Introduction
Keloids, a pathological response to cutaneous wound
healing, are characterized by the abnormal proliferation of
fibroblasts and the excessive deposition of collagen (1). Clinically, keloids represent a thick
tumor-like scar tissue that outgrows the original wound edges,
invades the adjacent normal derma and rarely regresses over time
(2). The effectiveness of the
numerous treatments for keloids, including excision and
intralesional corticosteroid injections, is still not satisfactory
due to their high recurrence rate (2,3).
Accumulating evidence suggests that a hypoxic
microenvironment is associated with keloids due to an abnormally
large number of occluded microvessels and that hypoxia plays a
crucial role in keloid pathogenesis (4,5).
Hypoxia has been found to increase the expression of vascular
endothelial growth factor (VEGF) in keloid fibroblasts (KFs)
(6). The level of
hypoxia-inducible factor-1α (HIF-1α) is consistently higher in
freshly biopsied keloid tissues than their associated normal skin
borders, which provides direct evidence of a local hypoxic state in
keloids (7). HIF-1, the major
transcription factor in response to hypoxia, is a heterodimeric
molecule of 2 subunits, HIF-1α and HIF-1β. HIF-1α has an
oxygen-dependent degradation domain and its expression and activity
are regulated by the cellular oxygen concentration, while HIF-1β is
constitutively expressed. HIF-1α binds to DNA on hypoxia response
elements (HREs) in promoters of more than 60 target genes, such as
VEGF and matrix metalloproteinases (MMPs), many involved in keloid
formation (8,9).
Periostin, a secreted extracellular matrix (ECM)
protein, was initially identified in MC3TC-E1 mouse osteoblasts as
a putative bone adhesion molecule (10). It was found to be upregulated in
cutaneous wound healing, cutaneous fibrosis and tumor progression
and is involved in cell survival, differentiation, metastasis and
ECM remodeling (11,12). Previously, we used suppression
subtractive hybridization and found the upregulated expression of
periostin among genes differentially expressed between keloids and
hypertrophic scars (13). The
expression of periostin is increased in keloids as compared with
normal skin and may influence fibroblast proliferation (14).
Based on the above data, we hypothesized that
periostin may play an important role in hypoxia-stimulated keloid
pathogenesis. In the present study, we examined this hypothesis by
examining the expression of periostin under hypoxic conditions (2%
O2), as well as the association between periostin and
HIF-1α. Furthermore, we investigated the effects of periostin on
hypoxia-stimulated KF bioactivity in terms of proliferation, cell
cycle, apoptosis, collagen synthesis, migration and invasion; in
addition, we aimed to identify the regulatory pathways
involved.
Materials and methods
All experiments in this study were performed in
compliance with the regulations of the Medical Ethics Committee of
Peking University Third Hospital, Beijing, China.
Primary cell culture and treatment
KFs were isolated from discarded keloid tissues of
patients who were undergoing surgery (n=6). The characteristics of
the study subjects are shown in Table
I. All keloid tissue originated from untreated primary lesions.
The KFs were maintained in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA) containing 100 U/ml penicillin
and 100 U/ml streptomycin (Invitrogen, Carlsbad, CA, USA). KFs at
passages 4–8 were used for the following experiments. For hypoxic
exposure, fibroblasts were placed in a modulator incubator in an
atmosphere of 93% N2, 5% CO2 and 2%
O2. Normoxic conditions were defined as 20%
O2. Recombinant human periostin (rhPN; BioVendor, Brno,
Czech) and echinomycin (Calbiochem, La Jolla, CA, USA) were applied
at 10 ng/ml and 10 nM, respectively. For inhibition experiments,
the cells were pre-treated with 30 μM LY294002 (inhibitor of PI3K;
Cell Signaling Technology, Beverly, MA, USA) or 20 μg/ml each of
αvβ3 or αvβ5-integrin antibody (Millipore, Billerica, MA, USA) for
1 h at 37°C.
 | Table ICharacteristics of patients enrolled
in this study. |
Table I
Characteristics of patients enrolled
in this study.
| Patient | Age (years) | Gender | Biopsy site | Duration
(years) |
|---|
| K1 | 24 | Female | Chest | 2 |
| K2 | 33 | Female | Earlobe | 2 |
| K3 | 50 | Male | Earlobe | 4 |
| K4 | 25 | Female | Chest | 1 |
| K5 | 34 | Female | Chest | 1 |
| K6 | 8 | Male | Instep | 0.5 |
Reverse transcription (RT)-polymerase
chain reaction (PCR) and quantitative PCR
Total RNA was extracted using TRIzol reagent
(Tiangen, Beijing, China), and cDNA was acquired using reverse
transcriptase (Thermo Scientific, Waltham, MA, USA). Quantitative
PCR was carried out using the Maxima SYBR-Green I qPCR Master Mix
(Fermentas, Waltham, MA, USA) with a qPCR/Real-Time PCR Instrument
(Bio-Rad, Hercules, CA, USA). cDNA was amplified with the primer
sequences for periostin (forward, 5′-tcattggaaaaggatttgaacc-3′ and
reverse, 5′-caggtgtgtctgctggatagag-3′; 189 bp); collagen I
(forward, 5′-ttctgtacgcaggtgattgg-3′ and reverse, 5′-catgttcag
ctttgtggacc-3′; 129 bp) and β-actin (forward, 5′-agcgagcat
cccccaaagtt-3′ and reverse, 5′-gggcacgaaggctcatcatt-3′; 285 bp).
For quantification, target gene expression was normalized to that
of β-actin in each sample. Data analysis was carried out using the
ΔΔCt method.
Western blot analysis
The KFs were washed twice with ice-cold
phosphate-buffered saline (PBS), then harvested with lysis buffer
containing phosphatase and protease inhibitors. The protein
concentration was quantified using the BCA Protein Assay kit
(CWbiotech, Beijing, China). Proteins in lysates were separated by
SDS-PAGE, then transferred onto nitrocellulose membranes (Applygen
Technologies, Inc., Beijing, China), and incubated with primary
antibodies to rabbit anti-periostin (1:1,000 dilution), rabbit
anti-HIF1α (1:500 dilution), rabbit anti-collagen I (1:1,000
dilution), rabbit anti-Akt (1:1,000 dilution) or anti-phospho-Akt
(1:1,000 dilution) (all from Abcam, Cambridge, MA, USA), or mouse
anti-β-actin (ZsBio, Beijing, China), then corresponding IgG
secondary antibodies (1:10,000 dilution; LI-COR Biosciences,
Lincoln, NE, USA). The membranes were scanned using the Odyssey
Infrared Imaging System (LI-COR Biosciences).
ELISA
Secreted periostin was measured with use of an ELISA
kit (R&D Systems, Minneapolis, MN, USA). Briefly, 96-well
microplates were coated overnight with capture antibody, washed 3
times with wash buffer, then blocked with reagent diluent for 1 h.
An amount of 100 μl of all standards and cell medium samples was
added to the 96-well plate for incubation for 2 h. After 2 h of
incubation with detection antibody, a 20-min incubation with a
working dilution of horseradish peroxidase-conjugated streptavidin
and a 20-min incubation with substrate solution in a
light-resistant container, stop solution was added to each well.
The absorbance of periostin was calculated by measuring the
absorbance at 450 nm, correcting for plate artifact at 570 nm and
utilizing a log-transformed standard curve.
Lentiviral-mediated stable gene knockdown
in KFs
Short hairpin RNA (shRNA) was prepared and a
vector-based shRNA plasmid was constructed. Briefly, one 21-nt
siRNA duplex (aaactgaaggacccacactaa) against the human periostin
consensus coding sequence (GenBank accession no. NM006475) or one
non-silencing luciferase sequence was inserted into an shRNA
oligonucleotide template, which was subcloned into the pGCsi shRNA
expression vector encoding green fluorescent protein (GFP;
GeneChem, Shanghai, China). The lentivirus harboring shRNA
periostin or non-silencing shRNA and the GFP gene were transfected
into the cells in serum-free DMEM for 10 h. The medium was then
replaced with DMEM and 10% FBS. The GFP-positive cells were
separated by flow cytometry. The KFs were divided into 3 groups:
cells transfected with PBS as a normal control, non-silencing shRNA
as a negative control (NC group), or shRNA periostin (sh-pn group).
Periostin mRNA and intracellular protein levels were reduced by 64
and 60%, respectively, as compared with the NC cells, with no
difference between the normal and NC cells.
Cell proliferation assay
Cells at 70% confluence were serum-starved in 0.1%
FBS for 24 h for synchronization, then plated at 5×103
cells/well in 96-well plates under hypoxic conditions. The cells
were incubated with fresh DMEM containing 10% FBS. The cell
counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) was used to
determine KF viability at various time points. For CCK-8 assay, the
cells were incubated with 10 μl CCK-8 solution for 1 h and the
absorbance was measured at 450 nm.
Flow cytometry
For cell cycle analysis, KFs transfected with or
without lentivirus were cultured in 60-mm dishes under hypoxic
conditions for 48 h, then harvested in PBS. The cell suspension was
stained with propidium iodide (PI; eBioscience, San Diego, CA, USA)
and the cell cycle was analyzed using the FACSCalibur system (BD
Biosciences, San Jose, CA, USA). Apoptosis was measured using an
Annexin V apoptosis detection kit with APC (eBioscience). The cells
were serum-starved for 24 h, then complete medium was added.
Following exposure to hypoxia, the cells stained with
APC-conjugated Annexin V and PI were pelleted and analyzed
according to the manufacturer’s instructions, and data were
analyzed using FlowJo software (Tree Star, Inc., Ashland, OR,
USA).
Migration and invasion assay
Migration and invasion in vitro were measured
in a Transwell chamber. An amount of 100 μl Matrigel (BD
Biosciences) was coated onto the upper chambers of the Transwell
inserts (6.5 mm, 8 μm pore size; Millipore) for the invasion assay
but not for the migration assay. Following pre-incubation with
serum-free medium for 12 h, 1×104 cells (migration
assay) or 5×104 cells (invasion assay) per well were
seeded for 24 h in 0.1% FBS medium into the upper chamber, and the
lower chamber contained 10% FBS medium. The cells were fixed in
paraformaldehyde for 15 min, and the cells at the bottom of the
membrane were stained with crystal violet for 30 min. Cells in
migration and invasion assay stained with crystal violet from 6
randomly selected fields were counted under a microscope (x100
magnification) and the mean for each chamber was determined.
Statistical analysis
Statistical analysis was carried out using SPSS 12.0
software (SPSS Inc., Chicago, IL, USA). Each experiment was
performed in triplicate. Quantitative data are presented as the
means ± standard deviation (SD). One-way ANOVA followed by
Scheffe’s post-hoc test were used to compare multiple groups.
Values of P<0.05 were considered to indicate statistically
significant differences.
Results
Increased expression of periostin in KFs
under hypoxic conditions
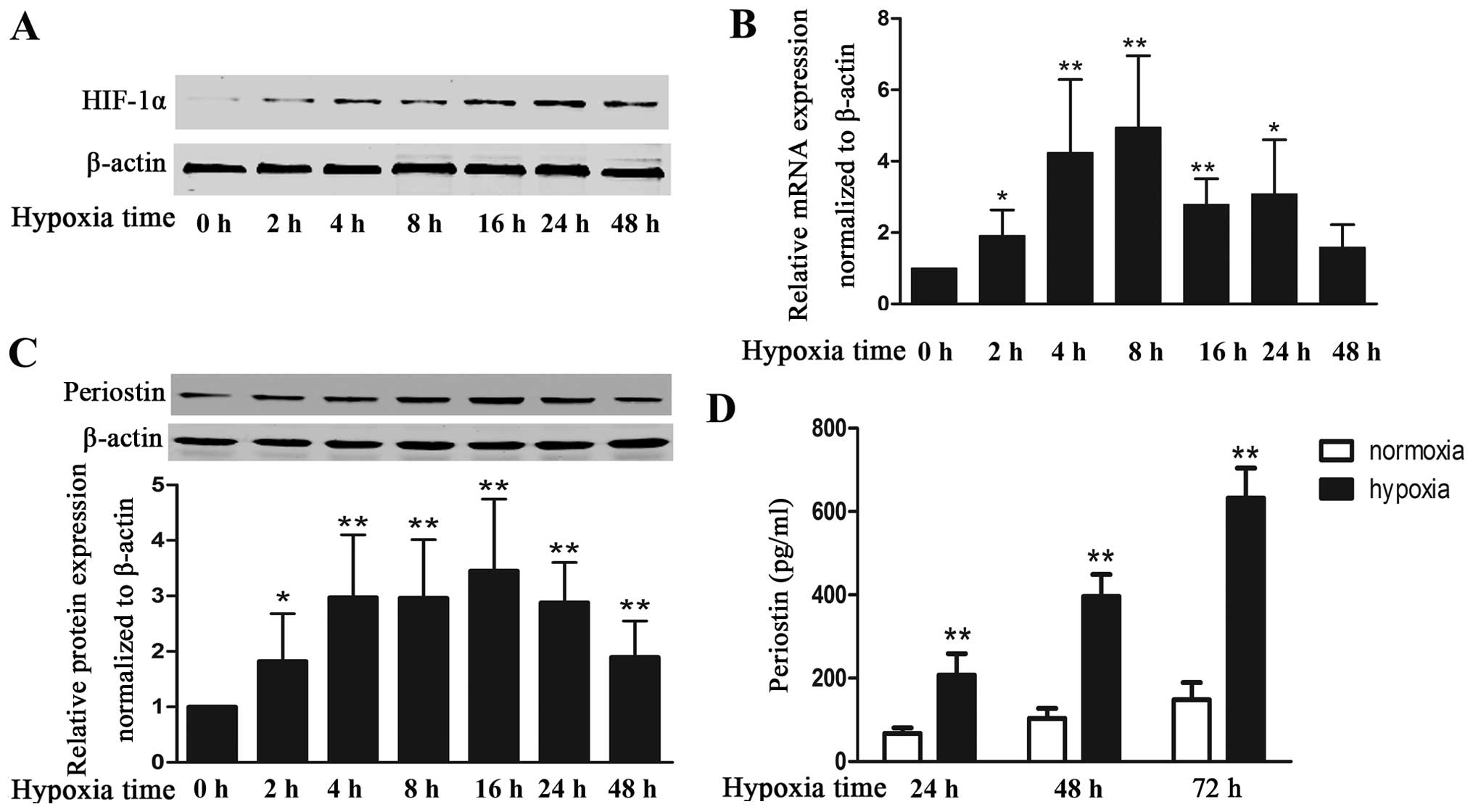
To examine the effects of hypoxia on the expression
of periostin in KFs, we cultured KFs under hypoxic conditions and
measured the HIF-1α and periostin levels. As expected, exposure to
hypoxia markedly increased the protein expression of HIF-1α, which
indicates that KFs exist within a hypoxic environment (Fig. 1A). Hypoxia also increased the
expression of periostin; mRNA level (4.9-fold increase) and the
intracellular protein level (3.1-fold increase) peaked at 8 and 16
h, respectively (Fig. 1B and C).
Periostin mRNA and intracellular protein levels were maintained at
relatively high levels even with 48 h of exposure to hypoxia. The
secreted protein in the cell supernatant under hypoxic conditions
was increased by 3-, 3.8- and 4.2-fold at 24, 48 and 72 h as
compared with cells cultured under normoxic conditions (Fig. 1D).
Upregulation of periostin expression
induced by hypoxia is HIF-1α-dependent
To investigate the association between periostin and
HIF-1α in the KFs, we incubated cultured KFs under hypoxic or
normoxic conditions with 10 nM echinomycin, a small-molecule
cyclic-peptide antibiotic known to specifically inhibit HIF
transcriptional activity by suppressing its binding to the HRE site
of target genes (15). The
periostin mRNA level under normoxic conditions was not affected by
echinomycin as compared with the controls (no echinomycin). Hypoxia
enhanced the periostin mRNA expression, and echinomycin abolished
this effect (Fig. 2A). The levels
of intracellular protein (Fig.
2B) and secreted protein in the cell supernatant (Fig. 2C) showed a similar effect.
Effects of periostin on
hypoxia-stimulated KF proliferation, cell cycle distribution and
apoptosis
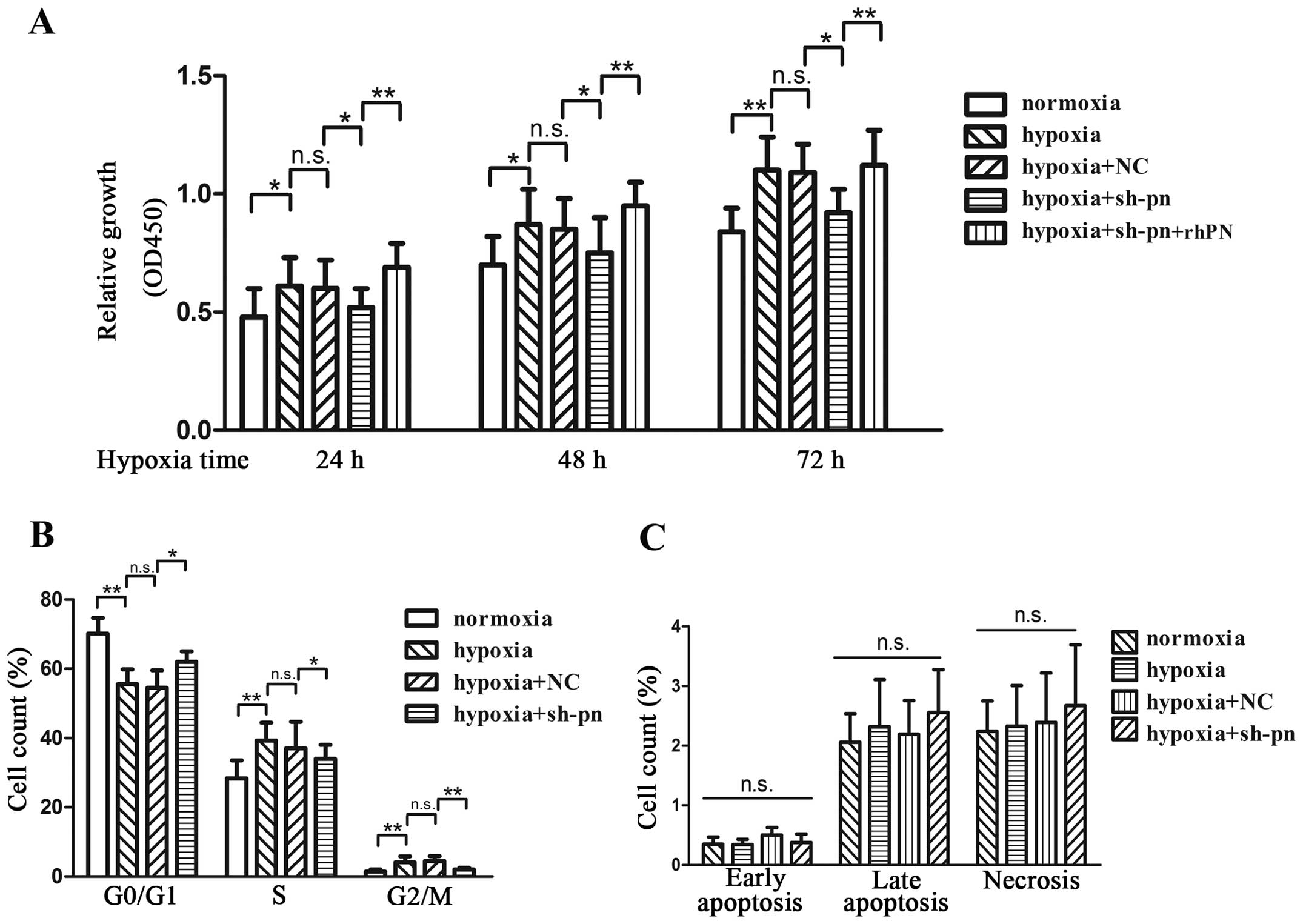
Hypoxia increased the proliferation of KFs by 26, 19
and 29% at 24, 48 and 72 h, respectively (Fig. 3A). To further examine the effects
of periostin on KF proliferation, we transfected the KFs with shRNA
against periostin or control shRNA. Fluorescence microscopy,
quantitative PCR and western blot analyses were performed to ensure
the efficiency of transfection before each experiment (Fig. 4). The knockdown of periostin
significantly decreased cell proliferation by 13, 11 and 16% at 24,
48 and 72 h, respectively, which was reversed by incubation with
rhPN (Fig. 3A). The KFs cultured
under hypoxic conditions showed an increased number of cells in the
G2/M phase and a decreased number of cells in the G0/G1 phase
(Fig. 3B). The knockdown of
periostin by shRNA resulted in G1/S cell cycle arrest, which was
reversed by rhPN treatment. We also evaluated the anti-apoptotic
role of periostin in KFs by Annexin V treatment (Fig. 3C). Neither hypoxia nor the
knockdown of periostin affected the apoptosis of KFs.
Effects of periostin on
hypoxia-stimulated collagen synthesis, migration and invasion of
KFs
Keloids exhibit aberrant, exuberant collagen
synthesis and deposition (16).
Type I collagen at both the mRNA (Fig. 5A) and protein (Fig. 5B) level was increased in the KFs
cultured under hypoxic conditions as compared with those cultured
under normoxic conditions. The inhibition of periostin by shRNA
decreased the expression of type I collagen, and this effect was
reversed by exogenous rhPN treatment.
The notable migratory activity of KFs and the
invasive nature of keloids are a hallmark in keloids (17). In our study, we found that a
greater number of KFs migrated to and invaded the lower compartment
of Transwell chambers under hypoxic conditions as compared with
normoxic conditions (Fig. 6). The
knockdown of periostin decreased the number of KFs migrating and
invading, which was reversed by rhPN treatment.
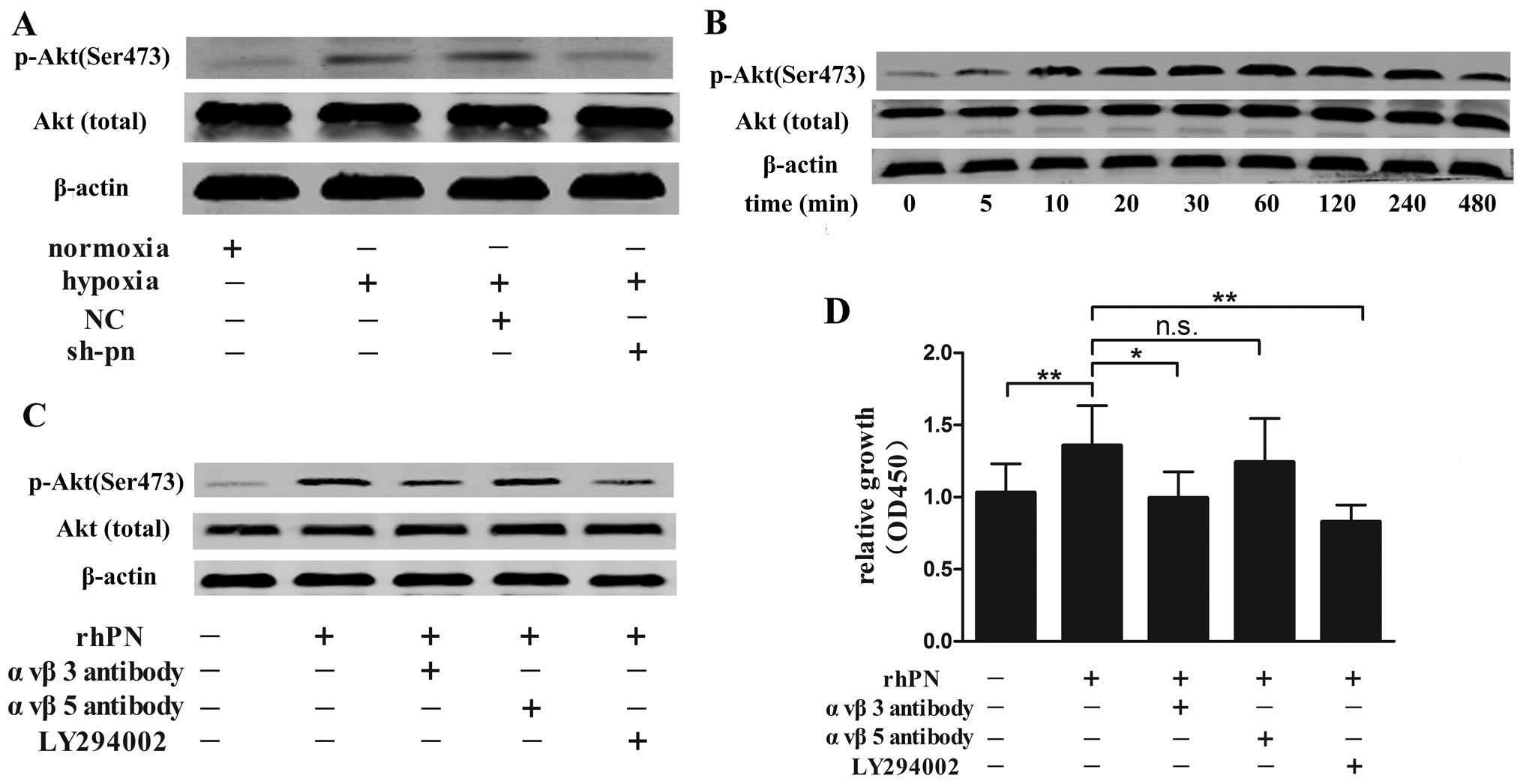
Role of integrin-Akt pathway in
periostin-induced proliferation of KFs
The PI3K/Akt pathway is one of most potent
proliferative signaling pathways in human fibroblasts and is
involved in keloid formation (18). In our study, we found that hypoxia
increased the level of phosphorylated Akt, and the phosphorylated
Akt level was lower in the KFs transfected with shRNA targeting
periostin than in the normal and negattive control-transfected
(non-silenced) KFs under hypoxic conditions (Fig. 7A). Akt phosphorylation was
increased 5 min following rhPN stimulation, with a maximal increase
observed at 240 min, and then decreased gradually for up to 12 h
but remained at a higher level compared with the control (no rhPN
stimulation; Fig. 7B). Periostin
is a ligand for selected integrins (19); thus, we pre-incubated the KFs with
αvβ3- or αvβ5-integrin antibody or the PI3K inhibitor, LY294002,
prior to exposure to hypoxia. The αvβ3 antibody and LY294002 but
not αvβ5 antibody decreased Akt phosphorylation (Fig. 7C) and the proliferation of the KFs
(Fig. 7D) as compared with the
controls (untreated cells).
Discussion
Normal wound healing has stop signals to end the
course of repair when the defective dermis is repaired and
epithelialization is complete. When a microenvironment or genetic
change results in futile or ineffective signals, the repair process
continues and results in excessive scar tissue (20). Keloid scar tissue most frustrating
to physicians due to the lack of ineffective therapy. Keloids,
generally considered benign hyperplastic dermal tumors, exhibit
abnormalities in cell proliferation, migration and invasion, they
escape from apoptosis, and are disproportionately accumulated in
the ECM (2). Keloids thrive in a
hypoxic microenvironment, In this study, we reported that the
periostin level was increased in KFs in response to a hypoxic
environment, and that its expression was regulated by HIF-1α. In
addition, the upregulated periostin expression promoted
hypoxia-stimulated KF bioactivity in terms of cell proliferation,
collagen synthesis, migration and invasion, and altered the cell
cycle and activated the αvβ3 integrin-PI3K/Akt signaling pathway.
The inhibition of periostin by shRNA decreased the
hypoxia-stimulated KF bioactivity. Thus, periostin may be a target
for the treatment of keloids.
A relative hypoxic state exists within the zone of
injury in early-stage healing wounds and initiates wound repair by
inducing fibroblast proliferation and angiogenesis (21). Kisher (22) hypothesized that keloids, resulting
from excessive repair, may be an aberration of the hypoxic
microenvironment. The hypoxic microenvironment in keloids was
confirmed by observing microvascular occlusion by a transmission
electron microscope and HIF-1α expression in keloid tissue
(23). In this study, cultured
KFs exposed to a hypoxic environment showed a marked time-dependent
increase in both the mRNA and protein levels of periostin. The
result was consistent with previous findings that hypoxic stress
increases the expression of periostin in human periodontal ligament
cells and rat pulmonary arterial smooth muscle cells (24,25). Furthermore, we found that the
HIF-1α level was upregulated along with the periostin level and
increased its expression. HIF-1α regulates the expression of
ECM-associated proteins. Deschene et al (26) showed that the secretion of
collagen and MMP2 by fibroblasts was mediated by the
transcriptional regulatory activity of HIF. In addition, HIF-1α has
been shown to increase the expression of plasminogen activator
inhibitor 1 at both the transcriptional and post-transcriptional
level in KFs (7). Thus, hypoxia
may contribute to keloid formation by activating HIF-1α and its
downstream proteins, including periostin, to remodel the ECM.
Although keloids are a benign disorder, they invade
the adjacent normal derma and KFs display a bioactivity similar to
that of tumors (27). Hypoxia is
thought to promote the growth and invasion of tumors (28). In this study, we found that
hypoxia stimulated the proliferation, mobility and collagen
production of KFs, which provides additional evidence of the
involvement of hypoxia in keloid pathogenesis. Fibroblasts are
responsible for the synthesis, deposition and remodeling of the
ECM, and the release and activation of growth factors and cytokines
by KFs are essential to keloid formation, in which the roles of
VEGF, insulin-like growth factor and transforming growth factor-β
(TGF-β) have been identified (6,29,30). TGF-β, the most potent profibrotic
cytokine, increases the proliferation of and collagen synthesis in
KFs (31). In this study, we
found that the inhibition of periostin by shRNA decreased
hypoxia-stimulated cell proliferation, migration, invasion and
collagen synthesis; these effects are similar to those of TGF-β
(32). Periostin has a similar
structure as βigH3, which also retains the 4 conserved fasciclin I
domains and conserved motifs to bind to the integrin receptor
family (33), and periostin is
upregulated by TGF-β1 in lung fibroblast cells (34). Thus, the association between
periostin and TGF-β requires further study.
Apoptosis mediates the transition from granulated
tissue to scar atrophy (35).
Periostin exerts an anti-apoptosis effect in many tumors under
hypoxic conditions (36).
However, in this study, we found that KF apoptosis showed no
significant difference between the NC group and the sh-pn group
under hypoxic conditions. We hypothesized that this is due to the
following reasons: i) in general, only severe and prolonged hypoxia
initiates apoptosis, whereas cells can adapt to and survive under
acute and mild hypoxia (37); the
hypoxic conditions used in our study (2% O2) may not be
sufficient to mimic the stress-induced hypoxic conditions of keloid
formation in vivo; ii) we only partially decreased the level
of periostin; the remaining periostin may prevent KFs from
undergoing apoptosis; and iii) in another study, we found that
various concentrations of periostin did not prevent the apoptosis
of fibroblasts under serum-free conditions (unpublished data); thus
periostin may have little effect on the regulation of apoptosis of
KFs.
Periostin has been studied as a ligand for certain
integrins, such as αvβ3 and αvβ5, and its downstream PI3K/Akt
signaling pathway has been well documented to be involved in
fibroblast cell proliferation, survival and migration (38). Our data showed that periostin can
significantly promote the phosphorylation of Akt in KFs.
Furthermore, αvβ3 integrin antibody and LY294002, but not αvβ5
antibody inhibited the periostin-stimulated Akt phosphorylation and
proliferation of KFs. Our results suggest that the upregulation of
periostin under hypoxic conditions affects the proliferation of KFs
by activating the αvβ3 integrin-PI3K/Akt signaling pathway.
Wound repair initiates a cascade of pathological
events, including inflammation, tissue formation and tissue
remodeling (39). Previously, we
(40), as well as others
(11) found that periostin was
located in the granulation tissue beneath the extended epidermal
wound edges and in the dermal-epidermal junctions and promoted
cutaneous wound healing by enhancing the proliferation and
migration of dermal fibroblasts. Periostin regulates myofibroblast
differentiation and collagen matrix contraction, and
periostin-deficient animal models exhibit delayed wound repair
(41). In addition, it affects
collagen production by fibroblasts following acute myocardial
infarction (42). The normal
regulation of periostin may be required throughout the repair
process, and the dysregulation of periostin may tip the balance
toward excessive repair, resulting in keloid formation and other
fibroproliferative disorders. Our results suggest that a hypoxic
microenvironment unduly prolongs the skin repair process due to the
overexpression of certain factors, including HIF-1α and periostin,
which may contribute to keloid formation.
Thus, we conclude that hypoxia initiates KF
hyperplasia, and that periostin may be a novel factor contributing
to the abnormal biological behaviour of KFs under hypoxic
conditions and to the progression of keloids. Our findings may
contribute to a better understanding of keloid pathogenesis and the
development of novel therapeutic approaches for keloids and other
fibroproliferative disorders.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30973126) and the Ministry
of Education doctoral foundation of the People’s Republic of China
(no. 20130001110095). We thank the teachers of the Medical Research
Center of Peking University Third Hospital for their excellent
technical assistance and Laura Smales for providing assistance with
the language.
References
|
1
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: a review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bran GM, Goessler UR, Hormann K, Riedel F
and Sadick H: Keloids: Current concepts of pathogenesis (Review).
Int J Mol Med. 24:283–293. 2009.PubMed/NCBI
|
|
3
|
Love PB and Kundu RV: Keloids: an update
on medical and surgical treatments. J Drugs Dermatol. 12:403–409.
2013.PubMed/NCBI
|
|
4
|
Zhang Q, Wu Y, Ann DK, et al: Mechanisms
of hypoxic regulation of plasminogen activator inhibitor-1 gene
expression in keloid fibroblasts. J Invest Dermatol. 121:1005–1012.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueda K, Yasuda Y, Furuya E and Oba S:
Inadequate blood supply persists in keloids. Scand J Plast Reconstr
Surg Hand Surg. 38:267–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinbrech DS, Mehrara BJ, Chau D, et al:
Hypoxia upregulates VEGF production in keloid fibroblasts. Ann
Plast Surg. 42:514–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami
CN and Le AD: Crosstalk of hypoxia-mediated signaling pathways in
upregulating plasminogen activator inhibitor-1 expression in keloid
fibroblasts. J Cell Physiol. 199:89–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahluwalia A and Tarnawski AS: Critical
role of hypoxia sensor - HIF-1alpha in VEGF gene activation.
Implications for angiogenesis and tissue injury healing. Curr Med
Chem. 19:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben-Yosef Y, Lahat N, Shapiro S, Bitterman
H and Miller A: Regulation of endothelial matrix
metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 90:784–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horiuchi K, Amizuka N, Takeshita S, et al:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou HM, Wang J, Elliott C, Wen W,
Hamilton DW and Conway SJ: Spatiotemporal expression of periostin
during skin development and incisional wound healing: lessons for
human fibrotic scar formation. J Cell Commun Signal. 4:99–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyutoku M, Taniyama Y, Katsuragi N, et al:
Role of periostin in cancer progression and metastasis: Inhibition
of breast cancer progression and metastasis by anti-periostin
antibody in a murine model. Int J Mol Med. 28:181–186.
2011.PubMed/NCBI
|
|
13
|
Wang Q, Nie FF, Zhao X and Qin ZL: The
expression of periostin in hyperplasic scars and the relations to
TGF-beta1 and its receptors. Zhonghua Zheng Xing Wai Ke Za Zhi.
23:229–232. 2007.(In Chinese).
|
|
14
|
Liu C, Song ZH and Qin ZL: Construction of
periostin shRNA vectors and their effects on the expression of
periostin in fibroblasts. Beijing Da Xue Xue Bao. 42:503–508.
2010.PubMed/NCBI
|
|
15
|
Kong D, Park EJ, Stephen AG, et al:
Echinomycin, a small-molecule inhibitor of hypoxia-inducible
factor-1 DNA-binding activity. Cancer Res. 65:9047–9055. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alster TS and Tanzi EL: Hypertrophic scars
and keloids: etiology and management. Am J Clin Dermatol.
4:235–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song J, Xu H, Lu Q, et al: Madecassoside
suppresses migration of fibroblasts from keloids: involvement of
p38 kinase and PI3K signaling pathways. Burns. 38:677–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Syed F, Sherris D, Paus R, Varmeh S,
Pandolfi PP and Bayat A: Keloid disease can be inhibited by
antagonizing excessive mTOR signaling with a novel dual TORC1/2
inhibitor. Am J Pathol. 181:1642–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
20
|
Chung KC, Disa JJ, Gosain AK, Kinney BM
and Rubin JP: Plastic Surgery. 1st edition. Elsevier; New York:
2009
|
|
21
|
Lokmic Z, Musyoka J, Hewitson TD and Darby
IA: Hypoxia and hypoxia signaling in tissue repair and fibrosis.
Int Rev Cell Mol Biol. 296:139–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kischer CW: The microvessels in
hypertrophic scars, keloids and related lesions: a review. J
Submicrosc Cytol Pathol. 24:281–296. 1992.PubMed/NCBI
|
|
23
|
Kischer CW, Thies AC and Chvapil M:
Perivascular myofibroblasts and microvascular occlusion in
hypertrophic scars and keloids. Hum Pathol. 13:819–824. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Yasue A, Fujihara S and Tanaka
E: PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK
pathway in human periodontal ligament cells. Arch Oral Biol.
57:52–59. 2012.PubMed/NCBI
|
|
25
|
Li P, Oparil S, Feng W and Chen YF:
Hypoxia-responsive growth factors upregulate periostin and
osteopontin expression via distinct signaling pathways in rat
pulmonary arterial smooth muscle cells. J Appl Physiol.
1985.97:1550–1558; discussion 1549, 2004.
|
|
26
|
Deschene K, Céleste C, Boerboom D and
Theoret CL: Hypoxia regulates the expression of extracellular
matrix associated proteins in equine dermal fibroblasts via HIF1. J
Dermatol Sci. 65:12–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vincent AS, Phan TT, Mukhopadhyay A, Lim
HY, Halliwell B and Wong KP: Human skin keloid fibroblasts display
bioenergetics of cancer cells. J Invest Dermatol. 128:702–709.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mucaj V, Shay JE and Simon MC: Effects of
hypoxia and HIFs on cancer metabolism. Int J Hematol. 95:464–470.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Zeng B, Yao H and Xu J: The effect
of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in
human keloid. Burns. 39:465–472. 2013.PubMed/NCBI
|
|
30
|
Bran GM, Goessler UR, Schardt C, Hormann
K, Riedel F and Sadick H: Effect of the abrogation of TGF-β1 by
antisense oligonucleotides on the expression of TGF-β-isoforms and
their receptors I and II in isolated fibroblasts from keloid scars.
Int J Mol Med. 25:915–921. 2010.
|
|
31
|
Wu CS, Wu PH, Fang AH and Lan CC: FK506
inhibits the enhancing effects of transforming growth factor
(TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid
fibroblasts: implication for new therapeutic approach. Br J
Dermatol. 167:532–541. 2012.
|
|
32
|
Bettinger DA, Yager DR, Diegelmann RF and
Cohen IK: The effect of TGF-beta on keloid fibroblast proliferation
and collagen synthesis. Plast Reconstr Surg. 98:827–833. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindsley A, Li W, Wang J, Maeda N, Rogers
R and Conway SJ: Comparison of the four mouse fasciclin-containing
genes expression patterns during valvuloseptal morphogenesis. Gene
Expr Patterns. 5:593–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naik PK, Bozyk PD, Bentley JK, et al:
Periostin promotes fibrosis and predicts progression in patients
with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol
Physiol. 303:L1046–L1056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang L, Wong YP, Cai YJ, Lung I, Leung CS
and Burd A: Low-dose 5-fluorouracil induces cell cycle G2 arrest
and apoptosis in keloid fibroblasts. Br J Dermatol. 163:1181–1185.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang G, Liu M, Ruan K, Song G, Mao Y and
Bao S: Upregulated expression of periostin by hypoxia in
non-small-cell lung cancer cells promotes cell survival via the
Akt/PKB pathway. Cancer Lett. 281:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bao S, Ouyang G, Bai X, et al: Periostin
potently promotes metastatic growth of colon cancer by augmenting
cell survival via the Akt/PKB pathway. Cancer Cell. 5:329–339.
2004. View Article : Google Scholar
|
|
39
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou JJ, Nie FF, Li BL, et al: The
expressions of periostin and the related factors during healing
process of full-thickness cutaneous wound in rat. Zhongguo Wei
Zhong Bing Ji Jiu Yi Xue. 24:334–337. 2012.(In Chinese).
|
|
41
|
Elliott CG, Wang J, Guo X, et al:
Periostin modulates myofibroblast differentiation during
full-thickness cutaneous wound repair. J Cell Sci. 125:121–132.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimazaki M, Nakamura K, Kii I, et al:
Periostin is essential for cardiac healing after acute myocardial
infarction. J Exp Med. 205:295–303. 2008. View Article : Google Scholar : PubMed/NCBI
|