumor response, while
minimizing toxicity to surrounding normal tissues. A number of
extranuclear and intranuclear factors have been identified to
influence the radiation responsiveness of melanoma cells, including
breast cancer 1, early onset (BRCA1), excision repair
cross-complementing rodent repair deficiency, complementation group
1 (ERCC1), poly(ADP-ribose) polymerase (PARP), transglutaminase 2
(TGM2) and SLUG, as well as chromosomal characteristics, such as
telomere length (8,9).
Telomeres, the specialized structures located at the
chromosome terminus, play important roles in the cellular response
to DNA damage and chromosomal stability. They consist of telomeric
DNA and a suite of telomere-binding proteins (TBPs), both of which
are responsible for telomere function. Six TBPs forming shelterin
have been reported as the telomeric core complex, telomeric
repeat-binding factor 1 and 2 (TRF1 and 2), protection of telomeres
1 (POT1), repressor/activator protein 1 (RAP1), TRF1-interacting
nuclear protein 2 (TIN2), and tripeptidyl peptidase I (TPP1;
formerly known as TINT1, PTOP, or PIP1). They play essential roles
in the regulation of telomere length by inhibiting excessive
nuclease activity at the chromosome ends and by regulating
telomerase, the enzyme that elongates telomeres (10–13). Other TBPs include Ku70/80, the MRN
complex (MRE11/Rad50/Nbs1), DNA-PKcs and tankyrase 1/2.
The newly identified mammalian conserved telomere
maintenance component 1 (CTC1)-STN1-TEN1 (CST) complex binding to
the telomeres has also been demonstrated to promote telomere
integrity (14). Mammalian STN1
and TEN1 are the sequence homologues of budding and fission yeast
proteins (14–16). The third member of the complex,
CTC1, is not a sequence homologue of Cdc13, although it shares
functional similarities. In addition, CTC1 binds to the telomeric
single-stranded DNA (ssDNA) in a sequence-independent manner
together with STN1 and TEN1 (14). A genome-wide meta-analysis has
pointed to CTC1 as a gene regulating telomere homeostasis in humans
(17). The depletion of human
CTC1 by RNAi in vitro triggers a DNA damage response,
chromatin bridges, the accumulation of G-overhangs, the inhibition
of telomerase and sporadic telomere loss (18,19). However, the role of human CTC1 in
the response of melanoma cells to ionizing radiation remains
unknown. In this study, we established a
radiosensitive/radioresistant human melanoma cell model,
MDA-MB-435/MDA-MB-435R, in order to investigate the mechanisms
responsible for radioresistance. Our data demonstrate that CTC1
expression is markedly decreased in the radiosensitive melanoma
cells compared with the radioresistant cells. Moreover, the
knockdown of CTC1 imparts radiosensitivity to human melanoma cells
by enhancing telomere shortening and inducing cell apoptosis.
Materials and methods
Cell culture and transfection
The human melanoma cell line, MDA-MB-435, was
purchased from the Cell Bank of the Chinese Academy of Sciences,
Shanghai, China. The relative radioresistant cell line,
MDA-MB-435R, was established in our laboratory by the repeated
irradiation of MDA-MB-435 cells (unpublished data). Irradiation, 6
MV X-ray, was produced by a linear accelerator (Siemens, Munich,
Germany) at a dose rate of 2 Gy/min. The D0 value of the
MDA-MB-435R cells (3.266±0.072) markeldy increased significantly
compared to that of the MDA-MB-435 cells (2.093±0.131).
The cells were maintained in Roswell Park Memorial
Institute (RPMI)-1640 medium (Life Technologies, Grand Island, NY,
USA) supplemented with 10% heat-inactivated fetal bovine serum, 100
U/ml penicillin and streptomycin (Life Technologies) at 37°C in a
humidified atmosphere of 5% CO2.
siRNA was designed against human CTC1 (GenBank
accession no. NM_025099.5) with the following sequences:
5′-CCAGAUCUCACAAUGUUUATT-3′ synthesized by GenePharma (Shanghai,
China). The sequence, 5′-GTTCTCC GAACGTGTCACGT-3′, was used as the
non-silencing control (negative control) in all the experiments.
Transfection was performed using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
Cells transfected with siCTC1#1–3, non-silencing siRNA and
transfection reagents alone were termed the siCTC1#1–3, siNC and
mock group, respectively. Cells without any treatment were used as
the untreated group. At 48 h post-transfection, the cells were
harvested for the following assays.
RNA extraction and quantitative reverse
transcription PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen) following the manufacturer’s instructions.
First-strand cDNA was obtained using the RevertAid™ First-Strand
cDNA Synthesis kit (Fermentas International, Inc., Burlington, ON,
Canada). For the quantitative analysis of CTC1 mRNA, the human
GAPDH gene was used as an internal control. Primer sequences were
designed as follows: CTC1 sense, 5′-TGGACC TTTCTTGGTTGGG-3′ and
antisense, 5′-AGGACAGGGAT AGGCGTGA-3′; GAPDH sense,
5′-ATCACTGCCACCCAG AAGAC-3′ and antisense, 5′-AGCGTCAAAGGTGGAGG
AGT-3′. The resulting cDNA was detected using SYBR-Green PCR Master
mix (Takara, Shiga, Japan) with Mx3000P (Stratagene, La Jolla, CA,
USA). For the measurements of relative telomere length, the single
copy gene, 36B4 (encodes acidic ribosomal phosphoprotein), was used
as an endogenous control. The primers used for the telomeres were
as follows: sense, 5′-GTTTTTGAGGGTGAGGGTGAGGGTGAGGG TGAGGGT -3′ and
antisense, 5′-TCCCGACTATCCCTAT CCCTATCCCTATCCCTATCCCTA-3′. The
primers used for 36B4 were sense, 5′-CAGCAAGTGGGAA GGTGTAATCC-3′
and amtisense, 5′-CCCATTCTATCATC AACGGGTACAA-3′. The Mx3000P
analysis program was used to analyze the results.
Western blot analysis
Cells in a 100-mm dish were rinsed twice with cold
phosphate-buffered saline (PBS), and lysed with 200 μl radio
immunoprecipitation assay (RIPA) lysis buffer and 1 mM PMSF
(Beyotime Institute of Biotechnology, Shanghai, China). A scraper
was used to remove the cells into an Eppendorf tube. After the
cells were ultrasonicated and centrifuged at 12,000 rpm for 10 min,
the supernatant was collected and incubated in boiled water for 3–5
min. Protein concentration was determined using a BCA protein assay
kit (Beyotime Institute of Biotechnology). Protein extracts (50 μg)
were electrophoresed on 8% SDS-PAGE gels and then transferred on to
PVDF membranes. The blots were blocked for 1 h at room temperature
in 5% non-fat milk in Tris-buffered saline with 0.1% Tween-20, and
incubated at 4°C overnight with the following antibodies: anti-CTC1
and anti-GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). After washing and incubating with secondary antibodies, the
bands were visualized using the ECL Plus kit (Beyotime Institute of
Biotechnology) and recorded onto X-Omat AR film (Eastman Kodak Co.,
Rochester, NY, USA). The density of each band was quantified using
ImageJ software.
Clonogenic survival assay
Teh cells were plated onto 6-well plates with
different cell numbers (100–2,000) for each dose group overnight
and subjected to 6 MV X-ray at the dose of 0–10 Gy as indicated.
After 14 days of incubation, the colonies were fixed and stained
with 1% crystal violet in absolute alcohol. The surviving colonies
(≥50 cells/colony) were scored while being viewed under an inverted
phase contrast microscope (Olympus, Tokyo, Japan). A single-hit
multi-target model (20) was used
to analyze the data. The survival curve of each group was plotted
as the log of the survival fraction vs. the radiation dose using
GraphPad Prism 5.0 software.
Immunofluorescence assay by confocal
laser scanning microscopy
The immunofluorescence detection of γH2AX foci was
used to determine the residual DNA double-strand breaks (DSBs).
Cells grown on round coverslips (Fisher Scientific, Hampton, NH,
USA) were divided into 4 groups: siCTC1#3, ionizing radiation (IR),
siCTC1#3 + IR and the untreated group. The IR and siCTC1#3 + IR
cell groups were exposed to 6 Gy X-ray at 36 h post-transfection.
Twelve hours later, the cells were fixed in ice-cold 4%
formaldehyde for 20 min, treated with 0.2% Triton X-100 for 15 min,
blocked in 5% (w/v) BSA for 1 h and incubated with antibody to
γH2AX (ser139, dilution 1:400; Millipore Corp., Billerica, MA, USA)
overnight at 4°C. After washing with PBS, the secondary
FITC-conjugated antibody (Millipore Corp.) was added for 45 min at
37°C, and the slides were washed with PBS and stained with DAPI.
Subsequently, images were recorded using a confocal microscope
(Leica Microsystems GmbH, Wetzlar, Germany). The percentage of
γH2AX foci-positive cells and the number of γH2AX foci per cell
were determined by analyzing 100 randomly selected cells.
Flow cytometric analysis of
apoptosis
For apoptosis assay, the cells were divided into 6
groups: siCTC1#3, negative control siRNA (siNC), IR, siCTC1#3 + IR,
siNC + IR and the untreated group. Cells in the IR, siCTC1#3 + IR
and siNC + IR groups were exposed to 6 Gy X-ray at 24 h
post-transfection. Another 24 h later, both Anexin V-fluorescein
isothiocyanate and propidium iodide (PI) were used to stain the
cells according to manufacturer’s instructions (Beyotime Institute
of Biotechnology). The occurrence of apoptosis was quantified by
flow cytometry (Cytomics FC 500; Beckman Coulter, Fullerton, CA,
USA).
Statistical analysis
All the experiments were repeated at least 3 times.
Data are presented as the means ± standard deviation (SD). The
quantification of band density was performed using ImageJ software.
Statistical analysis was performed with one way ANOVA using SPSS
18.0 and GraphPad Prism 5.0 software. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
siRNA against CTC1 effectively reduces
CTC1 mRNA and protein expression
qRT-PCR and western blot analysis were used to
analyze the altered relative mRNA and protein expression of CTC1.
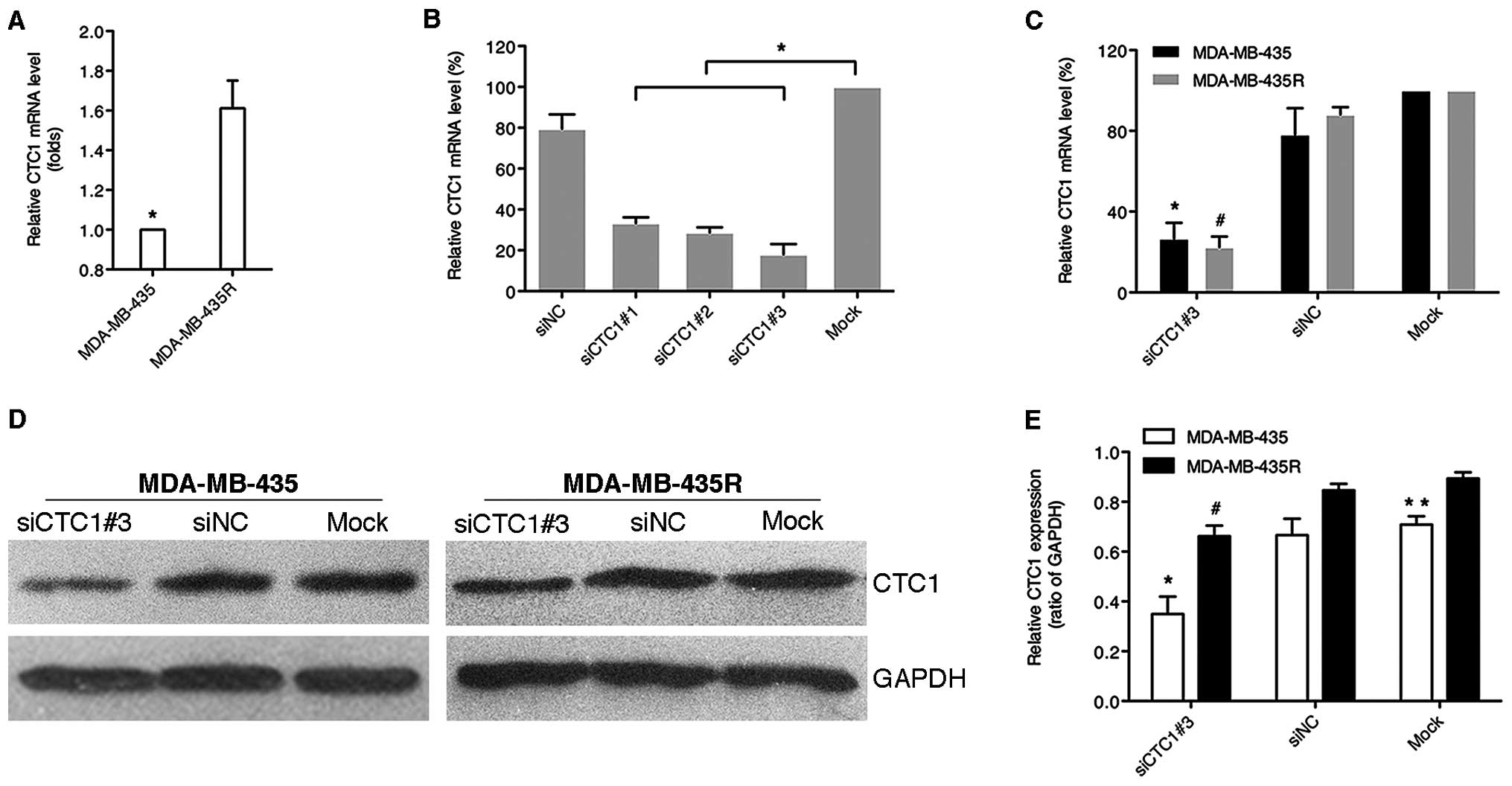
The MDA-MB-435R cells showed higher CTC1 mRNA and protein
expression levels compared with the MDA-MB-435 cells (Fig. 1A). Three siRNAs against CTC1
effectively reduced the relative mRNA levels of CTC1 in the
MDA-MB-435 cells (P<0.05), among which siCTC1#3 was the most
effective (Fig. 1B). In contrast
to the siNC and mock group, the relative CTC1 mRNA and protein
levels of both cell lines were markedly decreased in siCTC1#3 group
(P<0.05), while the control groups showed no apparent changes
(Fig. 1C–E). These results
indicated that siCTC1#3 effectively suppressed the CTC1 mRNA and
protein levels in the MDA-MB-435 and MDA-MB-435R cells.
Downregulation of CTC1 sensitizes cells
to radiation
In order to investigate the role of CTC1 in
modulating the radiosensitivity of the MDA-MB-435 and MDA-MB-435R
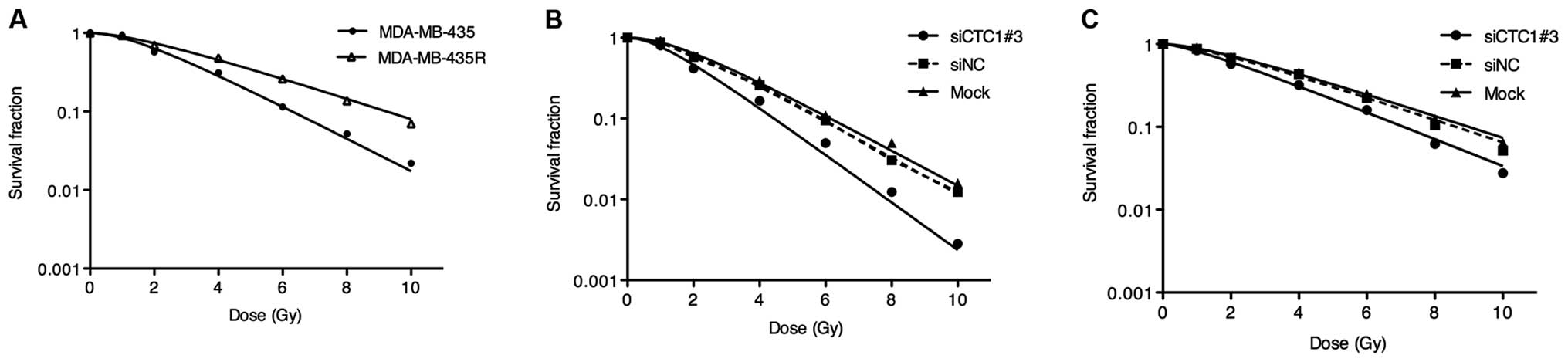
cells, CTC1 was downregulated by siRNA. The survival curves
described the radiobiological parameters of each group. As shown in
Fig. 2A, the survival fractions
of the MDA-MB-435R cells increased significantly at the 2, 4, 6, 8
and 10 Gy dose point compared with those of the MDA-MB-435 cells
(P<0.05). Compared to the siNC and mock group, the survival
fractions of the siCTC1#3 group markedly decreased at the 2, 4, 6,
8 and 10 Gy dose point (Fig. 2B and
C). The radiobiological parameters calculated according to the
curves are presented in Table I.
The D0, Dq and SF2 values in the cells transfected with
siCTC1#3 were significantly lower than the siNC and mock groups in
both cell lines (P<0.05), while the siNC groups showed no
significant differences with the mock groups. We concluded that the
downregulation of CTC1 enhances the radiosensitivity of MDA-MB-435
and MDA-MB-435R cells.
 | Table IRadiobiological parameters in the
different groups |
Table I
Radiobiological parameters in the
different groups
| Group | D0 | Dq | SF2 |
|---|
| MDA-MB-435 |
| Untreated | 2.093±0.131a | 2.088±0.046 | 0.574±0.018a |
| siCTC1#3 | 1.475±0.153b | 1.472±0.095b | 0.417±0.016b |
| siNC | 1.929±0.099 | 2.135±0.106 | 0.578±0.029 |
| Mock | 1.993±0.164 | 2.258±0.094 | 0.595±0.017 |
| MDA-MB-435R |
| Untreated | 3.266±0.072 | 2.379±0.108 | 0.701±0.016 |
| siCTC1#3 | 2.356±0.109c | 1.331±0.074c | 0.565±0.031c |
| siNC | 3.153±0.156 | 2.247±0.089 | 0.677±0.014 |
| Mock | 3.231±0.155 | 2.324±0.101 | 0.696±0.022 |
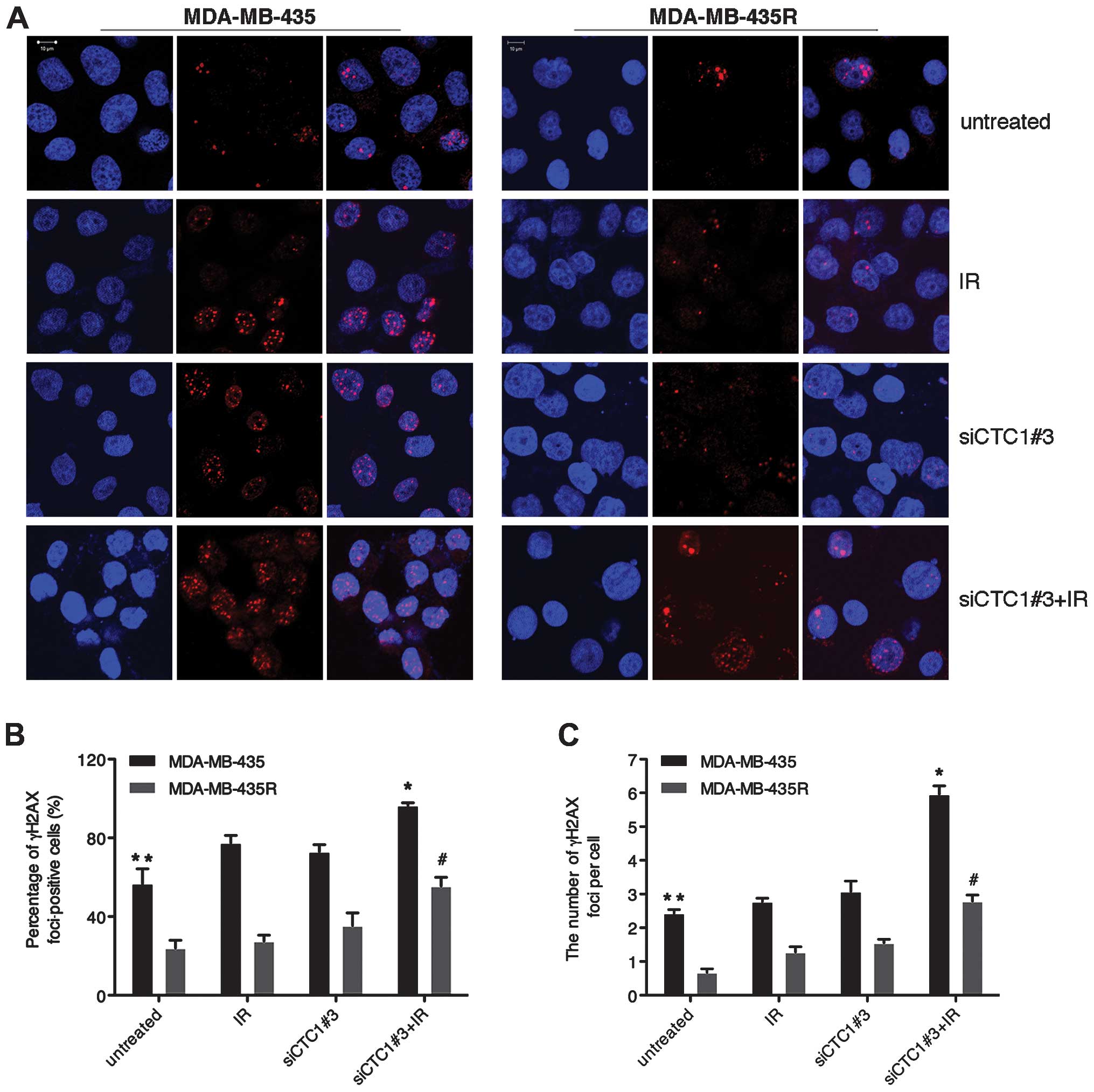
CTC1 increases the γH2AX-mediated repair
of DNA DSBs
As a histone H2A variant, H2AX plays an essential
role in the cellular response to DNA DSBs. H2AX senses DSBs through
rapid serine 139 phosphorylation, and forms phospho-γH2AX foci with
various proteins. In the cells with different sensitivity to
IR-induced DSBs, γH2AX selectively recruites specific proteins to
decide different cell fates (21). As shown in Fig. 3, γH2AX foci in the MDA-MB-435R
cells significantly decreased compared with the MDA-MB-435 cells
(P<0.05). Treatment with siCTC1#3 led to increased IR-induced
γH2AX foci in both cell lines (P<0.05). Our data indicate that
CTC1 is actively involved in repairing DSBs to reduce γH2AX foci in
MDA-MB-435 and MDA-MB-435R cells.
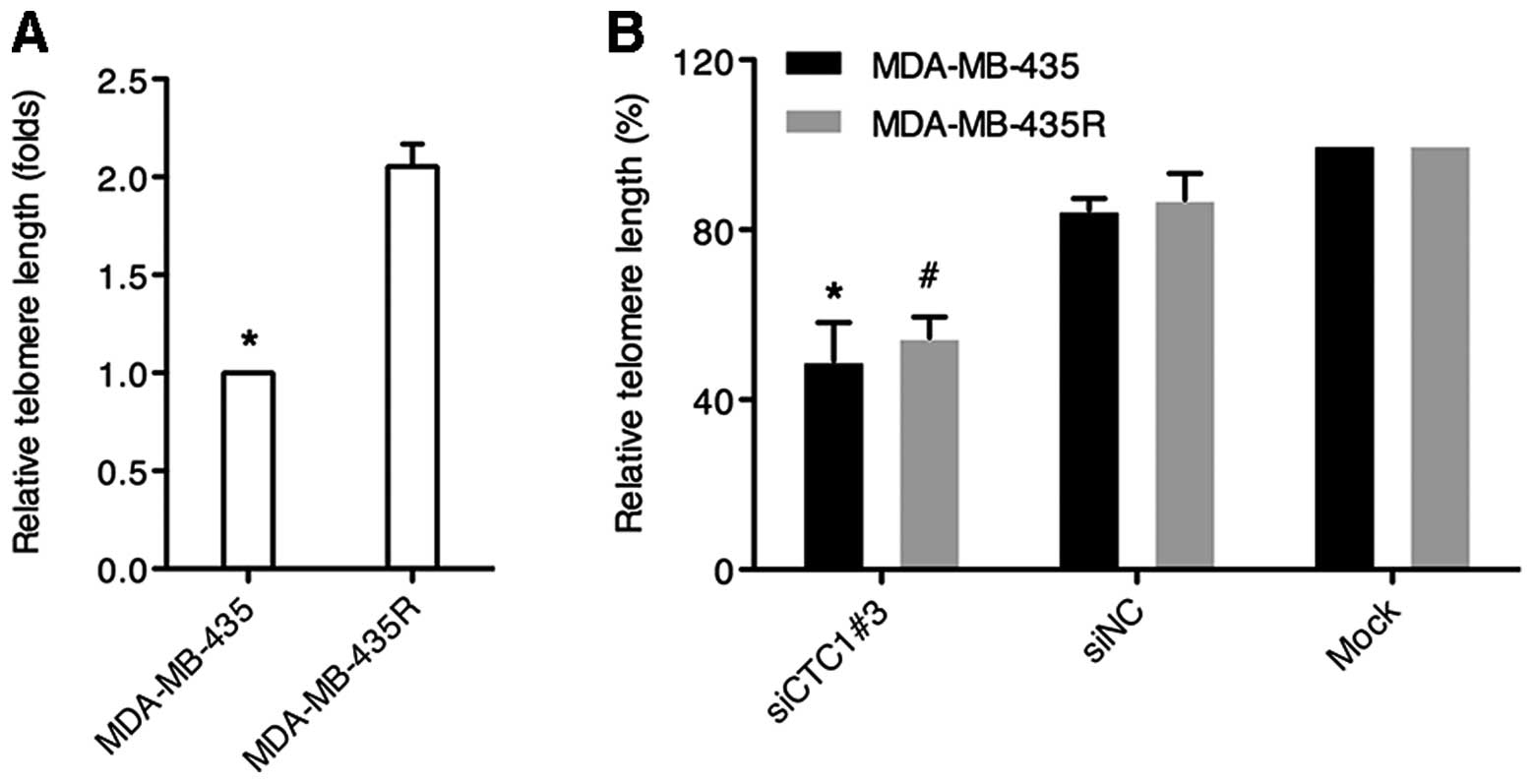
Depletion of CTC1 causes telomere length
attrition
Relative telomere lengths were determined by
quantitative PCR. The dissociation curves showed a unique peak from
the PCR amplification of the telomeres and the single copy gene,
36B4. Our data demonstrated that the relative telomere length in
the MDA-MB-435R cells was almost twice that in the MDA-MB-435 cells
(P<0.05) (Fig. 4A). As shown
in Fig. 4B, the cells (both cell
lines) transfected with siCTC1#3 exhibited obvious telomere
shortening compared with the cells transfected with siNC or the
mock-transfected cells (P<0.05). These results suggest that CTC1
is involved in telomere maintenance in MDA-MB-435 and MDA-MB-435R
cells.
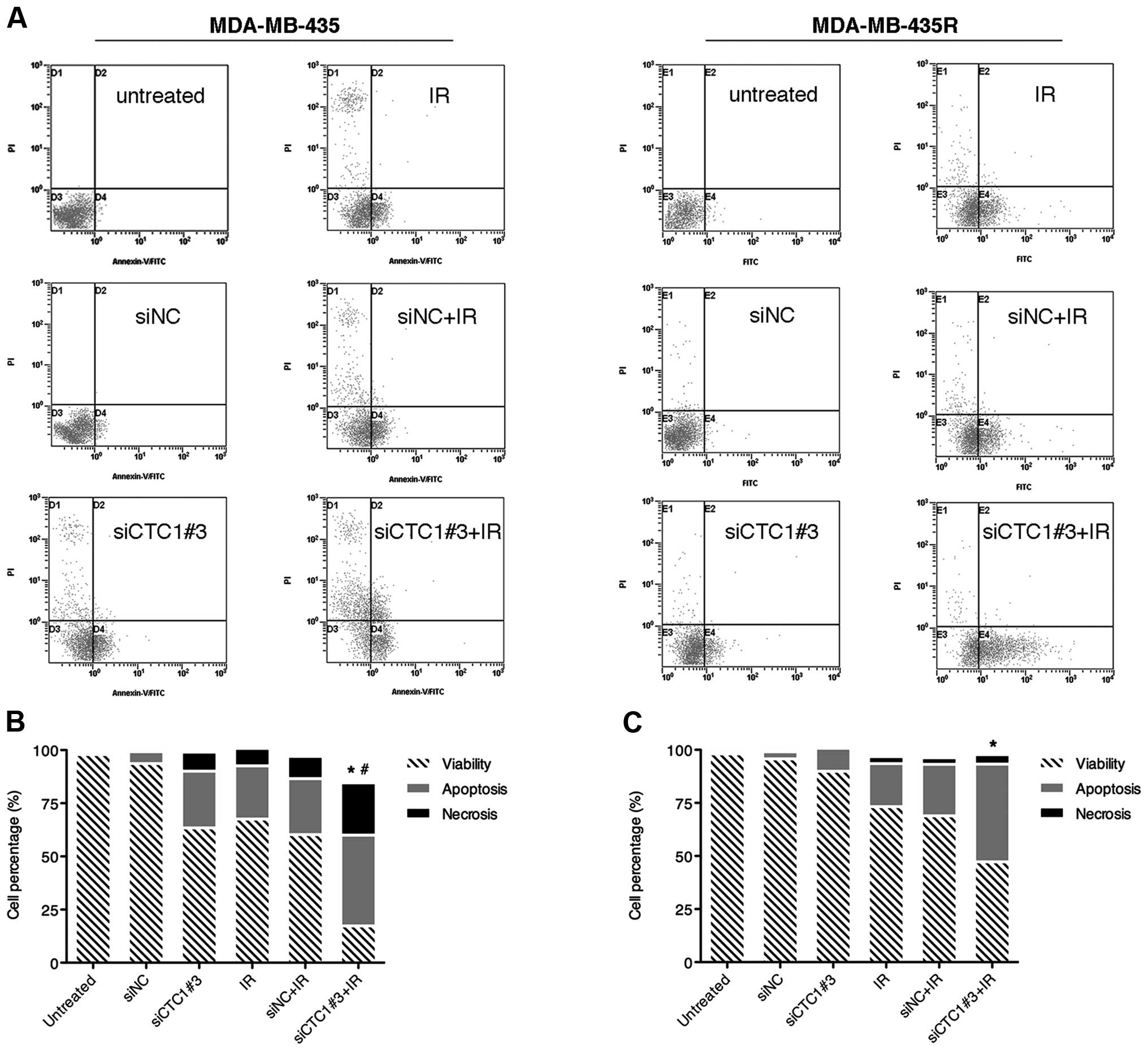
Combination of siCTC1 and radiation
increases cell apoptosis
It is known that irradiation can cause DNA damage.
If DNA damage is not adquately repaired after irradiation, cells
may progress towards apoptosis and/or necrosis. Since siRNA against
CTC1 sensitized the MDA-MB-435 and MDA-MB-435R cells to
irradiation, we further investigated the effects of siCTC1 on cell
death post-irradiation. Twenty-four hours after irradiation, the
cells were harvested and stained with Annexin V-fluorescein
isothiocyanate and PI and classified into 4 subpopulations as
follows: viable cells (Annexin V and PI double-negative), apoptotic
cells (Annexin V-positive), early dead cells (Annexin V and PI
double-positive) and dead cells (PI-positive). The results
(Fig. 5) revealed that while the
single treatment with siCTC1#3 or IR increased the apoptotic rates
in both cell lines, the combined treatment produced an even greater
number of apoptotic cells (P<0.05). Moreover, the combined
treatment of MDA-MB-435 cells significantly increased the rate of
necrosis compared to the groups with the single treatment
(P<0.05). siCTC1, in conjunction with IR, enhances the killing
effect on MDA-MB-435 and MDA-MB-435R cells.
Discussion
In this study, to the best of our knowledge, we
demonstrate for the first time that CTC1 expression is associated
with radioresistance in human melanoma cells.
Although melanoma is a relatively radioresistant
tumor type, our greater radiobiological understandings may provide
better control for certain clinical situations. In order to improve
the therapeutic ratio, there has been increasing interest in
studying the difference between radioresistant cells and their
parental counterparts. The cell lines used in this study were
derived from the same origin, which may have similar tumor
characteristics. Thus, this may be a good model to investigate
determinant factors for radiosensitivity. Our results demonstrated
that the MDA-MB-435 cells were more radiosensitive than the
MDA-MB-435R cells and that the knockdown of CTC1 increased the
radiosensitivity of both cell lines (Fig. 2). Thus, we infer that the
knockdown of CTC1 may act as a potent radiosensitizer in human
melanoma cell lines.
Telomere maintenance has been implicated in ageing
and cancer, and requires the cooperation of a multitude of
telomeric proteins. Some of these proteins are associated
exclusively with telomeres, whereas others localize to additional
subnuclear or subcellular sites (22). The altered expression of telomeric
proteins may disrupt the capping complex, facilitating telomere
degradation and shortening, regardless of the telomerase status
(23,24). The prevailing view has been that
two distinct telomeric capping complexes evolved, shelterin in
vertebrates and a trimeric complex termed CST (Cdc13, STN1 and
TEN1) in yeast. However, the recently discovered CST-like complex
in plants and humans raises new questions as to the composition of
telomeres and their regulatory mechanisms in multicellular
eukaryotes (14,15,18). Genetic data argue that CST and
shelterin act in distinct pathways to maintain telomere integrity
in human cells. The significant increase in the frequency of
telomere dysfunction-induced foci (TIF) in STN1/POT1-knockdown
cells compared to the STN1 or POT1 single knockdown cells, suggests
that CST and POT1 play redundant roles in telomere protection
(14). It is now widely accepted
that telomere length acts as a hallmark of radiosensitivity
(25–30). Consequently, we investigated
whether the radiosensitization of CTC1 downregulation was the
result of telomere length attrition. As expected, our data revealed
that telomere length in the MDA-MB-435R cells was almost twice that
in the MDA-MB-435 cells. The knocking down of CTC1 significantly
decreased telomere length in both cell lines (Fig. 4).
Recent studies have revealed that CTC1 null mice
undergo a rapid onset of global cellular proliferative defects and
die prematurely from complete bone marrow failure by activating an
ATR-dependent G2/M checkpoint (31). Mutations in CTC1 underlie the rare
human genetic disorder, Coats plus, characterized by
gastrointestinal and neurological defects, as well as shortened
telomeres and evidence of an ongoing DNA damage response (32). These clinical results suggest that
similar to the phenotypes observed in CTC1 null mice, human CTC1
missense mutations also confer haematopoietic defects. In addition,
previous studies have addressed the role of apoptosis in
determining radiation response. The disabling of apoptotic
responses may be a major contributor to radioresistance (33). Thus, we hypothesized that the
radioresistance of MDA-MB-435R cells imparted by CTC1 may be
related to the induction of apoptosis. Our results demonstrated
that the knockdown of CTC1 increased the rate of apoptosis in both
melanoma cell lines (Fig. 5).
Overall, we know that the second mechanism responsible for CTC1
modulating radiosensitivity is through the regulation of
apoptosis.
However, in our current study, the pro-apoptotic
activity of anti-CTC1 in tumor cells makes it difficult to
establish a stable clone to constitutively suppress CTC1.
Therefore, this hindered our study of a clear-cut mechanism of CTC1
in response to radiation. In the future, we may use a
Tet-on/Tet-off inducible system in stable clones, which can
eliminate the problem of transfection efficiency, and may be a
powerful tool for further investigation of the underlying
mechanisms of CTC1 on radiation response in human melanoma cell
lines.
In conclusion, the cohabitation at telomeres of the
CST and shelterin components adds to the complexity of telomere
biology. By establishing a radiosensitive-radioresistant human
melanoma cell model, we found that CTC1 enhances the
radioresistance of human melanoma cells by inhibiting telomere
shortening and apoptosis. High levels of CTC1 seem to be directly
associated with radioresistance in human melanoma cell lines as an
independent predictive factor. Likewise, further studies on the
role of CTC1 in regulating radiosensitivity in other
radiosensitive-radioresistant cancer cell lines are another avenue
to pursue.
Acknowledgements
We sincerely appreciate Professor Xucheng Jiang
(Department of Pathology, Shanghai Jiaotong University, Shanghai,
China) for editing our manuscript linguistically.
References
|
1
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Little JB, Hahn GM, Frindel E and Tubiana
M: Repair of potentially lethal radiation damage in vitro and in
vivo. Radiology. 106:689–694. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jenrette JM: Malignant melanoma: the role
of radiation therapy revisited. Semin Oncol. 23:759–762.
1996.PubMed/NCBI
|
|
5
|
Stevens G and McKay MJ: Dispelling the
myths surrounding radiotherapy for treatment of cutaneous melanoma.
Lancet Oncol. 7:575–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rofstad EK: Radiation biology of malignant
melanoma. Acta Radiol Oncol. 25:1–10. 1986. View Article : Google Scholar
|
|
7
|
Overgaard J, Overgaard M, Hansen PV and
von der Maase H: Some factors of importance in the radiation
treatment of malignant melanoma. Radiother Oncol. 5:183–192. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan N, Khan MK, Almasan A, Singh AD and
Macklis R: The evolving role of radiation therapy in the management
of malignant melanoma. Int J Radiat Oncol Biol Phys. 80:645–654.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arienti C, Tesei A, Carloni S, et al: SLUG
silencing increases radiosensitivity of melanoma cells in vitro.
Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Lange T: Shelterin: the protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005.PubMed/NCBI
|
|
11
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baumann P and Price C: Pot1 and telomere
maintenance. FEBS Lett. 584:3779–3784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F and Lei M: Human telomere POT1-TPP1
complex and its role in telomerase activity regulation. Methods Mol
Biol. 735:173–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyake Y, Nakamura M, Nabetani A, et al:
RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded
DNA and protects telomeres independently of the Pot1 pathway. Mol
Cell. 36:193–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song X, Leehy K, Warrington RT, Lamb JC,
Surovtseva YV and Shippen DE: STN1 protects chromosome ends in
Arabidopsis thaliana. Proc Natl Acad Sci USA.
105:19815–19820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Price CM, Boltz KA, Chaiken MF, Stewart
JA, Beilstein MA and Shippen DE: Evolution of CST function in
telomere maintenance. Cell Cycle. 9:3157–3165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangino M, Hwang SJ, Spector TD, et al:
Genome-wide meta-analysis points to CTC1 and ZNF676 as genes
regulating telomere homeostasis in humans. Hum Mol Genet.
21:5385–5394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surovtseva YV, Churikov D, Boltz KA, et
al: Conserved telomere maintenance component 1 interacts with STN1
and maintains chromosome ends in higher eukaryotes. Mol Cell.
36:207–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen LY, Redon S and Lingner J: The human
CST complex is a terminator of telomerase activity. Nature.
488:540–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ning S, Shui C, Khan WB, Benson W, Lacey
DL and Knox SJ: Effects of keratinocyte growth factor on the
proliferation and radiation survival of human squamous cell
carcinoma cell lines in vitro and in vivo. Int J Radiat Oncol Biol
Phys. 40:177–187. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cook PJ, Ju BG, Telese F, Wang X, Glass CK
and Rosenfeld MG: Tyrosine dephosphorylation of H2AX modulates
apoptosis and survival decisions. Nature. 458:591–596. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campisi J, Kim SH, Lim CS and Rubio M:
Cellular senescence, cancer and aging: the telomere connection. Exp
Gerontol. 36:1619–1637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith CD and Blackburn EH: Uncapping and
deregulation of telomeres lead to detrimental cellular consequences
in yeast. J Cell Biol. 145:203–214. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maes L, Van Neste L, Van Damme K, et al:
Relation between telomerase activity, hTERT and telomere length for
intracranial tumours. Oncol Rep. 18:1571–1576. 2007.PubMed/NCBI
|
|
25
|
Castella M, Puerto S, Creus A, Marcos R
and Surralles J: Telomere length modulates human radiation
sensitivity in vitro. Toxicol Lett. 172:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang T, Zhou FX, Lei H, et al: Increased
expression of telomere-related proteins correlates with resistance
to radiation in human laryngeal cancer cell lines. Oncol Rep.
21:1505–1509. 2009.PubMed/NCBI
|
|
27
|
Ayouaz A, Raynaud C, Heride C, Revaud D
and Sabatier L: Telomeres: hallmarks of radiosensitivity.
Biochimie. 90:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou FX, Xiong J, Luo ZG, et al: cDNA
expression analysis of a human radiosensitive-radioresistant cell
line model identifies telomere function as a hallmark of
radioresistance. Radiat Res. 174:550–557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drissi R, Wu J, Hu Y, Bockhold C and Dome
JS: Telomere shortening alters the kinetics of the DNA damage
response after ionizing radiation in human cells. Cancer Prev Res
(Phila). 4:1973–1981. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berardinelli F, Nieri D, Sgura A,
Tanzarella C and Antoccia A: Telomere loss, not average telomere
length, confers radiosensitivity to TK6-irradiated cells. Mutat
Res. 740:13–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu P, Min JN, Wang Y, et al: CTC1 deletion
results in defective telomere replication, leading to catastrophic
telomere loss and stem cell exhaustion. EMBO J. 31:2309–2321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anderson BH, Kasher PR, Mayer J, et al:
Mutations in CTC1, encoding conserved telomere maintenance
component 1, cause Coats plus. Nat Genet. 44:338–342. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eriksson D and Stigbrand T:
Radiation-induced cell death mechanisms. Tumour Biol. 31:363–372.
2010. View Article : Google Scholar
|